Abstract
Compound exocytosis is found in many cell types and is the major form of regulated secretion in acinar and mast cells. Its key characteristic is the homotypic fusion of secretory granules. These then secrete their combined output through a single fusion pore to the outside. The control of compound exocytosis remains poorly understood. Although soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) such as syntaxin 2, SNAP23 (synaptosome-associated protein of 23 kDa), and SNAP25 have been suggested to play a role, none has been proven. Vesicle-associated membrane protein 8 (VAMP8) is a SNARE first associated with endocytic processes but more recently has been suggested as an R-SNARE in regulated exocytosis. Secretion in acinar cells is reduced when VAMP8 function is inhibited and is less in VAMP8 knock-out mice. Based on electron microscopy experiments, it was suggested that VAMP8 may be involved in compound exocytosis. Here we have tested the hypothesis that VAMP8 controls homotypic granule-to-granule fusion during sequential compound exocytosis. We use a new assay to distinguish primary fusion events (fusion with the cell membrane) from secondary fusion events (granule-granule fusion). Our data show the pancreatic acinar cells from VAMP8 knock-out animals have a specific reduction in secondary granule fusion but that primary granule fusion is unaffected. Furthermore, immunoprecipitation experiments show syntaxin 2 association with VAMP2, whereas syntaxin 3 associates with VAMP8. Taken together our data indicate that granule-to-granule fusion is regulated by VAMP8 containing SNARE complexes distinct from those that regulate primary granule fusion.
Keywords: Calcium Signaling, Exocytosis, Membrane Fusion, Secretion, SNARE Proteins
Introduction
The precise role of compound exocytosis has not been determined, but it is thought that it might enhance secretion by enabling fusion of, and release of contents from, granules that lie deeper within the cell (1). For example, in the case of the massive exocytosis observed during mast cell degranulation (2), compound exocytosis would ensure that secretion occurs both through fusion of granules close to the plasma membrane and from deeper lying granules. This avoids the need to transport deeper granules up to the cell membrane and so would accelerate the secretory response.
In the case of acinar cells the apical plasma membrane area is relatively small compared with the total membrane area and is defined by tight junctional boundaries (3). Regulated exocytosis occurs exclusively at the apical membrane. Secretory (zymogen) granules are tightly packed in the apical region of acinar cells (see Fig. 1) and do not move over the minute timescales we use for stimulation. This means that only a few granules have direct access to the apical plasma membrane. Furthermore, granule fusion is so slow (many minutes) that close granules would limit access of deeper-lying granules to docking sites at the plasma membrane. Compound exocytosis provides a mechanism to enhance secretion by enabling deeper granules to fuse with peripheral granules and so release their content to the outside (4).
FIGURE 1.
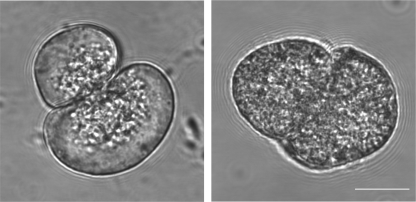
Isolated pancreatic acinar cells show a greatly increased number of zymogen granules in the VAMP8 knock-out mice. Morphological differences in the isolated exocrine pancreas from WT versus VAMP8 knock-out mice have previously been described at low magnification. Here, at high magnification, phase images of isolated single cells (all functional experiments are with pancreatic tissue fragments, but the differences in granule distribution are difficult to see in multicellular fragments) show the clustering of granules in the apical region (where the two cells are touching) of WT cells (left) compared with the distribution of granules across the whole of the cell in VAMP8 knock-out cells (right).
Two forms of compound exocytosis are recognized (1). Granule-to-granule fusion can form large multigranular structures within the cell that then fuse with the plasma membrane. Capacitance measurements in mast cells show very large step increases that may be due to multigranular fusion (2). In contrast, in acinar cells (5) a mechanism termed sequential compound exocytosis occurs where the first (primary) granules fuse with the cell membrane, and this is followed by sequential fusion of other (secondary and tertiary) granules onto these primary granules (5, 6). There is no evidence in acinar cells that granules fuse with each other before fusion with the cell membrane (5). The regulation of either form of compound exocytosis is not well understood. However, given that the process is dependent on membrane fusion, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE)2 proteins are likely to be involved.
SNARE proteins are thought to provide a common mechanism for membrane-membrane recognition before membrane fusion. Complementary SNAREs, Gln (Q) on one membrane and Arg (R) on the other, form part of the docking machinery thought to hold membranes in close apposition before fusion (7). The best studied example of regulated secretion is in neurons where the minimal SNAREs required for fusion include syntaxin 1, SNAP25, and a synaptobrevin/VAMP. In non-excitable cells homologous proteins are thought to provide similar machinery (8), although in many cases the identity of the actual proteins engaged in regulated exocytosis is not known.
Fusion of secondary granules during compound exocytosis in acinar cells is strictly sequential. This suggests that fusion of a primary granule changes the properties of that granule such that it is now recognized as fusion competent by adjacent secondary granules (1). One such change may be movement of SNAREs from the plasma membrane into the granule membrane. Three lines of evidence support this idea. First, there is evidence that syntaxin 2 moves into the primary granule membrane in acinar cells (9). Second, evidence in pancreatic β cells that shows that another SNARE, SNAP25, also moves selectively into primary granules as a prelude to secondary fusion (10). Finally, perhaps the best evidence for SNARE control of compound exocytosis is the observation in mast cells that function blocking antibodies inhibit translocation of SNAP25 into secretory granules and significantly knock down the secretory response (11). However, none of these studies provides direct proof that these SNAREs are necessary for homotypic granule-to-granule fusion during compound exocytosis.
VAMP8/endobrevin was first described as an R-SNARE of an early endosomal compartment (12–14) mediating homotypic endosomal fusion (15). Subsequently it has been shown that VAMP8 is involved in exocytosis in pancreatic acinar cells (16), mast cells (17, 18), cytotoxic T lymphocytes (19), platelets (20, 21), and kidney collecting duct epithelia (22).
