Abstract
Rationale
Phosphorylation of β2-adrenergic receptor (β2AR) by a family of serine/threonine kinases known as G protein-coupled receptor kinase (GRK) and protein kinase A (PKA) is a critical determinant of cardiac function. Upregulation of G protein-coupled receptor kinase 2 (GRK2) is a well-established causal factor of heart failure, but the underlying mechanism is poorly understood.
Objective
We seek to determine the relative contribution of PKA- and GRK-mediated phosphorylation of β2AR to the receptor coupling to Gi signaling that attenuates cardiac reserve and contributes to the pathogenesis of heart failure in response to pressure overload.
Methods and Results
Overexpression of GRK2 led to a Gi-dependent decrease of contractile response to βAR stimulation in cultured mouse cardiomyocytes and in vivo. Importantly, cardiac-specific transgenic overexpression of a mutant β2AR lacking PKA phosphorylation sites (PKA- TG), but not the wild type β2AR (WT TG) or a mutant β2AR lacking GRK sites (GRK- TG), led to exaggerated cardiac response to pressure overload, as manifested by markedly exacerbated cardiac maladaptive remodeling and failure, and early mortality. Furthermore, inhibition of Gi signaling with pertussis toxin restores cardiac function in heart failure associated with increased β2AR to Gi coupling induced by removing PKA phosphorylation of the receptor and in GRK2 transgenic mice, indicating that enhanced phosphorylation of β2AR by GRK and resultant increase in Gi-biased β2AR signaling play an important role in the development of heart failure.
Conclusions
Our data show that enhanced β2AR phosphorylation by GRK, in addition to PKA, leads the receptor to Gi-biased signaling which, in turn, contributes to the pathogenesis of heart failure, marking Gi-biased β2AR signaling as a primary event linking upregulation of GRK to cardiac maladaptive remodeling, failure and cardiodepression.
Keywords: β2-adrenergic receptor, G protein-coupled receptor kinase, Heart failure, hypertrophy
Introduction
Despite major developments in both diagnosis and treatment, heart failure (HF) continues to be a leading cause of death and disability in western countries and will become the number one killer worldwide in 20201. It has been controversial as to whether increased cAMP-PKA signaling is beneficial or detrimental in the context of heart failure. Patients with HF exhibit chronically enhanced PKA signaling,2, 3 and transgenic mouse models with cardiac specific overexpression of β1AR,3 the a-subunit of Gs,4 and the catalytic subunit of PKA5 display HF phenotypes, suggesting overtly enhanced PKA signaling is cardiac detrimental. Over the past two decades, compelling evidence indicates that phosphorylation of βARs by another family of serine/threonine kinases known as GPCR kinases (GRKs) in the heart is a critical determinant of cardiac function and has been implicated in many pathological conditions including HF.6 In humans or animal models with HF, chronic catecholamine elevation causes marked dysregulation of βARs, resulting in various molecular abnormalities, including upregulation of GRK2 and PTX-sensitive Gi proteins. Upregulation of both of these proteins have been implicated as causal factors in the development of HF. In particular, GRK2 is the most abundant and best-characterized GRK in the heart.7 GRK2 expression and activity are markedly elevated and play a central role in the HF-associated defect in βAR signaling and cardiac dysfunction. Myocardial ischemia and hypertension in humans and animal models have also been associated with elevated GRK2 expression and activity.6 These previous studies have defined GRK2 upregulation as an early common event in cardiac maladaptive remodeling and HF.
It has been shown that phosphorylation of β2AR plays a crucial role in regulating differential G protein coupling of the receptor. Specifically, βb2AR phosphorylation by PKA mediates the switch of coupling from Gs to Gi.8, 9 Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway β2AR physiologic signaling.10 Further studies have demonstrated that β2AR coupling to Gi may be also dependent on the receptor internalization and recycling.11–13 However, it is unclear whether GRK-mediated phosphorylation of β2AR is involved in the regulation of β2AR-coupled Gi signaling in heart. Because both GRK2 and Gi proteins are significantly elevated in HF caused by a multitude of etiologies,14–19 we hypothesize that the well-documented detrimental effects of GRK2 in the failing heart may causatively link to enhanced β2AR-coupled Gi signaling.
In the present study, we explored the mechanism which links pathological upregulation of GRK2 to the development of HF. We found both in vivo and in vitro that enhanced β2AR phosphorylation by GRK2 leads the receptor to Gi-biased signaling, and that inhibition of the Gi signaling blocks heart failure in transgenic mice with cardiac-specific overexpression of GRK2 (GRK2-TG) or of a β2AR mutant lacking all of the PKA phosphorylation sites (PKA- TG) subjected to pressure overload, marking Gi-biased β2AR signaling as a primary event linking upregulation of GRK2 to cardiac maladaptive remodeling and failure.
Methods and Materials
Generation of Transgenic Mice
Flag-tagged human β2AR mutants lacking either the putative GRK phosphorylation sites (GRK-) or the putative PKA phosphorylation sites (PKA-) (Fig. 2A&B) were subcloned into a pBluescript-based transgenic vector downstream of α-myosin heavy chain (α-MHC) gene promoter and upstream of the SV40 polyadenylation site. The detail sequences of the β2AR PKA- and GRK- mutants were presented in Fig. 2B. Transgenic mice with cardiac-specific overexpression of wild type human β2AR (WT TG) were imported from Dr. Gerald Dorn Lab.
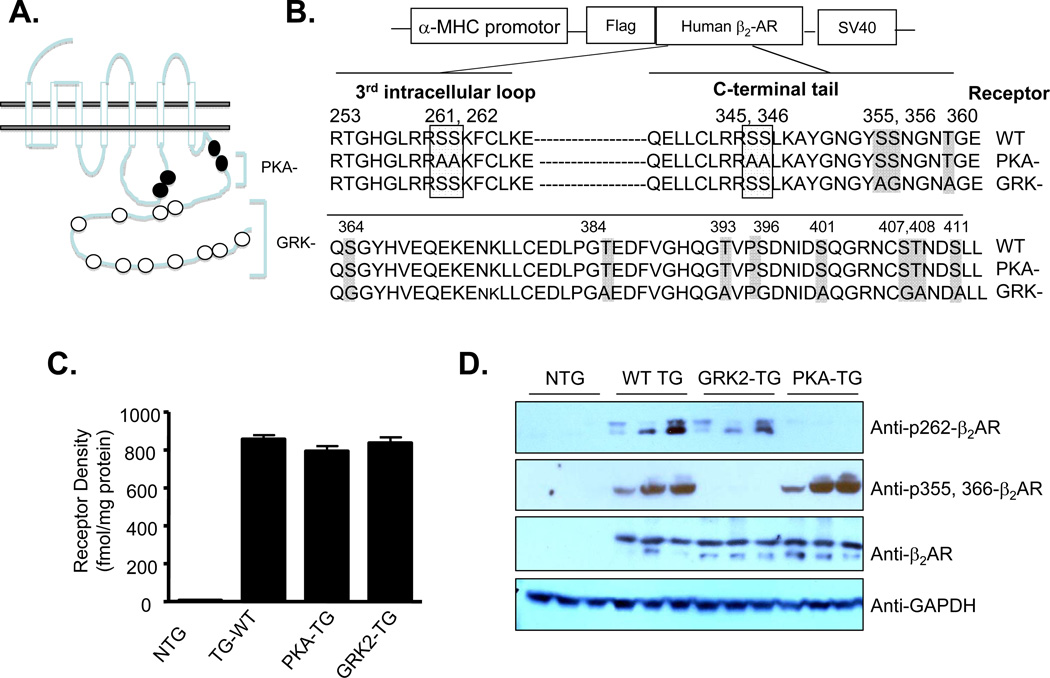
Figure 2. Transgenic mice with cardiac-specific overexpression of wild type (WT) human β2AR or its mutants lacking PKA or GRK phosphorylation sites.
