Abstract
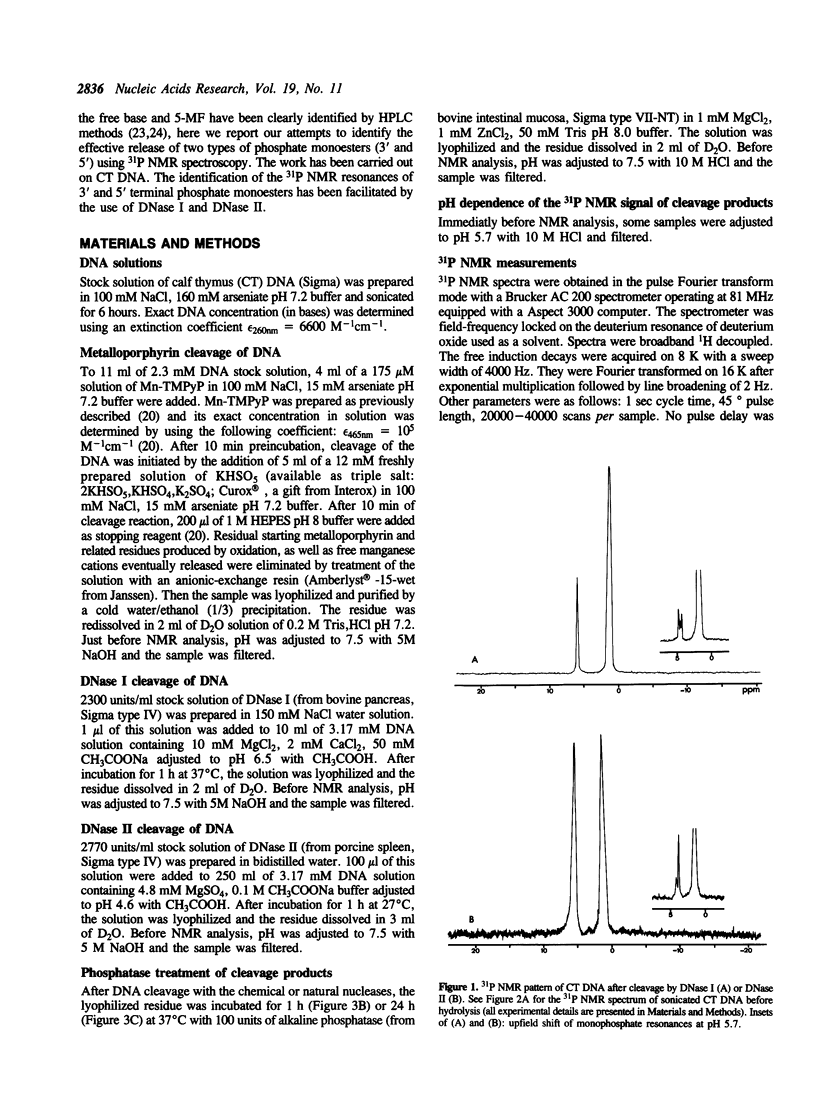
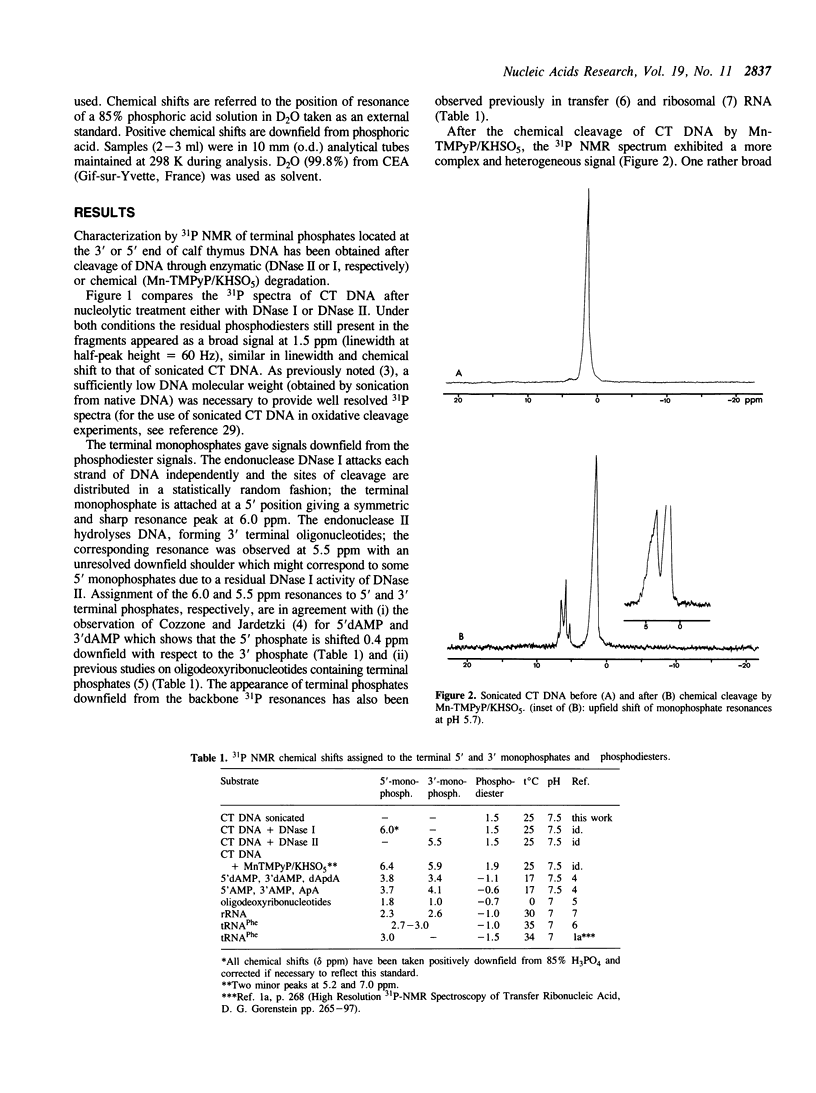
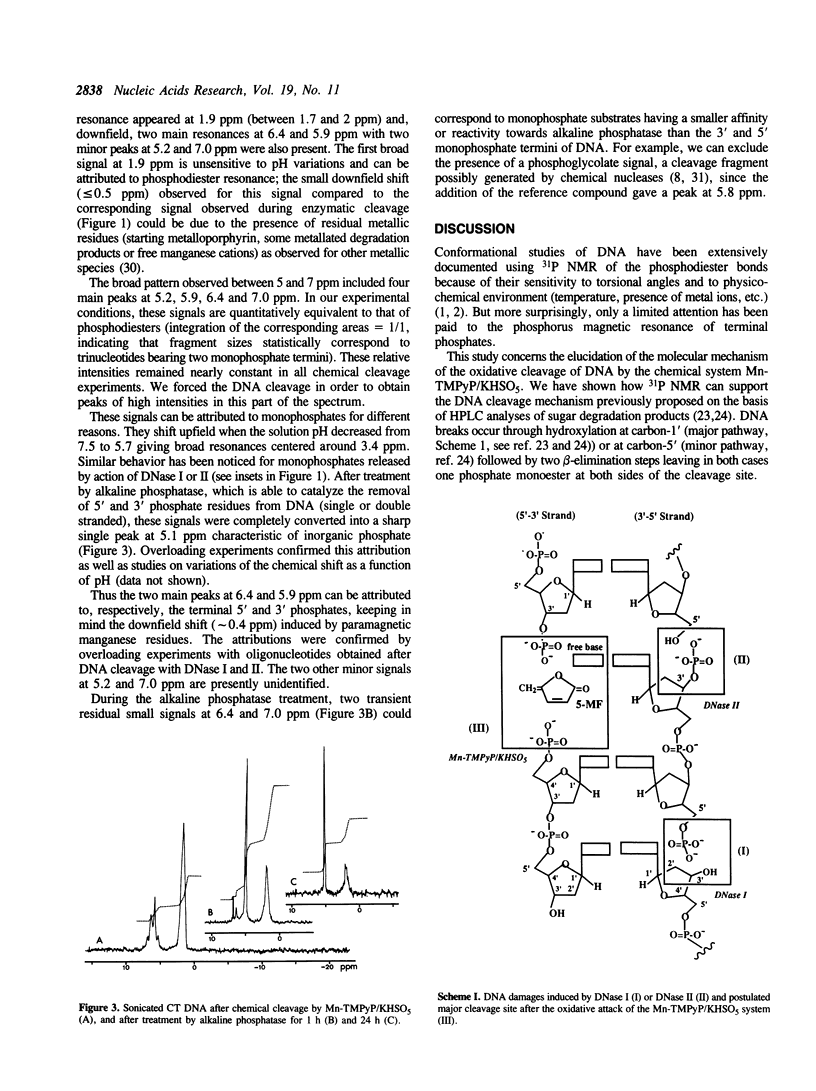
Phosphorus-31 NMR has been applied to the characterization of terminal phosphates on fragments of calf thymus DNA induced by three different nuclease systems: DNase I, DNase II and the artificial nuclease 'Mn-TMPyP/KHSO5'. In this last case, the oxidative damage to deoxyribose leads to two monophosphates esters (at the 3' and 5' ends) on both sides of the cleavage site. This method constitutes a promising approach to visualise the phosphate termini generated in DNA or RNA cleavage by cytotoxic drugs or chemical nucleases and provides a novel insight into the molecular aspects of their mechanism of action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aft R. L., Mueller G. C. Hemin-mediated DNA strand scission. J Biol Chem. 1983 Oct 10;258(19):12069–12072. [PubMed] [Google Scholar]
- Barton J. K. Metals and DNA: molecular left-handed complements. Science. 1986 Aug 15;233(4765):727–734. doi: 10.1126/science.3016894. [DOI] [PubMed] [Google Scholar]
- Bernadou J., Lauretta B., Pratviel G., Meunier B. Sur l'hydroxylation du carbone-1' du déoxyribose comme mécanisme de coupure du poly(dA) par une porphyrine de manganèse associée au monopersulfate de potassium. C R Acad Sci III. 1989;309(10):409–414. [PubMed] [Google Scholar]