Recent evidence suggests that VAMP8 might be important in compound exocytosis (23). Here it was shown by electron microscopy that in VAMP8 knock-out mice the of extent granule-to-granule fusion was much reduced (23). However, in that study, because of the inherent limitations of the assays used, it was not possible to determine whether the VAMP8 knock-out animals had defects in primary granule fusion and whether VAMP8 played a role in sequential exocytosis.
In our study on mouse pancreatic acinar cells we set out to determine whether VAMP8 is a specific SNARE controlling granule to granule fusion underlying sequential exocytosis. We employ novel methods to record single granule fusion in real time and determine whether that fusion event is due to a primary or secondary granule. In VAMP8 knock-out mice we show a reduced number of granule fusion events over time in response to agonists and to ionomycin stimulation. This reduction is only significant for secondary fusion with no effect on the numbers or kinetics of fusion of primary granules. In immunoprecipitation experiments we show that VAMP8 and VAMP4 form distinct SNARE complexes. We conclude that VAMP8 is a SNARE selectively required for granule-to-granule fusion during sequential exocytosis.
EXPERIMENTAL PROCEDURES
Solutions
Experiments were performed in NaCl-rich extracellular solution (135 mm NaCl, 5 mm KCl, 10 mm glucose, 2 mm MgCl2, 2 mm CaCl2, and 10 mm HEPES) adjusted to pH 7.4 with NaOH.
Mice
VAMP8 knock-out mice were produced as described previously (16) and maintained on a mixed genetic background of 29 SvJ and C57/BL6 mice. All animals were genotyped with PCR and VAMP8 knock-out animals compared with wild type (WT) litter mates.
Cell Preparation
Mice were humanely killed according to local animal ethics procedures. Isolated mouse pancreatic tissue was prepared by a collagenase digestion method in normal NaCl-rich extracellular solution modified to reduce the time in collagenase and limit mechanical trituration. The resulting preparation was composed mainly of pancreatic lobules and fragments (50–100 cells), which were plated onto poly-l-lysine-coated glass coverslips.
Two-photon Imaging
We used a custom-made, video-rate, 2-photon microscope with a 60× oil immersion objective (NA 1.42, Olympus), providing an axial resolution (full width, half-maximum) of ∼1 μm. We imaged exocytotic events using sulforhodamine B (400 μm) as a membrane-impermeant fluorescent extracellular marker excited by femtosecond laser pulses at 950 nm, with fluorescence emission detected at 550–650 nm. To image pH changes we used HPTS (400 μm) excited at 950 nm and fluorescence detected at 420–520 nm.
Images (resolution of 10 pixels/μm, average of 15 video frames) were analyzed with the Metamorph program (Molecular Devices Corp.). Exocytotic event kinetics were measured from regions of interest (0.78 μm2, 78 pixels) centered over individual granules. Traces were rejected if extensive movement was observed. All data are shown as the mean ± S.E.
Immunocytochemistry
Cells attached to glass coverslips were washed in PBS and fixed in methanol; 10 min at −20 °C. The preparation was then permeabilized with 0.2% saponin and blocked overnight in 2% donkey serum plus 2% fish skin gelatin in PBS. Cells were then incubated in primary antibody for 1 h and secondary antibodies for 30 min.
Primary antibodies were chymotrypsinogen (1:1000, Serotec 2100-0657), VAMP8 (1:500, in house), and inositol trisphosphate receptor (1:100, BD Biosciences 610312). Secondary antibodies were Alexa Fluor 488 (1:100, donkey anti-mouse, Invitrogen A-21072), and Alexa Fluor 633 (1:100, goat anti-rabbit, Invitrogen A-21071).
Images were obtained on an Olympus FV1000 LSM confocal microscope with a 60× oil, 1.4 NA objective. Images were acquired sequentially, and 2× Kalman averaging was applied. For Alexa-488, excitation was set at 473 nm, and emission was collected at 490–590 nm. For Alexa-633, excitation was set at 635 nm, and emission was collected 655–755 nm.
Immunoprecipitation and Immunoblotting
Immunoprecipitation with rabbit polyclonal syntaxin 2 or syntaxin 3 antibodies (SYSY, Goettingen, Germany) was performed similarly to our previous report (23) to detect CCK-8-induced apical and ZG-ZG trans-SNARE complex formation in dispersed rat pancreatic acinar cells.
Briefly, dispersed pancreatic acinar cells from male Sprague-Dawley rats (125 g, Willington, MA) were initially stimulated in Krebs-Ringer buffer with HEPES, pH 7.4, with or without 100 pm CCK-8 (maximal stimulatory concentration) for 1.0 h at 37 °C. After stimulation cells were harvested and lysed by sonication in lysis buffer (25 mm HEPES, 100 mm KCl, 1% Triton X-100 with protease inhibitors). Lysates were clarified by centrifugation (12,000 × g, 10 min) at 4 °C, and 1.0 mg of protein extracts (4 μg/μl) from each condition was initially precleared with 50 μl of protein A-agarose beads (Molecular Probes, Carlsbad, CA) for 2.0 h at 4 °C and then subjected to immunoprecipitation with 1.0 μg of either syntaxin 2 or syntaxin 3 antibody cross-linked to 50 μl of protein A-agarose beads overnight at 4 °C. Beads were washed twice with lysis buffer. After washes, beads were resuspended in 30 μl of glycine-HCl, pH 2.5, for 10 min at 37 °C to elute precipitated proteins and adjusted to pH 7.0 with 1.0 n NaOH. The samples were finally dissolved in Laemmli buffer, boiled for 3 min, separated on 12–15% gradient SDS-PAGE, and transferred to nitrocellulose membranes (Amersham Biosciences).
