Abstract
Drosophila serrata is a member of the montium group, which contains more than 98 species and until recently was considered a subgroup within the melanogaster group. This Drosophila species is an emerging model system for evolutionary quantitative genetics and has been used in studies of species borders, clinal variation and sexual selection. Despite the importance of D. serrata as a model for evolutionary research, our poor understanding of its genome remains a significant limitation. Here, we provide a first-generation gene-based linkage map and a physical map for this species. Consistent with previous studies of other drosophilids we observed strong conservation of genes within chromosome arms homologous with D. melanogaster but major differences in within-arm synteny. These resources will be a useful complement to ongoing genome sequencing efforts and QTL mapping studies in this species.
Keywords: linkage map, montium, RNA-Seq
Species of the genus Drosophila have a long history as experimental organisms in genetics research (Bridges 1916; Painter 1934) because of their worldwide distribution, easy maintenance in the laboratory, short generation times, and polytene chromosomes. Polytene chromosome maps constructed initially for Drosophila melanogaster (Bridges 1935; Lefevre 1976) and later for numerous other Drosophila species allowed the physical placement of genes in many types of genetic studies and provided the basis for modern genomics (Schaeffer et al. 2008). D. melanogaster has been developed as a standard species for genomics research, and its genome has now been extensively mapped and sequenced (Adams et al. 2000), as have the genomes of an additional eleven Drosophila species (Clark et al. 2007). Despite the continued development of genomic resources for the genus, the montium group has not yet been considered genomically. This group contains an estimated 98 species (Brake and Bachli 2008) and has long been considered a subgroup within the melanogaster group (Lemeunier et al. 1986). This relationship is now being reevaluated and it has been proposed to raise its status to that of species group (Da Lage et al. 2007). Its ancestors are thought to have separated from the group giving origin to D. melanogaster about 40MY ago (Tamura et al. 2004). It has been suggested that montium species have retained the ancestral genomic organization compared with D. melanogaster (Scouras 1996).
One member of the montium group, D. serrata, has been particularly well studied from an evolutionary standpoint. D. serrata has a broad geographical distribution, ranging from Papua New Guinea to south eastern Australia, and has emerged as a powerful model for addressing evolutionary questions such as the evolution of species borders (Blows and Hoffmann 1993; Hallas et al. 2002; Magiafoglou et al. 2002; Van Heerwaarden et al. 2009) and adaptation to climatic variables (Frentiu and Chenoweth 2010; Kellermann et al. 2009). The species has also been used to investigate the consequences of sexual selection (Hine et al. 2002), the evolution of mate recognition (Higgie et al. 2000), the evolution of sexual dimorphism (Chenoweth et al. 2008), sexual conflict (Delcourt et al. 2009), and the evolution of male mate choice (Chenoweth and Blows 2003; Chenoweth et al. 2007). Its multiple cuticular hydrocarbons, which serve as contact pheromones, have been extensively studied in the development of multivariate quantitative genetic approaches for exploring genetic constraints on adaptation (Blows et al. 2004; Chenoweth et al. 2010; McGuigan et al. 2011).
Despite the importance of this species as a model for evolutionary research, our poor understanding of its genome remains a significant limitation. Although a polytene chromosome map has been available for over two decades (Mavragani-Tsipidou and Scouras 1990), physical mapping has been confined to a small number of genes. Most of the genes are distributed on two chromosome arms with comparisons being made with other closely related species (Drosopoulou et al. 1996, 1997, 2002; Drosopoulou and Scouras 1995, 1998; Pardali et al. 1996; Scouras 1996). Inversion polymorphisms have been studied in D. serrata (Stocker et al. 2004) and the closely related D. birchii (Baimai 1970) and an expressed sequence tag (EST) library has recently been developed (Frentiu et al. 2009). Here, we provide a greatly expanded physical map, constructed by using in situ hybridizations to D. serrata polytene chromosomes of gene regions from D. melanogaster, D. serrata EST clones and polymerase chain reaction (PCR)-amplified D. serrata sequences. In addition we provide a first-generation linkage map based on EST-derived SNPs that will guide future quantitative trait locus (QTL) mapping studies and genome sequencing efforts in this emerging evolutionary model species.
Materials and Methods
Physical map construction
Laboratory lines of D. melanogaster and D. serrata were maintained at 19° in a constant light incubator. Several D. melanogaster lines were used. One was a chromosomal inversion-free long-term laboratory line, D20, whereas other lines had some of the common inversions found in this species, which helped in arm identification. The standard D. melanogaster cytogenetic map published on Flybase was used to localize the D. melanogaster probe sequences. The D. serrata line, W31, was collected from Wollongong, in eastern Australia, and determined to be inversion-free based on cytological comparisons (Stocker et al. 2004). Its polytene chromosome banding sequence was identical to the map published by Mavragani-Tsipidou and Scouras (1990) and used in the current study for allocating physical markers. However, for ease in this and future comparative mapping studies, we have changed the chromosome arm numbering from that of the original D. serrata map to better correspond to the D. melanogaster map. In the original map, the 2L and 3R arms of D. serrata were switched with respect to gene positions in D. melanogaster (Mavragani-Tsipidou and Scouras 1990). This was initially noted in the study by Drosopoulou et al. (1996). We have, therefore, exchanged the names of those two arms and changed the sequence of region numbers so that they are in better agreement with the D. melanogaster map.
Slides of D. melanogaster and D. serrata salivary gland chromosomes were prepared according to Ashburner (1989) and stored in 100% ethanol at −20°. They were removed from the ethanol, dried, and checked for chromosome spreads just before use. We used two different techniques for probe labeling. The most frequently used was nick translation. The plasmid and insert as well as some of the PCR-amplified probes were labeled according to the Invitrogen BioNick labeling system. These PCR-generated probes were amplified with primers designed from D. serrata ESTs. Some probes were also labeled by DOP PCR using the Roche Dig High Prime Biosynthesis Kit and primers designed for D. melanogaster intron/exon regions that had generated sequences in molecular studies on D. serrata genomic DNA.
The first amplification was a normal PCR using the specific primers and D. serrata genomic DNA. The amplified band of the expected size was excised from the gel and the DNA eluted. A second PCR was then performed on the amplified product with Dig-11-dUTP substituted for part of the dTTP and the specific primers. An aliquot of the product was run on a polyacrylamide gel together with the non-Dig−substituted PCR product to check for the presence and size shift of the Dig substituted sequence. The Dig substituted product was cleaned by spin column and used in the in situ technique.
In cases in which several bands were observed on the polyacrylamide gel after the Dig-substituted amplification, the band of the expected size was excised from the gel and the DNA extracted by mashing the gel slice in diffusion buffer (0.5M ammonium acetate, 10mM magnesium acetate, 1mM EDTA, pH 8.0, 0.1% sodium dodecyl sulfate), soaking it at 50° for 30 min in this buffer, vortexing briefly, and centrifuging the supernatant through a shredder column (QIAGEN) to remove gel fragments. This supernatant was subsequently used in the in situ hybridizations.
