Abstract
OBJECTIVE
Olanzapine (OLZ) is an atypical antipsychotic whose clinical efficacy is hampered by side effects including weight gain and diabetes. Recent evidence shows that OLZ alters insulin sensitivity independent of changes in body weight and composition. The present study addresses whether OLZ-induced insulin resistance is driven by its central actions.
RESEARCH DESIGN AND METHODS
Sprague-Dawley rats received an intravenous (OLZ-IV group) or intracerebroventricular (OLZ-ICV group) infusion of OLZ or vehicle. Glucose kinetics were assessed before (basal period) and during euglycemic-hyperinsulinemic clamp studies.
RESULTS
OLZ-IV caused a transient increase in glycemia and a higher rate of glucose appearance (Ra) in the basal period. During the hyperinsulinemic clamp, the glucose infusion rate (GIR) required to maintain euglycemia and the rate of glucose utilization (Rd) were decreased in OLZ-IV, whereas endogenous glucose production (EGP) rate was increased compared with vehicle-IV. Consistent with an elevation in EGP, the OLZ-IV group had higher hepatic mRNA levels for the enzymes glucose-6-phosphatase and phosphoenolpyruvate carboxykinase. Phosphorylation of hypothalamic AMP-activated protein kinase (AMPK) was increased in OLZ-IV rats compared with controls. Similarly, an intracerebroventricular infusion of OLZ resulted in a transient increase in glycemia as well as a higher Ra in the basal period. During the hyperinsulinemic period, OLZ-ICV caused a decreased GIR, an increased EGP, but no change in Rd. Furthermore, OLZ-ICV rats had increased hepatic gluconeogenic enzymes and elevated hypothalamic neuropeptide-Y and agouti-related protein mRNA levels.
CONCLUSIONS
Acute central nervous system exposure to OLZ induces hypothalamic AMPK and hepatic insulin resistance, pointing to a hypothalamic site of action for the metabolic dysregulation of atypical antipsychotics.
Atypical antipsychotics, such as olanzapine (OLZ), account for the majority of antipsychotic drugs prescribed for the treatment of schizophrenia and bipolar disorders. Furthermore, a multicenter double-blind comparison between several antipsychotics has underscored the efficacy of OLZ compared with other earlier-generation antipsychotics (1). However, atypical antipsychotics, in particular OLZ, have been associated with serious metabolic side effects, including weight gain, dyslipdemia, and diabetes (2,3).
Although it is established that atypical antipsychotics, especially OLZ, are strongly associated with increased weight gain in humans, little is known regarding the mechanisms responsible for this effect (4). Acutely, two atypical antipsychotics, clozapine and OLZ, increase the consumption of a high-calorie fat emulsion in rodents (5). Further, weight gain in rats treated for 7 days with OLZ was accounted for by increased food intake (6). However, hyperphagia and weight gain are not universally observed in animal models of antipsychotic treatment, but increased body adiposity is strongly associated with chronic OLZ administration (7,8).
In general, body adiposity positively correlates with insulin resistance. Indeed, short-term (4 weeks) treatment with OLZ caused increased visceral adiposity, which was associated to markedly reduced hepatic insulin sensitivity (9,10), a common feature of obesity and type 2 diabetes. However, recent evidence (11–13) suggests that OLZ can acutely impair insulin sensitivity, in the absence of changes in adiposity. OLZ, as well as other atypical antipsychotics, acts as an antagonist for many neurotransmitter receptors, including dopamine, serotonin, histamine, and acetylcholine (14,15). To date, the identity and location of the receptors blocked by OLZ that are implicated in the disruption of glucose metabolism remain unknown. The hypothalamus has long been implicated in the regulation of energy balance as well as glucose homeostasis. Circulating hormones and nutrients are acting in the hypothalamus, and specifically the arcuate nucleus (ARC), to modulate glucose metabolism (16). One likely site of action for the deleterious effects of OLZ on glucose homeostasis is in the ARC, since several receptors for which OLZ has moderate to high affinity are expressed in this hypothalamic area. Both serotonin and dopamine can inhibit firing in ARC neurons (17), whereas D2 receptor agonists reduce and antagonists increase the expression of ARC neuropeptide-Y (NPY) (18). In addition, serotoninergic inputs in ARC are shown to regulate NPY/AgRP neurons (19), supporting the notion that OLZ might affect energy balance and glucose homeostasis by modulating the activity of these neurons. In this regard, peripheral administration of OLZ in mice is able to acutely induce phosphorylation of hypothalamic AMP-activated protein kinase (AMPK), whose activation has been linked to increased expression of NPY/AgRP. NPY/AgRP neurons have been implicated in the control of glucose homeostasis and, more specifically, the regulation of hepatic insulin sensitivity (20). This study examines whether central nervous system (CNS) delivery of OLZ can affect peripheral glucose homeostasis and insulin action.
RESEARCH DESIGN AND METHODS
Animal model and surgical procedures.
Male Sprague-Dawley rats (250–275 g), purchased from Charles River Laboratories (Wilmington, MA), were acclimated to our facilities for 7 days before undergoing surgical catheterizations of the left jugular vein and right carotid artery, as previously described (21). A set of rats also received intracerebroventricular cannulae targeting the third ventricle (from Bregma-anterior-posterior: −2.2 mm; dorsal-ventral: −7.5 mm from sagital sinus surface; medial-lateral: 0.0 mm). The jugular catheter served for infusions and the carotid catheter for blood sampling. Rats were allowed to recover for at least 6–10 days after surgery before the clamp studies. The correct placement of the intracerebroventricular cannulae was verified by the induction of a drinking response to 30 ng angiotensin II intracerebroventricularly performed 4 days after the surgery. All animal procedures were approved by the institutional animal care and use committee of the University of Cincinnati.