(A) Schematic presentation showing β2AR phosphorylation sites for PKA or GRK. (B) The specific sequences of WT β2AR or its mutants lacking PKA (PKA-) or GRK (GRK-) phosphorylation sites. (C) The β2AR density was 9.6 ± 1.3 fmol/ mg protein and total βAR density was 28.6 ± 3.4 fmol/ mg protein in NTG mice (n=6). In transgenic mice, β2AR density was 856 ± 45, 828 ± 33 and 850 ± 23 fmol/mg protein for WT TG, PKA- TG, and GRK- TG mice, respectively, (n=6 for each group). (D) Phosphorylation of β2AR in PKA or GRK sites were assayed by Western blot using a site-specific antibody reacting with phosphorylated β2AR at a PKA site (aa262) or GRK sites (aa355 and aa366). The antibodies were raised against the peptides CDRTGHGLRRSpSKF-NH2 for the anti-pSer262 PKA site (clone 2G3) and CKAYGNGYpSpSNGN-NH2 for the anti-pS (Ser355, 356) (clone 5C3). Total expression of β2AR in transgenic mouse hearts was detected by Western blot using an antibody reacting with β2ARs.
Animal models
We used male non-transgenic mice (NTG), transgenic mice with cardiac-specific overexpression of wild type human β2AR (WT TG), or PKA-phosphodeficient β2AR (PKA- TG), or GRK-phosphodeficient β2AR (GRK- TG), and their littermate controls at 12–16 weeks of age. In addition, male transgenic mice with cardiac-specific overexpression of GRK2 (GRK2 TG) and their littermate control mice (LC) were used in a subset of experiments. Pressure overload was produced by transverse aortic constriction (TAC) as previously described 20.
Supplemental Materials on Detailed Methods
See the online supplemental materials for detailed methods regarding in vivo assessment of mouse cardiac contractility by echocardiography (ECHO) and Millar system, radioligand binding assay, western blot analysis, adult mouse cardiac myocyte culture and adenoviral gene transfer, cardiomyocyte contraction measurements, histological analysis, cAMP assay, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), and statistical analysis.
Results
Overexpression of GRK2 Causes βAR Dysfunction by Enhancing Gi Signaling in Cultured Cardiomyocytes And in Vivo
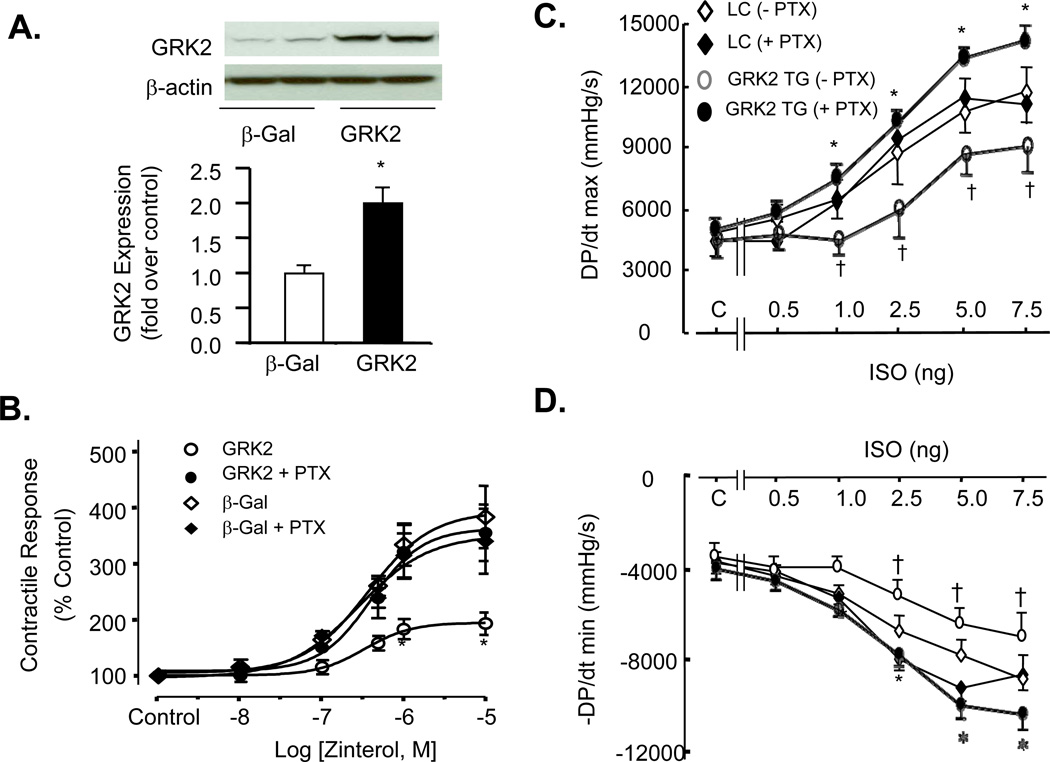
We have recently demonstrated that Gi-biased β2AR signaling is dependent on agonist stimulation, and that prolonged absence of agonist stimulation leads to uncoupling of β2AR from the Gi signaling, as is the case in adult mouse cardiomyocytes cultured for 24 hours.21 Consistent with the previous notion, in adult mouse cardiomyocytes cultured and infected by Adv-β-Gal for 24 hours, β2AR stimulation with zinterol led to a full contractile response which was insensitive to pertussis toxin (PTX) treatment (Figure 1 A&B). Importantly, overexpression of GRK2 with adenoviral gene transfer suppressed β2AR-mediated contractile response and the inhibitory effect of GRK2 was fully abolished by disrupting Gi signaling with PTX (Figure 1A & B). Prolonged stimulation of cardiomyocytes with isoproterenol (ISO, 1 nM) in the presence or absence of PTX did not change the expression of GRK2 (Supplemental material, online Figure I). These results demonstrate, for the first time, that overexpression of GRK2 enhances Gi-biased β2AR signaling. To further determine whether in vivo overexpression of GRK2 in the heart can facilitate β2AR-couled Gi signaling and, if so, whether the enhanced Gi signaling is involved in GRK2-mediated βAR dysfunction, we took advantage of transgenic mice with cardiac-specific overexpression of GRK2.22 Consistent with our previous studies on these mice,22 in vivo experiments revealed that βAR-induced increases in cardiac contractility and relaxation, as measured by left ventricular (LV) +dP/dtmax and −dP/dtmin, respectively, were markedly suppressed in mice overexpressing GRK2 (GRK2 TG mice) compared to wild type littermate controls (LC mice) (Figure 1C&D). Remarkably, disruption of Gi signaling with PTX fully restored cardiac contractile response to βAR stimulation with ISO in GRK2 TG mice without altering the effect of ISO in LC mice (Figure 1C&D), indicating that overexpression of GRK2-induced cardiac βAR dysfunction is mediated by enhanced Gi signaling.
Figure 1. Inhibition of Gi by pertussis toxin (PTX) enhances βAR-mediated contractile response in cultured adult mouse cardiomyocytes overexpressing GRK2 and in GRK2 transgenic mice (GRK2 TG) in vivo.
(A) A representative Western blot (top panel) of GRK2 in cultured adult mouse cardiomyocytes infected with adenovirus expressing GRK2 (Adv-GRK2) or β-Gal (Adv-β-Gal) at m.o.i. 100 for 24 h and quantified data (bottom panel) (n=4; *p<0.01 v.s. β-Gal). (B) Increased GRK2 expression enhances β2AR to Gi signaling in adult mouse cardiomyocytes cultured for 24 h. In cultured cardiomyocytes infected with Adv-GRK2 or Adv-β-Gal (m.o.i. 100), overexpression of GRK2 enhances the receptor to Gi signaling, as evidenced by PTX-induced augmentation of β2AR contractile response. *P<0.001 v.s. other three groups with a two-way repeated measures analysis of variance (ANOVA); (C, D) Disruption of Gi signaling with PTX abolishes GRK2-induced dysfunction of βAR contractile response in GRK2 TG mice. In vivo assessment of left ventricular (LV) function of GRK2 TG mice and littermate control (LC) mice (n=6–7 in each group; *P<0.001 v.s. GRK2 TG mice without PTX with a two-way repeated measures analysis of variance (ANOVA); †p<0.05 GRK2 TG v.s. LC in the absence of PTX with post hoc testing and Bonferroni’s F test). For single cell experiments (panel B), cells were pretreated with PTX (1.5 µg/ml) 3 hours before agonist stimulation. For in vivo studies in panels C&D, one dose of PTX was administered i.p. 72 hours before hemodynamic measurements.