The in situ hybridization technique has been described previously (Stocker et al. 1993, 2006). Probe solution contained 40% or 50% formamide, 2× SSPE, and 0.05 µg/µl salmon sperm DNA. Five percent dextran sulfate or polyethylene glycol was often added to facilitate hybridization. Chromosome preparations were steam denatured together with the probe at 75° for 10 min followed by overnight incubation in a humid chamber at either 37° or 32° (for some heterologous probes). Posthybridization washes were as follows: 2 times, 5 min each in 2× SSC at room temperature; 5 min in 2× SSC at 42°; 5 min in 50% formamide/2× SSC at 42°; 2 times, 5 min each in 2× SSC at room temperature and then storage in 1× TBS overnight at 4°. Blocking and antibody reactions were subsequently carried out as in Stocker et al. (1993, 2006), either using Superblock, goat α-biotin and rabbit anti-goat rhodamine (Pierce) for the nick-translated probes, or a Fluorescent Antibody Enhancer Set for Dig Detection containing blocking solution, mouse α-Dig, anti-mouse Ig Dig, and anti-Dig fluorescein (Roche). Chromosome preparations were mounted in Vectashield (Vector Laboratories) containing 1.5 µg/ml DAPI (Roche) and examined with a Zeiss Photomicroscope using the Axiovision analysis program.
The locations of all D. melanogaster probes were checked on D. melanogaster chromosomes. Many of the probe sequences were in pBluescript. Dca and white were in a pUAST vector. The position of Dca was identified by comparing signals obtained with and without the Dca insert. Only one signal was obtained on D. serrata chromosomes with this vector minus Dca. This signal was located on the X-chromosome, and its strength as well as its position near a heterochromatic band suggested that it was identifying the white gene that is a large component of this vector. ATP7 full length was in the Pac vector, RpLP2 in pBR 332, eve in pGem, and engrailed in λgt10. pAGEN1 plasmids containing D. serrata sequences were also labeled by nick translation and came from an EST library (Frentiu et al. 2009). They were inserted in pAGEN1. These sequences were also used in the linkage map. Some of the D. serrata EST probes were hybridized to D. melanogaster chromosomes and gave signals in the anticipated region.
Sequences labeled by DOP PCR were CG14616 (lethal(1) Go196), CG4717 (knirps), CG9734 (globin1), CG8740, CG17559 (donut), and CG2165 (C07) whereas all remaining sequences were labeled using nick translation. Some sequences were labeled by both methods and were verified to hybridize to the same chromosome site. CG8740 gave a strong hybridization signal at a site on the D. melanogaster-homologous arm of D. serrata. However, another hybridization site for this probe was observed within the chromocenter. It would appear that some similar sequences are located in chromocentric heterochromatin.
Linkage map construction
Crossing and genotyping:
We performed a reciprocal F2 intercross between two highly inbred lines of D. serrata derived from two natural populations spanning extremes of the species’ eastern Australian distribution (Cooktown: CTN42, Forster: FORS4). Because a large number of chromosomal inversions have been reported in D. serrata (Stocker et al. 2004), we verified that the lines were homosequential by using polytene chromosome squashes before crossing using the protocols described previously. For SNP discovery, we used Illumina RNA-sequation (35-bp reads) on cDNA created from RNA extracted from three-day-old adult flies from each of the two lines (CTN42: 14.9 million reads, FORS4: 3.8 million reads). We assembled ESTs from the two lines separately using Edena 2.0 (Hernandez et al. 2008) in “strict” mode using a minimum overlap value of 21 bp resulting in 23,081 ESTs from CTN42 (N50 = 322 bp) and 922 from FORS4 (N50 = 276 bp). Initial SNP discovery was achieved by then aligning the ESTs to each other using MUMmer version 3.2 (Kurtz et al. 2004). We then designed oligos for a subset of 65 SNPs and validated them via bidirectional Sanger sequencing of PCR amplicons from CTN42 and FORS4 lines. We annotated the ESTs using BLAST against the genome of D. melanogaster following the procedure in Frentiu et al. (2009).
SNP genotyping was performed using the SEQUENOM MassARRAY platform. Two multiplexes were used for genotyping 61 SNPS. For each multiplex assay approximately 10 ng of genomic DNA was used. DNA was extracted from whole fly bodies using a standard phenol-chloroform method. We removed three invariant SNPs from the 61 markers: s10, s36, and s37. We did not detect any difference in genotype calls between three replicates of each parental DNA sample, which is consistent with a genotyping error below 1% for these assays.
Analyses:
Markers were assigned to linkage groups using Joinmap 4.0 (Van Ooijen 2006) beginning at LOD = 4.0 and ending at 10.0. This approach assigned 58 markers to four linkage groups encompassing the X and the second, third, and fourth (dot) chromosomes. Map construction was performed using a least squares approach implemented in the regression option in Joinmap. We used a Kosambi (1943) mapping function. All other parameters were retained as default. Linkage maps were plotted with MapChart (Voorrips 2002).
We tested for transmission ratio distortion (TRD) using χ2 tests with a significance threshold of α = 0.05. Bonferroni corrections were not applied as physical linkage between markers on the same chromosome violates the assumption of independence between tests. We did not exclude markers showing TRD on the basis of χ2 test results for three reasons. First, many of the typed autosomal SNPs showed distortion from Mendelian expectations 1:2:1 at the nominal significance level. Second, we were unable to map large portions of the genome when the α = 0.05 criterion was used, resulting in unusually short maps. Third, one of our ultimate goals was to perform QTL mapping on the F2 cross. QTL analysis is not necessarily negatively affected by distortion (Xu 2008) and distortion can, in some circumstances, increase power. The Joinmap 4.0 program uses as default, a method for assessing linkage (“Independence LOD”) that is not sensitive to TRD. We therefore followed an approach similar to Muchero et al. (2009) that involved setting a minor allele frequency cut-off criterion for each SNP as a basis for marker exclusion. We used a minor allele frequency cut-off of 0.25.