Separated proteins were immunodecorated and identified by the indicated primary antibodies (Munc18b (rabbit, 1:1000, a gift from Dr. Y. Tamori, Kobe University Graduate School of Medicine, Kobe, Japan); rabbit antibodies against syntaxin 2 (1:1000), syntaxin 3 (1:1000), and SNAP23 (1:1000) were from SYSY) and visualized with enhanced chemiluminescence (Amersham Biosciences). VAMP2 and VAMP8 were probed in the same blot with a mixture of VAMP2 (1:1000, a gift from Dr. Anson Lowe, Stanford University) and VAMP8 (1:1300, in house) antibodies as these two proteins are well separated, and this enabled us to assess accurately which of the two v-SNAREs and the relative amounts were co-precipitated.
Statistical Analyses
All numerical data are presented as the means ± S.E. of the mean. Statistical analysis was performed using Microsoft Excel and GraphPad Prism. Data sets with just two groups were subjected to a two-tailed, unpaired Student's t test. A critical value for significance of p < 0.05 was used throughout. Denotation by asterisks (*) represents a significance of p < 0.05.
RESULTS
The anatomical phenotype of VAMP8 knock-out has been described as an increase in the numbers of zymogen granules as assessed by hematoxylin and eosin staining of tissue sections and electron microscopy (16). We confirmed this with single-cell phase contrast imaging and observe that the tight clustering of zymogen granules in the luminal region of the acinar cells in WT animals changes to a cell-wide distribution of granules in VAMP8 knock-out acinar cells (Fig. 1).
Identification of Compound Exocytosis
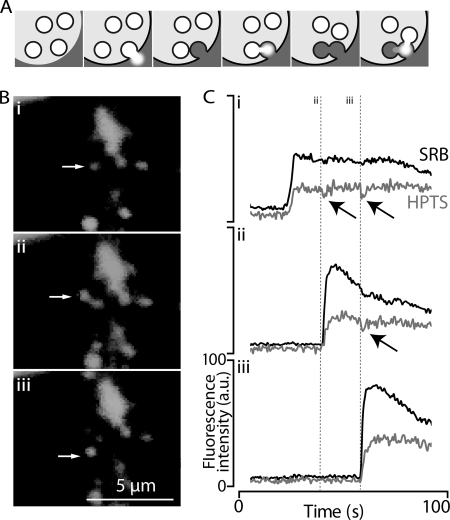
To image single-granule fusion events, we employ two-photon imaging of the entry of extracellular dyes in to fused granules, a method that can identify compound exocytic events (5, 6). In these studies compound exocytosis was determined on the basis of the spatial appearance of granule fusion events, i.e. fusion of granules apparently far away from the cell membrane is ascribed as a compound exocytic event. However, plasma membrane invaginations that go deep into the cell or unseen luminal processes could lead to incorrect identification of distant fusion events as secondary when, in fact, they are primary. We have, therefore, developed a method to positively identify secondary granule fusion on the basis of pH changes in the primary granules. All granules are acidic, and on fusion, proton release from the granule acidifies the local extracellular environment (24). In the case of primary fusion events this acidification is localized to the lumen, this contrasts with compound exocytic events in which the protons released from secondary granules enter and acidify the primary granule (Fig. 2, upper schematic). We track these pH changes through the use of an inert extracellular dye (sulforhodamine B) and a pH-sensitive dye (HPTS, which decreases its fluorescence on acidification). Fig. 2 (upper) shows fusion of a primary granule leads to a drop in pH in the lumen (cell stimulated with 600 nm acetylcholine). Subsequent fusion of two secondary granules each lead to a drop in pH measured in the primary granule (Fig. 2C). In control experiments, a pH-insensitive analog of HPTS, methoxy-pyrene 1,3,6 trisulfonate, showed no such changes and behaved like sulforhodamine B. This example experiment was conducted in VAMP8 knock-out mice and proves compound exocytosis can still occur in the absence of this SNARE.
FIGURE 2.
A new method to positively identify the fusion of secondary granules during compound exocytosis. A, shown is a schematic representation of compound exocytosis. The primary granule releases its content into the lumen, and a secondary granule releases its content into the primary. In our method two extracellular dyes are simultaneously imaged, inert sulforhodamine B (SRB), which tracks fusion, and pH-sensitive HPTS, which reports the movement of protons in the granule content. B, shown is a sequence of images of granule fusion events with fusion of a primary granule (i, arrow) and then fusion of two putative secondary granules (ii and iii, arrows). C, fluorescence signals in regions of interest placed over the primary (i) and secondary granules (ii and iii) show the sudden increase in fluorescence at the point of each granule fusion. In i each secondary fusion event is associated with a transient drop in the HPTS signal reflecting proton release from the secondary granules into the primary granule. A similar drop in HPTS is also seen in ii, reflecting the fusion of the second secondary granule. These data indicate the primary granule acts as a conduit for secretion from the secondary granules and is direct evidence for compound exocytosis. a.u., arbitrary units.
Stimulated Exocytosis in VAMP8 Knock-out Mice Is Selectively Deficient in Secondary Fusion Events
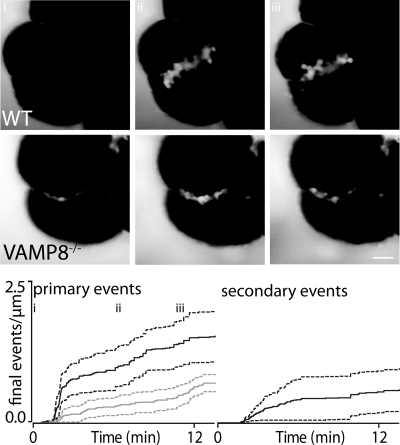
Stimulation of pancreatic fragments with 20 pm CCK induces moderate exocytosis that includes both primary and secondary fusion events (Fig. 3, upper). We quantified the numbers of fusion events over time per μm of lumen length and expressed them as a cumulative graph. In total we used 7 separate pancreatic fragments from 4 mice for both WT and for VAMP8 knock-out and 285 fusion events (primary and secondary). The time-course of primary fusion events in WT animals shows an initial flurry of exocytosis followed by a longer-lasting phase with a lower frequency of fusion events. In contrast, the time-course of secondary fusion events was relatively constant throughout the stimulation. Application of an identical CCK stimulus to pancreatic fragments from VAMP8 knock-out mice shows a distinctly different pattern of exocytosis. The numbers of primary exocytic events were reduced, and secondary exocytic events were completely abolished (Fig. 3, lower).
