Abstract
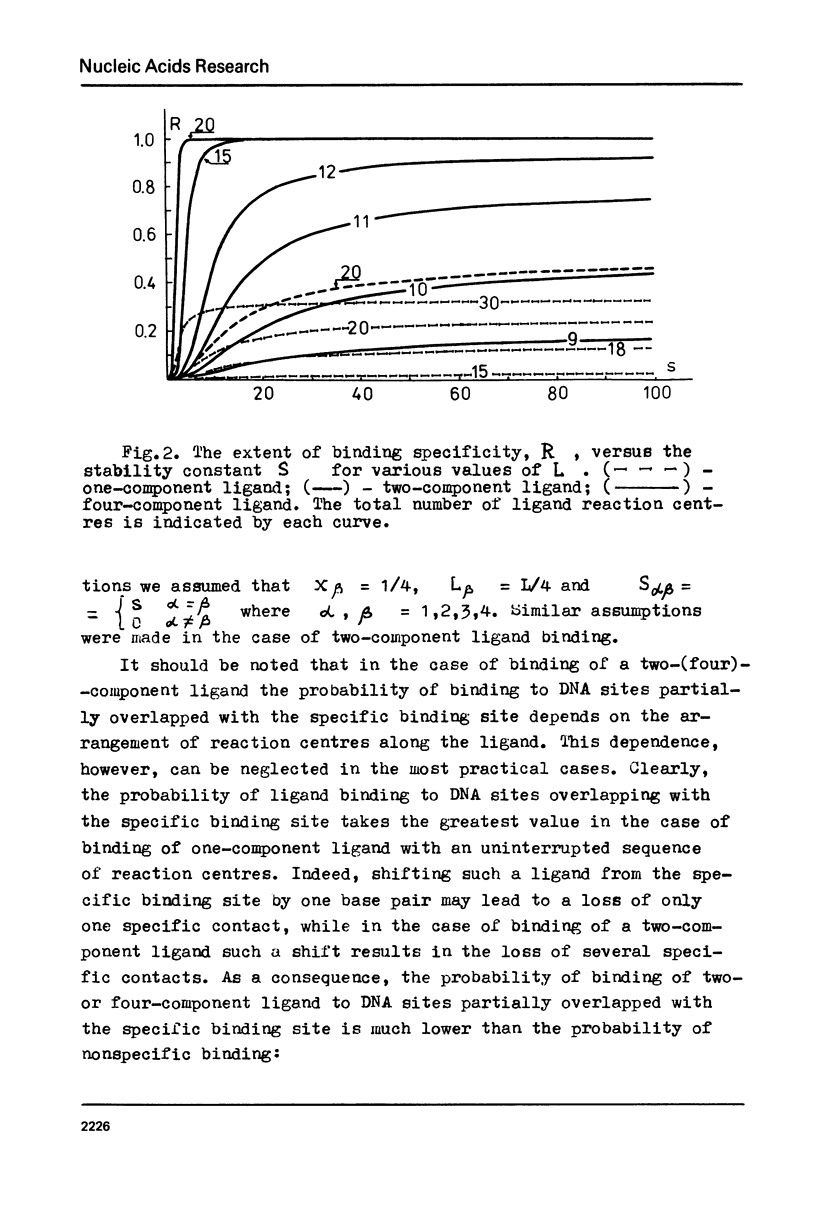
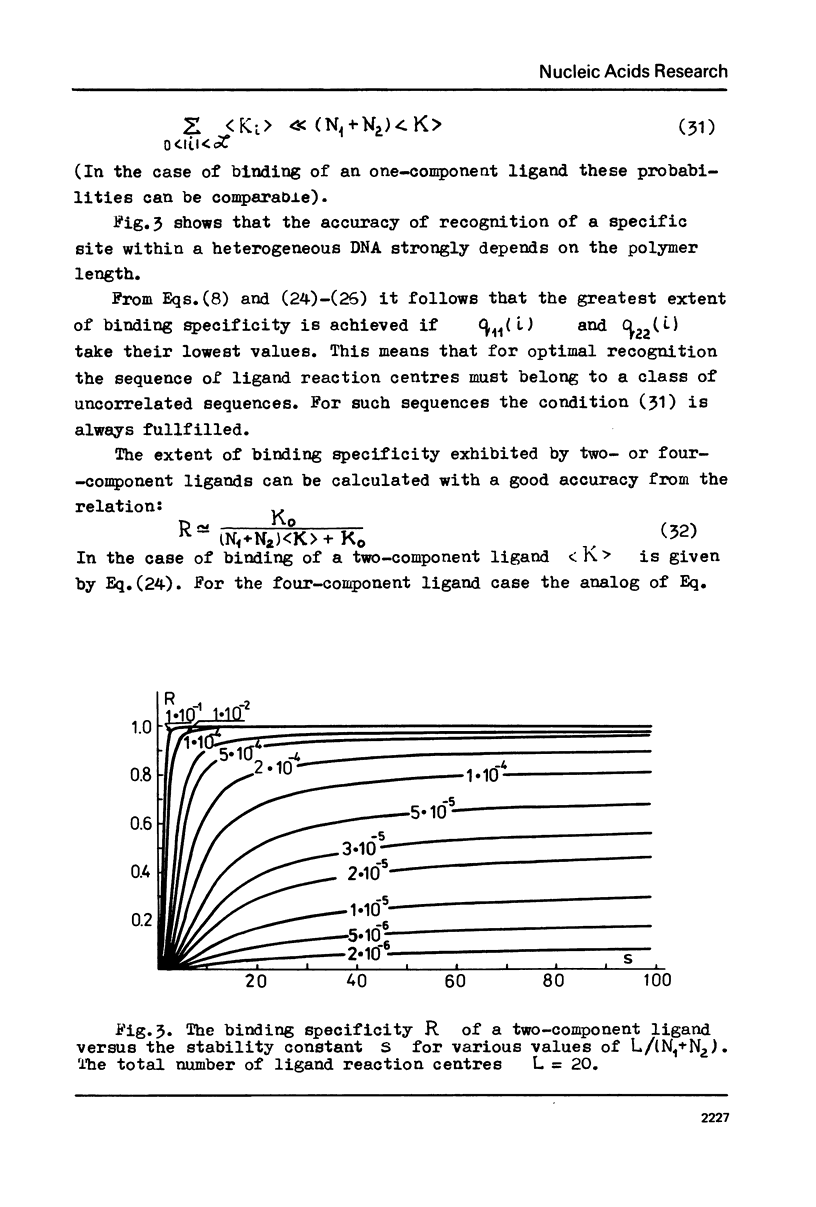
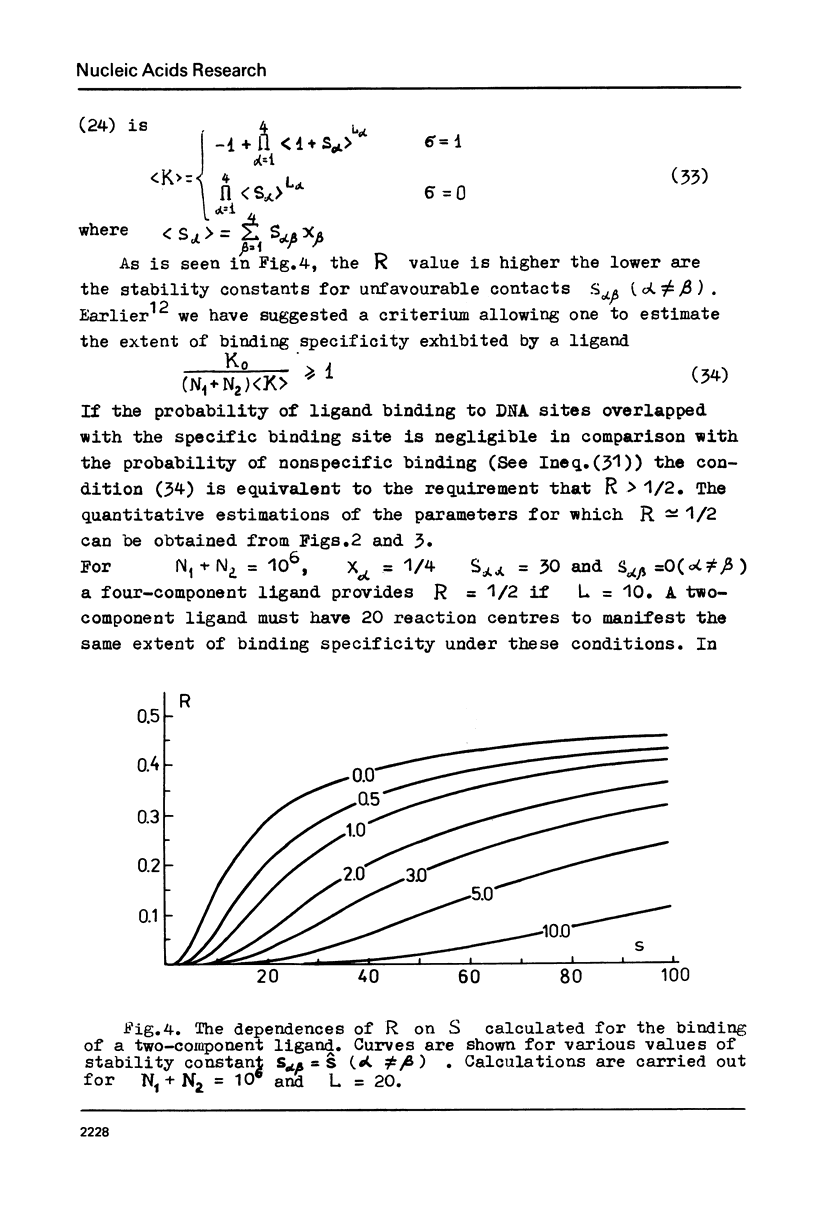
The specificity of regulatory protein binding to DNA is due to a complementarity between the sequence of reaction centres on the protein and the base pair sequence in the specific DNA site allowing the formation of a number of specific noncovalent bonds between the interacting entities. In the present communication the thermodynamic and kinetic aspects of these interactions are considered. The extent of binding specificity is shown to increase with an increase of the bond stability constants and with an increase in the number of ligand reaction centres. Kinetic analysis is carried out assuming that association process is very fast and that dissociation of nonspecific complexes is a rate-limiting step in the recognition of a specific binding site on DNA. The calculations show that a ligand can recognize its specific binding site on DNA within a reasonably limited time interval if the number of its reaction centres and the corresponding stability constants are strongly limited.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg O. G., Blomberg C. Association kinetics with coupled diffusion III. Ionic-strength dependence of the lac repressor-operator association. Biophys Chem. 1978 Sep;8(4):271–280. doi: 10.1016/0301-4622(78)80010-6. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Caruthers M. H. Binding of synthetic lactose operator DNAs to lactose represessors. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3292–3296. doi: 10.1073/pnas.74.8.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P. H., Poonian M. S., Nussbaum A. L., Tobias L., Garfin D. E., Boyer H. W., Goodman H. M. Restriction and modification of a self-complementary octanucleotide containing the EcoRI substrate. J Mol Biol. 1975 Dec 5;99(2):237–261. doi: 10.1016/s0022-2836(75)80143-4. [DOI] [PubMed] [Google Scholar]
- Gurskii G. V., Zasedatelev A. S. Tochnye sootnosheniia dlia rascheta sviazyvaniia reguliatornykh belkov i drugikh reshetochnykh ligandov na dvukhtiazhevykh polinukleotidakh. Biofizika. 1978 Sep-Oct;23(5):932–946. [PubMed] [Google Scholar]
- Gursky A. V., Tumanyan V. G., Zasedatelev A. S., Zhuze A. L., Grokhovsky S. L., Gottikh B. P. A code controlling specific binding of regulatory proteins to DNA. Mol Biol Rep. 1976 Apr;2(5):413–425. doi: 10.1007/BF00366264. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. II. The kinetics of the binding reaction. J Mol Biol. 1972 Sep 28;70(2):187–195. doi: 10.1016/0022-2836(72)90532-3. [DOI] [PubMed] [Google Scholar]
- Jobe A., Riggs A. D., Bourgeois S. Lac repressor-operator interaction. V. Characterization of super- and pseudo-wild-type repressors. J Mol Biol. 1972 Feb 28;64(1):181–199. doi: 10.1016/0022-2836(72)90328-2. [DOI] [PubMed] [Google Scholar]