Olanzapine dosing.
The dose of intravenous infusion of OLZ (OLZ-IV group) was selected according to two criteria: 1) the extent of occupancy of the D2 receptors (80–90%) achieved with a single dose (14,22) and 2) it was experimentally determined to cause only moderate sedation (up to grade 2 according to the Sedation Rating Scale in Salamone et al. [ 23]). In addition, since the plasma half-life of OLZ in rats is eightfold shorter than humans (22,24), we used a primed-continuous infusion in order to study its effect on steady-steady condition and minimize pharmacokinetic fluctuations (24). A stock solution of OLZ (provided by Eli Lilly, Indianapolis, IN, and the National Institute of Mental Health Chemical Synthesis and Drug Supply Program) was prepared in HCl (0.1 N). Working solutions of OLZ were prepared daily by dissolving the stock solution in saline (0.9%) or artificial cerebrospinal fluid (Harvard Apparatus, Holliston, MA) for intravenous (3 ml) or intracerebroventricular (15 μl) infusions, respectively. Final solutions were adjusted to pH 5.5–6.0 with sodium hydroxide (0.5 N). Control animals received saline (0.9%) or artificial cerebrospinal fluid after pH adjustment to 5.5–6.0 as well.
Hyperinsulinemic-euglycemic clamp.
Sixty minutes before the drug infusion (t = −60), a prime-continuous infusion of [3H-3]-glucose (Perkin-Elmer NEN, Boston, MA) was initiated and maintained throughout the study (prime 5.2 μCi/min for 4 min, then 0.52 μCi/min). At 0 min, a prime-continuous infusion of OLZ or vehicle was started and maintained throughout the study. A total of 4.5 mg/kg was delivered intravenously (prime 375 μg/kg/min for 4 min, then 25 μg/kg/min) and 330 μg/rat was delivered intracerebroventricularly (prime 22 μg/min for 5 min, then 1.9 μg/min). At +60 min, after collecting the last blood sample for the basal period, all rats received 1) a prime-continuous infusion of human insulin (prime 44.3 mU/kg for 1 min, then 2.5 mU/kg/min) and 2) a variable infusion of 25% glucose solution to maintain euglycemia. Blood samples for glucose concentration and specific activity were taken every 10 min during the basal period and the last hour of the clamp period. Larger blood samples were taken at 0, 30, 60, 120, 150, and 180 min and stored for subsequent analysis. To prevent anemia, erythrocytes from the blood samples were washed, resuspended in saline solution, and reinfused. The rate of glucose infusion was adjusted as needed in order to clamp the plasma glucose concentration at 130–140 mg/dl. Plasma radioactivity from [3H-3]-glucose was determined after deproteinization with Ba(OH)2 and ZnSO4 and subsequent evaporation to remove tritiated water, as described (25).
Clamp parameters calculations.
Since OLZ administration induced a transient increase in the plasma glucose levels during the basal period, we applied the non–steady-state Steele equation (26) to determine the rate of glucose appearance (Ra) and disappearance (Rd):
 |
where F equals tracer infusion rate (dpm/min/kg), p equals correction factor (0.65), and V equals volume of distribution of glucose (0.25 l/kg). Glu1 and Glu2 equal glucose concentration (mg/ml) at time t1 and t2 (min). SA1 and SA2 (dpm/mg) equal glucose-specific activities at times t1 and t2. The mean values of Ra and Rd during basal (60–120 min) and clamp (140–180 min) periods were used for comparison between groups. The endogenous glucose production (EGP) rate was calculated as the difference between Rd and the glucose infusion rate (GIR).
Biochemistry methods.
Plasma glucose concentrations were measured by the glucose oxidase method using a GM7 Micro-Stat Analyzer (ANALOX Instruments, London, U.K.). Insulin levels in plasma were determined by radioimmunoassay. Plasma free fatty acid (FFA) concentration was determined using a colorimetric kit (Wako Chemicals).
Gene expression methods.
Liver and hypothalamus were harvested immediately after the completion of the hyperinsulinemic clamps. Total RNA was isolated by using a TRIzol reagent (Invitrogen) and reverse transcribed into cDNA by using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's directions. Quantitative real-time PCR was carried out on the iCycler (Bio-Rad). Briefly, each 20 μl SYBR green reaction consisted of 1–5 μl cDNA (2∼10 ng/μl), 10 μl iQ SYBR green Supermix (Bio-Rad), 1 μl of 4 μmol/l forward and reverse primers and 3–7 μl nuclease-free water. PCR was performed with the following cycling conditions: 40 cycles of 10 s at 95°C and 30–60 s at 56–62°C. Specificity of the PCR product was confirmed by examination of dissociation reaction plots. A distinct single peak indicated that a single DNA sequence was amplified during PCR. Primers were as follows: α-tubulin: forward TACCCTCGCATCCACTTCCCT, reverse CGCTTGGTCTTGATGGTGGCA; glucose-6-phosphatase (G6Pase): forward CTTGTCAGGCATTGCTGTGGC, reverse CAGGAGGTCCACCCCTAGCC; PEPCK: forward ATTCTGCACCCCTGCCAGC, reverse AACACCCCATGCTGCCAGC; AgRP: forward GCAGAAGGCAGAAGCTTTGGC, reverse CCCAAGCAGGACTCGTGCAG; NPY: forward TACTCCGCTCTGCGACACTACATC, reverse CACATGGAAGGGTCTTCAAGCC; proopiomelanocortin: forward AGTGCCAGGACCTCACCACG, reverse AAGCGGTCCCAGCGGAAG; prepro-melanocyte-stimulating hormone (MCH): forward TTCCCAG CTGAGAATGGAGTTCAG, reverse CCCAGCATACACCTGAGCATGTC; and prepro-orexin: forward AGAAGACGTGTTCCTGCCGT, reverse CGTGGTT ACCGTTGGCCTGAA.
