Abstract
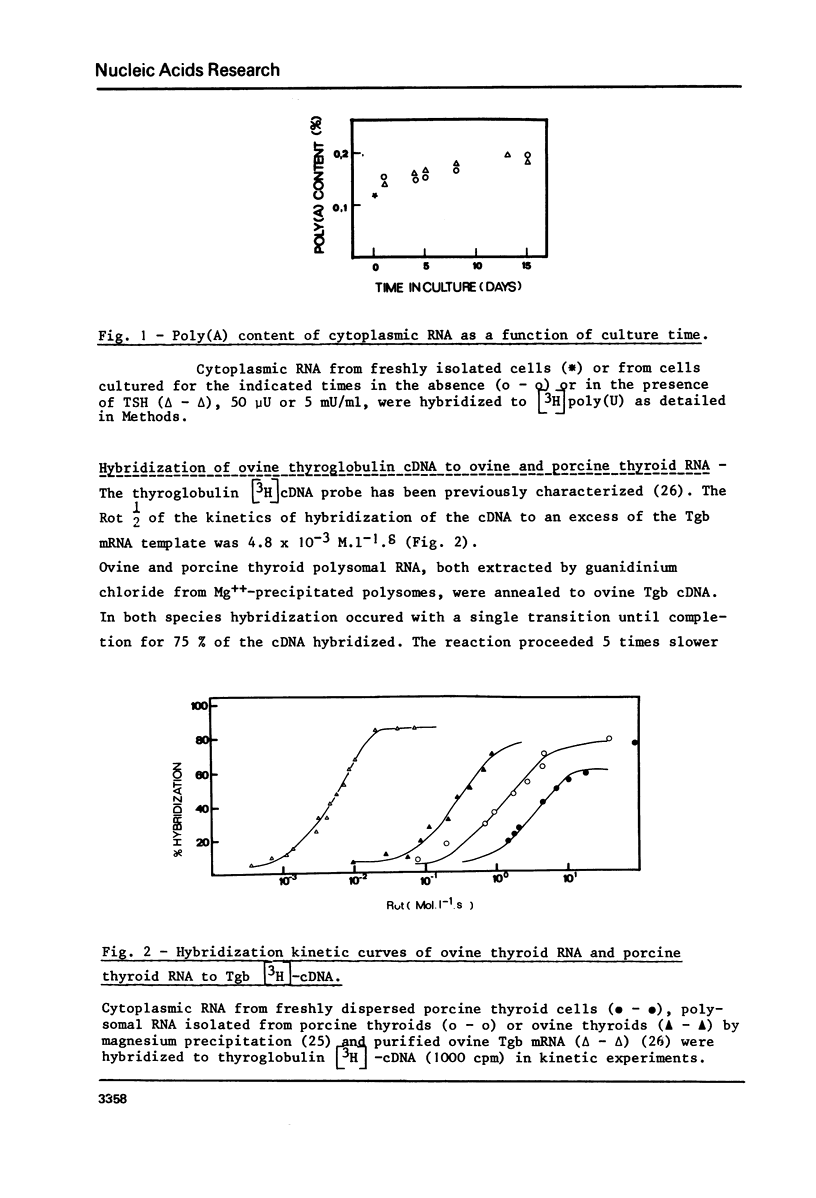
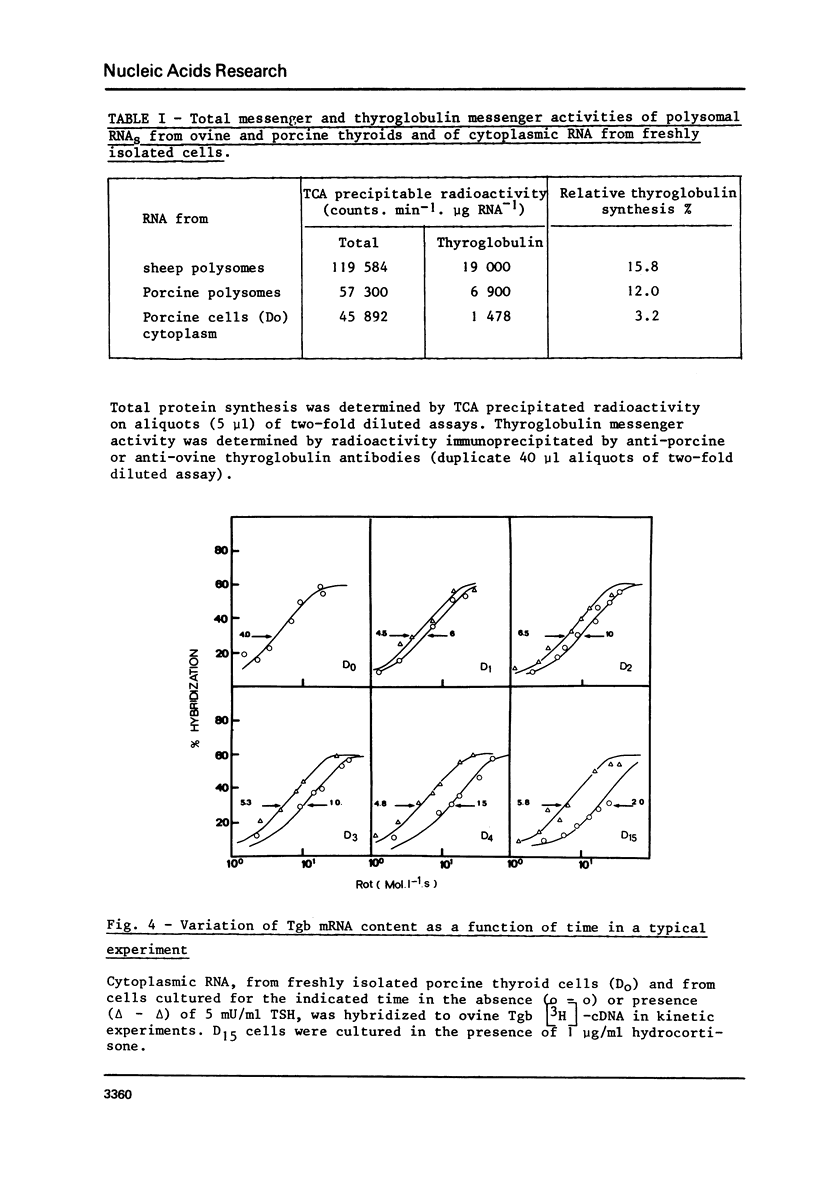
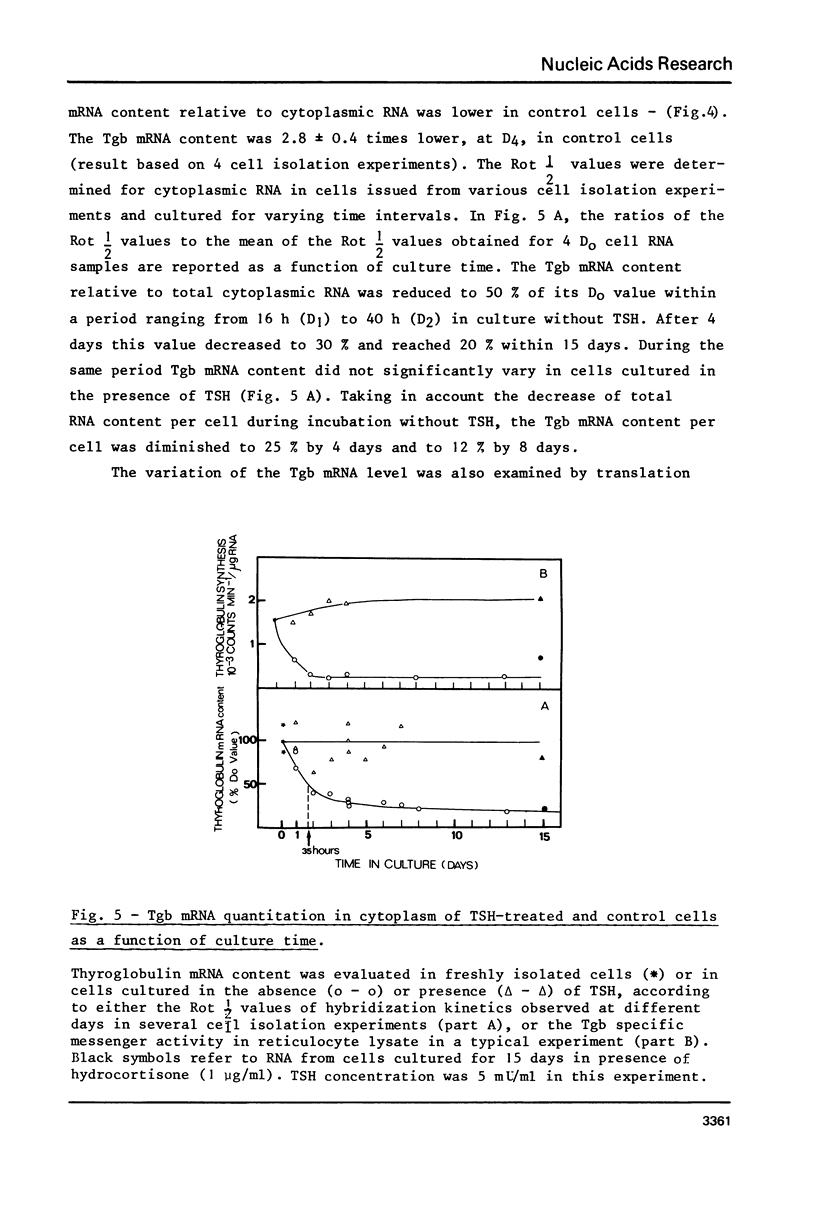
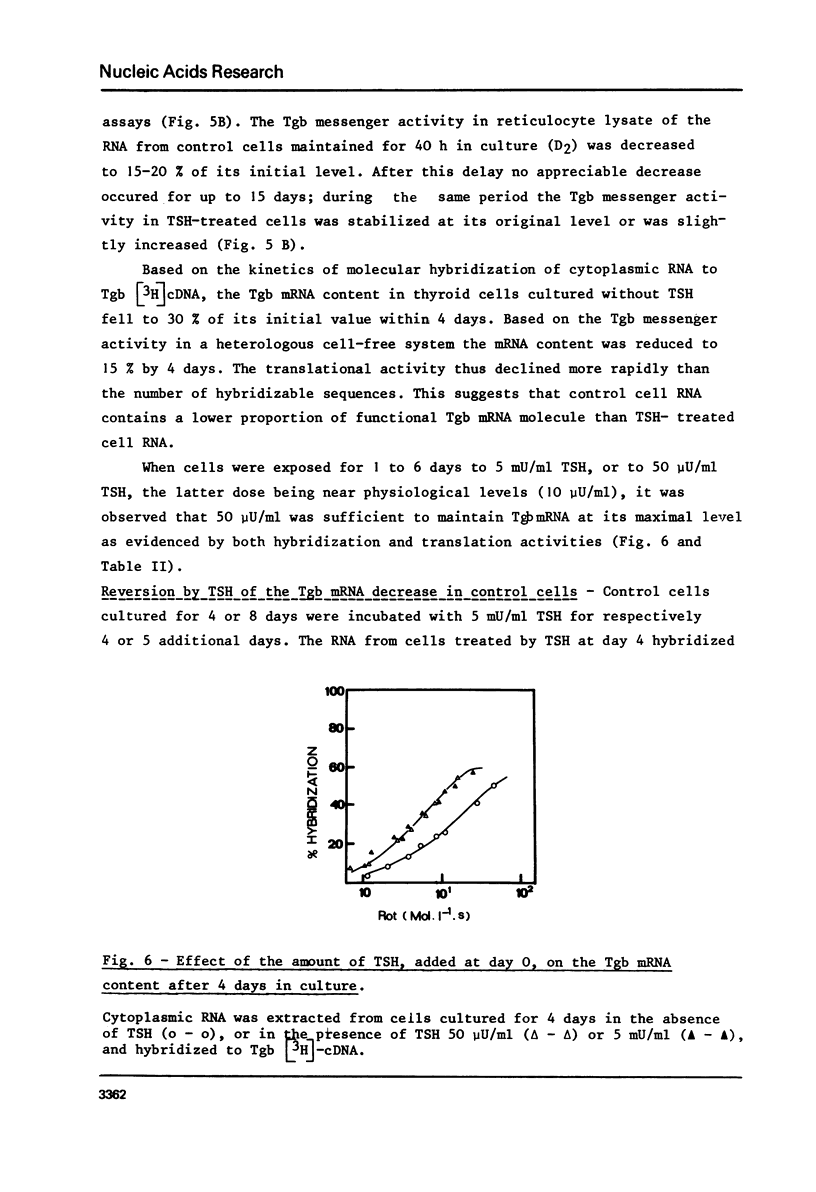
To examine the influence of thyrotropin (TSH) on the thyroglobulin (Tgb) mRNA content, the latter was evaluated in the cytoplasm of hog thyroid cells cultured in the absence (control cells) or presence of TSH. The Tgb mRNA levels were determined by, (i) kinetics of hybridization to sheep Tgb cDNA, (ii) capacity of coding for peptides immunologically related to Tgb in reticulocyte lysate. In cells cultured for 4 days in the absence of TSH, the content of Tgb mRNA sequences decreased to 30% of its initial value and the messenger activity to 15%. Conversely, TSH maintained the initial Tgb mRNA level in cells cultured in its presence, and TSH concentrations 50 micronU/ml or 5 mU/ml gave identical results. At each period tested poly (A) content was the same in TSH-treated and control cells. When TSH was added to media after 4 or 8 days culture without TSH, the Tgb mRNA level was partially restored. These results suggest that TSH exerts a positive control on Tgb gene expression through modulation of Tgb mRNA content of thyroid cells.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker H. J., Shapiro D. J. Rapid accumulation of vitellogenin messenger RNA during secondary estrogen stimulation of Xenopus laevis. J Biol Chem. 1978 Jul 10;253(13):4521–4524. [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Machen T. E., Bern H. A. Mouse mammary epithelial cells on floating collagen gels: transepithelial ion transport and effects of prolactin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):536–540. doi: 10.1073/pnas.76.