Results and Discussion
Physical map
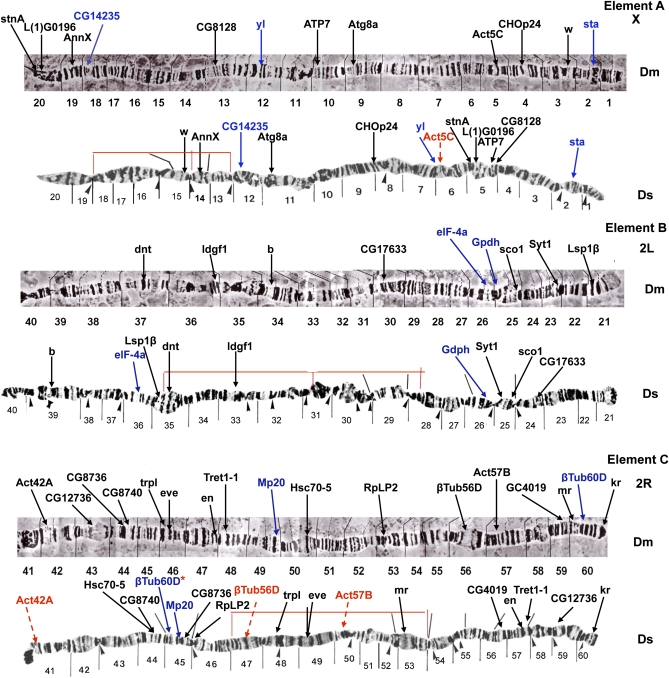
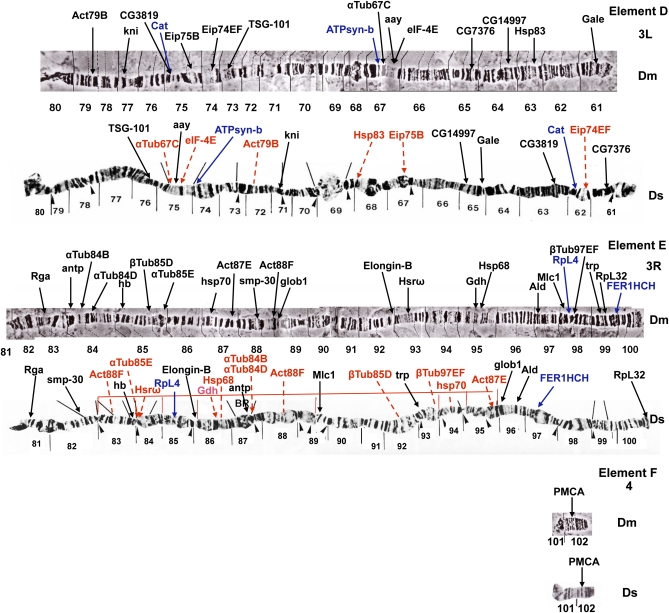
In terms of large-scale genome architecture, arm number and chromosome shape have been maintained between D. serrata and D. melanogaster. When combined with previous efforts, our physical map places 78 genes on the D. serrata genome (Drosopoulou et al. 1996, 1997, 2002; Drosopoulou and Scouras 1995, 1998; Pardali et al. 1996), (Mueller element A (X): 12, Mueller element B (2L): 9, Mueller element C (2R):17, Mueller element D (3L): 15, Mueller element E (3R): 24, Mueller element F(4):1, Table S1). When D. serrata and D. melanogaster are compared, almost complete chromosomal arm synteny is observed for the genes mapped (Figure 1, Figure 2, and Figure 3). Such arm level synteny is common, even in distantly related Drosophila species, because uncommon pericentric inversions and translocations cause duplications and deletions in gametes, but more common paracentric inversions usually do not (Krimbas and Powell 1992; Ranz et al. 2001). The actin gene family showed one additional signal on element E in D. serrata that was not present in D. melanogaster (Drosopoulou et al. 1997) (Act88F; Figure 2). This could be attributable to the use of a heterologous probe in mapping this gene family. Differences in signals for heterologous and homologous probes were observed for the β-tubulin genes in montium species (Drosopoulou et al. 2002; Drosopoulou and Scouras 1995; Scouras et al. 1992).
Figure 1 .
D. serrata and D. melanogaster polytene chromosomes showing mapped location of genes via in situ hybridization arranged in order of Mueller elements (Elements A–C in Figure 1 and Elements D–F in Figure 2). The D. melanogaster map is from Lefevre (1976), and the D. serrata map has been adapted from Mavragani-Tsipidou and Scouras (1990). Gene locations are indicated by arrows. Genes in red were mapped by Drosopoulou and Scouras (1995), Drosopoulou et al. (1996, 1997, 2002), Pardali et al. (1996), Drosopoulou and Scouras (1995, 1998) and are included for completeness. These genes were originally mapped to regions only. We have sometimes been able to give them a more defined position through examination of the original photographs. Genes in blue are SNP sequences also included on the linkage map. One of these genes, βTub60D, was also mapped by Drosopoulou et al. (2002). Thin red lines indicate inversions found along the eastern Australia D. serrata cline (Stocker et al. 2004). Black lines over D. serrata chromosomes and arrowheads beneath the chromosomes were designated as inverted repeat regions and weak points respectively by Mavragani-Tsipidou and Scouras (1990).
Figure 2 .
D. serrata and D. melanogaster polytene chromosomes showing mapped location of genes via in situ hybridization arranged in order of Mueller elements (Elements D–F). The D. melanogaster map is from Lefevre (1976), and the D. serrata map has been adapted from Mavragani-Tsipidou and Scouras (1990). Gene locations are indicated by arrows. Genes in red were mapped by Drosopoulou and Scouras (1995), Drosopoulou et al. (1996, 1997, 2002), Pardali et al. (1996), Drosopoulou and Scouras (1995, 1998) and are included for completeness. These genes were originally mapped to regions only. We have sometimes been able to give them a more defined position through examination of the original photographs. Genes in blue are SNP sequences also included on the linkage map. Thin red lines indicate inversions found along the eastern Australia D. serrata cline (Stocker et al. 2004). Black lines over D. serrata chromosomes and arrowheads beneath the chromosomes were designated as inverted repeat regions and weak points respectively by Mavragani-Tsipidou and Scouras (1990).
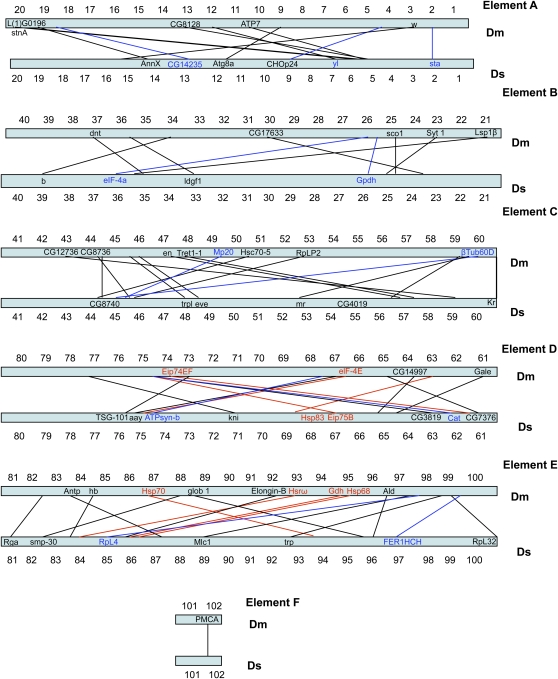
Figure 3 .
Schematic drawing showing conservation of synteny with shuffling of genes between D. serrata and D. melanogaster physical maps for the five chromosome arms. The numerous actin and tubulin genes mapped by Drosopoulou and Scouras (1995), Drosopoulou et al. (1997, 2002) are not included in this drawing.
Although chromosomal arm synteny has been conserved, there has been substantial positional gene change between D. serrata and D. melanogaster during the 40MY that have separated them (Figure 3). Extensive shuffling is also apparent when the positions of genes mapped in different members of the montium group are compared (Drosopoulou et al. 1997; Drosopoulou and Scouras 1995). This shuffling is most likely attributable to the occurrence of numerous large and small paracentric inversions. Some of these inversions could be caused by movements of transposable elements, which have been observed to be involved in restructuring events in chromosomes of Drosophila and other organisms (Gray 2000; Lim and Simmons 1994). Most transposable element–induced chromosomal rearrangements reported in Drosophila have been within-arm changes, possibly because of conformational restrictions of the chromatin (Lim and Simmons 1994) or lack of viability of the products of inter-arm arrangements (Gray 2000).