- Bernadou J., Pratviel G., Bennis F., Girardet M., Meunier B. Potassium monopersulfate and a water-soluble manganese porphyrin complex, [Mn(TMPyP)](OAc)5, as an efficient reagent for the oxidative cleavage of DNA. Biochemistry. 1989 Sep 5;28(18):7268–7275. doi: 10.1021/bi00444a019. [DOI] [PubMed] [Google Scholar]
- Bower M., Summers M. F., Kell B., Hoskins J., Zon G., Wilson W. D. Synthesis and characterization of oligodeoxyribonucleotides containing terminal phosphates. NMR, UV spectroscopic and thermodynamic analysis of duplex formation of [d(pGGAATTCC)]2, [d(GGAATTCCp)]2 and [d(pGGAATTCCp)]2. Nucleic Acids Res. 1987 Apr 24;15(8):3531–3547. doi: 10.1093/nar/15.8.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzone P. J., Jardetzky O. Phosphorus-31 Fourier transform nuclear magnetic resonance study of mononucleotides and dinucleotides. 1. Chemical shifts. Biochemistry. 1976 Nov 2;15(22):4853–4859. doi: 10.1021/bi00667a016. [DOI] [PubMed] [Google Scholar]
- Dabrowiak J. C., Ward B., Goodisman J. Quantitative footprinting analysis using a DNA-cleaving metalloporphyrin complex. Biochemistry. 1989 Apr 18;28(8):3314–3322. doi: 10.1021/bi00434a029. [DOI] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Ding L., Etemad-Moghadam G., Cros S., Auclair C., Meunier B. Syntheses and in vitro evaluation of water-soluble "cationic metalloporphyrin-ellipticine" molecules having a high affinity for DNA. J Med Chem. 1991 Mar;34(3):900–906. doi: 10.1021/jm00107a005. [DOI] [PubMed] [Google Scholar]