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebath J., Chabaud O., Becarevic A., Cartouzou G., Lissitzky S. Thyroglobulin messenger ribonucleic acid translation in vitro. Eur J Biochem. 1977 Jul 15;77(2):243–252. doi: 10.1111/j.1432-1033.1977.tb11663.x. [DOI] [PubMed] [Google Scholar]
- Chebath J., Chabaud O., Bergé-Lefranc J. L., Cartouzou G., Lissitzky S. Molecular weight of the thyroglobulin messenger RNA of sheep thyroid gland. Biochem Biophys Res Commun. 1977 Nov 7;79(1):267–273. doi: 10.1016/0006-291x(77)90090-0. [DOI] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. F. Estrogen withdrawal in chick oviduct. Selective loss of high abundance classes of polyadenylated messenger RNA. Biochemistry. 1977 Jul 26;16(15):3433–3443. doi: 10.1021/bi00634a022. [DOI] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Dumont J. E. The action of thyrotropin on thyroid metabolism. Vitam Horm. 1971;29:287–412. doi: 10.1016/s0083-6729(08)60051-5. [DOI] [PubMed] [Google Scholar]
- Garcia Ruiz J. P., Ingram R., Hanson R. W. Changes in hepatic messenger RNA for phosphoenolpyruvate carboxykinase (GTP) during development. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4189–4193. doi: 10.1073/pnas.75.9.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D. Insulin receptors and the site of action of insulin. Life Sci. 1978 Dec 31;23(27-28):2639–2648. doi: 10.1016/0024-3205(78)90643-4. [DOI] [PubMed] [Google Scholar]