Although many of the genes studied now occur at very different positions on their chromosome arm, some groupings are still maintained in D. melanogaster and D. serrata (Figures 1 and 2). On the X-chromosome, stnA and L(1)G0196 appear to have changed position but are still located next to each other in both species. This is also true for Syt, sco1, and gdph of arm 2L, Tret1-1 and en, and trpl and eve of 2R; ATPsyn-b, αTub67C, aay, and eIF-4E of 3L; and Hsp68 and Gdh of 3R. Hsp68 and Gdh have maintained a close relationship in all but one of the six montium species compared by Drosopoulou et al. (1997). These two genes are located near an inversion breakpoint in both D. melanogaster and D. serrata, and this may be one factor keeping them together.
Duplication/deletion events may also have played a role in the chromosomal changes that have occurred between the two species, and transposable elements have been implicated in some of these (Gray 2000; Lim and Simmons, 1994). For example, trpl and eve on element C (Figure 1) appear to be located closer together in D. melanogaster than in D. serrata. The region separating these two genes in D. serrata has the appearance of a repeated region. It would be interesting to know the position of these two genes in other members of the montium group.
Certain genes that we examined localize to the same chromosomal positions in D. melanogaster and D. serrata. On the X-chromosome, sta is located in region 2 of both species, and the region itself has a similar appearance (Figure 1). On the 2R arm, kr is located near the nonchromocentric telomere in both species (Figure 1). This telomeric location suggested that genes at the ends of chromosomes might not be easily shuffled by paracentric inversions. However, RpL32 is located near the end of the D. serrata 3R arm but has a more internal location in D. melanogaster (Figure 2). Despite the gene movements that have occurred between D. melanogaster and D. serrata, the polytene chromosome structure of short regions around some homologous genes and noncentromeric telomeres often has a similar appearance. Examples are short regions around AnnX, yl, and CHOp24 on the X-chromosome; regions around dnt, ldgf1, b, and CG17633 on arm 2L; regions around Act57B, Tret1-1, en, and Act42A on arm 2R; regions around Act79B and kni and aay, eIF-4E, αTub67C, and ATPsyn-b on chromosome 3L; and regions around Rga, Ald, and Gdh and Hsp68 on arm 3R (Figures 1 and 2). However, no extensive homosequential regions are apparent in these two species.
Broad-scale synteny with other Drosophila species:
Although our primary goal was to compare D. serrata and D. melanogaster, the availability of a further 11 sequenced Drosophila genomes enabled us to also compare syntenic relationships, at least in terms of within-arm order, in a wider phylogenetic context. Using Flybase, we compared the order of genes on the D. serrata physical map to the order of their orthologs in the 12 available genomes (Schaeffer et al. 2008) (Table S3). Our results were largely consistent with those obtained by Bhutkar et al. (2008) with the exception that we did not observe greater positional rearrangements in the Sophophoran subgenera than in the Drosophila subgenera, likely because of the smaller number of genes analyzed. Arm-level synteny has been conserved for almost all genes included on the D. serrata physical map, except where identifiable chromosome structural changes have occurred in other species. For example, the pericentric and paracentric inversions in two members of the melanogaster species group, D. erecta and D. yakuba have mixed genes from the B and C elements.
When D. serrata is compared with the species in the melanogaster group, gene order is poorly conserved for almost all regions of the genome. Despite this poor conservation, some similarities are apparent. For example, the similarity at the telomeric end of element A extends through the melanogaster group to D. serrata. The change caused by inversions in this region can also be observed in D. yakuba. There appear to be more changes caused by paracentric inversions between D. serrata and the melanogaster group species; however, they cannot be followed by banding similarities. From an examination of gene order, it appears that there has been an inversion between CG12500 and the centromere, with another possible break above CG32672, which has exchanged the position of genes in the two regions of the A element (Table S3). A subsequent inversion could be proposed between CG14792 and CG2759 to bring CG2759 nearer the centromere. However, numerous other inversions in these regions must have occurred to explain the shuffling of most other genes in this element.
In the C element, D. serrata shows greater similarity with D. ananassae than with the melanogaster group species. However, the inversions that have positioned the genes in this arm have obviously been different in the lines leading to the two species. For element E, the most densely mapped chromosome, a small group of genes are syntenic in the melanogaster species group and D. serrata. This group, in the order CG1028, CG1913, CG2512 telomere to centromere, in D. melanogaster, D. simulans, D. sechelia, and D. erecta has been maintained in D. serrata, but the genes reside in different regions. In D. yakuba, pericentric inversions appear to have reversed the order to CG2512, CG1913, CG1028 (Table S3). In D. ananassae and species more distantly related to D. melanogaster, this group of genes has been completely broken up. Although our comparison of syntentic relationships in this study was limited to relative gene order, future sequencing of the serrata genome should give a much more accurate picture of the gene changes that have occurred and their relationship to gene positions across the Drosophila phylogeny.
Linkage map
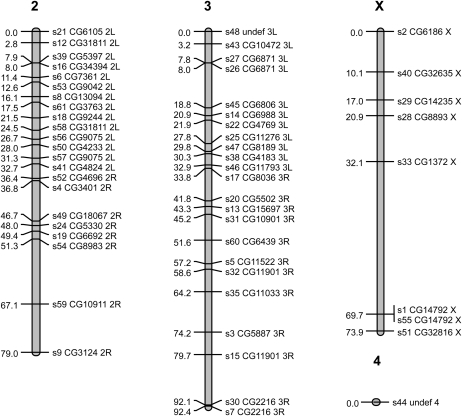
We genotyped a total of 417 flies, comprising 113 females and 104 males from the CTN42 × FORS4 cross, and 111 females and 89 males from the FORS4 × CTN42 cross. Of the initial 61 SEQUENOM assays designed, three were invariant in our mapping population and uninformative despite earlier checks (s10, s36, s37); these were removed and the remaining 58 were assigned to four linkage groups corresponding to the four chromosomes of D. serrata at a linkage LOD of 10.0 (X: 8 SNPs; Chr 2: 26 SNPs; Chr 3: 23 SNPs; and Chr 4: 1 SNP). We observed no differences in linkage group assignment between sexes or the two reciprocal crosses. No SNPs remained unassigned (Table 1; see File S1 for raw genotype data).