FIGURE 3.
CCK-induced exocytic responses are reduced in VAMP8 knock-out pancreas compared with WT. The images in the upper panel were taken at the time points indicated on the lower graph (i, ii, iii) and show the progression of granule fusion in WT (upper) and VAMP8 knock-out mouse pancreatic fragments. The responses were then measured as the number of primary (left graph) and secondary (right graph) fusion events observed over time and normalized to the length of the lumen (in μm). In our experiments the lumen before stimulation is seen as a dye-filled space between adjoining cells and can be seen in the far left images of the upper panel. The graphs show the cumulative numbers of fusion events in WT (black lines; S.E., black dotted lines) and VAMP8 knock-out (gray lines; S.E.; gray dotted lines) exocrine pancreatic fragments. In the VAMP8 knock-out mice there is a reduction in primary fusion events (left graph) and a complete absence of secondary fusion events (right graph). Scale bar, 10 μm.
Analysis of this data showed that at the end of the recording (800s after start of recording) the numbers of primary events in WT was 1.55 ± 0.44 events·(μm lumen)−1 compared with 0.71 ± 0.15 in VAMP8 knock-out mice (Student's t test p = 0.12, not significant).
We also analyzed the maximum slope in the events per time data. In the WT the slope was 0.07297 ± 0.02 events·(μm lumen)−1·min−1 compared with 0.03309 ± 0.003 in VAMP8 knock-out mice (Student's t test not significant). Because we saw no secondary events in the VAMP8 knock-out mice, tests of significance were not possible.
These data are suggestive of a defect in exocytosis in the VAMP8 knock-out exocrine pancreas, but the large variability in the CCK-evoked responses make it difficult to draw firm conclusions. We, therefore, conducted experiments with acetylcholine (ACh) and with ionomycin, both of which in our hands elicit more consistent secretory responses.
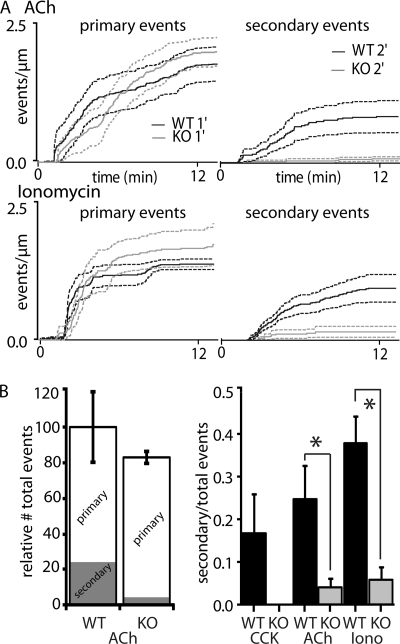
Application of 600 nm ACh to WT pancreatic fragments induced an initial rapid and subsequent longer-lasting slower response, measured in primary exocytosis (we recorded a total of 471 fusion events (primary and secondary) from at least 4 WT and VAMP8 knock-out mice). Secondary exocytic events appeared at a consistent rate after stimulation before reaching a maximum (Fig. 4A, upper). In the VAMP8 knock-out pancreatic fragments the rate of primary exocytosis was very similar to WT, but secondary exocytic events were almost completely abolished (Fig. 4A, upper).
FIGURE 4.
Ach- and ionomycin-induced exocytic responses in VAMP8 knock-out pancreas show a selective reduction in secondary fusion compared with WT. A, shown are plots of cumulative numbers of fusion events per length of lumen for stimulation with ACh (upper graph) and ionomycin (lower graph). WT primary events (black lines; S.E., black dotted lines) show little difference compared with primary events in VAMP8 knock-out mice (gray lines; S.E., gray dotted lines). In contrast, comparison of the secondary fusion events shows a dramatic and significant reduction in VAMP8 knock-out (gray lines; S.E., gray dotted lines) compared with WT (black lines; S.E., black dotted lines). B, shown is a histogram of the relative reduction infusion events for ACh stimulation (left graph). This shows no significant overall reduction in total fusion events in the VAMP8 knock-out mice but a significant drop in the proportion of secondary fusion events (gray block within the histogram). The ratios of secondary to total fusion events for all conditions (right graph) show this loss of secondary fusion is consistent with all three of the stimuli we used.
Analysis showed that the total numbers of fusion events (both primary and secondary) were reduced in VAMP8 knock-out compared with WT by about 20%. Most of this loss was accounted for by an 89% reduction in the numbers of secondary events (Fig. 4B).
Similar findings were obtained with ionomycin (5 μm) stimulation. Ionomycin bypasses the G-protein signal cascade of the agonist and directly elevates intracellular calcium. The exocytic responses induced by ionomycin in VAMP8 knock-out mice show a decrease in secondary exocytic events with no effect on primary exocytosis (Fig. 4A, lower) (we recorded 377 fusion events from at least 4 WT and VAMP8 knock-out mice).
The numbers of primary fusion events at the end of the record for ACh stimulation was 1.79 ± 0.31 events·(μm lumen)−1 in WT compared with 2.02 ± 0.26 events·(μm lumen)−1 in VAMP8 knock-out mice (mean ± S.E., Student's t test, p = 0.59, not significant). For ionomycin stimulation, 1.38 ± 0.095 events·(μm lumen)−1 in WT were observed at the end of recording compared with 1.74 ± 0.377 events·(μm lumen)−1 in VAMP8 knock-out mice (Student's t test p = 0.39, not significant). We also measured the maximum slopes in each condition for the primary fusion events, and these were not significantly different (data not shown), and conclude that VAMP8 knock-out mice show no deficit in primary granule fusion.
