Background: Slc26a2 is an SO42− transporter, mutations in which cause diastrophic dysplasia. How Slc26a2 transports SO42− is unknown.
Results: We found that Slc26a2 exchanges SO42− for 2OH− or 2Cl− and is regulated by a promiscuous extracellular anion site.
Conclusion: Slc26a2 functions as SO42−/2OH− or SO42−/2Cl− exchanger, regulated by extracellular Cl−o.
Significance: The findings should help in understanding aberrant SLC26A2 function in diastrophic dysplasia.
Keywords: Anion transport; Chloride transport; Membrane transport; Sulfur; Transporters; SLC26A2; SO42−/2OH−, SO42−/2Cl−, and SO42−/OH−/Cl−; Diastrophic dysplasia; Exchanger
Abstract
Slc26a2 is a ubiquitously expressed SO42− transporter with high expression levels in cartilage and several epithelia. Mutations in SLC26A2 are associated with diastrophic dysplasia. The mechanism by which Slc26a2 transports SO42− and the ion gradients that mediate SO42− uptake are poorly understood. We report here that Slc26a2 functions as an SO42−/2OH−, SO42−/2Cl−, and SO42−/OH−/Cl− exchanger, depending on the Cl− and OH− gradients. At inward Cl− and outward pH gradients (high Cl−o and low pHo) Slc26a2 functions primarily as an SO42−o/2OH−i exchanger. At low Cl−o and high pHo Slc26a2 functions increasingly as an SO42−o/2Cl−i exchanger. The reverse is observed for SO42−i/2OH−o and SO42−i/2Cl−o exchange. Slc26a2 also exchanges Cl− for I−, Br−, and NO3− and Cl−o competes with SO42− on the transport site. Interestingly, Slc26a2 is regulated by an extracellular anion site, required to activate SO42−i/2OH−o exchange. Slc26a2 can transport oxalate in exchange for OH− and/or Cl− with properties similar to SO42− transport. Modeling of the Slc26a2 transmembrane domain (TMD) structure identified a conserved extracellular sequence 367GFXXP371 between TMD7 and TMD8 close to the conserved Glu417 in the permeation pathway. Mutation of Glu417 eliminated transport by Slc26a2, whereas mutation of Phe368 increased the affinity for SO42−o 8-fold while reducing the affinity for Cl−o 2 fold, but without affecting regulation by Cl−o. These findings clarify the mechanism of net SO42− transport and describe a novel regulation of Slc26a2 by an extracellular anion binding site and should help in further understanding aberrant SLC26A2 function in diastrophic dysplasia.
Introduction
Protein sulfation, and thus SO42− , is essential for cellular and tissue survival. Many proteins undergo post-translational modification by sulfation. Tyrosine sulfation of signaling molecules, like the G protein-coupled receptor chemokine receptors (1), modifies signaling pathways. Protein sulfation contributes to detoxification of endogenous compounds (2). A critical role of protein sulfation is sulfation of proteoglycans (3). Proteoglycans are constituents of the extracellular matrix that mediate the cell response to growth factors (4). Several disorders are caused by mutations in genes that affect proteoglycan synthesis or sulfation. The sulfate groups in proteoglycans are critical in formation of active domains, and the high polyanionic charge density of the proteoglycans is neutralized by SO42− (5). Sulfation of secretory proteins, like digestive enzymes and mucins, is essential for their synthesis, processing through the biosynthetic pathway and packaging in secretory granules (6). Hence, understanding SO42− homeostasis is essential for understanding cell development and function.
Cells have two sources of SO42−, a minor source from degradation of cysteine and methionine and active uptake of SO42− mediated largely by the SO42− transporters Slc26a1 and Slc26a24 (7, 8). Slc26a1 and Slc26a2 belong to the family of the SLC26 transporters, which includes 11 genes with Slc26a10 being a pseudogene (9). Members of the family transport remarkably diverse substrates, including Cl−, HCO3−, I−, SO42−, formant, and oxalate, and can function as coupled electroneutral or electrogenic transporters or as ion channels (9, 10). Mutations in several members of the family are associated with human diseases, including autosomal recessively inherited chondrodysplasias (SLC26A2) (11, 12), congenital chloride diarrhea (SLC26A3) (13), Pendred syndrome (SLC26A4) (14), deafness (SLC26A5) (15), and perhaps reduced fertility (SLC26A8) (16). In addition, deletion of Slc26a6 in mice resulted in nephrolithiasis due to aberrant oxalate transport (17) and in aberrant pancreatic and parotid ducts HCO3− transport (18, 19).
Although Slc26a1 has limited tissue distribution, Slc26a2 is ubiquitously expressed with particularly high levels in developing and mature cartilage as well as in epithelial tissues like pancreas, salivary glands, colon, bronchial glands, tracheal epithelium, and eccrine sweat glands (20, 21). The central role of Slc26a2 in supplying the bulk of cellular SO42− is evident from the lethality of deletion of the SLC26A2 gene in humans and mice (20, 22), mainly due to under-sulfation of proteoglycans leading to aberrant development (23). Indeed, measurement of SO42− uptake in fibroblast from patients with a severe form of the disease showed reduced or lack of SO42− uptake (20, 24). Most mutations causing diastrophic dysplasia are missense mutations that affect either trafficking to the plasma membrane or showed reduced SO42− transport (25, 26).
The phenotype of chondrodysplasias is highly variable, ranging from mild (27) to lethal before or shortly after birth (11). To better understand the disease and cellular SO42− homeostasis, it is necessary to understand transport and regulation of Slc26a2. To date, characterization of transport by Slc26a2 was based on measurement of isotopic fluxes (24, 25, 28) that are the sum of both net and exchange fluxes, with the exchange dominating the fluxes. These studies revealed that Slc26a2 can transport SO42−, Cl−, and oxalate (24, 25, 28), and a recent detailed characterization of the fluxes suggested that Slc26a2 functions as an electroneutral transporter when mediating isotopic fluxes. SO42− fluxes appeared to be sensitive to intracellular and extracellular pH (24). An unusual finding was that inhibition of SO42− and oxalate isotopic uptake by external Cl− exhibited simple saturation, whereas Slc26a2-mediated exchange of intracellular SO42−, oxalate, or Cl− for external Cl− was non-saturable (24), suggesting that the measured fluxes, at least isotopic efflux, is mostly exchange rather than net fluxes.
The available information is not sufficient to determine the mode of SO42− and other ions transport by Slc26a2 and the cellular ionic gradients that drive net transport. We used Xenopus oocytes expressing Slc26a2 to report that Slc26a2 functions as SO42−/2OH−, SO42−/2Cl−, and SO42−/OH−/Cl− exchanger, depending on the cellular Cl− and OH−(H+) gradients. Slc26a2 can also mediate Ox2−o/OH−i/Cl−i exchange and transport I−, Br−, and NO3−. Slc26a2 activity is regulated by an extracellular anion binding site, which is not involved in ion transport. Modeling of the Slc26a2 transmembrane sector identified an extracellular loop, which contains the conserved sequence 367GFXXP371 in the vicinity of the gating Glu417 as a potential part of the permeation pathway. These findings should help in further understanding ion transport by the SLC26 transporters and aberrant SLC26A2 function in diastrophic dysplasia.