Table 1 . EST derived SNP markers used to construct the D. serrata linkage map.
| Marker | D. serrata Linkage Group | D. melanogaster Chr. Arm | CG No. | Flybase Locus Name | FlyBaseID | MAF | P Value |
|---|---|---|---|---|---|---|---|
| s1 | X | X | CG14792 | Dmel\sta | FBgn0003517 | 0.45 | 0.000 |
| s2 | X | X | CG6186 | Dmel\Tsf1 | FBgn0022355 | 0.40 | 0.000 |
| s3 | 3 | 3R | CG5887 | Dmel\desat1 | FBgn0086687 | 0.49 | 0.000 |
| s4 | 2 | 2R | CG3401 | Dmel\BTub60D | FBgn0003888 | 0.35 | 0.107 |
| s5 | 3 | 3R | CG11522 | Dmel\RpL6 | FBgn0039857 | 0.26 | 0.012 |
| s6 | 2 | 2L | CG7361 | Dmel\RFeSP | FBgn0021906 | 0.43 | 0.001 |
| s7 | 3 | 3R | CG2216 | Dmel\Fer1HCH | FBgn0015222 | 0.43 | 0.000 |
| s8 | 2 | 2L | CG13094 | Dmel\Dh31 | FBgn0032048 | 0.37 | 0.290 |
| s9 | 2 | 2R | CG3124 | Dmel\CG3124 | FBgn0034840 | 0.29 | 0.000 |
| s10 | Not mapped | 2L | CG9042 | Dmel\Gpdh | FBgn0001128 | 0.00 | 0.000 |
| s11 | 2a | 2L | CG31811 | Dmel\cenG1A | FBgn0028509 | 0.12 | 0.000 |
| s12 | 2 | 2L | CG31811 | Dmel\cenG1A | FBgn0028509 | 0.29 | 0.000 |
| s13 | 3 | 3R | CG15697 | Dmel\RpS30 | FBgn0038834 | 0.31 | 0.546 |
| s14 | 3 | 3L | CG6988 | Dmel\Pdi | FBgn0014002 | 0.31 | 0.345 |
| s15 | 3 | 3R | CG11901 | Dmel\Ef1γ | FBgn0029176 | 0.33 | 0.000 |
| s16 | 2 | 2L | CG34394 | Dmel\CG34394 | FBgn0085423 | 0.39 | 0.042 |
| s17 | 3 | 3R | CG8036 | Dmel\CG8036 | FBgn0037607 | 0.29 | 0.046 |
| s18 | 2 | 2L | CG9244 | Dmel\Acon | FBgn0010100 | 0.46 | 0.000 |
| s19 | 2 | 2R | CG6692 | Dmel\Cp1 | FBgn0013770 | 0.38 | 0.084 |
| s20 | 3 | 3R | CG5502 | Dmel\RpL4 | FBgn0003279 | 0.29 | 0.138 |
| s21 | 2 | 2L | CG6105 | Dmel\I(2)06225 | FBgn0010612 | 0.49 | 0.000 |
| s22 | 3 | 3L | CG4769 | Dmel\CG4769 | FBgn0035600 | 0.31 | 0.478 |
| s23 | 2a | 2R | CG5330 | Dmel\Nap1 | FBgn0015268 | 0.18 | 0.000 |
| s24 | 2 | 2R | CG5330 | Dmel\Nap1 | FBgn0015268 | 0.37 | 0.338 |
| s25 | 3 | 3L | CG11276 | RpS4 | FBgn0011284 | 0.28 | 0.026 |
| s26 | 3 | 3L | CG6871 | Dmel\Cat | FBgn0000261 | 0.29 | 0.210 |
| s27 | 3 | 3L | CG6871 | Dmel\Cat | FBgn0000261 | 0.30 | 0.347 |
| s28 | X | X | CG8893 | Dmel\Gapdh2 | FBgn0001091 | 0.43 | 0.000 |
| s29 | X | X | CG14235 | Dmel\CG14235 | FBgn0031066 | 0.42 | 0.000 |
| s30 | 3 | 3R | CG2216 | Dmel\Fer1HCH | FBgn0015222 | 0.44 | 0.000 |
| s31 | 3 | 3R | CG10901 | Dmel\osk | FBgn0003015 | 0.30 | 0.482 |
| s32 | 3 | 3R | CG11901 | Dmel\Ef1γ | FBgn0029176 | 0.30 | 0.364 |
| s33 | X | X | CG1372 | Dmel\yl | FBgn0004649 | 0.43 | 0.000 |
| s34 | 2a | 2R | CG3161 | Dmel\Vha16-1 | FBgn0262736 | 0.17 | 0.000 |
| s35 | 3 | 3R | CG11033 | Dmel\Kdm2 | FBgn0037659 | 0.48 | 0.000 |
| s36 | Not mapped | 3R | CG7610 | Dmel\ATPsyn-γ | FBgn0020235 | 0.00 | 0.000 |
| s37 | Not mapped | 3L | CG6988 | Dmel\Pdi | FBgn0014002 | 0.00 | 0.000 |
| s38 | 3 | 3L | CG4183 | Dmel\Hsp26 | FBgn0001225 | 0.29 | 0.061 |
| s39 | 2 | 2L | CG5397 | Dmel\CG5397 | FBgn0031327 | 0.40 | 0.021 |
| s40 | X | X | CG32635 | Dmel\CG32635 | FBgn0052635 | 0.41 | 0.000 |
| s41 | 2 | 2L | CG4824 | Dmel\BicC | FBgn0000182 | 0.37 | 0.205 |
| s42 | 2a | 2R | CG9364 | Dmel\Treh | FBgn0003748 | 0.22 | 0.000 |
| s43 | 3 | 3L | CG10472 | Dmel\CG10472 | FBgn0035670 | 0.30 | 0.421 |
| s44 | 4 | 4 | No definition line found | 1095281-1095303 | _ | 0.32 | 0.226 |
| s45 | 3 | 3L | CG6806 | Dmel\Lsp2 | FBgn0002565 | 0.31 | 0.507 |
| s46 | 3 | 3L | CG11793 | Dmel\Sod | FBgn0003462 | 0.29 | 0.065 |
| s47 | 3 | 3L | CG8189 | Dmel\ATPsyn-b | FBgn0019644 | 0.28 | 0.032 |
| s48 | 3 | 3L | No definition line found | 18976884-18976901 | _ | 0.33 | 0.485 |
| s49 | 2 | 2R | CG18067 | Dmel\CG18067 | FBgn0034512 | 0.37 | 0.113 |
| s50 | 2 | 2L | CG4233 | Dmel\Got2 | FBgn0001125 | 0.46 | 0.000 |
| s51 | X | X | CG32816 | Dmel\CG32816 | FBgn0052816 | 0.46 | 0.000 |
| s52 | 2 | 2R | CG4696 | Dmel\Mp20 | FBgn0002789 | 0.35 | 0.081 |
| s53 | 2 | 2L | CG9042 | Dmel\Gpdh | FBgn0001128 | 0.40 | 0.025 |
| s54 | 2 | 2R | CG8983 | Dmel\ERp60 | FBgn0033663 | 0.40 | 0.017 |
| s55 | X | X | CG14792 | Dmel\sta | FBgn0003517 | 0.45 | 0.000 |
| s56 | 2 | 2L | CG9075 | Dmel\eIF-4a | FBgn0001942 | 0.43 | 0.000 |
| s57 | 2 | 2L | CG9075 | Dmel\eIF-4a | FBgn0001942 | 0.36 | 0.213 |
| s58 | 2 | 2L | CG31811 | Dmel\cenG1A | FBgn0028509 | 0.47 | 0.000 |
| s59 | 2 | 2R | CG10911 | Dmel\CG10911 | FBgn0034295 | 0.43 | 0.000 |
| s60 | 3 | 3R | CG6439 | Dmel\CG6439 | FBgn0038922 | 0.32 | 0.807 |
| s61 | 2 | 2L | CG3763 | Dmel\Fbp2 | FBgn0000640 | 0.37 | 0.279 |
Included are D. melanogaster gene names corresponding to the top BLAST hit of the full EST to the D. melanogaster genome.