- Ding L., Etemad-Moghadam G., Meunier B. Oxidative cleavage of DNA mediated by hybrid metalloporphyrin-ellipticine molecules and functionalized metalloporphyrin precursors. Biochemistry. 1990 Aug 28;29(34):7868–7875. doi: 10.1021/bi00486a013. [DOI] [PubMed] [Google Scholar]
- Fiel R. J., Beerman T. A., Mark E. H., Datta-Gupta N. DNA strand scission activity of metalloporphyrins. Biochem Biophys Res Commun. 1982 Aug;107(3):1067–1074. doi: 10.1016/0006-291x(82)90630-1. [DOI] [PubMed] [Google Scholar]
- Hertzberg R. P., Dervan P. B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): reaction conditions and product analyses. Biochemistry. 1984 Aug 14;23(17):3934–3945. doi: 10.1021/bi00312a022. [DOI] [PubMed] [Google Scholar]
- Kessel D. Porphyrin localization: a new modality for detection and therapy of tumors. Biochem Pharmacol. 1984 May 1;33(9):1389–1393. doi: 10.1016/0006-2952(84)90403-9. [DOI] [PubMed] [Google Scholar]
- Moan J. Porphyrin photosensitization and phototherapy. Photochem Photobiol. 1986 Jun;43(6):681–690. doi: 10.1111/j.1751-1097.1986.tb05647.x. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Gaudemer A., Verlhac J. B., Kraljic I., Sissoëff I., Guillé E. Photocleavage of DNA in the presence of synthetic water-soluble porphyrins. Photochem Photobiol. 1986 Dec;44(6):717–724. doi: 10.1111/j.1751-1097.1986.tb05529.x. [DOI] [PubMed] [Google Scholar]
- Roongta V. A., Jones C. R., Gorenstein D. G. Effect of distortions in the deoxyribose phosphate backbone conformation of duplex oligodeoxyribonucleotide dodecamers containing GT, GG, GA, AC, and GU base-pair mismatches on 31P NMR spectra. Biochemistry. 1990 Jun 5;29(22):5245–5258. doi: 10.1021/bi00474a005. [DOI] [PubMed] [Google Scholar]