Tissue extraction and Western blotting.
Hypothalami were homogenized in freshly prepared ice-cold buffer (150 mmol/l NaCl, 50 mmol/l Tris pH 8.0, 0.1% SDS, 0.5% deoxycholate, and 1% Triton X-100, radioimmunoprecipitation assay buffer modified) with one tablet of protease inhibitor cocktail (catalog no. 04693124001; Roche) and 100 μl of phosphatase inhibitor cocktail Set II (catalog no. 524625; Calbiochem) per 10 ml of buffer. The samples were spun down for 10 min at 14,000 rpm and the supernatant sonicated in a fresh tube four times for 5 s. The insoluble material was removed by centrifugation (14,000 rpm) for 20 min at 4°C. Aliquots of the resulting supernatants containing 400 μg total proteins were diluted in Laemmli sample buffer and boiled for 5 min before electrophoresis separation. For immunoblot analysis, 40 μg of protein from denatured extracts was separated by SDS-PAGE (150 min at 110 V constant) using a miniature-slab gel apparatus (Bio-Rad). Electrotransfer of proteins from the gel to polyvinylidene fluoride membranes (Bio-Rad) was performed (60 min at 100 V constant at 4°C) and blotted with specific antibodies, anti–phospho-AMPK and anti–AMPK (catalog no. 2535 and no. C2532, respectively; Cell Signaling Technology) and anti–β-tubulin (catalog no. MAB380; Millipore). Band intensities were quantified by optical densitometry of developed radiographs (Quantity One 1-D Analysis Software; Bio-Rad).
Statistical analysis.
All data are presented as means ± SE. Plasma glucose, insulin, and FFA levels were analyzed by two-way ANOVA, followed by the Duncan post hoc test for multiple comparisons when appropriate. Student t test for independent samples was performed to the others parameters described here. The statistical calculations were performed using Statistica 6.1 software (StatSoft, Tulsa, OK).
RESULTS
Intravenous infusion of OLZ before and during a hyperinsulinemic-euglycemic clamp.
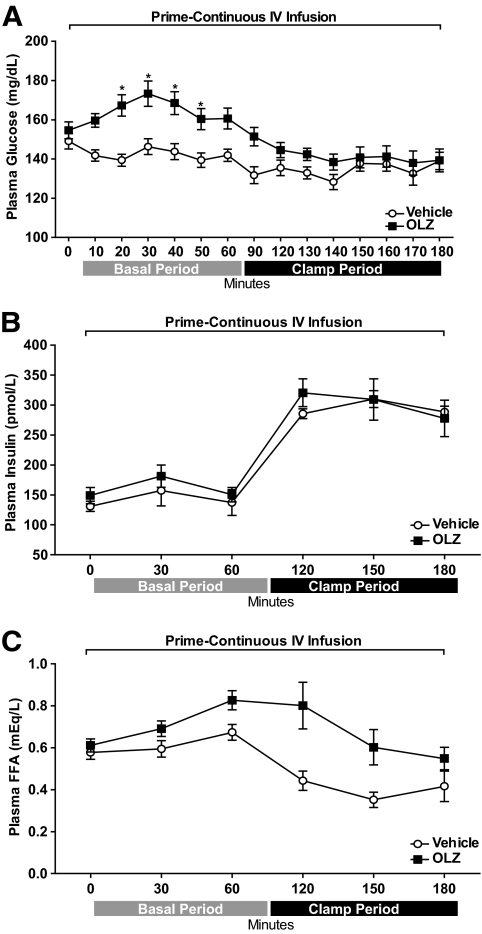
Both OLZ and vehicle groups had similar body weight (vehicle: 317.0 ± 6.3 g vs. OLZ: 316.1 ± 6.0 g), food intake (vehicle: 123.6 ± 4.9 Kcal vs. OLZ: 114.8 ± 5.9 Kcal), and adiposity (epididymal pads, vehicle: 2.165 ± 0.18 g vs. OLZ: 2.198 ± 0.08 g). Intravenous infusion of OLZ transiently increased plasma glucose levels during the basal period compared with rats receiving vehicle [two-way repeated-measures ANOVA group effect, OLZ-IV vs. vehicle: F(1,224) = 6.0, P = 0.02; group × time interaction effect: F(14,224) = 5.7, P < 0.0001] (Fig. 1A). Although the peak in plasma glucose levels was observed 30 min after the start of OLZ administration, there were no differences in the plasma insulin levels during basal or clamp periods between OLZ-IV– and vehicle-treated groups [two-way repeated-measures ANOVA group effect, OLZ-IV vs. vehicle: F(1,70) = 0.61, P > 0.05; group × time interaction effect: F(5,70) = 0.42, P > 0.05] (Fig. 1B). In addition, OLZ increased plasma FFAs, although at the end of the hyperinsulinemic period there was no difference between vehicle- and OLZ-IV–treated groups [two-way repeated-measures ANOVA group effect, OLZ-IV vs. vehicle: F(1,80) = 15.84, P < 0.01; group × time interaction effect: F(5,80) = 2.06, P = 0.07] (Fig. 1C).
FIG. 1.
Time course (means ± SE) of plasma levels of glucose (A), insulin (B), and FFAs (C) during basal and hyperinsulinemic-euglycemic clamp periods. Minute 0 represents sampling before a prime-continuous (a bolus of 1.5 mg/kg and 1.0 mg/kg/h) intravenous (IV) infusion of OLZ (OLZ-IV, n = 10) or vehicle (n = 8) *Statistics denote comparison to respective 0 min and vehicle time point. Two-way ANOVA followed by Duncan test, P < 0.05.