Augmentation of GRK-mediated β2AR Phosphorylation Leads To Exacerbated Cardiac Hypertrophy, Heart Failure, and Early Mortality in Response to Pressure Overload
Since β2AR can be phosphorylated by at least two types of protein kinases, PKA and GRK (Figure 2A), we sought to distinguish the potential role of GRK- versus PKA-mediated β2AR phosphorylation in the pathogenesis of HF in vivo. The experiments took advantage of three different transgenic mouse models we developed: mice cardiac-specifically overexpressed the WT human β2AR (WT TG) or β2AR mutants in which all of the PKA phosphorylation sites or all of the GRK phosphorylation sites were substituted by alanine (A) or glycine (G) (namely PKA-TG or GRK2- TG, respectively) (Figure 2 A&B) at a matched receptor density (Figure 2C). The receptor densities in hearts from WT TG, PKA- TG, and GRK- TG mice were detected by radioligand binding assay (856±45, 828±33, and 850±23 fmole/mg protein, n=3–4, respectively) (Figure 2C). As compared to non-transgenic (NTG) mice, overexpression of the WT β2AR led to profoundly increased phosphorylation of β2AR at both GRK and PKA sites assayed by site-specific antibodies (Figure 2D). As expected, PKA- TG mice showed a clear increase in phosphorylation of β2AR by GRK but not PKA, whereas GRK- TG mice exhibited augmented phosphorylation of β2AR by PKA but not GRK (Figure 2D). Thus, GRK- and PKA-mediated phosphorylation of β2AR was selectively enhanced in PKA- TG and GRK- TG mice, respectively.
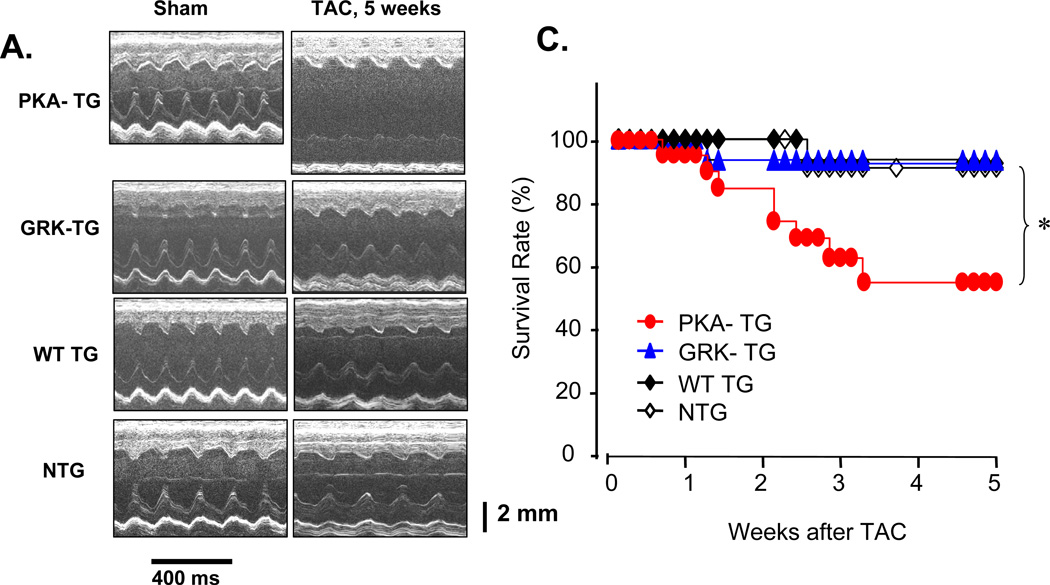
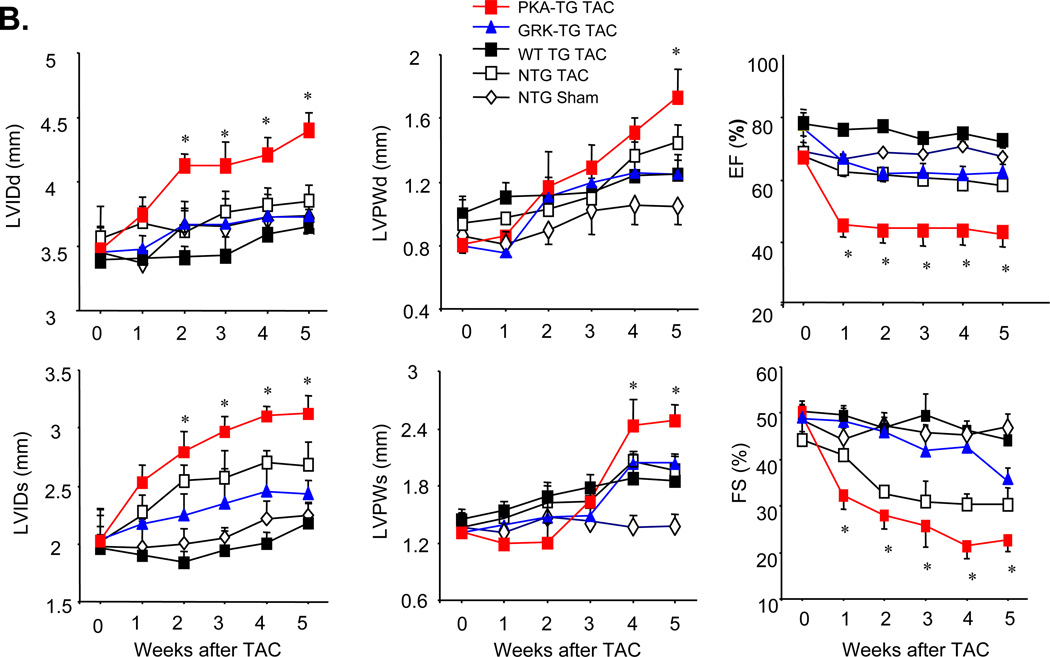
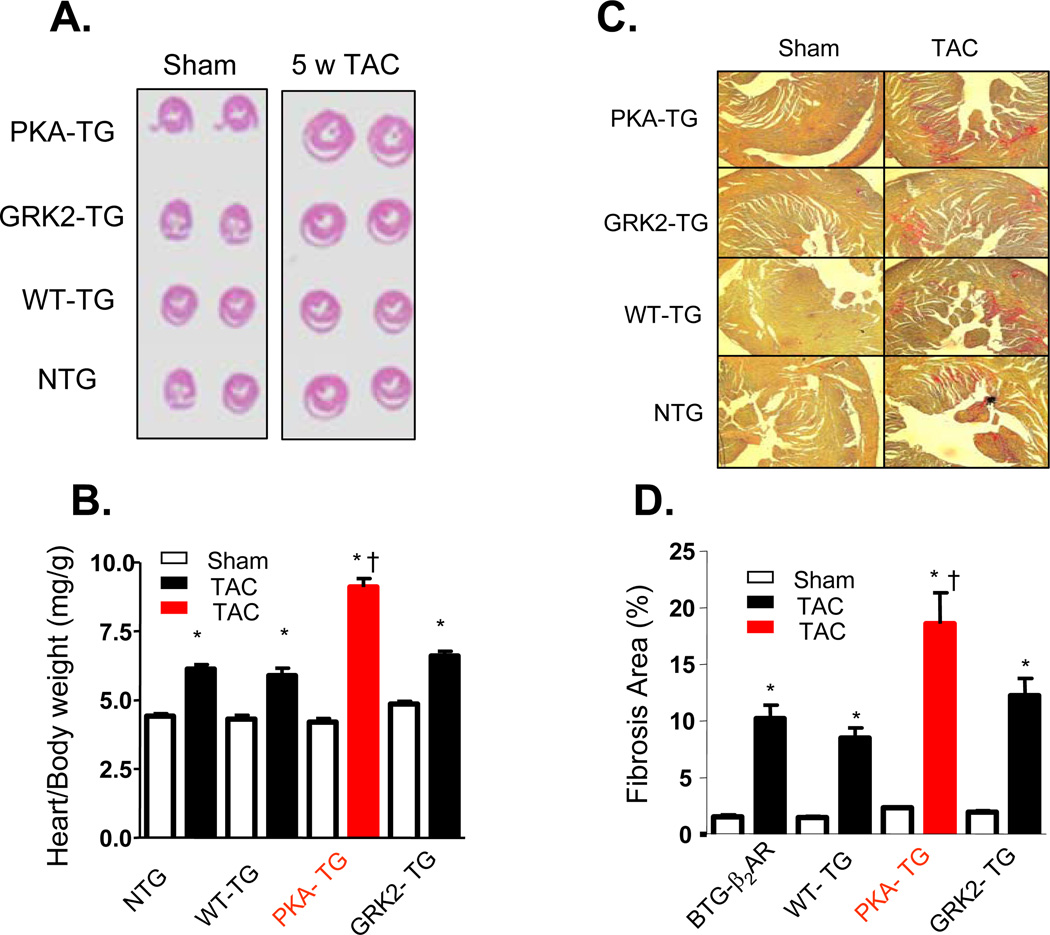
To investigate whether β2AR phosphorylation by GRK and PKA is differentially involved in the regulation of cardiac structure and function, NTG or PKA- TG, GRK- TG, or WT TG mice were subjected to pressure overload by transverse aortic constriction (TAC). There was no obvious phenotypic difference in cardiac anatomy and function among the genotypes under resting conditions at 4–5 months old of age, as assessed by echocardiography (ECHO) (Figure 3A&B). However, after TAC for 5 weeks, PKA- TG mice developed severe left ventricular dilation and contractile dysfunction (Figure 3 A&B) and increased mortality rate (Figure 3C). Cardiac dysfunction occurred as early as one week after TAC and progressively worse as TAC continued only in PKA- TG mice (Figure 3B). In contrast, GRK- TG mice were not different from NTG or WT TG mice in terms of cardiac anatomy and function in response to TAC. Furthermore, PKA- TG mice displayed markedly exaggerated cardiac hypertrophic response to TAC, as manifested by significantly increased heart size (Figure 4A), heart/body ratio (Figure 4B), and cardiomycoyte size (supplemental material, online Figure II). It is also important to note that PKA- TG mice, but not NTG or WT TG or GRK- TG, showed increased fibrosis after TAC for 5 weeks (Figure 4C&D). Thus, pressure overload led to more severe ventricular dilation with increased end-diastolic and end-systolic dimensions, reduced fractional shortening (FS) (Figure 3), and exacerbated cardiac maladaptive remodeling (Figure 4) in PKA- TG mice but not in other groups including NTG, WT TG and GRK- TG mice.