- Krylov A. S., Grokhovskii S. L., Zasedatelev A. S., Zhuze A. L., Gurskii G. V. Kolichestvennaia otsenka vklada pirrokarboksamidnykh grupp antibiotika distamitsina A v obespechenie spetsifichnosti ego sviazyvaniia s AT-parami DNK. Dokl Akad Nauk SSSR. 1978;239(3):732–735. [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B. Lac repressor and lac operator. Prog Biophys Mol Biol. 1975;30(2-3):227–252. doi: 10.1016/0079-6107(76)90011-0. [DOI] [PubMed] [Google Scholar]
- Richter P. H., Eigen M. Diffusion controlled reaction rates in spheroidal geometry. Application to repressor--operator association and membrane bound enzymes. Biophys Chem. 1974 Oct;2(3):255–263. doi: 10.1016/0301-4622(74)80050-5. [DOI] [PubMed] [Google Scholar]
- Sobell H. M. Symmetry in protein-nucleic acid interaction and its genetic implications. Adv Genet. 1973;17:411–490. doi: 10.1016/s0065-2660(08)60175-3. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., McGhee J. D. DNA-protein interactions. Annu Rev Biochem. 1972;41(10):231–300. doi: 10.1146/annurev.bi.41.070172.001311. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Barkley M. D., Bourgeois S. Measurements of unwinding of lac operator by repressor. Nature. 1974 Sep 20;251(5472):247–249. doi: 10.1038/251247a0. [DOI] [PubMed] [Google Scholar]
- Yarus M. Recognition of nucleotide sequences. Annu Rev Biochem. 1969;38:841–880. doi: 10.1146/annurev.bi.38.070169.004205. [DOI] [PubMed] [Google Scholar]