- Greif R. L., Eich E. F. Alkaline ribonuclease inhibitor in rat thyroid. Biochim Biophys Acta. 1972 Dec 29;286(2):350–359. doi: 10.1016/0304-4165(72)90270-x. [DOI] [PubMed] [Google Scholar]
- HALLINAN T., FLECK A., MUNRO H. N. Loss of ribonucleic acid into lipid solvents after acid precipitation. Biochim Biophys Acta. 1963 Jan 29;68:131–133. doi: 10.1016/0006-3002(63)90121-5. [DOI] [PubMed] [Google Scholar]
- Harding J. D., Rutter W. J. Rat pancreatic amylase mRNA. Tissue specificity and accumulation during embryonic development. J Biol Chem. 1978 Dec 25;253(24):8736–8740. [PubMed] [Google Scholar]
- Keller G. H., Taylor J. M. Effect of hypophysectomy and growth hormone treatment on albumin mRNA levels in the rat liver. J Biol Chem. 1979 Jan 25;254(2):276–278. [PubMed] [Google Scholar]
- Land H., Hofer E., Sekeris C. E. Differential behavior of two dexamethasone induced mRNA activities in HTC cells in response to cordycepin and to withdrawal of hormone. Biochem Biophys Res Commun. 1978 Jul 28;83(2):607–615. doi: 10.1016/0006-291x(78)91033-1. [DOI] [PubMed] [Google Scholar]
- Lecocq R. E., Dumont J. E. In vivo and in vitro effects of thyrotropin on ribosomal pattern of dog thyroid. Biochim Biophys Acta. 1973 Mar 19;299(2):304–311. doi: 10.1016/0005-2787(73)90354-7. [DOI] [PubMed] [Google Scholar]
- Lecocq R. E., Dumont J. E. Stimulation by thyrotropin of amino acid incorporation into proteins in dog thyroid slices in vitro. Biochim Biophys Acta. 1972 Oct 27;281(3):434–441. doi: 10.1016/0005-2787(72)90459-5. [DOI] [PubMed] [Google Scholar]