Markers that were assigned to chromosomal arms but not mapped on the linkage map because of transmission ratio distortion. MAF is the minor allele frequency in the entire dataset of 414 individuals and P-values correspond to χ2 for deviation from Mendelian expectations in the combined datasets (d.f. = 2 for autosomal markers and d.f. = 1 for X-linked markers).
As observed on the physical map, the linkage group assignments had tight correspondence with the chromosomal locations of the D. melanogaster genes, which further suggests arm-level gene conservation between the two species (Table 1). We observed one instance of a marker being assigned to a different chromosome as reflected by its best BLAST hit to D. melanogaster. The top D. melanogaster BLAST hit for the EST from which we designed this assay was Gapdh1 (e-value = 6.45381e-54), which resides on 2R in D. melanogaster. However in D. serrata the s28 marker co-segregated with all of the X-linked markers. An absence of male heterozygotes in our F2 mapping population also strongly suggested that this marker is indeed X-linked in D. serrata. Closer inspection of the BLAST table for this marker indicated a near equal quality hit to Gapdh2 (e-value = 2.55014e-53). This gene is X-linked in D. melanogaster and therefore may be the most likely ortholog of the marker. The strong conservation of genes within homologous chromosomal arms observed for D. serrata is entirely consistent with previous studies of the genus Drosophila (Sturtevant and Novitski 1941).
Despite some differences in the location, but not direction, of TRD in the reciprocal crosses, we observed very few differences between linkage maps constructed from each reciprocal and therefore we constructed the final linkage map on the combined data set. After removing highly distorted markers (MAF < 0.25 threshold) we were able to order 54 on our linkage map. From chromosome 2 we removed four assigned markers (s11, s23, s34, s42). No markers were removed from the third or X-chromosomes. The placement of markers along the two autosomes corresponded to the chromosomal arm assignments in D. melanogaster; we did not observe any instance of a marker moving chromosomal arms on the basis of its expected arm designation in D. melanogaster (Figure 3).
The total map length was 245.3 cM (Figure 4), which is similar to D. melanogaster (290 cM) (Catcheside 1977) but shorter than estimates reported for other species such as D. pseudoobscura (447cM) (Anderson 1990) or D. simulans (376.2 cm) (Barker and Moth 2001). At least two SNPs on the linkage map were physically mapped to each chromosome arm with the exception of the fourth (dot) chromosome, where only one SNP was assigned (Figures 1 and 2, blue text). Their positions on the physical map were comparable with their relative positions on the linkage map (Figure 3). The average marker coverage across the entire genome was one marker per 4.5 cM (X: 9.2 cM; Chr 2: 3.6 cM; Chr 3: 4.0 cM).
Figure 4 .
First-generation linkage map for D. serrata. Shown are the locations of EST-derived SNP markers on four chromosomes. SNP labels give the marker name followed by the D. melanogaster gene corresponding to the top BLAST hit of the EST from which the SNP assay was developed followed by the chromosomal arm on which it resides in D. melanogaster.
When we assayed two SNPs in the same gene, both usually mapped to the same location and were separated by very small distances (e.g., s7 and s30, s26, and 27 and s1 and s55). However, in a few cases we observed discrepancies in the location of SNPs as determined by sequence homology with D. melanogaster and the linkage map. Markers s15 and s32 BLAST to different locations within the Ef1γ gene in D. melanogaster. Although both these SNPs mapped to 3L, they mapped to different locations along it, separated by approximately 21.1 cM. It is possible that there are multiple copies of the oligo binding sites for one of these assays in the D. serrata genome. A similar pattern was observed in two cases on 2L (s12 and s58, s57 and s56). Although it is also true that these cases of discrepancy in marker location may represent problems with the genotyping assay, because we cannot currently exclude the multiple copy hypothesis we have retained them on the map.
We observed significant TRD in the F2 crosses. When analyzing the reciprocals separately by sex, several of the autosomal markers showed significant deviations from Mendelian expectations at α = 0.05 (FORS4 × CTN42: males 48%, females 32%; CTN42 × FORS4: males 30%, females 28%). There was generally a large degree of overlap in the identity of distorted markers in the reciprocal crosses but there were also markers showing distortion in only one cross (Table S2). For X-linked markers, we only detected two instances of apparent but weak distortion in FORS4 × CTN42 (males for s28 and s29). We observed consistency in the direction of TRD with an overrepresentation of CTN 42 alleles in all but 2 of the 69 individual significant tests (Table S2).
This result suggests that the apparent distortion was not a consequence of genotyping error but instead reflects a biological phenomenon. TRD is a common observation in both interspecific and intraspecific Drosophila crosses and may indicate segregation distortion (Lyttle 1991), although its demonstration requires additional information. TRD is more common in interspecific than intraspecific crosses but may arise from genetic incompatibilities among populations. The Cooktown and Forster populations crossed in this study represent near-extreme ends of the natural distribution of D. serrata along the eastern coast of Australia and are separated by more than 2000 km. Previous work has shown that population structure tends to be weak in this species (Magiafoglou et al. 2002, Van Heerwaarden et al. 2009), but it does exhibit a pattern of isolation by-distance at neutral markers (Chenoweth and Blows 2008), suggesting that the evolution of gametic incompatibilities between distant populations of D. serrata is possible. Although chromosomal inversions are sometimes associated with TRD (Lyon 2003), we karyotyped the CTN42 and FORS4 lines before crossing and verified that they were homosequential and therefore are able to exclude large inversions as cause of the TRD. However, it remains possible that smaller inversions contributing to TRD were undetected by our screening procedures. The pattern could also be explained by the fixation of a greater number of deleterious mutations in the FORS4 line during inbreeding as compared with CTN42. However, as many of these alleles would be expected to be recessive, this pattern predicts a deficiency of CTN42 homozygotes in the F2 population. The fact that in many cases we observed a deficiency of heterozygotes rather than homozygotes to some extent precludes this explanation, leaving genomic incompatibilities a more likely explanation for the observed pattern.
We have presented an extensive physical map and a first-generation linkage map for D. serrata, a model species for studying climatic adaptation and sexual selection, and we have provided a comparison of the location of genes with D. melanogaster. There was strong conservation of genes within chromosomal arms but a lack of macrosynteny within chromosomal arms. This work suggests that it will be relatively straightforward to assign genome scaffolds to chromosomal arms on the basis of homology with D. melanogaster but establishing scaffold order will remain challenging. However as in many species of Drosophila, where small regions of microsynteny are often highly conserved, phylogenetically-informed bioinformatic approaches (Bhutkar et al. 2006) can exploit this microsynteny to great effect to assign scaffold orders (Schaeffer et al. 2008). The linkage map presented here will be useful for initial QTL mapping, and as a basis for guiding marker development for fine mapping efforts.