In contrast, the proportion of secondary granule fusion events with ACh stimulation was very different comparing WT with VAMP8 knock-out (Student's unpaired t test, p < 0.05, n = 7 fragments WT, n = 6 fragments VAMP8−/−, 4 animals each, Fig. 4B, right graph). A similar significant drop in the proportion of secondary exocytosis was obtained with ionomycin stimulation (Student's unpaired t test, p < 0.05, n = 8 fragments WT, n = 6 fragments VAMP8−/−, 4 animals each, Fig 4B). This reduction was accounted for by an 84% reduction in secondary fusion events. We conclude there is a significant and selective deficit in secondary granule fusion in the acinar cells of VAMP8 knock-out mice.
VAMP8 Is Located throughout the Subapical Domain
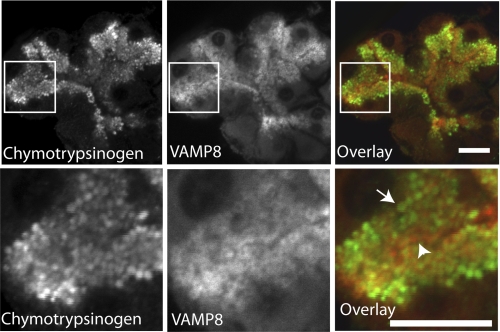
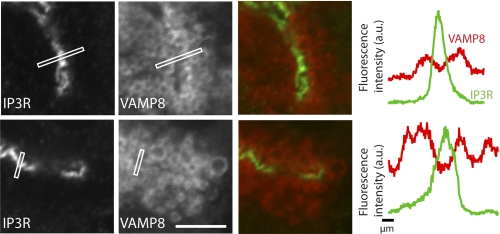
In our hands VAMP8 immunostaining was found throughout the apical region, surrounding each individual zymogen granule that was identified by chymotrypsinogen counter-immunostaining (Fig. 5). VAMP8 also appeared to be along the apical plasma membrane, but immunocytochemistry shows that VAMP8 is enriched in regions distal from inositol trisphosphate receptors (Fig. 6), indicating a location away from the apical membrane. Our images cannot distinguish if VAMP8 is actually present on zymogen granule membranes, but this has been shown by cell fractionation studies (16), which we have confirmed,3 and by proteomic analysis of granule membranes (25).
FIGURE 5.
Chymotrypsinogen and VAMP8 is enriched in the apical pole of mouse pancreatic fragments. Chymotrypsinogen is a zymogen granule content protein; our images (green) show granules across the apical pole. VAMP8 immunostaining (red) surrounds individual zymogen granules (arrow), which close to the lumen is intense (arrowhead). Scale bar, 10 μm.
FIGURE 6.
VAMP8 localizes close to the apical plasma membrane of mouse pancreatic fragments. Inositol trisphosphate receptors (IP3R) are known to be placed right beneath the apical plasma membrane. VAMP8 (red) is enriched close to the inositol trisphosphate receptors staining (green), confirming enrichment close to the apical plasma membrane. However, line scan graphs (drawn across the lines shown in the images) of the average fluorescence intensity show that inositol trisphosphate receptors enrichment is in a distinct narrow region that lies between peaks of VAMP8 enrichments. a.u., arbitrary units.
Vamp 8 Forms a Distinct SNARE Complex with Syntaxin 3
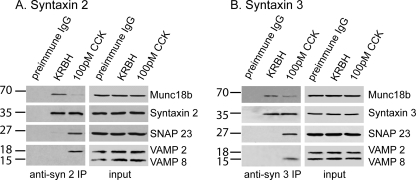
If VAMP8 is selectively required for granule-to-granule fusion, this would imply that secretion in acinar cells utilizes one SNARE complex for primary granule exocytosis and a distinct SNARE complex for granule-to-granule fusion. To explore this possibility we performed immunoprecipitation experiments using antibodies against either syntaxin 2 or syntaxin 3. We previously reported syntaxin 2 to be the putative Q-SNARE on the apical PM (8, 9, 25, 26), whereas syntaxin 3 is a Q-SNARE most abundant on zymogen granules (8, 26, 27); hence, syntaxin 2 and 3 would be the likely Q-SNAREs for primary and secondary exocytosis, respectively. Because of the requirement for larger amounts of tissue, we used rat pancreas in these experiments.
As previously shown, at basal state syntaxin 2 strongly associates with Munc18b (23) (Fig. 7), likely with syntaxin 2 in closed conformation unable to mediate fusion (7). After stimulation with 100 pm CCK-8, Munc18b would be activated to induce syntaxin 2 into open conformation to assemble with cognate SNAREs capable of mediating fusion but that may reduce the affinity of syntaxin to Munc18b (7), Indeed, less Munc18b is immunoprecipitated (23), and now an association with SNAP23 and VAMP2 is seen. Immunoprecipitation using antibodies against syntaxin 3 again shows a strong association with Munc18b at rest, but after stimulation the evidence indicates that syntaxin 3 complexes with SNAP23 and VAMP8 and also with some Munc18b (Fig. 7).
FIGURE 7.
Maximal CCK stimulation of rat pancreatic acini causes formation of distinct Munc18b-SNARE complexes that mediate apical exocytosis. Dispersed rat pancreatic acini were treated with control Krebs-Ringer buffer with HEPES (KRBH, 1 h) or 100 pm CCK (1 h). 1 mg of protein of acini lysates was immunoprecipitated (IP) with anti-syntaxin 2 (A) or anti-syntaxin 3 (B) antibodies as described under “Experimental Procedures” or with preimmune IgG as an additional control. The precipitated proteins were separated on 12–15% gradient SDS-PAGE and identified with the indicated antibodies. Total lysates (25 μg of protein) serving as input controls showed similar levels of SNARE and Munc18b proteins in the various treatments. These blots are representative of two independent experiments. CCK typically gives responses of variable latency; we used 1 h of stimulation to ensure maximal coordinated recruitment of these dispersed acini.
These data indicate that upon stimulation Munc18b could activate Q-SNAREs syntaxin 2 and syntaxin 3, but these Q-SNAREs would partner with different R-SNAREs, VAMP2 and VAMP8, respectively, to form distinct SNARE complexes we have postulated to mediate primary (granule-to-apical plasma membrane) and secondary (granule-to-granule) exocytosis, respectively.