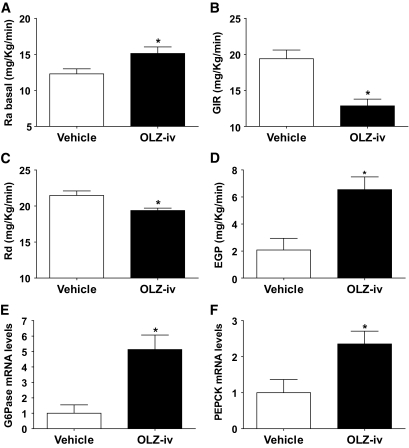
During the basal period, the rate of glucose appearance (Ra) was higher (t test = 2.40, P = 0.02) in OLZ-infused animals compared with vehicle (Fig. 2A). During the clamp period, the infusion rate of exogenous glucose (GIR) required to maintain euglycemia was significantly lower (t test = 4.33, P < 0.001) in the OLZ group (Fig. 2B), indicating that OLZ decreased insulin sensitivity. Tracer analysis during the clamp period showed that the insulin resistance in OLZ-IV rats was the result of a reduced glucose disposal rate (Rd) (t test = 3.10, P < 0.01) and an increased EGP (t test = 3.4, P < 0.01) compared with the vehicle group (Fig. 2C and D).
FIG. 2.
Effects of intravenous infusion of OLZ (OLZ-IV, n = 10) or vehicle (n = 8) on (means ± SE) glucose appearance rate (Ra) (A), GIR (B), glucose disappearance rate (Rd) (C), and EPG (D) before and during stead-state hyperinsulinemic-euglycemic clamp condition. Liver (E), G6Pase, and PEPCK (F) mRNA levels (means ± SE) of rats intravenously (iv) infused with OLZ following the hyperinsulinemic-euglycemic clamp. *Statistics denote comparison to vehicle group; t test, P < 0.05.
Consistent with the increased EGP, the mRNA levels of hepatic G6Pase (t test = 3.55, P < 0.01) and PEPCK (t test = 2.62, P = 0.01) were higher in the OLZ-IV group compared with control (Fig. 2E and F).
Since these studies were conducted in male rats, in a separate experiment in mice, we tested whether the acute effect of OLZ on glucose and FFA levels is sex specific. Intraperitoneal injection of OLZ cause significant hyperglycemia and increased FFA levels in both male and females mice (supplemental Fig. 1 in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0449/DC1).
Intracerebroventricular infusion of OLZ before and during a hyperinsulinemic-euglycemic clamp.
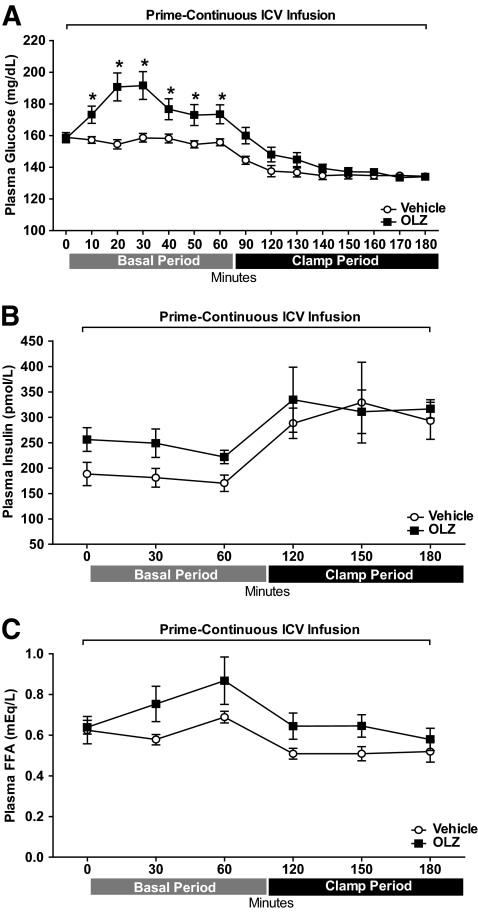
Similar to the OLZ-IV studies, intracerebroventricular infusion of OLZ (OLZ-ICV) transiently increased plasma glucose levels during the basal period compared with rats receiving ICV vehicle [two-way repeated-measures ANOVA group effect, OLZ-ICV vs. vehicle: F(1,224) = 12.23, P < 0.01; group × time interaction effect: F(14,224) = 7.57, P < 0.0001] (Fig. 3A). Both groups had similar body weight (vehicle: 329.3 ± 4.4 g vs. OLZ: 325.0 ± 4.5 g), food intake (vehicle: 123.7 ± 2.8 Kcal vs. OLZ: 132.3 ± 4.4 Kcal), and adiposity (vehicle: 2.30 ± 0.11 g vs. OLZ: 2.248 ± 0.09 g). Like in the intravenous studies, OLZ-ICV increased plasma glucose levels in the basal period, with peak values observed 30 min after the bolus of OLZ. Plasma insulin levels during basal or clamp periods were not changed significantly between OLZ-ICV and vehicle groups [two-way repeated-measures ANOVA group effect, OLZ-ICV vs. vehicle: F(1,50) = 1.25, P = 0.28; group × time interaction effect: F(5,50) = 0.54, P = 0.74] (Fig. 3B). Additionally, there were no significant differences in the plasma FFAs between the groups during the basal or hyperinsulinemic periods [two-way repeated-measures ANOVA group effect, OLZ-ICV vs. vehicle: F(1,60) = 3.51, P = 0.08; group × time interaction effect: F(5,60) = 1.10, P = 0.37] (Fig. 3C).