Figure 3. Overexpression of β2AR mutant lacking PKA phosphorylation sites increases adverse pressure overload-induced ventricular remodeling.
(A) Representative serial M-mode echocardiography in conscious NTG, WT TG, PKA- TG or GRK2- TG mice measured before and 5 weeks after TAC. (B) Summary data of Left Ventricular Internal Dimension-Diastole (LVIDd), Left Ventricular Internal Dimension-Systole (LVIDs), Left Ventricular Posterior Wall Dimensions-diastole (LVPWd), Left Ventricular Posterior Wall Dimensions-Systole (LVPWDs), Ejection Fraction (EF), and Fractional Shorting (FS). Data are presented as means ± SE (n = 8–12 in each group) and data was analyzed with a two-way repeated measures analysis of variance (ANOVA). Bonferroni correction was applied for multiple comparisons. *p<0.05 PKA- TG v.s. other four groups. (C) Survival curves. Kaplan-Meier Survival Analysis, *P<0.05 PKA- TG v.s. other three groups (GRK2- TG, WT TG, and NTG) (n=28, 25, 24 and 25 for PKA- TG, WT TG, GRK2- TG, and NTG, respectively).
Figure 4. Overexpression of β2AR mutant lacking PKA phosophorylation sites exaggerated cardiac maladaptive remodeling after pressure overload.
(A) Cross sections of hearts subjected to Sham operation (Sham) or TAC for 5 weeks (5w TAC); (B) The ratio of heart/body weight (n= 8–12, *p<0.01 v.s. Sham; †p<0.01 v.s. other three groups with TAC). (C) Representative examples of myocardial connective tissue staining (E.P.S. R.) to show cardiac fibrosis (top) and average data of fibrosis area (bottom) (n=5 for each group, *p<0.01 v.s. Sham; †p<0.01 v.s. other three groups with TAC).
Accelerated Heart Failure of PKA- TG Mice Is Associated With Enhanced β2AR-coupled Gi Signaling
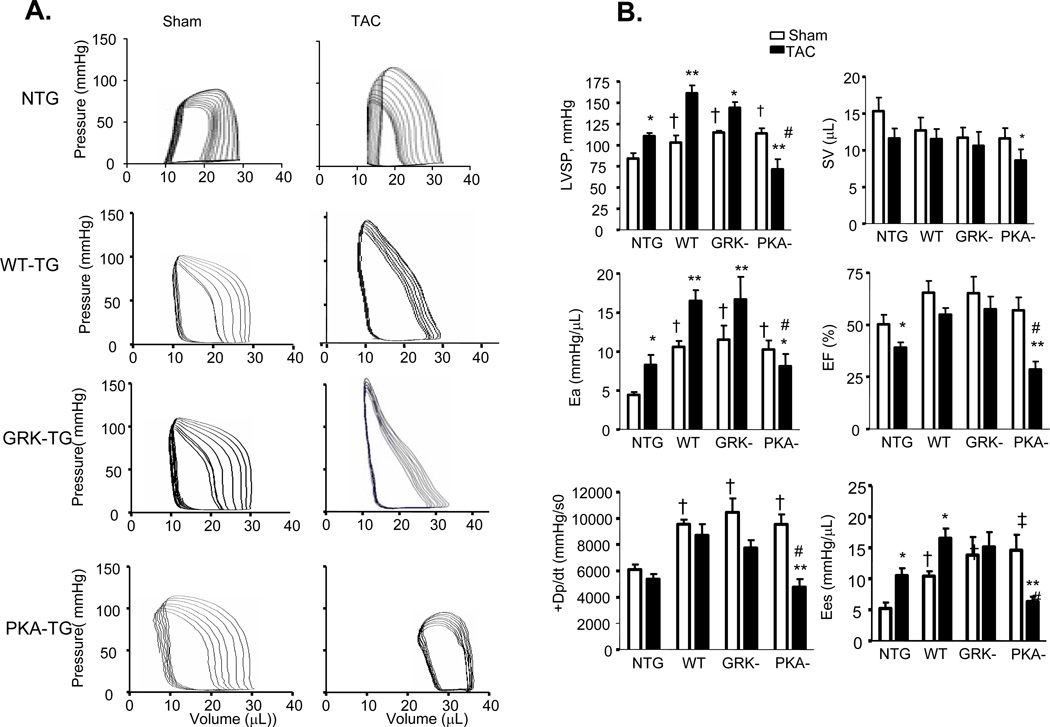
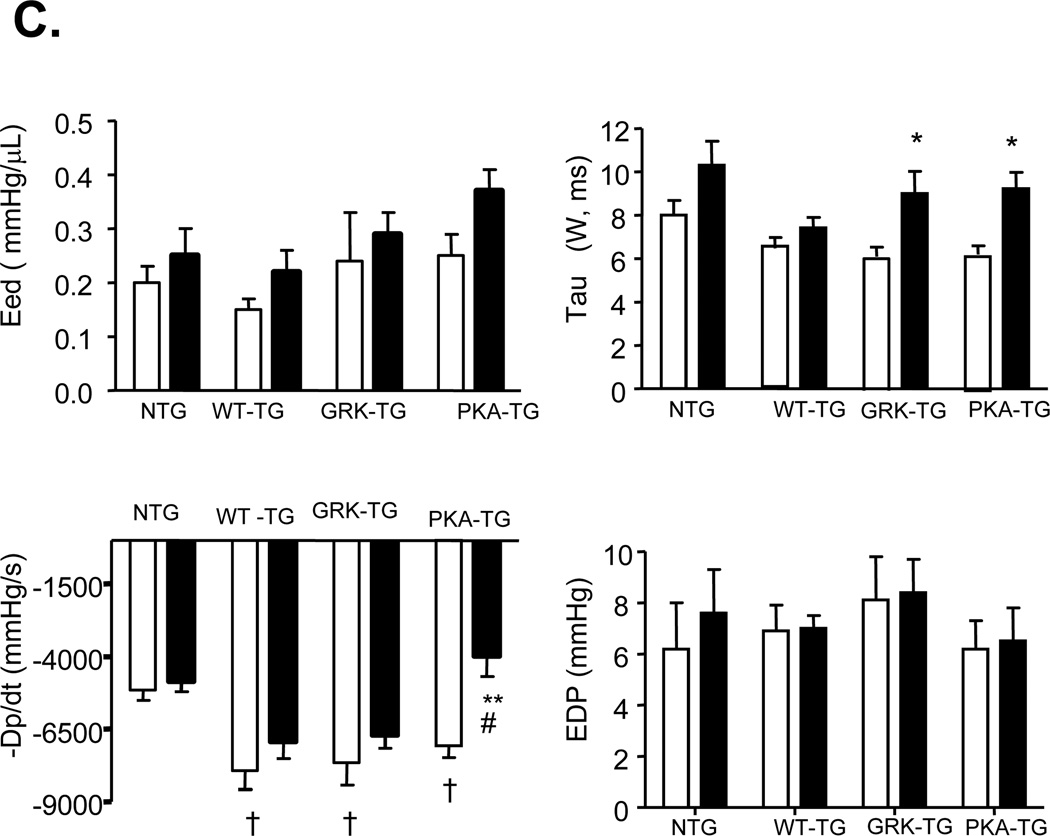
More detailed examination of cardiac function was carried out by invasive pressure-volume analysis. Pressure-volume loops were measured before and during transient reduction of chamber preload to generate specific end-systolic (ESPVR) and end-diastolic (EDPVR) pressure volume relations (Figure 5). After TAC for 5 weeks, NTG hearts had enhanced effective arterial elastance (Ea; an index of total ventricular afterload) and slope of end-systolic pressure-volume relation with reduced ejection fraction (EF), characteristics of hypertrophy induced by sustained pressure overload. Under resting conditions, PKA- TG, GRK- TG, and WT TG mice had similar increases in LV peak systolic pressure and basal systolic function with a slight upward and leftward shift of their ESPV compared to NTG mice. TAC for 5 weeks triggered distinct phenotypes between PKA- TG mice and GRK- TG or WT TG mice in terms of the progression of cardiac dysfunction. As shown by the representative examples, in the GRK-TG and WT TG mice, enhancements in resting Ea and slopes of ESPVR (before TAC) were slightly increased after 5 weeks of TAC, consistent with a functional response with feature of cardiac hypertrophy (Figure 5A). This did not occur in PKA- TG mice. Notably, PKA- TG mice displayed ventricular dilation, suppressed +dP/dt, reduced Ea, decreased EF associated with a rightward shift of pressure-volume relation, and a decrease in the slope of end systolic pressure-volume relation (Figure 5A&B). After 5 weeks of TAC, PKA- mice demonstrated a more significant decrease in maximal rate of pressure decline (−dP/dt), although a relaxation index tau was comparable in GRK- TG, and PKA- TG mice (Figure 5C).