Supplementary Material
Acknowledgments
We thank Thomas Naderer, Monash University, for his patience and helpfulness in advising us on the operation of the photomicroscope and Ronald Lee, Department of Genetics, University of Melbourne, Melbourne, Victoria, Australia, for help with synteny comparisons. The following people generously provided vectors containing D. melanogaster sequences that were used as probes on D. serrata chromosomes: Valeria Valente, Federal University of Ribeiraõ Preto (UFRP), Ribeirao Preto, Brazil: CG3060 (morula) and CG7376; Maria Luisa Paco-Larson, UFRP, Ribeirao Preto, Brazil: CG4918 (RpLP2), CG7939 (RpL32), and CG14616 (lethal(1) Go196); Raphaela Martins Maia, UFRP, Ribeirao Preto, Brazil: CG2165; Luis Paulo Andrioli, Depto de Biologia, University of Saõ Paulo, Sao Paulo, Brazil: CG2329 (even-skipped), CG3440 (kruppel), CG4717 (knirps), CG9786 (hunchback), and CG1028 (antennapedia); Richard Burke, Monash University, Melbourne, Victoria, Australia: CG1816 (ATP7 full-length and short sequence) and CG8885 (Sco 1); Eliana Abdelhay, Department of Genetics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil: CG9015 (engrailed); Steve McKechnie, Department of Genetics, Monash University, Melbourne, Victoria, Australia: CG7390 (Dca) and CG2757 (white); Marie Phillips, Department of Genetics, University of Melbourne, Melbourne, Victoria, Australia: CG7811 (black), CG12500 (stonedA), CG3139 (syt1), CG7875 (trp), and CG18345 (trpl). This research was supported with funding from the Australian Research Council and The University of Queensland.
Footnotes
Communicating editor: R. Kulathinal
Literature Cited
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., et al. , 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195 [DOI] [PubMed] [Google Scholar]
- Anderson, W., 1990 Linkage map of the fruit fly Drosophila pseudoobscura, pp. 3.188–3.189 in Genetic Maps, edited by S. J. O’Brien. Cold Spring Harbor Laboratory Press, New York.
- Ashburner M., 1989. Drosophila: A Laboratory Manual, Cold Spring Harbor Press, New York [Google Scholar]
- Baimai V., 1970. Chromosomal polymorphisms in Drosophila birchii. J. Hered. 66: 109–126 [PubMed] [Google Scholar]
- Barker J. S. F., Moth J. J., 2001. Linkage maps of D. simulans: An update of Sturtevant (1929) with additional loci. Drosoph. Inf. Serv. 84: 205–206 [Google Scholar]
- Bhutkar A., Russo S., Smith T. F., Gelbart W. M., 2006. Techniques for multi-genome synteny analysis to overcome assembly limitations. Genome Inform. 17: 152–161 [PubMed] [Google Scholar]
- Bhutkar A., Schaeffer S. W., Russo S. M., Xu M., Smith T. F., et al. , 2008. Chromosomal rearrangement inferred from comparisons of 12 Drosophila genomes. Genetics. 179: 1657–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blows M. W., Chenoweth S. F., Hine E., 2004. Orientation of the genetic variance-covariance matrix and the fitness surface for multiple male sexually selected traits. Am. Nat. 163: E329–E340 [DOI] [PubMed] [Google Scholar]
- Blows M. W., Hoffmann A. A., 1993. The genetics of central and marginal populations of Drosophila serrata. 1. Genetic variation for stress resistance and species borders. Evolution 47: 1255–1270 [DOI] [PubMed] [Google Scholar]
- Brake I., Bachli G., 2008. World Catalogue of Insects, Volume 9: Drosophilidae (Diptera). Apollo Books, Stenstrup, Denmark [Google Scholar]
- Bridges C., 1916. Non-disjunction as proof of the chromosomal theory of heredity (Concluded). Genetics 1: 107–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C., 1935. Salivary chromosome maps. J. Hered. 26: 60–64 [Google Scholar]
- Catcheside D. W., 1977. Genetics of Recombination, University Park Press, Baltimore, MD [Google Scholar]
- Chenoweth S. F., Blows M. W., 2003. Signal trait sexual dimorphism and mutual sexual selection in Drosophila serrata. Evolution 57: 2326–2334 [DOI] [PubMed] [Google Scholar]
- Chenoweth S. F., Blows M. W., 2008. Qst meets the G matrix: the dimensionality of adaptive divergence in multiple correlated quantitative traits. Evolution 62: 1437–1449 [DOI] [PubMed] [Google Scholar]
- Chenoweth S. F., Petfield D., Doughty P., Blows M. W., 2007. Male choice generates stabilizing sexual selection on a female fecundity correlate. J. Evol. Biol. 20: 1745–1750 [DOI] [PubMed] [Google Scholar]
- Chenoweth S. F., Rundle H. R., Blows M. W., 2008. Genetic constraints and the evolution of display trait sexual dimorphism by natural and sexual selection. Am. Nat. 171: 22–34 [DOI] [PubMed] [Google Scholar]
- Chenoweth S. F., Rundle H. D., Blows M. W., 2010. The contribution of selection and genetic constraints to phenotypic divergence. Am. Nat. 175: 186–196 [DOI] [PubMed] [Google Scholar]
- Clark A. G., Eisen M. B., Smith D. R., Bergman C. M., Oliver B., et al. , 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450: 203–218 [DOI] [PubMed] [Google Scholar]
- Da Lage J.-L., Kergoat G. J., Maczkowiak F., Silvain J.-F., Cariou M.-L., et al. , 2007. A phylogeny of Drosophilidae using the Amyrel gene: questioning the Drosophila melanogaster species group boundaries. J. Zoological Syst. Evol. Res. 45: 47–63 [Google Scholar]
- Delcourt M., Blows M. W., Rundle H. D., 2009. Sexually antagonistic genetic variance for fitness in an ancestral and a novel environment. Proc. Biol. Sci. 276: 2009–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosopoulou E., Scouras Z. G., 1995. The Beta-tubulin gene family evolution in the Drosophila montium subgroup of the melanogaster species group. J. Mol. Evol. 41: 293–298 [PubMed] [Google Scholar]
- Drosopoulou E., Scouras Z. G., 1998. The organization of the alpha-tubulin gene family in the Drosophila montium subgroup of the melanogaster species group. Genome 41: 504–509 [DOI] [PubMed] [Google Scholar]
- Drosopoulou E., Konstantopoulou I., Scouras Z. G., 1996. The heat shock genes in the Drosophila montium subgroup: Chromosomal localization and evolutionary implications. Chromosoma 105: 104–110 [DOI] [PubMed] [Google Scholar]
- Drosopoulou E., Tsiafouli M., Mavragani-Tsipidou P., Scouras Z. G., 1997. The glutamate dehydrogenase, E74 and putative actin gene loci in the Drosophila montium subgroup - Chromosomal homologies among the montium species and D. melanogaster. Chromosoma 106: 20–28 [DOI] [PubMed] [Google Scholar]
- Drosopoulou E., Wiebauer K., Yiangou M., Mavragani-Tsipidou P., Domdey H., et al. , 2002. Isolation, characterization, and localization of beta-tubulin genomic clones of three Drosophila montium subgroup species. Genome 45: 604–607 [DOI] [PubMed] [Google Scholar]