EXPERIMENTAL PROCEDURES
Solutions and Reagents
For experiments in oocytes, the standard HEPES-buffered ND96 solution contained (in mm): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, pH 7.5. Cl−-free solutions were prepared by replacing chloride with gluconate in the presence of calcium cyclamate substituted for CaCl2. A 100 mm solution of diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) (Invitrogen) dissolved in DMSO was prepared freshly and diluted to a final concentrations of 10 or 50 μm in the relevant solutions. All other chemicals and reagents were purchased from Sigma.
cRNA Preparation
The pCMV-Sport6-Slc26a2 (GenBankTM/EMBL/DDBJ, accession no. BC028345) was purchased from Open Biosystems and was used as template for cRNA preparation. The plasmid was linearized with NotI and used to transcribe cRNA with an mMESSAGE mMACHINE Sp6 kit (Life Technologies, Applied Biosystems), respectively. Mutation in Slc26a2 were generated by a site-directed mutagenesis kit (Agilent Technologies) and verified by sequencing.
Biotinylation and Western Blot Analysis
To monitor surface expression of Slc26a2 WT, E417A, and E417K, HEK cells transfected with vector alone or Myc-tagged Slc26a2 constructs were incubated with EZ link Sulfo-NHS-LC-Biotin (0.5 mg/ml, Thermo Fisher Scientific) for 30 min at room temperature. Subsequent steps were as previously described (29) with the following modifications: 50 μl of 1:1 slurry of immobilized avidin beads (Thermo Fisher Scientific) was added to 300 μg of protein in 300 μl of cell extract, and the mixture was incubated overnight. To monitor protein expression the PVDF membranes were incubated overnight with anti-Myc antibodies diluted 1:1,000 (Cell Signaling) and for 1 h with HRP-conjugated goat anti-mouse (Invitrogen) diluted 1:2,000. For β-actin detection membranes were incubated for 1 h with monoclonal anti-β-actin peroxidase (Sigma-Aldrich) diluted 1:20,000.
Xenopus laevis Oocyte Preparation
All experiments in this study were conducted under the National Institutes of Health guidelines for research on animals, and experimental protocols were approved by the Institutional Animal Care and Use Committee. Oocytes were isolated by partial ovariectomy of anesthetized female X. laevis (Xenopus Express, Brooksville, FL) and treated by collagenase B (Roche Applied Science), as described previously (30). Stage V–VI oocytes were injected with 10 ng of cRNA using glass micropipettes and a microinjection device (Nanoliter 2000; World Precision Instruments) in a final volume of 27.6 nl. Control oocytes were injected with equal volumes of H2O. Oocytes were incubated at 18 °C in ND96 supplemented with 2.5 mm pyruvate and antibiotics and were studied 72–144 h after injection.
Voltage, pH, and Cl− Measurement in Oocytes
Voltage recordings were performed at room temperature with two-electrode voltage clamp, exactly as described previously (29, 30). Voltage, pHi, and Cl−i concentrations were measured as detailed previously (31, 32). In the present study, the Cl−-sensitive electrode was also used to record intracellular Br−, I−, and NO3− with the resin and the procedure used to measure Cl− (see “Results”).
Measurement of Buffer Capacity
To determine OH−(H+) fluxes by Slc26a2 we determined the buffer capacity of oocytes bathed in HEPES-buffered medium. Because we can measure both Cl−i and pHi, we determined the buffer capacity directly rather than relying on pHi changes induced by weak acids. Supplemental Fig. 1A shows that two consecutive injections of the oocytes with 13.8 nl of 100 mm HCl reduced pHi and increased Cl−i. Similar determination in five experiments and using the pHi and Cl−i changes of the first injection resulted in a buffer capacity of 17.1 ± 2.2/pH unit, which is similar to that reported by others (33).
Modeling and Prediction of the Slc26a2 Transmembrane Domains Structure
The transmembrane sector of the mouse Slc26a2 model was generated using the Deepview Swiss-PDB viewer by Raw sequence fit of the Slc26a2 sequence (NCBI accession no. NP_031911) onto the putative Slc26a6 model previously generated by us based on structural similarity to the bacterial ClC-ec protein (29). The predicted binding site of DIDS on the Slc26a2 model was performed with the AutoDockVina software (34), according to software tutorial instructions. Briefly, the box grid determining the Slc26a2 region of binding was set using the AutoDockTools software with the following coordinates (center: x = 0.472, y = 1.222, z = 0.472) (size: x = 30, y = 26, z = 24). Exhaustiveness level was set to default. AutoDockTools was further used to select all rotatable bonds of the DIDS molecule. The AutoDockVina software generated nine different models, and herein we present the best model as ranked by the software with a predicted affinity of −8.9 kcal/mol for the binding of Slc26a2 and DIDS. The final model (cartoon and surface representations) was generated using PyMOL (Schrödinger, LLC).
RESULTS AND DISCUSSION
Slc26a2 Functions as an SO42−/2OH−, SO42−/2Cl−, and Possibly SO42−/OH−/Cl− Exchanger
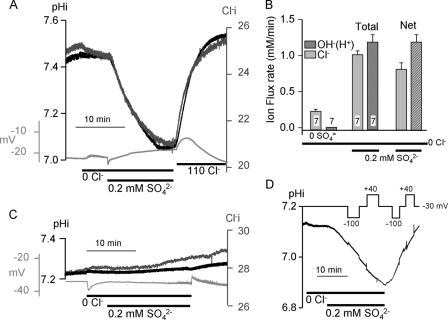
Slc26a2-mediated net fluxes were assayed in Xenopus oocytes by measuring intracellular pH (pHi) and Cl− (Cl−i) and the membrane potential in the same oocytes. Fig. 1A shows that removal of extracellular Cl− (Cl−o) had no effect on pHi and the membrane potential with a slow rate of reduction in Cl−i. Exposing Slc26a2-expressing oocytes bathed in Cl−-free solution to 0.2 mm SO42− resulted in a precipitous reduction in pHi and Cl−i. Removal of SO42−o with the concomitant addition of Cl−o resulted in increased pHi and Cl−i. Fig. 1C shows almost no change in Cl−i and pHi in water-injected oocytes under the same conditions.
FIGURE 1.
Slc26a2 functions as an electroneutral SO42−/OH−(H+)/Cl− exchanger. pHi (dark traces) and Cl−i (dark gray traces) and membrane potential (light gray traces) were simultaneously recorded in Slc26a2 (A) or H2O (C)-injected oocytes. Note that the membrane potential did not change following addition of SO42−. B, averaged (mean ± S.E. of the indicated number of experiments) transport rates of Cl− and OH−(H+) in the presence and absence of 0.2 mm SO42− were used to determine net transport (rightmost columns). D, pHi was recorded while clamping membrane potential at −100 , +40, or −30 mV, as indicated.