FIG. 3.
Time course (means ± SE) of plasma levels of glucose (A), insulin (B), and FFAs (C) during basal and hyperinsulinemic-euglycemic clamp periods. Minute 0 represents sampling before a prime-continuous (a bolus of 110 μg and 73.4 μg/h) intracerebroventricular (icv) infusion of OLZ (OLZ-ICV, n = 8) or vehicle (n = 10). *Statistics denote comparison to respective 0 min and vehicle time-point. Two-way ANOVA followed by Duncan test, P < 0.05.
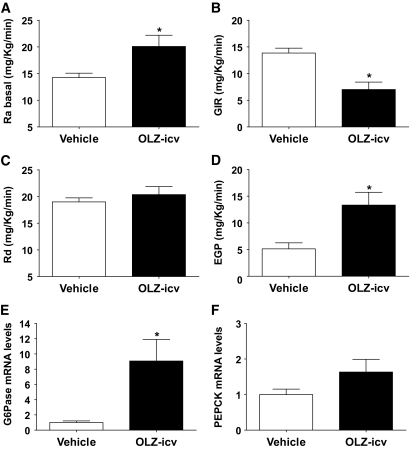
During the basal period, the OLZ-ICV group had a higher Ra (t test = 2.79, P = 0.01) compared with the vehicle group (Fig. 4A). During the clamp period, the GIR was decreased in the OLZ-ICV group compared with the vehicle group (t test = 4.24, P < 0.001) (Fig. 4B). The decreased insulin sensitivity of the OLZ-ICV group was accounted for by a higher EGP (t test = 3.30, P < 0.01) (Fig. 4D) compared with the vehicle group. However, unlike the OLZ-IV studies, the rate of glucose disposal (Rd) of the OLZ-ICV group was not changed compared with control (t test = 0.85, P = 0.40) (Fig. 4C).
FIG. 4.
Effects of intracerebroventricular (icv) infusion of OLZ (OLZ-ICV, n = 8) or vehicle (n = 10) on (means ± SE) glucose appearance rate (Ra) (A), GIR (B), glucose disappearance rate (Rd) (C), and EPG (D) before and during stead-state hyperinsulinemic-euglycemic clamp condition. Liver (E), G6Pase, and PEPCK (F) mRNA levels (means ± SE) of rats intracerebroventricularly infused with OLZ following the hyperinsulinemic-euglycemic clamp. *Statistics denote comparison to vehicle group; t test, P < 0.05.
Moreover, intracerbroventricular infusion of OLZ significantly increased gene expression of G6Pase (t test = 3.18, P < 0.01) and tended to increase PEPCK expression (t test = 1.75, P = 0.09) (Fig. 4E and F).
Hypothalamic effects of intravenous and intracerbroventricular OLZ.
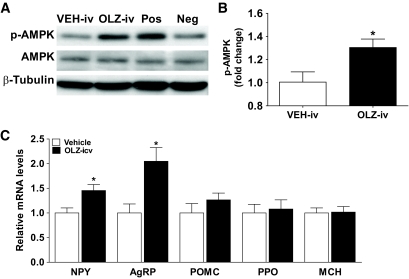
The phosphorylation of AMPK in hypothalamus of OLZ-IV rats was significantly increased (t test = 2.59, P = 0.01) (Fig. 5A and B), indicating that peripheral acute administration of OLZ activates hypothalamic AMPK signaling. Despite this activation, we did not detect any change in the levels of NPY or AgRP mRNAs (supplemental fig. 2). However, OLZ-ICV significantly increased expression of hypothalamic NPY (t test = 2.88, P = 0.01) and AgRP (t test = 3.27, P < 0.01) mRNA levels compared with vehicle rats without changing the mRNA levels of orexin, MCH, and proopiomelanocortin (Fig. 5C).
FIG. 5.
Immunoblot for phospho-AMPK, AMPK, and β-tubulin (A) and optical densitometry of the phospho-AMPK/AMPK ratio (B) from hypothalamus of intravenously infused rats with Vehicle (Veh-IV, n = 8) or OLZ (OLZ-IV, n = 10). Control samples from insulin-induced hypoglycemia (Positive; Pos) or ad libitum–fed (Negative; Neg) rats were also included. C: Relative gene expression (means ± SE) in the hypothalamus of rats intracerebroventricularly infused with OLZ (OLZ-ICV, n = 8) or vehicle (n = 10) following the hyperinsulinemic-euglycemic clamp. *Statistics denote comparison to vehicle group; t test, P < 0.05.
DISCUSSION
In the present study, we show that acute infusion of OLZ either intravenously or intracerbroventricularly induces hepatic insulin resistance. These metabolic alterations are accompanied by hypothalamic activation of AMPK and increased expression of NPY and AgRP. These findings are consistent with the notion that insulin resistance associated with the administration of OLZ could, at least in part, be secondary to its effects on the central nervous system.
Treatment with atypical antipsychotics increases the risk of weight gain, diabetes, and other metabolic disorders in comparison to first-generation antipsychotic drugs (1–3,27). Recent studies (28–30) have also shown that some atypical antipsychotics affect glucose homeostasis before changes in body weight are observed, suggesting that insulin resistance could occur before or independent of any change in adiposity. We showed here that in lean, male rats an acute intravenous infusion of OLZ induced a transient increase of plasma glucose levels (Fig. 1A) in the absence of a compensatory insulin response (Fig. 1B). In several studies, increases in plasma glucose levels have been observed after acute administration of several different atypical antipsychotics in mice. These effects were blocked by a glanglionic blocker, an α2 adrenergic receptor antagonist, or a glucocorticoid receptor antagonist, suggesting a central activation of the sympathetic system (31–33).