Figure 5. In vivo cardiac pressure-volume relations in mice with sham operation or 5 weeks after TAC.
(A) Left-ventricular pressure-volume loops show TAC-induced increase in systolic load. In NTG-, WT TG, GRK- TG mice, TAC leads to rightward shift of the loops and end-systolic pressure-volume relation, marking hypertrophy remodeling. But in PKA- TG mice, TAC induces hear failure (HF). (B, C) Summary data on systolic function (B) and diastolic function (C). LVSP, left ventricle systolic pressure. SV, stroke volume. Ea, arterial elastance (measure of ventricular afterload). EF, ejection fraction. +dp/dt, maximum dP/dt. Ees, endsystolic pressure-volume relationship (ESPVR), Eed, end-diastolic pressure-volume relationship (ESDPVR). EDP, end-diastolic pressure. Tau, Regression of Log (pressure) vs time (Weiss method). All values present means ± SE (n=8–13 for each group, *P<0.05, **P<0.01 v.s. respective Sham; †P<0.05, ‡P<0.01 v.s. respective TAC).
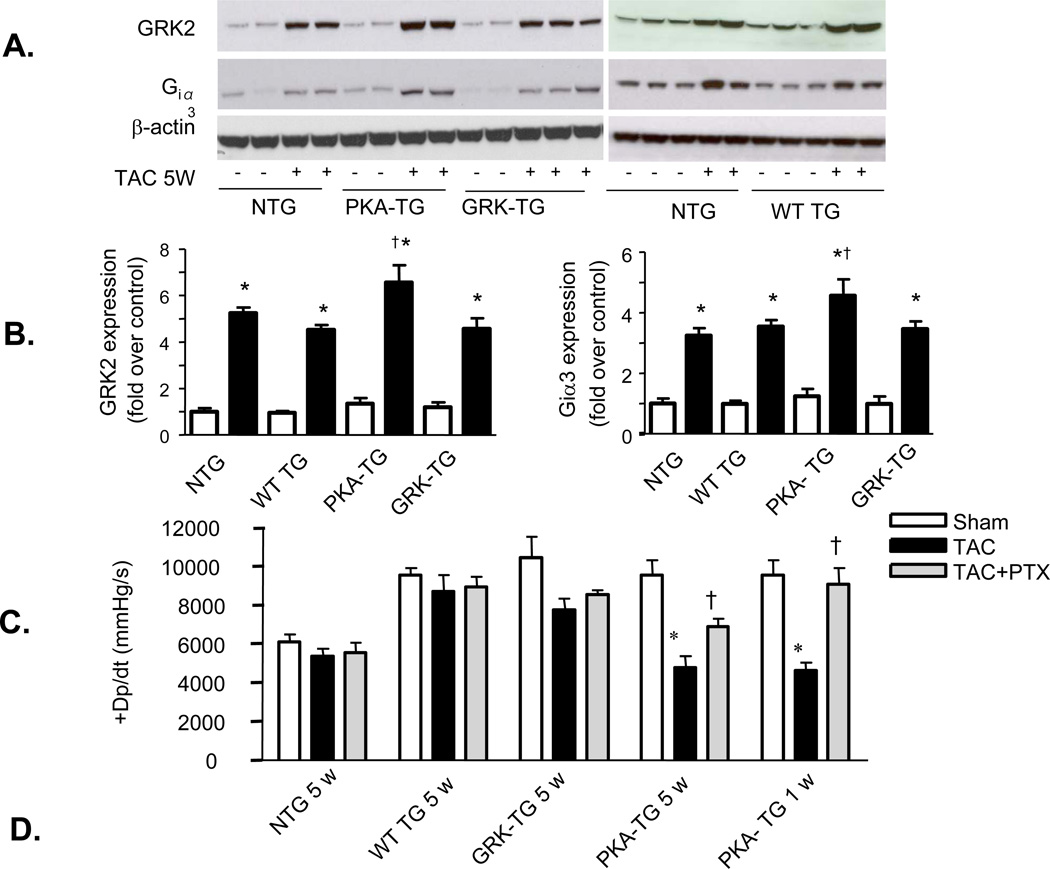
The aforementioned data clearly indicate that PKA- TG mice are more vulnerable to pressure overload, suggesting that selectively enhancing β2AR phosphorylation by GRK, but not PKA, profoundly exacerbates pressure overload-induced cardiac maladaptive remodeling and dysfunction. Next, we sought to decipher the mechanism underlying the distinct phenotypes of PKA- TG mice versus those of GRK- TG mice. Western blotting revealed that the increases in GRK2 and Gi protein abundance were significantly greater in hearts from PKA-TG mice relative to hearts from NTG or WT TG or GRK- TG mice, although both GRK2 and Gi proteins were markedly elevated in all genotypes after 5 weeks TAC (Figure 6A&B). Concomitantly, hearts from PKA- TG mice failed to compensate pressure overload even one week after TAC, resulting in significantly diminished cardiac contractility in PKA- TG mice compared to that in other genotypes (NTG or WT TG or GRK2- TG) (Figure 6C). Importantly, disruption of the Gi signaling with PTX treatment minimized the difference between PKA- TG mice and other groups (Figure 6C). In fact, in an early stage of heart failure (one week after TAC), PTX treatment fully restored cardiac function in PKA- TG mice, while in the later stage of heart failure (5 weeks after TAC), PTX substantially improved cardiac function in PKA- TG mice (Figure 6C), highlighting that enhanced GRK2 and subsequent enhancement of Gi-biased β2AR signaling play a crucial role in the triggering and worsening TAC-induced cardiac maladaptive remodeling and failure.