Reduction in pHi can be due to H+ influx or OH− efflux. Because some of the SO42− transport is coupled to Cl−, and the Cl− coupling is affected by pHo (see below), we will refer to the transported ion as OH−, although we cannot distinguish between the transport of OH− and H+. The average Slc26a2-mediated SO42−-coupled net Cl− and OH− transports are shown in Fig. 1B and indicate that under the conditions of Fig. 1A ∼40% of SO42− is transported in exchange for Cl− and ∼60% in exchange with OH−. SO42− transport is electroneutral, because it is not associated with a change in membrane potential (Fig. 1), and SO42−-coupled OH− (Fig. 1D) and Cl− (not shown) fluxes are the same at membrane potentials of +40 and −100 mV. This indicates that the coupling stoichiometry of SO42− exchange with Cl− and OH− is likely 1:2 with Slc26a2 functioning as SO42−/2OH−, SO42−/2Cl− and possible SO42−/OH−/Cl− exchanger.
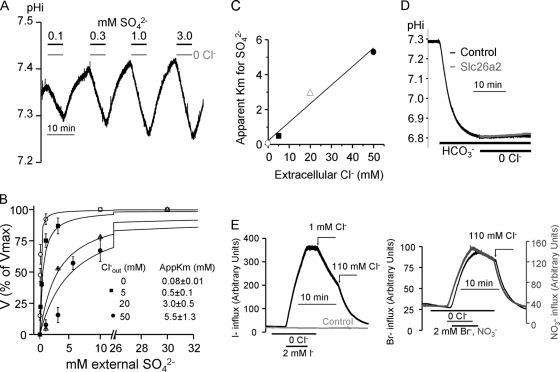
To further determine the relationship between SO42− and Cl− we measured the effect of Cl−o on the apparent affinity for SO42−o. Fig. 2A shows an example of the protocol used for these experiments. Oocytes expressing Slc26a2 were exposed to solutions containing the desired Cl−o (0, 5, 20 or 50 mm) and SO42− concentration for 5 min to obtain the rate of OH− efflux. Then the oocytes were incubated in Cl−-containing solution without SO42− to extrude the SO42− and recover pHi before exposure to the subsequent SO42− concentration. The plots in Fig. 2B obtained from these experiments were used to calculate the apparent Km for SO42− that were then plotted as a function of Cl−o (Fig. 2C). The linear relationship in Fig. 2C indicates that Cl−o competes with SO42−o for interaction with the external substrate site. This competition is different from the non-saturating Cl−o dependence reported for the isotopic exchange of Cl−o with intracellular Cl− (Cl−i) or intracellular oxalate (24). This may reflect the different dependence of Cl−o of the half (exchange) and full turnover cycle (net) of transport by Slc26a2.
FIGURE 2.
Effect of Cl−o on Slc26a2-mediated SO42−o/OH−i exchange. A, Slc26a2-expressing oocytes were alternately perfused with a solution containing 110 mm Cl− and Cl−-free solutions containing the indicated SO42− concentrations for ∼5 min. The plots in B are the relative average rates of OH− fluxes as a function SO42− at Cl−o of 0 (○), 5 (■), 20 (△), and 50 (●) mm. The rates were normalized to the rate at 30 mm SO42−, which was taken as 100%, and the plots were fitted to the Hill equation. The averages are the mean ± S.E. of 3–5 experiments at each Cl−o concentration. The resulting apparent Km values for SO42− are plotted as a function of Cl−o (C). D, control (H2O-injected, black trace) and Slc26a2-expressing oocytes (gray trace) bathed in HCO3−-buffered solution were exposed to Cl−-free solution at the indicated time to asses Cl−/HCO3− exchange activity. In E the exchange of I−, Br−, and NO3− with Cl−i was measured by incubating oocytes in Cl−-free solution containing 2 mm of I− (left traces), Br−, or NO3− (right traces). The influx was terminated by removal of the anions from the bath and anion efflux was initiated by addition of Cl− to the bath. The lack or minimal anion efflux in the absence of Cl−o indicates low net and exchange rate of the anions with OH− and the rapid efflux of the anions upon addition of Cl−o indicates fast exchange rate of the anions with Cl−. The control in the left panel is representative of oocytes injected with H2O.
Many of the SLC26 transporters can transport HCO3− in exchange for Cl− (9). However, Fig. 2D shows that Slc26a2 does not function as a Cl−/HCO3− exchanger. The capacity of Slc26a2 to transport other anions, such as I−, Br− and NO3−, in addition to SO42−, OH− and Cl− was further tested by measuring their intracellular concentration. supplemental Fig. 1B shows that the resin used to detect Cl− can also detect Br− and NO3− ∼10 times better than Cl− and I− ∼100 times better than Cl− (see also (29)). The left panel of Fig. 2D shows that exposing Slc26a2-expressing oocytes to Cl−-free solution containing 2 mm I− resulted in a rapid influx of I−. Removal of I− in the absence of Cl−o stopped the influx. To initiate I− efflux it was necessary to add Cl−o, with as little as 1 mm Cl−o resulting is nearly maximal rate of I− efflux. Similar behavior was observed with Br− and NO3− (Fig. 2D, right panel) and no I− (Fig. 2D, gray trace, left panel), Br− or NO3− (not shown) fluxes were observed in water-injected oocytes. These findings indicate that the Slc26a2 permeation pathway is not very selective and can accommodate I−, Br− and NO3− to mediate I−/Cl−, Br−/Cl− and NO3−/Cl− exchange.
The Ratio of SO42−/2OH− and SO42−/2Cl− Exchange Is Determined by pHo
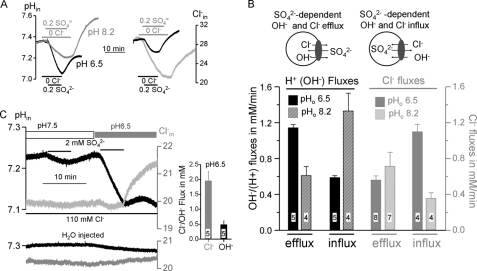
Coupling of SO42− transport to OH− and Cl− raised the question of how the availability of substrate would affect the coupling. We addressed this question by examining the effect of pHi and pHo on SO42− transport. Fig. 3A shows example traces of the changes in pHi (left panel) and of the Cl−i (right panel) as a result of SO42− transport at pHo of 6.5 (black traces) and 8.2 (gray traces). The rates of OH− and Cl− influx and efflux under both conditions are summarized in Fig. 3B. The models in Fig. 3B show the direction of ion fluxes during SO42− influx (left) and SO42− efflux (right) and the columns show the associated OH−(H+) and Cl− fluxes at pHo of 6.5 and 8.2. SO42− influx is coupled to Cl− and OH− efflux, while SO42− efflux initiated by removal of SO42−o and addition of Cl−o is coupled to Cl− and OH− influx. During SO42− influx acidic pHo increases OH− efflux with low Cl− efflux while alkaline pHo has the opposite effect. On the other hand, during SO42− efflux acidic pHo inhibits OH− efflux and increases Cl− efflux while alkaline pHo has the opposite effect.
FIGURE 3.
Effect of pHo on SO42−o influx and SO42−i efflux. A, example traces for measurement of pHi and Cl−i in solutions buffered to pHo of 6.5 (black traces) or 8.2 (gray traces). The models in B show the direction of the fluxes and the average efflux and influx rates of OH−(H+) (dark columns) and Cl− (gray columns) fluxes are summarized in B. The results are the mean ± S.E. of the number of experiments listed in the columns. In C pHi and Cl−i were measured at 110 mm Cl−o and first at pHo of pH 7.5 and then at pHo of 6.5. The columns on the right show the average rates (mean ± S.E.) of Cl− and OH− influx at pHi of 6.5 upon removal of SO42−, and the lower traces are example traces obtained in H2O-injected oocytes.