Although in humans OLZ-induced obesity and diabetes are not sex specific (34), early studies have preferentially used female rodents because of an apparent stronger sensitivity to OLZ in causing obesity (6,10). Recent findings and our data show that male rats, and mice of both genders, are similarly at risk for OLZ-induced alterations in body weight and glucose homeostasis (supplemental Fig. 1) (8,35).
OLZ can impair glucose-stimulated insulin secretion after chronic or acute peripheral administration, as shown in hyperglycemic clamp experiments (9,12). We did not detect a change in insulin levels during the basal period of OLZ infusion, despite the fact that intravenous OLZ increased glycemia. The lack of an increase in insulin secretion in response to increased plasma glucose during the OLZ infusion might be related to sympathetic inhibition of insulin release from pancreatic β-cells, as previously suggested (32).
Under hyperinsulinemic-euglycemic clamp conditions, which allowed us to override potential differences in insulin secretion, we found lower rates of exogenous glucose infusion and glucose utilization (GIR and Rd) and higher EGP in OLZ-IV–infused rats (Fig. 2B–D). These data support the hypothesis that acutely administered OLZ induces insulin resistance. Our observations agree with previous studies showing impairment of insulin action on hepatic glucose production and peripheral glucose utilization after a subcutaneous bolus injection of OLZ (11–13). In addition, here we showed that OLZ-induced insulin resistance was associated with higher gene expression levels of G6Pase and PEPCK, indicating that OLZ impairs the ability of insulin to suppress the expression of rate-limiting enzymes for hepatic glucose output (Fig. 2E and F).
To test the hypothesis that OLZ impairs insulin sensitivity through CNS action, we infused OLZ intracerebroventricularly before and during hyperinsulinemic-euglycemic clamps. Indeed, intracerebroventricular OLZ infusion increased the rate of glucose appearance (Ra) during the basal period, impaired the ability of insulin to suppress EGP (Fig. 4A and D), and decrease the expression of gluconeogenic enzymes in liver (Fig. 4E and F).
Interestingly, like OLZ-IV, OLZ-ICV markedly impaired hepatic insulin action but did not increase plasma FFAs or impair glucose disposal. Under basal and low insulin levels, OLZ-IV infusion increased plasma FFA levels. However, during hyperinsulinemia, the elevated FFA levels gradually return to control levels (Fig. 1C). This finding agrees with previous reports showing no differences in FFA levels after the hyperinsulinemic-euglycemic clamp condition in rats (11). Nonetheless, in our OLZ-IV studies, we observe a time-dependent and transient increase in FFA levels. Similarly, we found that acute intraperitoneal administration of OLZ in male and female mice increases plasma FFA levels, an effect that was not blocked by pretreatment with a β3-blocker (supplemental Fig. 1D and E). The effect of OLZ on FFA levels has been reported in studies with more prolonged treatment. Healthy men receiving daily OLZ treatment for 8 days display an impaired ability of hyperinsulinemia to reduce plasma FFA levels and increase Rd, in the absence of changes in body weight and adiposity (36). Thus, the reported lack of effect on FFA plasma levels (11) could be due to differences in time of sampling and/or OLZ route of administration.
It is known that elevated plasma FFA levels impair the ability of insulin to suppress hepatic glucose production and stimulate glucose uptake by skeletal muscle (37). However, we observed that only OLZ-IV administration increased plasma FFAs (Fig. 1C) and impaired insulin-stimulated Rd (Fig. 2C), while OLZ effects on hepatic glucose production occurred regardless of the route of administration and the presence of increased plasma FFA levels (Fig. 2D and Fig. 4D). These data suggest that increased FFA levels do not explain the deleterious effects of OLZ on glucose production.
Our data suggest that the effect of OLZ on FFA and glucose utilization may be mediated by peripheral receptors. In support of this notion, recent studies have shown that OLZ reduced basal and isoproterenol-stimulated release of FFAs in rat adipocytes, as well as glucose uptake (38,39).
Hepatic insulin resistance is a common feature of obesity and type 2 diabetes (37,40) and may result not only from alterations of insulin signaling in the liver but also from impaired insulin action in the brain. Neuronal circuitry responsive to insulin play an important role in modulating hepatic gluconeogenesis in response to physiologic elevations of plasma insulin (41). In ARC, insulin receptor activation inhibits NPY/AgRP neurons and stimulates proopiomelanocortin neurons in order to control energy homeostasis (42). Furthermore, insulin action on arcuate neurons suppresses hepatic glucose production by a mechanism dependent on phosphatidylinositol kinase–ATP-sensitive K+ channel activation (43).