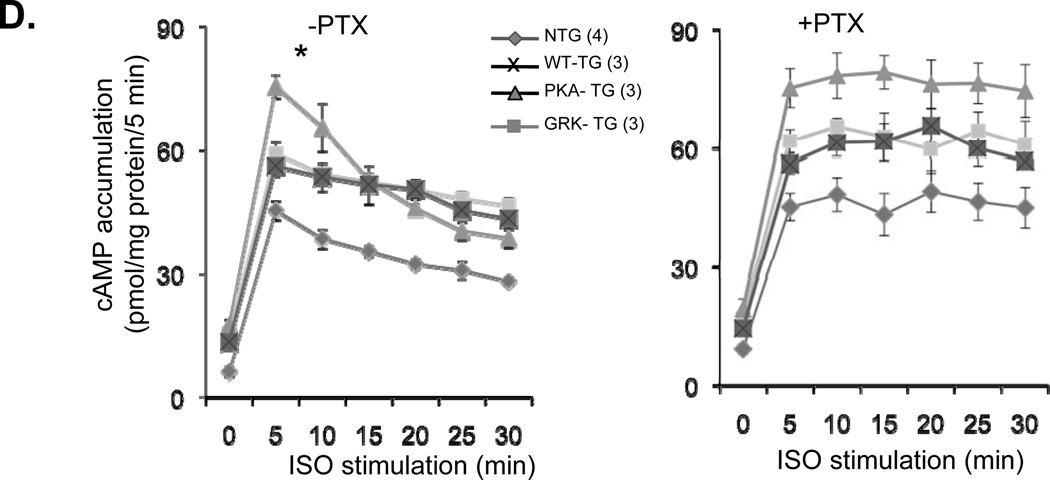
Figure 6. TAC-induced heart failure is associated with greater increases in Gi and GRK2 expression and inhibition of Gi signaling with PTX restores cardiac contractility and blocks the decay of cAMP accumulation by ISO in PKA- TG mouse hearts.
(A, B) Panel A shows representative Western bolts using a specific antibody reacting with GRK2 or Giα3 to assay GRK2 and Giα3 protein levels in hearts from NTG, WT TG, PKA- TG or GRK2- TG mice. β-actin was used as a control for protein loading. Panel B displays the average data (n=4–6 for each group, *p<0.01 v.s. respective Sham; †p<0.05 v.s. other three groups with TAC. (C) PTX restored the blunted cardiac contractility in PKA- TG mice subjected to 1 week or 5 weeks TAC (n=5–13, *p<0.05 v.s. respective Sham; †P<0.05 v.s. respective TAC). Note that PTX treatment fully restored contractile response in the early stage heart failure in PKA- TG mice subjected to 1 week TAC and significantly improved cardiac contractility even in the later stage heart failure in PKA- TG mice after 5 weeks TAC. (D) The decay of cAMP accumulation evoked by ISO (left panel) is prevented by PTX treatment (right panel). Cardiomyocytes from NTG, WT-, PKA-, GRK- TG mice were stimulated with isoproterenol (ISO, 10 µM). At each indicated time point, cells were treated with 200 µM of IBMX (3-isobutyl-1-methylxanthine) for 5 minutes before stopping reaction to accumulate cAMP. Note that the decay of cAMP response is accelerated in cardiomyocytes from PKA- TG mice compared to that of other groups, and that this genotype-specific difference is prevented by PTX treatment. *P<0.05 v.s. other three strains.
In principle, the cardiac dysfunction in PKA- TG mice could be due to an enhancement in the receptor to Gi signaling for attenuation of tonic cAMP signaling or due to cardiac adaptive remodeling for structure changes in myocardium or both alterations. To further investigate this issue, we measured basal and ISO-induced cAMP formation in cardiomyocytes from NTG or TG mice expressing WT or mutant β2AR. The present data demonstrated that spontaneous β2AR activity is increased in all of the transgenic mice expressing WT or mutant β2AR, as evidenced by their increased cAMP baselines (Figure 6D and supplementary data, online Figure III), consistent with the hemodynamic data (Figure 5). It is noteworthy that the decay of ISO-induced cAMP accumulation declines faster in cells from PKA- TG mice than that in cells from WT TG or GRK- TG mice. Since the decay of ISO-induced cAMP accumulation is sensitive to PTX, our data indicates that β2AR/Gi coupling is enhanced in PKA- TG mice. Thus, the cardiac dysfunction of PKA- TG mice is likely due to enhanced Gi signaling, which, in turn, contributes to cardiac adaptive remodeling and the progression of heart failure.
Discussion
The present study has revealed three major findings. First, phosphorylation of β2AR by GRK as well as PKA is a primary determinant of the receptor-coupled Gi signaling. Second, overexpression of GRK2 enhances Gi-biased β2AR signaling, which subsequently negates cardiac contractile response to βAR stimulation in a PTX-sensitive manner in GRK2 transgenic mice and cultured mouse cardiomyocytes. Third, selective augmentation of GRK-mediated β2AR phosphorylation in PKA- TG mice renders the heart more vulnerable to pressure overload and inhibition of Gi signaling can restore cardiac function of PKA- TG mice. In contrast, a selective increase in PKA-mediated phosphorylation of β2AR does not affect cardiac response to pressure overload in GRK- TG mice compared to NTG or WT TG mice. Therefore, we conclude that cardiac detrimental effects of GRK2 upregulation are mediated, at least in part, by enhanced β2AR-coupled Gi signaling which, in turn, contributes to the pathogenesis and progression of HF. Thus, both GRK2 and β2AR-coupled Gi signaling may offer novel therapeutic opportunities for the treatment of heart failure.
Role of GRK2 in Normal and Failing Hearts
In our previous studies, we have shown that cardiac-specific overexpression of GRK2 to the levels seen in human HF suppresses cardiac contractile response to βAR stimulation with ISO,22 indicating that myocardial overexpression of GRK2 triggers βAR desensitization in vivo. In contrast, overexpression of the peptide inhibitor of GRK2, GRK2-ct, enhances cardiac contractility and relaxation.22 Thus, GRK2 expression level has an important impact on cardiac performance under normal conditions. This perception has been further elucidated in recent studies on GRK2 knockout mouse models.32
In the failing heart, adrenergic overdriving occurs early in the progression to HF, as evidenced by increased catecholamine levels before the onset of HF.33 As a result, the expression and activity of GRK2 are elevated in the failing heart. Previous studies have shown upregulation of GRK2 is essentially involved in the pathogenesis of HF by further diminishing adrenergic responsiveness. Indeed, two recent clinical studies indicate that lymphocyte GRK2 decreases with angiotensin-converting enzyme (ACE) inhibitor therapy in class II HF,34 and that lymphocyte and myocardial GRK2 levels decrease, while bAR responsiveness increases, after mechanical left ventricular support in end-stage HF.26 Using heterozygous GRK2 knockouts with 50% diminished GRK2 expression, recent studies have established a dose-response between GRK2 levels and suppression of cardiac contractile function in HF.27, 28 Indeed, in various experimental models of HF, inhibition of GRK2 with GRK2-ct improves cardiac function by restoring βAR signaling (for review see refs 6, 35). Thus, upregulation of GRK2 and resultant βAR desensitization are initially adaptive compensation of the heart, but when exaggerated, causes cardiac maladaptive remodeling and HF.
The beneficial effect of restoration of βAR signaling through inhibiting GRK2 activity appears to contradict clinical convention that -blockade is widely used to treat patients with HF and chronic β-agonist (i.e., catecholamine) stimulation leads to deleterious effects. However, a close inspection reveals that the detrimental effects of catecholamines are mainly mediated by stimulation of β1ARs, which triggers myocyte apoptosis and arrhythmogenic events. In contrast, the present study has shown that inhibition of GRK2-mediated β2AR-coupled Gi signaling can normalize cardiac contractile response and ameliorate maladaptive remodeling. In this regard, our recent in vivo studies on a rat ischemic HF model have demonstrated that selective activation of β2AR can, indeed, improve cardiac function and reduce maladaptive remodeling as well as arrhythmogenic events.36 Taken together, we propose that a combination of GRK2 inhibition and β2AR activation with β1-blocker therapy may provide a more effective therapy for the treatment of HF.