- Salemink P. J., Swarthof T., Hilbers C. W. Studies of yeast phenylalanine-accepting transfer ribonucleic acid backbone structure in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1979 Aug 7;18(16):3477–3485. doi: 10.1021/bi00583a007. [DOI] [PubMed] [Google Scholar]
- Sigman D. S. Chemical nucleases. Biochemistry. 1990 Oct 2;29(39):9097–9105. doi: 10.1021/bi00491a001. [DOI] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A., Churchill M. E., Kam L. Hydroxyl radical footprinting: a high-resolution method for mapping protein-DNA contacts. Methods Enzymol. 1987;155:537–558. doi: 10.1016/0076-6879(87)55035-2. [DOI] [PubMed] [Google Scholar]
- Van Atta R. B., Bernadou J., Meunier B., Hecht S. M. On the chemical nature of DNA and RNA modification by a hemin model system. Biochemistry. 1990 May 22;29(20):4783–4789. doi: 10.1021/bi00472a006. [DOI] [PubMed] [Google Scholar]
- Ward B., Skorobogaty A., Dabrowiak J. C. DNA cleavage specificity of a group of cationic metalloporphyrins. Biochemistry. 1986 Nov 4;25(22):6875–6883. doi: 10.1021/bi00370a021. [DOI] [PubMed] [Google Scholar]
- Zhang P., Rycyna R., Moore P. B. A study of the conformation of 5S RNA by 31P NMR. Nucleic Acids Res. 1989 Sep 25;17(18):7295–7302. doi: 10.1093/nar/17.18.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]