Fig. 3C further illustrates the reciprocal effect of pHo on OH− and Cl− fluxes. Exposing Slc26a2-expressing oocytes to a solution buffered to pH 7.5 and containing 110 mm Cl− and 2 mm SO42− resulted in a reduction in pHi at a rate of ∼0.18 ± 0.03 mm/min (n = 8), with no change in Cl−i. Removal of SO42− resulted in recovery of pHi. H2O injected oocytes showed no response to SO42−. Hence, at high Cl−o and pHo of 7.4 all the Slc26a2-mediated SO42−iflux is mediated by SO42−/2OH− exchange (or SO42−/2H+ cotransport). When the same oocytes were exposed to the same solution containing 110 Cl− and 2 mm SO42−, but now buffered to pH of 6.5, SO42− uptake resulted in a large reduction in pHi with no change in Cl−i, while SO42− efflux initiated by removal of SO42−o resulted in a small increase in pHi and a large Cl− influx (Fig. 3C). Thus, at low pHo SO42− uptake is predominantly mediated by SO42−/2OH−(2H+) exchange, while SO42− efflux is dominated by SO42−i/Cl−o exchange.
The sulfate transported species can be SO42− or HSO3−. Although we did not examine this in great detail, the results in Figs. 1–3 favor SO42−. Thus, if the transported species is HSO3− then acidic pHo should markedly enhance Sulfate influx. Fig. 3B indicates that is not the case. Second, SO42− efflux after removal of SO42−o should be independent of pHo since pHo should have no effect of the transported SO42− species. Again, this is not the case. Third, changes on pHo have the same effect on SO42− and Ox2− transport (see below), suggesting that the transport rate follows the pH gradient rather than substrate species.
Coupling of SO42− transport to both Cl− and OH− may function to ensure SO42− uptake under acidic and alkaline conditions. Slc26a2 is expressed in the luminal membrane of polarized cells (31, 35) that can be exposed to acidic and alkaline pH. In the stomach and synovial fluid pH is acidic (36, 37) and SO42−o/2OH−i exchange mediates most SO42− uptake. On the other hand, in secretory glands, like the pancreas (38) and salivary glands (39), luminal pH is alkaline, which inhibits SO42−o/OH−i exchange (Fig. 3B) and most SO42− uptake is by SO42−o/2Cl−i exchange.
Regulation of Slc26a2 by Cl−o
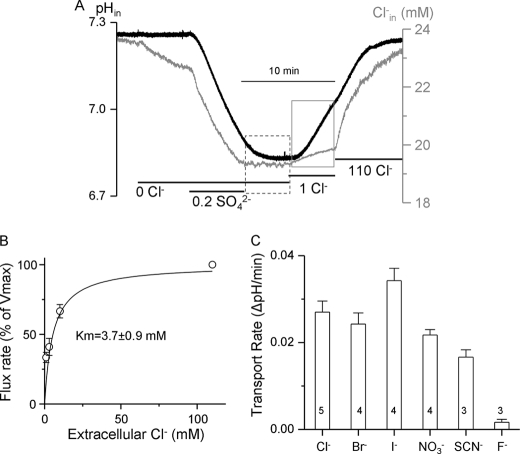
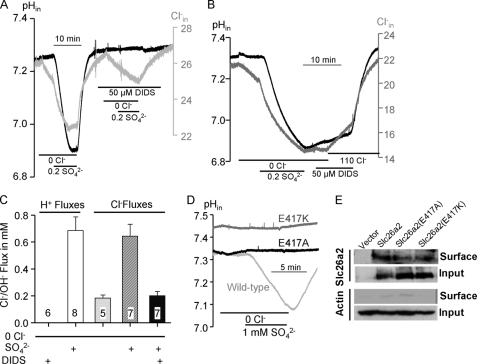
While measuring net SO42− efflux we noticed that removal of SO42−i in the continuous absence of Cl−o never resulted in SO42− efflux, as would be expected from SO42−i/2OH−o exchange. This is illustrated in the period bordered by the dashed box of Fig. 4A. However, addition of as little as 1 mm Cl−o triggered a robust SO42−i/2OH−o exchange and a small Cl− influx (Fig. 4A, period marked by gray box). The dependence of the SO42−i/2OH−o exchange rate of Cl−o followed simple saturation curve with apparent Km of 3.7 ± 0.9 mm (Fig. 4B). Activation of the exchange was not specific for Cl−. Fig. 4C shows that 1 mm external Cl−, Br−, I−, NO3− and SCN− similarly activated SO42−i/2OH−o exchange. Only 1 mm F− did not activate the exchange (Fig. 4C), but actually inhibited the exchange initiated by the other anions (not shown).
FIGURE 4.
Activation of Slc26a2-mediated SO42−i/OH−o exchange by Cl−o. A, example traces of Cl−i (black) and pHi (gray) in oocytes expressing Slc26a2. SO42−iflux was terminated by removal of SO42−o (period bordered by dashed line). SO42− efflux did not start until the addition of 1 mm Cl−o (period bordered by gray square), which triggered robust SO42−i/OH−o exchange with minimal SO42−i/Cl−o exchange. B, the relative rate of SO42−i/OH−o exchange is plotted as a function of the activating Cl−o. The rates at each Cl−o were normalized to the rate measured at 110 mm Cl−o, which was taken as 100%. The plot is the average of 3–5 experiments and fitted to the Hill equation. C, the protocol in A was used to measure activation of SO42−i/OH−o exchange by 1 mm of the indicated anions (period marked by gray square). The results are mean ± S.E. of the number of experiments indicated in the columns.
The findings in Fig. 4 suggest that SO42− transport by Slc26a2 is regulated by interaction of an anion with a regulatory site. The regulatory site is not selective for Cl−, but because Cl− is the major extracellular anion, Slc26a2 is likely regulated by Cl−o interaction with the regulatory site. The Cl−o regulatory site is likely different from the transport site since increased Cl−o should increase SO42−i/2Cl−o exchange while reducing SO42−i/2OH−o exchange. However, the opposite is observed. Activation of Slc26a2-mediated SO42−i/2OH−o by Cl−o may be by stabilization of an active Slc26a2 conformation. However, the exact mechanism remains to be elucidated. The physiological significance of regulation of Slc26a2 activity by Cl−o is not known at present. The Cl− content in the GI tract is high in the range of 100–150 mm and is determined largely by acid secretion (40). On the other hand, urine Cl− can be below 4 mm when prerenal azotemia occurres with metabolic alkalosis (41) and regulation of Slc26a2 by Cl−o can become significant. In addition, the luminal membrane-localized Slc26a2 is exposed to variable Cl− concentrations, as low Cl− in ducts that absorb the Cl−, such as the pancreatic (38) and salivary (32) ducts, the intestine (42) and the vas deferens (43). One possibility is that SO42− absorption take place only when there is some luminal Cl−. Perhaps in Cl− absorbing epithelia completion of Cl− absorption may be used to signal termination of the SO42− absorptive activity. Additionally, regulation by Cl−o may be used to stop reverse Slc26a2 transport to prevent SO42− loss by SO42− efflux across the luminal membrane due to SO42−i/2OH−o when luminal Cl− becomes very low due to Cl− absorption.