Role of GRK-mediated β2AR Phosphorylation in Promoting Gi-biased signaling and Its Implications in Heart Failure
While βAR is classically viewed as a prototypical Gs-coupled receptor, compelling evidence indicates that β2AR couples dually to Gs and Gi proteins.37 We and others have previously shown that Gi-biased β2AR signaling may protect cardiomyocytes against various insulting stimuli induced apoptosis.38, 39 In addition, it has been shown that inhibition of Gi signaling worsens cardiac outcomes in response to ischemia/reperfusion injury and myocardial infarction,40 and that GRK inhibition with the peptide inhibitor, GRK2-ct, is cardioprotective, at least in part, due to increasing Gi-biased β2AR signaling.41 Furthermore, recent studies have shown that ablation of β2AR, indeed, worsens pressure overload-induced cardiac hypertrophy and remodeling in mice.42 These previous studies seem to contradictory to the present conclusion that an augmentation in GRK-mediated β2AR-Gi signaling contributes to cardiac maladaptive remodeling and failure under TAC in PKA- TG mice. To address this question, we have examined the potential effect of TAC on myocardial cell apoptosis in all four groups of mice (NTG, WT-TG, GRK-TG, PKA-TG) and found no genotype-related difference in TAC-triggered myocardial apoptosis (supplemental material, online Figure IV). In addition, we have shown that PTX enhances ISO-induced cardiomyocyte death, whereas β2AR stimulation with zinterol protects cardiomyocytes in PTX-sensitive manner (supplemental material, online Figure V), consistent with the previous notion that β2AR-Gi signaling is cardiac protective. However, cardiomyocytes from PKA- TG mice are more vulnerable than those from other groups (supplementary data, online Figure V), although β2AR-Gi signaling is markedly enhanced in PKA- TG mice. Thus, it merits future investigation to elucidate the exact mechanism underlying cardiac maladaptive remodeling and failure associated with enhanced GRK-mediated β2AR/Gi signaling in PKA- TG mice under TAC.
During prolonged agonist stimulation, GRK2 plays a predominant role in desensitizing βARs and has been implicated as a causal factor of HF, but the underlying etiological mechanism is unknown until now. Traditionally, it has been shown that GRK-mediated phosphorylation of βAR inhibits the interaction between activated receptor and G proteins via recruiting β-arrestins, which bind to phosphorylated βAR and sterically block the receptor coupling to the β subunit of Gs protein.43 In this study, however, we have provided multiple lines of evidence to show that increased phosphorylation of β2AR by GRK facilitates the receptor to Gi signaling which, in turn, results in cardiac contractile dysfunction and maladaptive remodeling. The present findings have also unraveled a novel function of the prototypical GRK, GRK2, in switching Gs- to Gi-biased β2AR signaling. Thus, in addition to PKA, GRK plays an important role in sorting β2AR to Gi-biased signaling pathway in response to enhanced catecholamine stimulation, as is the case in the failing heart.
It is noteworthy that, while the β1AR subtype does not couple to Gi under normal conditions, β1AR-mediated contractile response is cross-inhibited by enhanced Gi-biased β2AR signaling in the failing heart.29, 44 The reinforced GRK-dependent Gi-biased β2AR signaling is likely also responsible for GRK2 overexpression-induced dysfunction of β1AR in addition to the defect of β2AR contractile response in these transgenic mice. This idea is, indeed, corroborated by the fact that disruption of Gi signaling fully, rather than partially, restores the nonselective βAR agonist, ISO, induced positive inotropic effect in transgenic mice overexpressing cardiac GRK2 (Fig. 1C&D). Thus, similar to the situation of the failing heart,14, 44, 45 enhanced β2AR-coupled Gi signaling is responsible for the defects of both β1AR and β2AR signaling in the GRK2 TG mice, and that the previously reported beneficial effects of GRK2-ct in improving the function of the failing heart 23 is mediated, at least in part, by attenuating GRK-dependent Gi-biasedβ2AR signaling.
It is known that overexpression of βARs may alter their signaling behavior and G protein coupling properties.46 Overexpression of the PKA-phosphodeficient β2AR mutant in cells and in transgenic mice may complicate our conclusion on the functional consequence of GRK-mediated phosphorylation of β2AR in facilitating Gi-biased β2AR signaling because of possible disruptions of signaling events mediated by other molecules such as Gs, β-arrestins, and receptor trafficking,13 although the present study has used WT TG and GRK2- TG for comparison. This technical limitation should be taken into consideration, when the present data is interpreted.
In summary, we have revealed a novel function of GRK in promoting Gi-biased β2AR signaling that compromises cardiac reserve and contributes to the pathogenesis of HF. In the failing heart, enhanced expression and activity of GRK2 and Gi proteins further promote Gi-biased β2AR signaling, thus negating β1AR- and β2AR-mediated cardiac reserve function, resulting in cardiac maladaptive remodeling and failure. These in vitro and in vivo results have not only revealed a fundamental function and new mechanism of action of GRK2, the best characterized GRKs, in facilitating Gi-biased β2AR signaling, but also defined the β2AR-Gi signaling as an essential link between pathologic upregulation of GRK and the development of heart failure. As a well-established pathogenic factor of heart failure, GRK2 and resultant Gi-biased β2AR signaling may present important therapeutic targets for the treatment of heart failure caused by various etiologies.
Novelty and Significance.
What is Known?
-
●
Phosphorylation of G-protein coupled receptor alters the selectivity of the receptor for G protein coupling.
-
●
β2-adrenergic receptor (β2-AR) phosphorylation by protein kinase A (PKA) is a critical determinant in its signaling to distinct G proteins such as Gs versus Gi.
-
●
β2-AR mediated Gi signaling elicits cardiac protective effects.
-
●
Upregulation of G protein-coupled receptor kinase (GRK) is a well-established causal factor of heart failure.
What New Information Does This Article Contribute?
-
●
Overexpression of GRK2 profoundly enhances Gi-biased β2AR Gi signaling in vivo and in vitro, whereas inhibition of GRK2 markedly suppresses the β2AR-coupled Gi signaling.
-
●
Phosphorylation of β2AR by GRK as well as PKA is an important determinant for the Gi-biased β2AR signaling.
-
●
In transgenic mice with cardiac-specific overexpression of a β2AR mutant lacking PKA phosphorylation sites (PKA- TG), augmentation of β2AR phosphorylation by GRK leads to exaggerated cardiac response to pressure overload, resulting in increased Gi-biased β2AR, markedly exacerbated cardiac maladaptive remodeling and failure, leading to early mortality.
-
●
In contrast, enhancement of PKA-mediated phosphorylation of β2AR in mice overexpressing a β2AR mutant lacking GRK phosphorylation sites (GRK- TG) does not alter cardiac anatomy or function when compared with non-transgenic mice (NTG) or transgenic mice overexpressing the wild type β2AR (WT TG) at a matched receptor density, indicating that phosphorylation of β2AR by GRK, but not by PKA, contributes to the pathogenesis of heart failure.
-
●
Inhibition of Gi signaling with pertussis toxin restores cardiac function in PKA- TG mice with pressure overload-induced heart failure and in GRK transgenic mice.
-
●
Enhanced β2AR coupling to Gi signaling may induce cardiac protective or detrimental effects.
Our in vitro and in vivo results reveal a fundamental function and new mechanism of action of GRK in facilitating Gi-biased β2AR signaling by phosphorylation of β2AR. We found that define β2AR-Gi signaling is an essential linker between pathologic upregulation of GRK and the development of heart failure. As a well-established pathogenic factor of heart failure, GRK and resultant Gi-biased β2AR signaling may be important therapeutic targets for the treatment of heart failure.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Robert J. Lefkowitz at Dept. of Medicine and Howard Hughes Medical Institute, Duke University Medical Center for kindly providing cDNAs encoding wild type human β2AR, β2AR mutant lacking putative phosphorylation sites for PKA (PKA-, point mutations of serine residues at 261, 262, 345, and 346) or for GRK (GRK-, 11 point mutations of serine or theronine residues in the C-terminus substituted with alanines or glycine) and Dr. Richard B. Clark at The University of Texas Health Science Center for anti-pSer262 PKA site and the anti-pS (Ser355, 356) GRK sites. The authors are also grateful to Dr. Hal Spurgeon and Mr. B. Ziman for their excellent technique supports.
Sources of Funding
This research is supported in part by Intramural Research Program of the NIH, National Institute on Aging (WZZ, KC, EGL, MT, NP, AZZ, and RPX), and by the National Basic Research Program of China (2012CB518000) and National Natural Science Foundation of China (81070674 and 81130073) (RPX), and by NIH grants R37 HL061690 and R01 HL56205 (WJK), P01 HL091799 (WJK and AMF).