DISCUSSION
Our data strongly support the hypothesis that VAMP8 is a key regulator of compound exocytosis in the exocrine pancreas. We have developed a novel method to positively identify secondary granule fusion events. Using this method we show that pancreatic acinar cells from VAMP8 knock-out mice show a selective deficit in secondary fusion with no significant effect on primary granule fusion. Immunoprecipitation experiments add further support to the idea that VAMP8 is part of a specific granule-to-granule SNARE complex with syntaxin 3. Our evidence indicates that this is distinct from a plasma membrane to granule VAMP2-syntaxin 2 SNARE complex.
We show an overall reduction in granule fusion of 20%, with the majority of this accounted for by a loss of secondary fusion events (Fig. 4B). We can compare our data to previous findings where amylase secretion has been measured. Here, with carbachol stimulation, WT acini secrete 19.4% of total amylase compared with 6.8% in VAMP8 knock-out mice. When the 2.1-fold increase in total amylase content found in the VAMP8 knock-out is taken into account, this gives a reduction of actual secretion of 26.3% (23). This number is consistent with our estimates of the loss of total granule fusion events.
Our immunolocalization experiments are consistent with those previously shown for VAMP8 (26) and demonstrate its presence on granules. VAMP2 staining is also localized in the apical regions (26–29), and comparison with VAMP2 distribution has suggested that VAMP2 is located more apically than VAMP8 (26). We have not immunostained for VAMP2, but such a distribution would be entirely consistent with the hypothesis that VAMP2 was the SNARE engaged in primary fusion and VAMP8 engaged in secondary fusion.
Which SNAREs Are Important in Acinar Cells?
There is general agreement on the localization of the SNARES in pancreatic acinar cells but little consistency as to their functional effects in controlling secretion.
It is clear that syntaxin 2 is the major SNARE on the apical membrane (8, 26, 30). Syntaxin 4 shows a more diffuse basal membrane distribution (8) with some possible overlap with syntaxin 2 in the apical membrane (26). The fact that syntaxin 2 is prominent on the apical membrane suggests but does not prove it is the principal Q-SNARE. It should be noted that the apical plasma membrane in these native tissues has a relatively small area circumscribed by tight and adherens junctions (3) and that none of these studies on SNAREs have used junctional markers to definitively identify the apical membrane.
On the granule membrane there is agreement that syntaxin 3 is present (8, 30, 31). There is also a consensus that the R-SNAREs VAMP2 (26, 32, 33) and VAMP8 are both present (8, 26, 30, 32) with a recent paper indicating these SNAREs might be separately located on different populations of granules (26). Finally, studies show that SNAP23 is present (23), although it has variously been localized to the cell membrane (30) or on the granule membrane (26).
So all the components of a minimal SNARE complex exist, but which of these is actually involved in regulating secretion is controversial. Functional evidence using competing antibodies (30) or botulinum neurotoxin C (31) supports the idea that syntaxin 2 is important, but conflicting data suggest syntaxin 4 as the crucial Q-SNARE (26). There is a diversity of evidence for a role of VAMP8 as the R-SNARE, including a reduction in secretion in VAMP8 knock-out mice (16) and partial inhibition of secretion with competing antibodies (30) and peptides (26). Further evidence suggests that VAMP8 may play a specific role in granule-granule fusion during the process of compound exocytosis (23). In addition to VAMP8, competing peptides (26) and treatment with tetanus toxin (33) suggest that VAMP2 may play a role in secretion, although Pickett et al. (30) failed to show actions of VAMP2 antibodies or peptides on secretion.
In most of these functional studies the experimental manipulations reduced secretion by only around 50% (26, 30). This suggests a number of possibilities; this could be due to lack of efficacy of the manipulation, it could be that some SNAREs are preformed and, therefore, cannot be functionally disrupted, or it could be that there is redundancy and that more than one set of SNAREs may be involved in secretion. Recent work (26) indicates that VAMP8 and VAMP2 are found on separate populations of granules. These populations may, therefore, subserve different functions and may be a confounding factor in the interpretation of experiments. A further problem is that the functional studies mostly measure enzyme secretion. But we now know that granule fusion dynamics are complicated. Transient fusion can occur (34) with the possibility of only partial release of granule content (35). Furthermore, as we describe here compound exocytosis is prevalent in these cells (5), and this cannot be measured in enzyme assays. Enzyme release assays give only incomplete insights into the process of SNARE control of granule fusion.
Immunoprecipitation experiments should help to resolve the composition of the SNARE complex, but instead they have led authors to distinct conclusions. A syntaxin 2-VAMP2 complex, shown to be formed after cell stimulation by Cosen-Binker et al. (23), is not supported in an earlier study by Wang et al. (16) who show that VAMP8 co-immunoprecipitated syntaxin 4. The major difference in these studies is that Cosen-Binker et al. (23) show that the syntaxin 2-VAMP2 complex only forms after cell stimulation. Interestingly, they also show that a VAMP8-syntaxin 4 complex can form but only in models of pancreatitis purported to have been caused by redirection of apical exocytosis to the basolateral plasma membrane (23).
The data we present here adds further insights. We show direct functional evidence that control of granule fusion to the plasma membrane is distinct from granule-to-granule fusion in that it does not require VAMP8. Our current data reinforces previous findings for a SNARE complex consisting of syntaxin 2, VAMP2, and SNAP23 (23). Because these SNAREs are enriched on the plasma membrane and granule membrane, respectively, we suggest this SNARE complex regulates primary granule exocytosis.
Control of Compound Exocytosis
One of the earliest descriptions (in 1965) of compound exocytosis in acinar cells concluded that the fusing primary granule “ … takes on the properties of the plasmalemma … . enabling other granules to coalesce with it” (4). Nearly 40 years later this idea was supported by evidence that, after fusion, SNAREs may move into the primary granule membrane (9, 10, 11). These studies have limited functional information, but the implied model is that the fused granule could take on the SNARE apparatus of the plasma membrane (1). In this way the same SNAREs that control plasma membrane to granule fusion would be involved in granule-to-granule fusion.