- Lissitzky S., Fayet G., Giraud A., Verrier B., Torresani J. Thyrotrophin-induced aggregation and reorganization into follicles of isolated porcine-thyroid cells. 1. Mechanism of action of thyrotrophin and metabolic properties. Eur J Biochem. 1971 Dec 22;24(1):88–99. doi: 10.1111/j.1432-1033.1971.tb19658.x. [DOI] [PubMed] [Google Scholar]
- Lissitzky S., Fayet G., Verrier B., Hennen G., Jaquet P. Thyroid-stimulating hormone binding to cultured thyroid cells. FEBS Lett. 1973 Jan 1;29(1):20–24. doi: 10.1016/0014-5793(73)80006-7. [DOI] [PubMed] [Google Scholar]
- MCINTIRE F. C., SPROULL M. F. A simple method for determination of desoxypentose nucleic acid in tissue cultures. Proc Soc Exp Biol Med. 1957 Jul;95(3):458–462. doi: 10.3181/00379727-95-23251. [DOI] [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Pavlovic-Hournac M., Delbauffe D. Action of TSH on the in vivo incorporation of labeled amino acid into thyroglobulin and other thyroidal proteins. Endocrinology. 1973 Apr;92(4):1273–1276. doi: 10.1210/endo-92-4-1273. [DOI] [PubMed] [Google Scholar]
- Pavlovic-Hournac M., Rappaport L., Nunez J. Incorporation of labeled amino acid into protein by thyroid glands from hypophysectomized rats. I. In vitro studies. Endocrinology. 1971 Dec;89(6):1477–1484. doi: 10.1210/endo-89-6-1477. [DOI] [PubMed] [Google Scholar]
- Pavlović-Hournac M., Rappaport L., Nunez J. Synthèse de la thyroglobuline dans les glandes thyroïdes en culture organotypique. I. Etudes qualitatives. Exp Cell Res. 1971 Oct;68(2):332–338. doi: 10.1016/0014-4827(71)90158-3. [DOI] [PubMed] [Google Scholar]