A Potential Slc26a2 Permeation Pathway
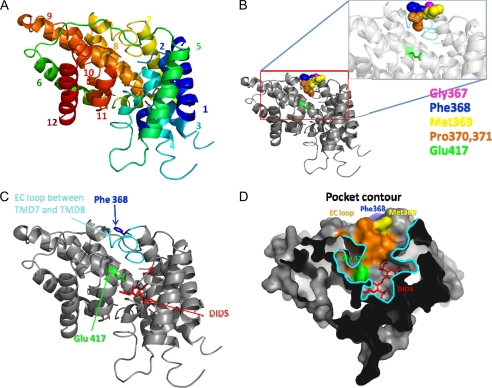
In a previous study we developed a model of the Slc26a6 transmembrane sector to search for motifs that determine the function of the electrogenic Slc26 transporters as coupled and uncoupled transporters (29). The modeling identified a glutamate (Glu−) conserved in all Slc26 transporters that has the same orientation as Glu− E148 in the Cl− permeation pathway of the ClC transporters (44–47). Interestingly, a recent study utilized the predicted Slc26a6 model and the crystal structure of the Slc26a5 STAS domain to assemble a detailed putative structure of Slc26 transporters (48). This structure showed a surprising similarity to the low resolution structure of a bacterial Slc26 homologue obtained using SANS (small angle neutron scattering) method in terms of symmetry and size. Notably, this study suggested that Slc26 functions as a dimer, as was previously suggested based on the predicted similarity of Slc26a6 to the bacterial ClC-ec dimeric crystal structure. Therefore, assuming a similar overall architecture for Slc26 transporters, we thread the Slc26a2 transmembrane sector on the Slc26a6 model to determine the localization of the conserved Glu− Glu417 (Fig. 5). Another purpose of the modeling was to identify additional determinants of the Slc26a2 ion permeation pathway and perhaps the extracellular Cl− regulatory site. The motif GSGIP was identified as a potential anion (Cl−) binding site that is conserved in the ClC transporters (44, 49). Mutations of residues within this motif altered ionic selectivity and coupling in the yeast and mammalian ClCs (50, 51). We searched for a similar motif in the Slc26 transporters. Although identical motif is not present in the Slc26 transporters, supplemental Fig. 2 shows the presence of the well conserved sequence GFXXP. The structural model in Fig. 5 shows the predicted localization of the Slc26a2 conserved Glu− Glu417 and phenylalanine Phe368 and of a potential DIDS binding site.
FIGURE 5.
In silico model of the putative structure of the Slc26a2 TMDs. The model was derived by threading the TMD sector of Slc26a2 on the TMDs of Slc26a6 reported before (29) (see “Experimental Procedures”). A, shows the predicted position of the 12 transmembrane helices of Slc26a2. B, shows the space filling of the GFMPG sequence. Highlighted in C are the positions of the extracellular loop located between TMDs 7 and 8 (light blue), the conserved phenylalanine Phe368 (blue), the conserved Glu− Glu417 (green), and the putative position of DIDS binding site (red). D, illustrates a cross-section (∼20 Å) through the surface representation of the putative Slc26a2 revealing a potential binding cavity. Interestingly, Glu− 417 (green) and the EC loop (orange) are constituents of the binding pocket (outlined in cyan) in which the inhibitor DIDS (red) is also bound.
To test the prediction in Fig. 5 we first determined the sensitivity of Slc26a2 to DIDS. Fig. 6A shows that 50 μm DIDS completely inhibited SO42−-driven OH−(H+) efflux and most of the SO42−-driven Cl− efflux. The residual DIDS-insensitive Cl− efflux is likely not mediated by Slc26a2 but by a DIDS-insensitive transporter native to the oocytes. Fig. 6B shows that DIDS inhibited SO42− efflux when added after SO42− uptake. Also in this case DIDS completely inhibited OH−(H+) influx, but with a residual Cl− influx. Similar results were obtained with 10 and 50 μm DIDS, indicating that the DIDS sensitivity of Slc26a2 is in the same range of that reported for Slc26a6 (52). Fig. 6C summarizes the rates of OH− and Cl− fluxes in the absence and presence of SO42− and DIDS, indicating that at pHo of 7.5 and the absence of Cl−o ∼60% of SO42− uptake is coupled to OH− efflux and 40% to Cl− efflux. Fig. 6D test another prediction of the model in Fig. 5 by neutralizing (Slc26a2(E417A)) or reversing (Slc26a2(E417K)) the charge of the conserved Glu417. Both mutations eliminated SO42− (Fig. 6D) and I− (not shown) transport activity. Inhibition of transport was not due to altered trafficking of the mutants to the plasma membrane (Fig. 6E).
FIGURE 6.
Inhibition of Slc26a2 by DIDS and by mutations of Glu417. Example traces depicting inhibition of SO42−iflux (A) and SO42− efflux (B) by 50 μm DIDS. Note the complete inhibition of the coupled OH− but not of Cl− fluxes. C, the average rates (mean ± S.E. of the indicated number of experiments) of OH− and Cl− fluxes. In D shown are examples of oocytes expressing either wild-type Slc26a2, Slc26a2(E417A), or Slc26a2(E417K) that were used to measure the SO42−-associated OH− and Cl− fluxes. E, shows the surface expression of Slc26a2 and mutants with actin used as a control for the biotinylation.
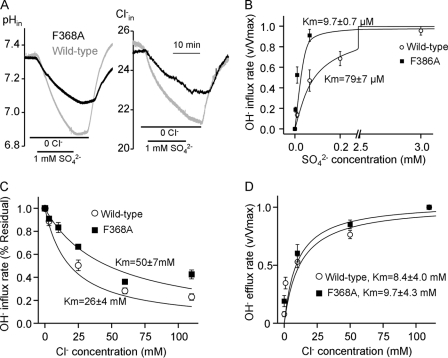
The sequence GFXXP is predicted to be in the extracellular loop between transmembrane domains (TMDs) 7 and 8, with Phe368 predicted to be in the entrance of the permeation pathway (Fig. 5). The mutations G367A and P371A had no effect on SO42− transport or its coupling to Cl− and OH− (not shown). However the F368A mutation had multiple effects. Fig. 7A shows that Slc26a2(F368A) is ∼50% less active than wild-type Slc26a2 in exchanging SO42− for OH− (left traces) and Cl− (right traces). Most notably, the F368A mutation increased the apparent affinity of Slc26a2 for SO42− by ∼8-fold to reduce the apparent Km for SO42−o from 79 ± 7 to 9.7 ± 0.7 μm. Unexpectedly from competition between SO42−o and Cl−o (Fig. 2), the F368A mutation increased the apparent Km for inhibition of SO42− uptake by Cl−o from 26 to 50 mm (Fig. 7C). Hence, Phe368 appears to control the access of SO42− and Cl− to the permeation pathway. Interestingly, Fig. 7D shows that the F368A mutation had no effect of the apparent affinity for the Cl−o regulatory site that activates SO42−i/OH−o exchange. This finding provides the strongest evidence that inhibition of SO42− uptake by Cl−o (Figs. 4B and 7C) and activation of SO42−i/OH−o exchange by Cl−o probably involves interaction of Cl−o with two separate sites.