Non-standard Abbreviations and Acronyms
- Adv
adenovirus
- GPCR
G protein-coupled receptor
- β-AR
β-adrenergic receptor
- ISO
isoproterenol
- FBS
fetal bovine serum
- CGP
CGP20712A
- ICI
ICI 118,551
- MEM
minimal essential medium
- [125I]-ICYP
[125I]-iodocyanopindolol
- m.o.i.
multiplicity of infection
- PKA
protein kinase A
- PBS
phosphate buffered saline
- PKI
protein Kinase inhibitor
- PTX
pertussis toxin
- NTG
non-transgenic mice
- WT TG
transgenic mice overexpressing wild type human β2-AR
- GRK- TG
transgenic mice expressing a β2AR mutant lacking GRK phosphorylation sites
- PKA- TG
transgenic mice expressing a β2AR mutant lacking PKA phosphorylation sites
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: Heart disease and stroke statistics-2010 update: A report from the american heart association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard CB, Dzavik V. Inotropes and vasopressors: Review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118:1047–1056. doi: 10.1161/CIRCULATIONAHA.107.728840. [DOI] [PubMed] [Google Scholar]
- 3.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwase M, Uechi M, Vatner DE, Asai K, Shannon RP, Kudej RK, Wagner TE, Wight DC, Patrick TA, Ishikawa Y, Homcy CJ, Vatner SF. Cardiomyopathy induced by cardiac gs alpha overexpression. Am J Physiol. 1997;272:H585–H589. doi: 10.1152/ajpheart.1997.272.1.H585. [DOI] [PubMed] [Google Scholar]
- 5.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 6.Hata JA, Koch WJ. Phosphorylation of g protein-coupled receptors: Gpcr kinases in heart disease. Mol Interv. 2003;3:264–272. doi: 10.1124/mi.3.5.264. [DOI] [PubMed] [Google Scholar]
- 7.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of g protein-coupled receptor kinases (grks) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different g proteins by protein kinase a. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 9.Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase a-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to gs and gi. Demonstration in a reconstituted system. J Biol Chem. 2002;277:31249–31256. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 10.Wang WC, Mihlbachler KA, Brunnett AC, Liggett SB. Targeted transgenesis reveals discrete attenuator functions of grk and pka in airway beta2-adrenergic receptor physiologic signaling. Proc Natl Acad Sci U S A. 2009;106:15007–15012. doi: 10.1073/pnas.0906034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, De Arcangelis V, Gao X, Ramani B, Jung YS, Xiang Y. Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by grk2 phosphorylation in cardiomyocytes. J Biol Chem. 2008;283:1799–1807. doi: 10.1074/jbc.M705747200. [DOI] [PubMed] [Google Scholar]
- 12.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Ramani B, Soto D, De Arcangelis V, Xiang Y. Agonist dose-dependent phosphorylation by protein kinase a and g protein-coupled receptor kinase regulates beta2 adrenoceptor coupling to g(i) proteins in cardiomyocytes. J Biol Chem. 2009;284:32279–32287. doi: 10.1074/jbc.M109.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte grk2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 15.Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial g protein receptor kinases in left ventricular cardiac diseases. Eur J Pharmacol. 2004;489:167–177. doi: 10.1016/j.ejphar.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of beta-arrestins and beta-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–213. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- 17.Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, Lefkowitz RJ, Koch WJ, Lakatta EG. Coupling of beta2-adrenoceptor to gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto H, Ohyanagi M, Nakamura K, Yamamoto J, Iwasaki T. Increased levels of inhibitory g protein in myocardium with heart failure. Jpn Circ J. 1994;58:913–924. doi: 10.1253/jcj.58.913. [DOI] [PubMed] [Google Scholar]
- 19.Fu LX, Feng QP, Liang QM, Sun XY, Hedner T, Hoebeke J, Hjalmarson A. Hypersensitivity of gi protein mediated muscarinic receptor adenylyl cyclase in chronic ischaemic heart failure in the rat. Cardiovasc Res. 1993;27:2065–2070. doi: 10.1093/cvr/27.11.2065. [DOI] [PubMed] [Google Scholar]
- 20.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: Effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 21.Chakir K, Zhu W, Tsang S, Woo AY, Yang D, Wang X, Zeng X, Rhee MH, Mende U, Koitabashi N, Takimoto E, Blumer KJ, Lakatta EG, Kass DA, Xiao RP. Rgs2 is a primary terminator of beta(2)-adrenergic receptor-mediated g(i) signaling. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ark inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 23.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ark1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci U S A. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by g beta gamma activation of p21ras. Proc Natl Acad Sci U S A. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a g protein-coupled receptor kinase attenuates g beta gamma-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 26.Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, Petrofski JA, Jakoi A, Milano CA, Koch WJ. Lymphocyte levels of grk2 (betaark1) mirror changes in the lvad- supported failing human heart: Lower grk2 associated with improved beta-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12:360–368. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. Control of myocardial contractile function by the level of beta-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem. 1998;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 28.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M, Gong H, Terracciano CM, Ranu H, Harding SE. Loss of beta-adrenoceptor response in myocytes overexpressing the na+/ca(2+)-exchanger. J Mol Cell Cardiol. 2004;36:43–48. doi: 10.1016/j.yjmcc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Xiao RP, Balke CW. Na+/ca2+ exchange linking beta2-adrenergic g(i) signaling to heart failure: Associated defect of adrenergic contractile support. J Mol Cell Cardiol. 2004;36:7–11. doi: 10.1016/j.yjmcc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhu W, Zeng X, Zheng M, Xiao RP. The enigma of beta2-adrenergic receptor gi signaling in the heart: The good, the bad, and the ugly. Circ Res. 2005;97:507–509. doi: 10.1161/01.RES.0000184615.56822.bd. [DOI] [PubMed] [Google Scholar]
- 32.Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW, 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted grk2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285:16378–16386. doi: 10.1074/jbc.M109.077859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 34.Oyama N, Urasawa K, Kaneta S, Sakai H, Saito T, Takagi C, Yoshida I, Kitabatake A, Tsutsui H. Angiotensin converting enzyme inhibitors attenuated the expression of g-protein coupled receptor kinases in heart failure patients. Circ J. 2006;70:362–363. doi: 10.1253/circj.70.362. [DOI] [PubMed] [Google Scholar]
- 35.Dorn GW., 2nd Grk mythology: G-protein receptor kinases in cardiovascular disease. J Mol Med. 2009;87:455–463. doi: 10.1007/s00109-009-0450-7. [DOI] [PubMed] [Google Scholar]
- 36.Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, Poosala S, Xiao RP, Lakatta EG, Talan MI. Cardioprotective and survival benefits of long-term combined therapy with beta2 adrenoreceptor (ar) agonist and beta1 ar blocker in dilated cardiomyopathy postmyocardial infarction. J Pharmacol Exp Ther. 2008;325:491–499. doi: 10.1124/jpet.107.135335. [DOI] [PubMed] [Google Scholar]
- 37.Xiao RP. Beta-adrenergic signaling in the heart: Dual coupling of the beta2-adrenergic receptor to g(s) and g(i) proteins. Sci STKE. 2001;2001:re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- 38.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through g(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 40.DeGeorge BR, Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, Chuprun JK, Harris DM, Kim GW, Soltys S, Eckhart AD, Koch WJ. Targeted inhibition of cardiomyocyte gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation. 2008;117:1378–1387. doi: 10.1161/CIRCULATIONAHA.107.752618. [DOI] [PubMed] [Google Scholar]
- 41.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of g protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res. 2010;107:1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao M, Fajardo G, Urashima T, Spin JM, Poorfarahani S, Rajagopalan V, Huynh D, Connolly A, Quertermous T, Bernstein D. Cardiac pressure overload hypertrophy is differentially regulated by {beta}-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2011;301:H1461–H1470. doi: 10.1152/ajpheart.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of g-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 44.He JQ, Balijepalli RC, Haworth RA, Kamp TJ. Crosstalk of beta-adrenergic receptor subtypes through gi blunts beta-adrenergic stimulation of l-type ca2+ channels in canine heart failure. Circ Res. 2005;97:566–573. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- 45.Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu W, Bond RA, Balke CW, Lakatta EG, Cheng H. Enhanced g(i) signaling selectively negates beta2-adrenergic receptor (ar)-- but not beta1-ar-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–1639. doi: 10.1161/01.CIR.0000087595.17277.73. [DOI] [PubMed] [Google Scholar]
- 46.Wellner-Kienitz MC, Bender K, Pott L. Overexpression of beta 1 and beta 2 adrenergic receptors in rat atrial myocytes. Differential coupling to g protein-gated inward rectifier k(+) channels via g(s) and g(i)/o. J Biol Chem. 2001;276:37347–37354. doi: 10.1074/jbc.M106234200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.