- Pavlović-Hournac M., Rappaport L., Nunez J. Synthèse de la thyroglobuline dans les glandes thyroïdes en culture organotypique. II. Etudes quantitatives. Exp Cell Res. 1971 Oct;68(2):339–346. doi: 10.1016/0014-4827(71)90159-5. [DOI] [PubMed] [Google Scholar]
- Peavy D. E., Taylor J. M., Jefferson L. S. Correlation of albumin production rates and albumin mRNA levels in livers of normal, diabetic, and insulin-treated diabetic rats. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5879–5883. doi: 10.1073/pnas.75.12.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pisarev M. A., Aiello L. O., Kleiman de Pisarev D. L. Action of KI, thyroxine and cyclic AMP on [3H]uridine incorporation into the RNA of thyroid slices. Acta Endocrinol (Copenh) 1976 Oct;83(2):313–320. doi: 10.1530/acta.0.0830313. [DOI] [PubMed] [Google Scholar]
- Regard E., Mauchamp J. Ultrastructure de la glande thyroïde du Xénope larvaire normal et hypophysectomisé: corrélations avec la biosynthése de la thyroglobuline. J Ultrastruct Res. 1971 Dec;37(5):664–678. doi: 10.1016/s0022-5320(71)80030-8. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Schimke R. T. Differential effects of estrogen and progesterone on ovalbumin mRNA utilization. J Biol Chem. 1978 Dec 25;253(24):8925–8934. [PubMed] [Google Scholar]
- Rosbash M. Polyadenylic acid-containing RNA in Xenopus laevis oocytes. J Mol Biol. 1974 May 5;85(1):87–101. doi: 10.1016/0022-2836(74)90131-4. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Dowbenko D. J. Role of growth hormone in the multihormonal regulation of messenger RNA for alpha2u globulin in the liver of hypophysectomized rats. Biochemistry. 1977 Aug 23;16(17):3918–3922. doi: 10.1021/bi00636a030. [DOI] [PubMed] [Google Scholar]