FIGURE 7.
Phe368 in Slc26a2 permeation pathway. A, example traces for pHi and Cl−i measurement in oocytes expressing either wild-type (gray traces) or Slc26a2(F368A) (black traces). This protocol was used to monitor Slc26a2-mediated the SO42− dependence of SO42−o/OH−i exchange (B) and inhibition of the exchange by Cl−o (C). SO42−i/OH−o exchange was used to monitor activation of the reverse exchange by Cl−o (D). All plots (B–D) were fitted to the Hill equation, and Km values are given as mean ± S.E.
The findings in Fig. 7, A–C provide additional evidence for the importance of the GSGIP or the GFXXP motifs in the function of the Cl− transporters, in addition to the two additional GXXXP motifs that participate in Cl− transport in the bacterial ClCs (44). The bacterial ClC-ec1 crystal structure shows that the permeation pathway has three Cl− interacting sites (44–46, 49). Ser107 and Gly108 in the GSGIP motif coordinate the Cl− ion in the internal substrate site, and the side chain of Ser107 participates in binding of the middle Cl− (45, 49). In Slc26a2 Phe368 appears to control the affinity for the substrate (SO42− ), suggesting that Phe368 may participate in the access of SO42− to the permeation pathway or in shaping the external SO42− binding site. The increased apparent affinity for SO42− and reduced apparent affinity for Cl− by the F368A mutation suggests that Phe368 may hinder access of SO42− and facilitate access of Cl− to the permeation pathway or reduces the time SO42− spends in the external binding site on its way across the plasma membrane. Perhaps this is necessary to allow SO42−i/Cl−o exchange at high SO42−i when SO42− efflux is required. Irrespective of the exact role of Phe368, the present findings further support the notion of similarities between the ClC and SLC26 transporters permeation pathways and that the opening of the permeation pathway is situated in the region of TMDs 7 and 8.
Properties of Slc26a2-mediated Oxalate Transport
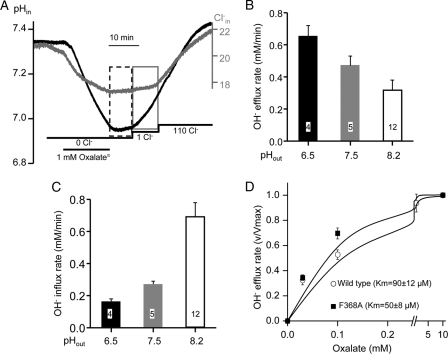
Slc26a2 was reported to transport Oxalate (Ox2−) by mediate Ox2−/SO42− exchange (8, 24–26, 28) and that Ox2−/SO42− exchange is 10 times slower than SO42−/Cl− exchange (24). However, the properties and mode of Ox2− transport and the capacity of net Ox2− transport by Slc26a2 are not known. We set to estimate net Ox2− transport by measuring Ox2−-mediated OH− and Cl− fluxes. Fig. 8A shows that Slc26a2 mediates net Ox2−o/OH−i and Ox2−o/Cl−i exchange in oocytes bathed in Cl−-free solution containing 1 mm Ox2−, pH 7.5. Removal of Ox2− was not followed by Ox2− efflux until the addition of 1 mm Cl−o to activate the efflux. Importantly, addition of 1 mm Cl−o resulted in minimal Ox2−i/Cl−o exchange but near maximal Ox2−i/OH−o exchange. Increasing Cl−o to 110 mm caused a small additional increase in Ox2−i/OH−o exchange and modest Ox2−i/Cl−o exchange. As expected, Fig. 8B shows that reducing pHo to 6.5 increased the rate of Ox2−o/OH−i exchange and increasing pHo to 8.5 inhibited the Ox2−o/OH−i exchange. Fig. 8C shows the opposite effect of pHo on the Ox2−i/OH−o exchange. Finally, the F368A mutation increased the apparent affinity for Ox2− and reduced the apparent Km for Ox2− from 90 ± 12 to 50 ± 8 μm. Although this was not as prominent as the increased apparent affinity for SO42− (Fig. 7), it was in the same direction. The results in Fig. 8, A–D indicate that the properties of Ox2− transport closely resemble those of SO42− transport, although at the same conditions the Ox2− transport rate was ∼50% slower than the SO42− transport rate.
FIGURE 8.
Properties of Slc26a2-mediated oxalate (Ox2− ) transport. A, example traces of the Slc26a2-mediated OH− (black trace) and Cl− (gray trace) efflux in response to addition of 1 mm Ox2−o to Cl−-free solution at pH 7.5. Removal of Ox2−o terminated the influx (dashed box) and addition of 1 mm Cl−o was required to initiate Ox2−i/OH−o exchange (gray box) with minimal Ox2−i/Cl−o exchange. B and C, effect of pHo of Ox2− fluxes was as in A, except that the Ox2− efflux was initiated by the addition of 110 mm Cl−o and pHo was set at 6.5 (dark columns), 7.5 (gray columns), or 8.2 (empty columns). The results are the mean ± S.E. of the number of experiments indicated in the columns. D, the dependence of Ox2−o/OH−i exchange on Ox2−o concentrations was measured with wild-type Slc26a2 (○) and Slc26a2(F368A) (■). The apparent Km values are given as the mean ± S.E. of 4–5 experiments.
In summary, the present study reports the mechanism of SO42− and Ox2− transport by Slc26a2. Both anions are transported in exchange for Cl− and OH− or by cotransport with H+. Based on the rate of the coupled OH−(H+) fluxes in the absence of Cl−o and at substrate concentration of 1 mm, net SO42− transport by Slc26a2 is about twice faster than net Ox2− transport. Under normal conditions plasma oxalate is in the micromolar range and even in patients with primary hyperoxaluria plasma oxalate is around 40 μm (53). Moreover, although Slc26a2 is expressed at high level in the luminal membrane of colonic crypts (21), SO42− in the colon can be in the millimolar range both in human (54) and animals (55) that will favor SO42− uptake by Slc26a2. Indeed, the colon is a major site of SO42− absorption (54, 56) that is likely mediated by Slc26a2. Similarly, although Slc26a2 is expressed in the proximal tubule luminal membrane (31), SO42− concentration in the proximal tubule is in the millimolar range, and although the role of Slc26a2 in the kidney is not know, if any it is likely to function mainly as an SO42− transporter (8). The only possible scenario where Slc26a2 can affect Ox2− homeostasis is by mediating Ox2− secretion in exchange for external SO42− when external Cl− is low and pH is high. Even then, this process will be inhibited by the high cytoplasmic Cl− typical of epithelia and by intracellular SO42−. Thus Slc26a2 is not likely to play a major role in oxalate metabolism in the colon or the kidney.