Our new data in this paper strongly argue that there is a greater level of complexity in terms of SNARE involvement in compound exocytosis. Our observations that VAMP8 knock-out mice are selectively deficient in granule-to-granule exocytosis and that VAMP8 makes a distinct complex with syntaxin 3 indicate a distinction between the SNARE regulation of primary granule fusion and granule-to-granule fusion. VAMP8 clearly plays a unique role in granule-to-granule exocytosis.
An appealing aspect of the model of SNARE movement into the primary granule is that it explains the sequential nature of compound exocytosis and why granules so crowded within the apical pole of the cell do not fuse with each other. If we are right about an exclusive role for VAMP8, then there must be a distinct mechanism that signals the initiating “fusion readiness” of a primary fused granule to plasma membrane. There are a number of possible changes that may signal the primary fused granule is available for fusion with secondary granules. These include the following. First, SNARE movement has been shown to occur and, although our data argue that plasma membrane Q-SNAREs are not directly involved in granule-to-granule fusion, they could play an indirect signaling role. Second, soon after fusion the primary granules become coated with F-actin (37–39). This F-actin coating is also observed in oocytes (40) and type 2 pneumocytes (41) and has been suggested to be triggered post-fusion by lipid and/or protein interchange between the plasma membrane and the granule membrane (42). We know that F-actin is intimately involved in exocytosis (34, 43). Therefore, the F-actin remodeling observed may make architectural changes that support the fusion machinery to enable granule-to-granule fusion to proceed.
Third, an obvious factor that changes after primary granule fusion is the loss of granule contents. Acidic granules lose protons on fusion, and the acid pH is rapidly neutralized (24). Granule protein content loss, such as GP2 (44), may also be a component in a mechanism that signals to the secondary granules. We conclude that the primary granule does undergo dramatic changes after fusion, any one of which could be used either to indicate that the granule is available for secondary granule fusion or as a mechanism to support that fusion.
Ca2+ Regulation of Compound Exocytosis
How is fusion of secondary granules actually triggered? Our data show that ionomycin can induce sequential compound exocytosis comparable in kinetics and extent to the agonists. This supports the idea that a rise in intracellular Ca2+ is the key trigger to secondary granule fusion and that additional signal cascades from engagement of G-protein-coupled receptors are not required. In the past there has been discussion about local control of exocytosis by elevation of Ca2+ within small-volume nanodomains (45). However, we have recently demonstrated that this is not the case and that exocytosis (both primary and secondary) in acinar cells is controlled by cytosolic Ca2+ elevation within a larger-volume microdomain in the apical region (46). Consistent with this idea, Nemoto et al. (5) show that compound exocytosis is observed during longer-lasting (>30 s) Ca2+ signals by which time the Ca2+ signal encompasses the whole of the apical region. We, therefore, conclude that it is the Ca2+ signal within this apical microdomain that controls the fusion of both primary and secondary granules.
These functional data point to a similarity in Ca2+-dependent control of primary and secondary granule fusion. However, it does not rule out that there may be differing molecular mechanisms. Synaptotagmin is a known Ca2+ sensor present in acinar cells (47) that could be modulated by proteins like cysteine string protein (25) and complexins (28). It could be that differential distribution of these accessory proteins may differentially regulate primary versus secondary exocytosis.
Acknowledgments
We thank Alexandra Hickey and Li Suan Chew for work in the early stages of this project.
This work was supported by Australian Research Council Grant DP110100642 (to P. T.) and National Health and Medical Research Council Grant APP1002520 (to P. T. and H. Y. G.).
S. Dolai and H. Y. Gaisano, unpublished data.
- SNARE
- soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- VAMP8
- vesicle-associated membrane protein 8
- SNAP23
- synaptosome-associated protein of 23 kDa
- SNAP25
- synaptosome-associated protein of 25 kDa
- HPTS
- hydroxypyrene 3,6,8-trisulfonate
- ACh
- acetylcholine
- CCK
- cholecystokinin.
REFERENCES
- 1. Pickett J. A., Edwardson J. M. (2006) Traffic 7, 109–116 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez de Toledo G., Fernandez J. M. (1990) J. Gen. Physiol 95, 397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larina O., Thorn P. (2005) J. Cell Sci. 118, 4131–4139 [DOI] [PubMed] [Google Scholar]
- 4. Ichikawa A. (1965) J. Cell Biol. 24, 369–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nemoto T., Kimura R., Ito K., Tachikawa A., Miyashita Y., Iino M., Kasai H. (2001) Nat. Cell Biol. 3, 253–258 [DOI] [PubMed] [Google Scholar]
- 6. Thorn P., Fogarty K. E., Parker I. (2004) Proc. Natl. Acad. Sci. U. S. A. 101, 6774–6779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Südhof T. C., Rothman J. E. (2009) Science 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaisano H. Y., Ghai M., Malkus P. N., Sheu L., Bouquillon A., Bennett M. K., Trimble W. S. (1996) Mol. Biol. Cell 7, 2019–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pickett J. A., Thorn P., Edwardson J. M. (2005) J. Biol. Chem. 280, 1506–1511 [DOI] [PubMed] [Google Scholar]