- SEED R. W., GOLDBERG I. H. BIOSYNTHESIS OF THYROGLOBULIN. II. ROLE OF SUBUNITS, IODINATION, AND RIBONUCLEIC ACID SYNTHESIS. J Biol Chem. 1965 Feb;240:764–773. [PubMed] [Google Scholar]
- Scheinman S. J., Burrow G. N., Theoharides T. C., Canellakis Z. N. Stimulation of ornithine decarboxylase synthesis in the rat thyroid. Life Sci. 1977 Oct 15;21(8):1143–1147. doi: 10.1016/0024-3205(77)90113-8. [DOI] [PubMed] [Google Scholar]
- Scherberg N. H. Isolation of thyroglobulin messenger RNA from rats: increased yield in propylthiouracil-induced hyperplasia. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1415–1423. doi: 10.1016/0006-291x(78)91161-0. [DOI] [PubMed] [Google Scholar]
- Sherwin J. R., Tong W. Stimulatory actions of thyrotropin and dibutyryl cyclic AMP on transcription and translation in the regulation of thyroidal protein synthesis. Biochim Biophys Acta. 1976 Apr 2;425(4):502–510. doi: 10.1016/0005-2787(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Takasu N., Charrier B., Mauchamp J., Lissitzky S. Modulation of adenylate cyclase/cyclic AMP response by thyrotropin and prostaglandin E2 in cultured thyroid cells. 2. Positive regulation. Eur J Biochem. 1978 Sep 15;90(1):139–146. doi: 10.1111/j.1432-1033.1978.tb12584.x. [DOI] [PubMed] [Google Scholar]
- Takasu N., Charrier B., Mauchamp J., Lissitzky S. Positive and negative regulation by thyrotropin of thyroid cyclic AMP response to thyrotropin in porcine thyroid cells. FEBS Lett. 1977 Dec 1;84(1):191–194. doi: 10.1016/0014-5793(77)81087-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]