- Frentiu F. D., Adamski M., McGraw E. A., Blows M. W., Chenoweth S. F., 2009. An expressed sequence tag (EST) library for Drosophila serrata, a model system for sexual selection and climatic adaptation studies. BMC Genomics 10: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentiu F. D., Chenoweth S. F., 2010. Clines in cuticular hydrocarbons in two Drosophila species with independent population histories. Evolution 64: 1784–1794 [DOI] [PubMed] [Google Scholar]
- Gray H. M., 2000. It takes two transposons to tango. Trends Genet. 16: 461–468 [DOI] [PubMed] [Google Scholar]
- Hallas R., Schiffer M., Hoffmann A. A., 2002. Clinal variation in Drosophila serrata for stress resistance and body size. Genet. Res. 79: 141–148 [DOI] [PubMed] [Google Scholar]
- Hernandez D., Francois P., Farinelli L., Osteras M., Schrenzel J., 2008. De novo bacterial genome sequencing: Millions of very short reads assembled on a desktop computer. Genome Res. 18: 802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgie M., Chenoweth S., Blows M. W., 2000. Natural selection and the reinforcement of mate recognition. Science 290: 519–521 [DOI] [PubMed] [Google Scholar]
- Hine E., Lachish S., Higgie M., Blows M. W., 2002. Positive genetic correlation between female preference and offspring fitness. Proc. R. Soc. Lond. B Biol. Sci. 269: 2215–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann V., Van Heerwaarden B., Sgro C. M., Hoffmann A. A., 2009. Fundamental evolutionary limits in ecological traits drive drosophila species distributions. Science 325: 1244–1246 [DOI] [PubMed] [Google Scholar]
- Kosambi D. D., 1943. The estimation of map distances from recombination values. Ann. Eugen. 12: 172–175 [Google Scholar]
- Krimbas C. B., Powell J. R., 1992. Drosophila inversion polymorphism, CRC Press, Boca Raton, FL [Google Scholar]
- Kurtz S., Phillippy A., Delcher A. L., Smoot M., Shumway M., et al. , 2004. Versatile and open software for comparing large genomes. Genome Biol. 5: R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre G., 1976. A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands, pp. 1–66 in The Genetics and Biology of Drosophila, edited by Ashburner M., Novitsky E. Academic Press, London [Google Scholar]
- Lemeunier F., David J. R., Tsacas L., Ashburner M., 1986. The Melanogaster species group. Genet. Biol. Drosophila 3e: 147–156 [Google Scholar]
- Lim J. K., Simmons M. J., 1994. Gross chromosome rearrangements mediated by transposable elements in Drosophila melanogaster. Bioessays 15: 269–275 [DOI] [PubMed] [Google Scholar]
- Lyttle T. W., 1991. Segregation distorters. Annu. Rev. Genet. 25: 511–557 [DOI] [PubMed] [Google Scholar]
- Lyon M. F., 2003. Transmission ratio distortion in mice. Annu. Rev. Genet. 37: 393–408 [DOI] [PubMed] [Google Scholar]
- Magiafoglou A., Carew M. E., Hoffmann A. A., 2002. Shifting clinal patterns and microsatellite variation in Drosophila serrata populations: a comparison of populations near the southern border of the species range. J. Evol. Biol. 15: 763–774 [Google Scholar]
- Mavragani-Tsipidou P., Scouras Z. G., 1990. Evolutionary implications of duplications and Balbani rings in Drosophila. A study of Drosophila serrata. Genome 33: 478–483 [DOI] [PubMed] [Google Scholar]
- McGuigan K., Rowe L., Blows M. W., 2011. Pleiotropy, apparent stabilizing selection and uncovering fitness optima. Trends Ecol. Evol. 26: 22–29 [DOI] [PubMed] [Google Scholar]
- Muchero W., Diop N. N., Bhat P. R., Fenton R. D., Wanamaker S., et al. , 2009. A consensus genetic map of cowpea Vigna unguiculata (L) Walp. and synteny based on EST-derived SNPs. Proc. Natl. Acad. Sci. USA 106: 18159–18164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter T., 1934. Salivary chromosomes and the attack on the gene. J. Hered. 25: 465–476 [Google Scholar]
- Pardali E., Feggou E., Drosopoulou E., Konstantopoulou I., Scouras Z. G., Mavragani-Tsipidou P., 1996. The Afrotropical Drosophila montium subgroup: Balbiani ring 1, polytene chromosomes, and heat shock response of Drosophila vulcana. Genome 39: 588–597 [DOI] [PubMed] [Google Scholar]
- Ranz J. M., Casals F., Ruiz A., 2001. How malleable is the eukaryotic genome? Extreme rate of chromosomal rearrangement in the genus Drosophila. Genome Res. 11: 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer S. W., Bhutkar A. U., McAllister B. F., Matsuda M., Matzkin L. M., et al. , 2008. Polytene chromosomal maps of 11 Drosophila species: The order of genomic scaffolds inferred from genetic and physical maps. Genetics 179: 1601–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scouras Z. G., 1996. The Drosophila montium subgroup species: recent cytogenetic, molecular, developmental and evolutionary studies. Bios 3: 125–158 [Google Scholar]
- Scouras Z. G., Kyrpides N., Mavragani-Tsipidou P., 1992. The prominent role of ectopic pairing in the toroidal conformation of Drosophila polytene chromosome bands. Cytobios 69: 47–54 [PubMed] [Google Scholar]
- Stocker A. J., Gorab E., Amabis J. M., Lara F. J. S., 1993. A molecular cytogenic compariosn between Rhynchosciara americana and Rhynchosciara hollaendreri (Diptera, Sciaridae). Genome 36: 831–843 [DOI] [PubMed] [Google Scholar]
- Stocker A. J., Foley B., Hoffmann A., 2004. Inversion frequencies in Drosophila serrata along an eastern Australian transect. Genome 47: 1144–1153 [DOI] [PubMed] [Google Scholar]
- Stocker A. J., Madalena C. R. G., Gorab E., 2006. The effects of temperature shock on transcription and replication in Rhynchosciara americana (Diptera: Sciaridae). Genetica 126: 277–290 [DOI] [PubMed] [Google Scholar]
- Sturtevant A. H., Novitski E., 1941. The homologies of the chromosome elements in the genus Drosophila. Genetics 26: 517–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Subramanian S., Kumar S., 2004. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol. Biol. Evol. 21: 36–44 [DOI] [PubMed] [Google Scholar]
- van Heerwaarden B., Kellermann V., Schiffer M., Blacket M., Sgro C. M., et al. , 2009. Testing evolutionary hypotheses about species borders: patterns of genetic variation towards the southern borders of two rainforest Drosophila and a related habitat generalist. Proc. Biol. Sci. 276: 1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen J., 2006. JoinMap: Software for the calculation of genetic linkage maps, version 4, Kyazma, Wageningen, Netherlands [Google Scholar]
- Voorrips R. E., 2002. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 93: 77–78 [DOI] [PubMed] [Google Scholar]
- Xu S., 2008. Quantitative trait locus mapping can benefit from segregation distortion. Genetics 180: 2201–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.