Intracerebroventricular administration of OLZ increased hypothalamic mRNA levels of NPY and AgRP (Fig. 5C) in rats killed after 2 h of hyperinsulinemic-euglycemic clamps. This result suggests a potential mechanism by which CNS OLZ reduced hepatic insulin sensitivity. Insulin inhibits arcuate NPY and AgRP gene expression and inhibits AMPK phosphorylation/activity (44), whereas inhibition of insulin action in the hypothalamic arcuate nucleus increases the expression of NPY and AgRP (45). Conversely, atypical antipsychotics, including clozapine and OLZ, increase phosphorylation/activity of AMPK (46). In our intravenous studies, OLZ increases hypothalamic AMPK phosphorylation, suggesting that this atypical antipsychotic might counteract the action of insulin on NPY/AgRP neurons by preventing the insulin-mediated inhibition of AMPK. However, unlike intracerebroventricular OLZ, we did not detect increased expression of NPY/AgRP in the OLZ-IV studies. Several studies with peripheral injection of OLZ have failed to find changes in NPY/AgRP levels (6,47), although several lines of evidence show that peripheral OLZ acutely activates ARC neurons (48). We speculate that our finding of increased NPY/AgRP in ICV-OLZ and not in OLZ-IV can be explained by a greater time of neuronal exposure to OLZ in the intracerebroventricular group. Since OLZ can transiently activate AMPK in hypothalamic explants (46), our results in OLZ-IV rats are consistent with an acute activation of AMPK that would later result in increased NPY/AgRP expression. Taken together, our data support the hypothesis that OLZ counteracts the inhibitory action of insulin on hypothalamic AMPK in NPY/AgRP neurons. Intracerebroventricular administration of NPY impairs insulin's ability to suppress hepatic glucose production (49,50). In addition, selective ablation of the insulin receptor in AgRP-positive neurons leads to hepatic insulin resistance (20). Taken together, these data support the notion that atypical antipsychotics alter hepatic insulin sensitivity by impairing insulin's action on NPY/AgRP neurons.
In conclusion, we have shown that acute administration of OLZ induces insulin resistance in the absence of changes in food intake and body composition. In agreement with previous studies (9,13), this effect is primarily due to hepatic insulin resistance. These effects are accompanied by the activation of hypothalamic AMPK. Moreover, we showed that CNS delivery of OLZ induces hepatic insulin resistance and activates hypothalamic NPY/AgRP neurons. These data are consistent with the notion that the deleterious effects of OLZ on hepatic insulin action are mediated at least in part by impaired hypothalamic control of hepatic glucose metabolism.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by a grant from Eli Lilly and Company; P.J.F.M. is recipient of a fellowship from CNPq-200863/2007-0 Brazil and the American Heart Association (0825677D). No other potential conflicts of interest relevant to this article were reported.
P.J.F.M. performed experiments and wrote the manuscript. M.H. performed surgeries and clamp studies. S.O. supervised experiments and reviewed/edited the manuscript.
We thank Kay Ellis in Dr. David D'Alessio laboratory, Department of Medicine, University of Cincinnati, for performing the plasma insulin assays. We thank Jack Magrisso, Department of Medicine, University of Cincinnati, for outstanding technical support.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209–1223 [DOI] [PubMed] [Google Scholar]
- 2. Keck PE, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003;64:1426–1435 [DOI] [PubMed] [Google Scholar]
- 3. Bergman RN, Ader M. Atypical antipsychotics and glucose homeostasis. J Clin Psychiatry 2005;66:504–514 [DOI] [PubMed] [Google Scholar]
- 4. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–1696 [DOI] [PubMed] [Google Scholar]
- 5. Hartfield AW, Moore NA, Clifton PG. Effects of clozapine, olanzapine and haloperidol on the microstructure of ingestive behaviour in the rat. Psychopharmacology (Berl) 2003;167:115–122 [DOI] [PubMed] [Google Scholar]
- 6. Davoodi N, Kalinichev M, Korneev SA, Clifton PG. Hyperphagia and increased meal size are responsible for weight gain in rats treated sub-chronically with olanzapine. Psychopharmacology (Berl) 2009;203:693–702 [DOI] [PubMed] [Google Scholar]
- 7. Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, Lynch CJ. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity (Silver Spring) 2006;14:36–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper GD, Pickavance LC, Wilding JP, Harrold JA, Halford JC, Goudie AJ. Effects of olanzapine in male rats: enhanced adiposity in the absence of hyperphagia, weight gain or metabolic abnormalities. J Psychopharmacol 2007;21:405–413 [DOI] [PubMed] [Google Scholar]
- 9. Ader M, Kim SP, Catalano KJ, Ionut V, Hucking K, Richey JM, Kabir M, Bergman RN. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes 2005;54:862–871 [DOI] [PubMed] [Google Scholar]
- 10. Chintoh AF, Mann SW, Lam TK, Giacca A, Remington G. Insulin resistance following continuous, chronic olanzapine treatment: an animal model. Schizophr Res 2008;104:23–30 [DOI] [PubMed] [Google Scholar]
- 11. Chintoh AF, Mann SW, Lam L, Lam C, Cohn TA, Fletcher PJ, Nobrega JN, Giacca A, Remington G. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol 2008;28:494–499 [DOI] [PubMed] [Google Scholar]
- 12. Chintoh AF, Mann SW, Lam L, Giacca A, Fletcher P, Nobrega J, Remington G. Insulin resistance and secretion in vivo: effects of different antipsychotics in an animal model. Schizophr Res 2009;108:127–133 [DOI] [PubMed] [Google Scholar]
- 13. Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology 2007;32:289–297 [DOI] [PubMed] [Google Scholar]
- 14. Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996;14:87–96 [DOI] [PubMed] [Google Scholar]
- 15. Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res 1999;37:107–122 [DOI] [PubMed] [Google Scholar]