- 10. Takahashi N., Hatakeyama H., Okado H., Miwa A., Kishimoto T., Kojima T., Abe T., Kasai H. (2004) J. Cell Biol. 165, 255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo Z., Turner C., Castle D. (1998) Cell 94, 537–548 [DOI] [PubMed] [Google Scholar]
- 12. Wong S. H., Zhang T., Xu Y., Subramaniam V. N., Griffiths G., Hong W. J. (1998) Mol. Biol. Cell 9, 1549–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steegmaier M., Lee K. C., Prekeris R., Scheller R. H. (2000) Traffic 1, 553–560 [DOI] [PubMed] [Google Scholar]
- 14. Advani R. J., Bae H. R., Bock J. B., Chao D. S., Doung Y. C., Prekeris R., Yoo J. S., Scheller R. H. (1998) J. Biol. Chem. 273, 10317–10324 [DOI] [PubMed] [Google Scholar]
- 15. Antonin W., Holroyd C., Tikkanen R., Höning S., Jahn R. (2000) Mol. Biol. Cell 11, 3289–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang C. C., Ng C. P., Lu L., Atlashkin V., Zhang W., Seet L. F., Hong W. J. (2004) Dev. Cell 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 17. Lippert U., Ferrari D. M., Jahn R. (2007) FEBS Lett. 581, 3479–3484 [DOI] [PubMed] [Google Scholar]
- 18. Sander L. E., Frank S. P., Bolat S., Blank U., Galli T., Bigalke H., Bischoff S. C., Lorentz A. (2008) Eur. J. Immunol. 38, 855–863 [DOI] [PubMed] [Google Scholar]
- 19. Loo L. S., Hwang L. A., Ong Y. M., Tay H. S., Wang C. C., Hong W. J. (2009) Eur. J. Immunol. 39, 3520–3528 [DOI] [PubMed] [Google Scholar]
- 20. Polgár J., Chung S. H., Reed G. L. (2002) Blood 100, 1081–1083 [DOI] [PubMed] [Google Scholar]
- 21. Ren Q., Barber H. K., Crawford G. L., Karim Z. A., Zhao C., Choi W., Wang C. C., Hong W., Whiteheart S. W. (2007) Mol. Biol. Cell 18, 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang C. C., Ng C. P., Shi H., Liew H. C., Guo K., Zeng Q., Hong W. J. (2010) Mol. Cell. Biol. 30, 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cosen-Binker L. I., Binker M. G., Wang C. C., Hong W., Gaisano H. Y. (2008) J. Clin. Investig. 118, 2535–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Behrendorff N., Floetenmeyer M., Schwiening C., Thorn P. (2010) Gastroenterology 139, 1711–1720 [DOI] [PubMed] [Google Scholar]
- 25. Chen X.., Walker A. K., Strahler J. R., Simon E. S., Tomanicek-Volk S. L., Nelson B. B., Hurley M. C., Ernst S. A., Williams J. A., Andrews P. C. (2006) Mol. Cell. Proteomics 5, 306–312 [DOI] [PubMed] [Google Scholar]
- 26. Weng N., Thomas D. D., Groblewski G. E. (2007) J. Biol. Chem. 282, 9635–9645 [DOI] [PubMed] [Google Scholar]
- 27. Braun J. E., Fritz B. A., Wong S. M., Lowe A. W. (1994) J. Biol. Chem. 269, 5328–5335 [PubMed] [Google Scholar]
- 28. Falkowski M. A., Thomas D. D., Groblewski G. E. (2010) J. Biol. Chem. 285, 35558–35566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaisano H. Y., Sheu L., Grondin G., Ghai M., Bouquillon A., Lowe A., Beaudoin A., Trimble W. S. (1996) Gastroenterology 111, 1661–1669 [DOI] [PubMed] [Google Scholar]
- 30. Pickett J. A., Campos-Toimil M., Thomas P., Edwardson J. M. (2007) Biochem. Biophys. Res. Commun. 359, 599–603 [DOI] [PubMed] [Google Scholar]
- 31. Hansen N. J., Antonin W., Edwardson J. M. (1999) J. Biol. Chem. 274, 22871–22876 [DOI] [PubMed] [Google Scholar]
- 32. Wang C. C., Shi H., Guo K., Ng C. P., Li J., Gan B. Q., Chien, Liew H., Leinonen J., Rajaniemi H., Zhou Z. H., Zeng Q., Hong W. (2007) Mol. Biol. Cell 18, 1056–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaisano H. Y., Sheu L., Foskett J. K., Trimble W. S. (1994) J. Biol. Chem. 269, 17062–17066 [PubMed] [Google Scholar]
- 34. Larina O., Bhat P., Pickett J. A., Launikonis B. S., Shah A., Kruger W. A., Edwardson J. M., Thorn P. (2007) Mol. Biol. Cell 18, 3502–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhat P., Thorn P. (2009) Mol. Biol. Cell 20, 1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deleted in proof. [Google Scholar]
- 37. Turvey M. R., Thorn P. (2004) Pflugers Arch. 448, 552–555 [DOI] [PubMed] [Google Scholar]
- 38. Nemoto T., Kojima T., Oshima A., Bito H., Kasai H. (2004) J. Biol. Chem. 279, 37544–37550 [DOI] [PubMed] [Google Scholar]
- 39. Valentijn J. A., Valentijn K., Pastore L. M., Jamieson J. D. (2000) Proc. Natl. Acad. Sci. U. S. A. 97, 1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sokac A. M., Co C., Taunton J., Bement W. (2003) Nat. Cell Biol. 5, 727–732 [DOI] [PubMed] [Google Scholar]
- 41. Miklavc P., Wittekindt O. H., Felder E., Dietl P. (2009) Ann. N. Y. Acad. Sci. 1152, 43–52 [DOI] [PubMed] [Google Scholar]
- 42. Sokac A. M., Bement W. M. (2006) Mol. Biol. Cell 17, 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eitzen G. (2003) Biochim. Biophys. Acta 1641, 175–181 [DOI] [PubMed] [Google Scholar]
- 44. Freedman S. D., Kern H. F., Scheele G. A. (1998) Eur. J. Cell Biol. 75, 163–173 [DOI] [PubMed] [Google Scholar]
- 45. Thorn P. (1996) Cell Calcium 20, 203–214 [DOI] [PubMed] [Google Scholar]
- 46. Low J. T., Shukla A., Behrendorff N., Thorn P. (2010) J. Cell Sci. 123, 3201–3208 [DOI] [PubMed] [Google Scholar]
- 47. Falkowski M. A., Thomas D. D., Messenger S. W., Martin T. F., Groblewski G. E. (2011) Am. J. Physiol. doi ajpgi.00108.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]