The permeation pathway includes the conserved SLC26 transporter Glu− and may lay between TMD7 and TMD8, where a phenylalanine conserved in the loop predicted to connect the TMDs may control SO42− and Cl− access to the permeation pathway. As yet, mutations of these residues, or even in the vicinity of these residues, have not been found in patients with diastrophic dysplasia (57). This is most likely because Slc26a2 is an essential gene and the mutations that markedly affect Slc26a2 activity may not be compatible with life. Indeed, analysis of several disease causing Slc26a2 mutations showed retention of some SO42− transport capacity by the mutants and a good correlation between loss of SO42− transport and disease severity (25, 26). The coupling of SO42− transport to both OH− and Cl− likely serves to ensure transport at both acidic pH when most SO42− uptake is mediated by SO42−/2OH− exchange and alkaline pH when most SO42− uptake is mediated by SO42−/2Cl− exchange. Slc26a2 is also regulated by an extracellular anion binding site different from the transport site, the physiological function of which remains to be determined, although it may control SO42− uptake when Cl−o is very low.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant Z1A-DE000735 from NIDCR.

This article contains supplemental Figs. S1 and S2.
The amino acid sequence of this protein can be accessed through NCBI Protein Database under NCBI accession number NP_031911.
- Slc26a1 and -a2
- solute carrier family 26 isoforms a1 and a2
- subscripts “i” and “o”
- used throughout as intracellular and extracellular, respectively
- DIDS
- diisothiocyanostilbene-2,2′-disulfonic acid
- TMD
- transmembrane domain.
REFERENCES
- 1. Neel N. F., Schutyser E., Sai J., Fan G. H., Richmond A. (2005) Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 16, 637–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alnouti Y. (2009) Bile acid sulfation. A pathway of bile acid elimination and detoxification. Toxicol. Sci. 108, 225–246 [DOI] [PubMed] [Google Scholar]
- 3. Gorsi B., Stringer S. E. (2007) Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 17, 173–177 [DOI] [PubMed] [Google Scholar]
- 4. Nakato H., Kimata K. (2002) Heparan sulfate fine structure and specificity of proteoglycan functions. Biochim. Biophys. Acta 1573, 312–318 [DOI] [PubMed] [Google Scholar]
- 5. Habuchi O. (2000) Diversity and functions of glycosaminoglycan sulfotransferases. Biochim. Biophys. Acta 1474, 115–127 [DOI] [PubMed] [Google Scholar]
- 6. Stone M. J., Chuang S., Hou X., Shoham M., Zhu J. Z. (2009) Tyrosine sulfation. An increasingly recognised post-translational modification of secreted proteins. N. Biotechnol. 25, 299–317 [DOI] [PubMed] [Google Scholar]
- 7. Elgavish A., Meezan E. (1991) Sulfation by human lung fibroblasts: SO4(2-) and sulfur-containing amino acids as sources for macromolecular sulfation. Am. J. Physiol. 260, L450-L456 [DOI] [PubMed] [Google Scholar]
- 8. Markovich D., Aronson P. S. (2007) Specificity and regulation of renal sulfate transporters. Annu. Rev. Physiol. 69, 361–375 [DOI] [PubMed] [Google Scholar]
- 9. Dorwart M. R., Shcheynikov N., Yang D., Muallem S. (2008) The solute carrier 26 family of proteins in epithelial ion transport. Physiology 23, 104–114 [DOI] [PubMed] [Google Scholar]
- 10. Ohana E., Yang D., Shcheynikov N., Muallem S. (2009) Diverse transport modes by the solute carrier 26 family of anion transporters. J. Physiol. 587, 2179–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Superti-Furga A., Hästbacka J., Wilcox W. R., Cohn D. H., van der Harten H. J., Rossi A., Blau N., Rimoin D. L., Steinmann B., Lander E. S., Gitzelmann R. (1996) Achondrogenesis type IB is caused by mutations in the diastrophic dysplasia sulphate transporter gene. Nat. Genet. 12, 100–102 [DOI] [PubMed] [Google Scholar]
- 12. Hästbacka J., de la Chapelle A., Mahtani M. M., Clines G., Reeve-Daly M. P., Daly M., Hamilton B. A., Kusumi K., Trivedi B., Weaver A. (1994) The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell 78, 1073–1087 [DOI] [PubMed] [Google Scholar]
- 13. Höglund P., Haila S., Socha J., Tomaszewski L., Saarialho-Kere U., Karjalainen-Lindsberg M. L., Airola K., Holmberg C., de la Chapelle A., Kere J. (1996) Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat. Genet. 14, 316–319 [DOI] [PubMed] [Google Scholar]
- 14. Everett L. A., Glaser B., Beck J. C., Idol J. R., Buchs A., Heyman M., Adawi F., Hazani E., Nassir E., Baxevanis A. D., Sheffield V. C., Green E. D. (1997) Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat. Genet. 17, 411–422 [DOI] [PubMed] [Google Scholar]
- 15. Liu X. Z., Ouyang X. M., Xia X. J., Zheng J., Pandya A., Li F., Du L. L., Welch K. O., Petit C., Smith R. J., Webb B. T., Yan D., Arnos K. S., Corey D., Dallos P., Nance W. E., Chen Z. Y. (2003) Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum. Mol. Genet. 12, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 16. Touré A., Lhuillier P., Gossen J. A., Kuil C. W., Lhôte D., Jégou B., Escalier D., Gacon G. (2007) The testis anion transporter 1 (Slc26a8) is required for sperm terminal differentiation and male fertility in the mouse. Hum. Mol. Genet. 16, 1783–1793 [DOI] [PubMed] [Google Scholar]
- 17. Jiang Z., Asplin J. R., Evan A. P., Rajendran V. M., Velazquez H., Nottoli T. P., Binder H. J., Aronson P. S. (2006) Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat. Genet. 38, 474–478 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y., Soyombo A. A., Shcheynikov N., Zeng W., Dorwart M., Marino C. R., Thomas P. J., Muallem S. (2006) Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion. Relevance to cystic fibrosis. EMBO J. 25, 5049–5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shcheynikov N., Yang D., Wang Y., Zeng W., Karniski L. P., So I., Wall S. M., Muallem S. (2008) The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3− exchanger. Role of Slc26a4 and Slc26a6 in I- and HCO3− secretion and in regulation of CFTR in the parotid duct. J. Physiol. 586, 3813–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forlino A., Piazza R., Tiveron C., Della Torre S., Tatangelo L., Bonafè L., Gualeni B., Romano A., Pecora F., Superti-Furga A., Cetta G., Rossi A. (2005) A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse. Morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum. Mol. Genet. 14, 859–871 [DOI] [PubMed] [Google Scholar]
- 21. Haila S., Hästbacka J., Böhling T., Karjalainen-Lindsberg M. L., Kere J., Saarialho-Kere U. (2001) SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J. Histochem. Cytochem. 49, 973–982 [DOI] [PubMed] [Google Scholar]
- 22. Ballhausen D., Bonafè L., Terhal P., Unger S. L., Bellus G., Classen M., Hamel B. C., Spranger J., Zabel B., Cohn D. H., Cole W. G., Hecht J. T., Superti-Furga A. (2003) Recessive multiple epiphyseal dysplasia (rMED). Phenotype delineation in eighteen homozygotes for DTDST mutation R279W. J. Med. Genet. 40, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Superti-Furga A., Rossi A., Steinmann B., Gitzelmann R. (1996) A chondrodysplasia family produced by mutations in the diastrophic dysplasia sulfate transporter gene. Genotype/phenotype correlations. Am. J. Med. Genet. 63, 144–147 [DOI] [PubMed] [Google Scholar]