- 16. Obici S, Martins PJF. The role of brain in glucose metabolism. In Principles of Diabetes Mellitus. Poretsky L. Ed. Springer U.S., New York, 2010, p. 89–104 [Google Scholar]
- 17. Lin JY, Li CS, Pan JT. Effects of various neuroactive substances on single-unit activities of hypothalamic arcuate neurons in brain slices. Brain Res Bull 1993;31:587–594 [DOI] [PubMed] [Google Scholar]
- 18. Pelletier G, Simard J. Dopaminergic regulation of pre-proNPY mRNA levels in the rat arcuate nucleus. Neurosci Lett 1991;127:96–98 [DOI] [PubMed] [Google Scholar]
- 19. Coll AP, Yeo GS, Farooqi IS, O'Rahilly S. SnapShot: the hormonal control of food intake. Cell 2008;135:572. [DOI] [PubMed] [Google Scholar]
- 20. Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007;5:438–449 [DOI] [PubMed] [Google Scholar]
- 21. Obici S, Feng ZH, Tan JZ, Liu LS, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest 2001;108:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther 2003;305:625–631 [DOI] [PubMed] [Google Scholar]
- 23. Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology (Berl) 1996;125:105–112 [DOI] [PubMed] [Google Scholar]
- 24. Aravagiri M, Teper Y, Marder SR. Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos 1999;20:369–377 [DOI] [PubMed] [Google Scholar]
- 25. Rossetti L, Stenbit AE, Chen W, Hu M, Barzilai N, Katz EB, Charron MJ. Peripheral but not hepatic insulin resistance in mice with one disrupted allele of the glucose transporter type 4 (GLUT4) gene. J Clin Invest 1997;100:1831–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, New Jersey, John Wiley & Sons, 2005, p. 1–474 [Google Scholar]
- 27. Nasrallah HA, Newcomer JW. Atypical antipsychotics and metabolic dysregulation: evaluating the risk/benefit equation and improving the standard of care. J Clin Psychopharmacol 2004;24:S7–S14 [DOI] [PubMed] [Google Scholar]
- 28. Ramankutty G. Olanzapine-induced destabilization of diabetes in the absence of weight gain. Acta Psychiatr Scand 2002;105:235–236 [DOI] [PubMed] [Google Scholar]
- 29. Varma MK, Connolly K, Fulton B. Life-threatening hyperglycemia and acidosis related to olanzapine: a case report and review of the literature. J Intensive Care Med 2007;22:52–55 [DOI] [PubMed] [Google Scholar]
- 30. Newcomer JW, Haupt DW, Fucetola R, Melson AK, Schweiger JA, Cooper BP, Selke G. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 2002;59:337–345 [DOI] [PubMed] [Google Scholar]
- 31. Dwyer DS, Donohoe D. Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav 2003;75:255–260 [DOI] [PubMed] [Google Scholar]
- 32. Savoy YE, Ashton MA, Miller MW, Nedza FM, Spracklin DK, Hawthorn MH, Rollema H, Matos FF, Hajos-Korcsok E. Differential effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in the mouse: evidence for the involvement of sympathetic regulation. Schizophr Bull 2008;36:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tulipano G, Rizzetti C, Bianchi I, Fanzani A, Spano P, Cocchi D. Clozapine-induced alteration of glucose homeostasis in the rat: the contribution of hypothalamic-pituitary-adrenal axis activation. Neuroendocrinology 2007;85:61–70 [DOI] [PubMed] [Google Scholar]
- 34. Ganguli R, Brar JS, Ayrton Z. Weight gain over 4 months in schizophrenia patients: a comparison of olanzapine and risperidone. Schizophr Res 2001;49:261–267 [DOI] [PubMed] [Google Scholar]
- 35. Minet-Ringuet J, Even PC, Lacroix M, Tome D, de Beaurepaire R. A model for antipsychotic-induced obesity in the male rat. Psychopharmacology (Berl) 2006;187:447–454 [DOI] [PubMed] [Google Scholar]
- 36. Vidarsdottir S, de Leeuw van Weenen JE, Frolich M, Roelfsema F, Romijn JA, Pijl H. Effects of olanzapine and haloperidol on the metabolic status of healthy men. J Clin Endocrinol Metab 2010;95:118–125 [DOI] [PubMed] [Google Scholar]
- 37. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vestri HS, Maianu L, Moellering DR, Garvey WT. Atypical antipsychotic drugs directly impair insulin action in adipocytes: effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology 2007;32:765–772 [DOI] [PubMed] [Google Scholar]
- 39. Minet-Ringuet J, Even PC, Valet P, Carpene C, Visentin V, Prevot D, Daviaud D, Quignard-Boulange A, Tome D, de Beaurepaire R. Alterations of lipid metabolism and gene expression in rat adipocytes during chronic olanzapine treatment. Mol Psychiatry 2007;12:562–571 [DOI] [PubMed] [Google Scholar]
- 40. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rother E, Konner AC, Bruning JC. Neurocircuits integrating hormone and nutrient signaling in control of glucose metabolism. Am J Physiol Endocrinol Metab 2008;294:E810–E816 [DOI] [PubMed] [Google Scholar]
- 42. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 43. Prodi E, Obici S. Minireview: the brain as a molecular target for diabetic therapy. Endocrinology 2006;147:2664–2669 [DOI] [PubMed] [Google Scholar]
- 44. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569–574 [DOI] [PubMed] [Google Scholar]
- 45. Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 2002;5:566–572 [DOI] [PubMed] [Google Scholar]
- 46. Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the cover: antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A 2007;104:3456–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang XF, Deng C, Zavitsanou K. Neuropeptide Y mRNA expression levels following chronic olanzapine, clozapine and haloperidol administration in rats. Neuropeptides 2006;40:213–219 [DOI] [PubMed] [Google Scholar]
- 48. Stefanidis A, Verty AN, Allen AM, Owens NC, Cowley MA, Oldfield BJ. The role of thermogenesis in antipsychotic drug-induced weight gain. Obesity (Silver Spring) 2009;17:16–24 [DOI] [PubMed] [Google Scholar]
- 49. Marks JL, Waite K. Intracerebroventricular neuropeptide Y acutely influences glucose metabolism and insulin sensitivity in the rat. J Neuroendocrinol 1997;9:99–103 [DOI] [PubMed] [Google Scholar]
- 50. van den Hoek AM, van Heijningen C, der Elst JPSV, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes 2008;57:2304–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.