- 24. Heneghan J. F., Akhavein A., Salas M. J., Shmukler B. E., Karniski L. P., Vandorpe D. H., Alper S. L. (2010) Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am. J. Physiol. Cell Physiol. 298, C1363-C1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karniski L. P. (2001) Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene. Correlation between sulfate transport activity and chondrodysplasia phenotype. Hum. Mol. Genet. 10, 1485–1490 [DOI] [PubMed] [Google Scholar]
- 26. Karniski L. P. (2004) Functional expression and cellular distribution of diastrophic dysplasia sulfate transporter (DTDST) gene mutations in HEK cells. Hum. Mol. Genet. 13, 2165–2171 [DOI] [PubMed] [Google Scholar]
- 27. Superti-Furga A., Neumann L., Riebel T., Eich G., Steinmann B., Spranger J., Kunze J. (1999) Recessively inherited multiple epiphyseal dysplasia with normal stature, club foot, and double layered patella caused by a DTDST mutation. J. Med. Genet. 36, 621–624 [PMC free article] [PubMed] [Google Scholar]
- 28. Satoh H., Susaki M., Shukunami C., Iyama K., Negoro T., Hiraki Y. (1998) Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J. Biol. Chem. 273, 12307–12315 [DOI] [PubMed] [Google Scholar]
- 29. Ohana E., Shcheynikov N., Yang D., So I., Muallem S. (2011) Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J. Gen. Physiol. 137, 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko S. B., Shcheynikov N., Choi J. Y., Luo X., Ishibashi K., Thomas P. J., Kim J. Y., Kim K. H., Lee M. G., Naruse S., Muallem S. (2002) A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 21, 5662–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chapman J. M., Karniski L. P. (2010) Protein localization of SLC26A2 (DTDST) in rat kidney. Histochem. Cell Biol. 133, 541–547 [DOI] [PubMed] [Google Scholar]
- 32. Melvin J. E., Yule D., Shuttleworth T., Begenisich T. (2005) Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 67, 445–469 [DOI] [PubMed] [Google Scholar]
- 33. Becker H. M., Bröer S., Deitmer J. W. (2004) Facilitated lactate transport by MCT1 when coexpressed with the sodium bicarbonate cotransporter (NBC) in Xenopus oocytes. Biophys. J. 86, 235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trott O., Olson A. J. (2010) AutoDock Vina. Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kujala M., Hihnala S., Tienari J., Kaunisto K., Hästbacka J., Holmberg C., Kere J., Höglund P. (2007) Expression of ion transport-associated proteins in human efferent and epididymal ducts. Reproduction 133, 775–784 [DOI] [PubMed] [Google Scholar]
- 36. Treuhaft P. S., McCarty D. J. (1971) Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 14, 475–484 [DOI] [PubMed] [Google Scholar]
- 37. Sachs G. (2003) Physiology of the parietal cell and therapeutic implications. Pharmacotherapy 23, 68S-73S [DOI] [PubMed] [Google Scholar]
- 38. Steward M. C., Ishiguro H., Case R. M. (2005) Mechanisms of bicarbonate secretion in the pancreatic duct. Annu. Rev. Physiol. 67, 377–409 [DOI] [PubMed] [Google Scholar]
- 39. Catalán M. A., Scott-Anne K., Klein M. I., Koo H., Bowen W. H., Melvin J. E. (2011) Elevated incidence of dental caries in a mouse model of cystic fibrosis. PLoS One 6, e16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guyton A. C., Hall J. E. (2006) Textbook of Medical Physiology, 11th Ed., Elsevier Saunders, Philadelphia [Google Scholar]
- 41. Anderson R. J., Gabow P. A., Gross P. A. (1984) Urinary chloride concentration in acute renal failure. Miner. Electrolyte Metab. 10, 92–97 [PubMed] [Google Scholar]
- 42. Kato A., Romero M. F. (2011) Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 73, 261–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu W. M., Shi Q. X., Chen W. Y., Zhou C. X., Ni Y., Rowlands D. K., Yi Liu G., Zhu H., Ma Z. G., Wang X. F., Chen Z. H., Zhou S. C., Dong H. S., Zhang X. H., Chung Y. W., Yuan Y. Y., Yang W. X., Chan H. C. (2007) Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc. Natl. Acad. Sci. U.S.A. 104, 9816–9821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dutzler R., Campbell E. B., Cadene M., Chait B. T., MacKinnon R. (2002) X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294 [DOI] [PubMed] [Google Scholar]
- 45. Feng L., Campbell E. B., Hsiung Y., MacKinnon R. (2010) Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science 330, 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Accardi A., Miller C. (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427, 803–807 [DOI] [PubMed] [Google Scholar]
- 47. Picollo A., Pusch M. (2005) Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436, 420–423 [DOI] [PubMed] [Google Scholar]
- 48. Compton E. L., Karinou E., Naismith J. H., Gabel F., Javelle A. (2011) Low resolution structure of a bacterial SLC26 transporter reveals dimeric stoichiometry and mobile intracellular domains. J. Biol. Chem. 286, 27058–27067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faraldo-Gómez J. D., Roux B. (2004) Electrostatics of ion stabilization in a ClC chloride channel homologue from Escherichia coli. J. Mol. Biol. 339, 981–1000 [DOI] [PubMed] [Google Scholar]
- 50. Bergsdorf E. Y., Zdebik A. A., Jentsch T. J. (2009) Residues important for nitrate/proton coupling in plant and mammalian CLC transporters. J. Biol. Chem. 284, 11184–11193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zdebik A. A., Zifarelli G., Bergsdorf E. Y., Soliani P., Scheel O., Jentsch T. J., Pusch M. (2008) Determinants of anion-proton coupling in mammalian endosomal CLC proteins. J. Biol. Chem. 283, 4219–4227 [DOI] [PubMed] [Google Scholar]
- 52. Shcheynikov N., Wang Y., Park M., Ko S. B., Dorwart M., Naruse S., Thomas P. J., Muallem S. (2006) Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J. Gen. Physiol. 127, 511–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coulter-Mackie M. B., White C. T., Hurley R. M., Chew B. H., Lange D. (1993) Primary Hyperoxaluria Type 1, in GeneReviews (Pagon R. A., Bird T. D., Dolan C. R., Stephens K., eds) Seattle, WA [Google Scholar]
- 54. Florin T., Neale G., Gibson G. R., Christl S. U., Cummings J. H. (1991) Metabolism of dietary sulphate. Absorption and excretion in humans. Gut 32, 766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kerr B. J., Weber T. E., Ziemer C. J., Spence C., Cotta M. A., Whitehead T. R. (2011) Effect of dietary inorganic sulfur level on growth performance, fecal composition, and measures of inflammation and sulfate-reducing bacteria in the intestine of growing pigs. J. Anim. Sci. 89, 426–437 [DOI] [PubMed] [Google Scholar]
- 56. Ruppin H., Bar-Meir S., Soergel K. H., Wood C. M., Schmitt M. G., Jr. (1980) Absorption of short-chain fatty acids by the colon. Gastroenterology 78, 1500–1507 [PubMed] [Google Scholar]
- 57. Rossi A., Superti-Furga A. (2001) Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene (SLC26A2). 22 novel mutations, mutation review, associated skeletal phenotypes, and diagnostic relevance. Hum. Mutat. 17, 159–171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.