Background: Tnrc6a is involved for microRNA-mediated gene silencing in vitro.
Results: Disruption of Tnrc6a results in defect in miRNAs pathway and growth arrest and apoptosis of yolk sac endoderm.
Conclusion: Tnrc6a-mediated miRNA function plays an important role in development of yolk sac endoderm.
Significance: Results from this study will significantly improve our understanding on the role of Tnrc6a in mouse development.
Keywords: Apoptosis, Cell Cycle, MicroRNA, mRNA Decay, P-body, Cdkn1a (p21), GW182 (Tnrc6a), Yolk Sac Endoderm
Abstract
Tnrc6 family members (Tnrc6a/b/c) are key components of the RNA-induced silencing complex in microRNA (miRNA)-mediated gene suppression. Here, we show that Tnrc6a, also known as GW182, is selectively expressed in the yolk sac endoderm and that gene trap disruption of GW182 leads to growth arrest and apoptosis. We found that targets of miRNAs highly expressed in the yolk sac are significantly derepressed in GW182gt/gt mutant mice, although levels of miRNAs are not altered. Specifically, growth arrest and apoptosis phenotype are associated with significant derepression of Cdkn1a (p21), Cdkn1c (P27), Lats1, Lats2, Rb1, Rbl, Bim, and Pten, known targets of miRNAs from miR-17/20/93/106 clusters highly expressed in yolk sac endoderm. Together, these data strongly suggest that GW182 is an essential functional component in the RNA-induced silencing complex for miRNA-mediated gene silencing in vivo, and selectively regulation of miRNA activity plays an important role in the proper development of yolk sac.
Introduction
miRNAs3 are small (∼21 nucleotides) non-protein-coding RNAs that regulate gene expression post-transcriptionally (1). The primary miRNA (pri-miRNA) is transcribed by RNA polymerase II from either a non-protein-coding gene or an intron of a protein-coding gene. This is followed by processing into a stem-loop intermediate miRNA precursor (pre-miRNA) of ∼70 bp by RNase III endonuclease Drosha and the double-stranded RNA-binding protein Pasha (Dgcr8). The pre-miRNA is transported into cytoplasm and further processed into a mature miRNA by another RNase III endonuclease Dicer and other currently uncharacterized components (2, 3). The mature miRNA is then incorporated into a multi-protein complex called the miRNA-induced silencing complex (RISC) that mediates translational repression or degradation of specific target mRNAs (4).
Tnrc6a was identified as an auto-antigen using serum of a patient suffering from motor and sensory neuropathy (5). It is a 182-kDa protein containing a large number of repeats of glycine (G):tryptophan (W) motifs that was originally designated as GW182 (5). GW182 belongs to an evolutionary conserved family with three paralogs in vertebrates including Tnrc6a/GW182, Tnrc6b, and Tnrc6c (6). Results from in vitro studies showed that all three GW182 family members associate with Argonaute family members in the RISC (7–10) to repress translation or promote mRNA degradation. In addition, knockdown of individual GW182 family members partially impaired miRNA- or siRNA-mediated gene silencing without affecting miRNA biogenesis or stability, indicating redundant roles of GW182 family members in controlling miRNA function (6, 11, 12). Extensive in vitro studies showed the interaction of GW182 with other components of the P-body (mRNA processing body), such as PAN2-PAN3 and CCR4-CAF1-NOT deadenylase complexes and promoting mRNA deadenylation (13–15). Further, GW182, when directly tethered to an mRNA of a reporter gene, represses the reporter expression, suggesting that GW182 acts at the final step of miRNA-mediated gene silencing (16, 17).
The Caenorhabditis elegans genome encodes two highly divergent members of GW proteins, including AlG-1-interacting protein 1 (AIN-1) and AIN-2. Both of them are interacted with Ago proteins and are required for miRNA function. A delay in vulval cell division in the double mutants of lin-31(lf) and ain-1(lf), one of GW182 homolog, suggesting a synergistic role in cell division and differentiation (18, 19). There is single GW protein in Drosophila, which is associated with RISC complex and required for miRNA activities (20). Increased expression of GW182 in Drosophila is associated with rapid cell proliferation both in early embryo and during pupal development. GW182 mutation in Drosophila resulted in defects of nuclear division and cellularization of early embryo (20). Similarly, higher GW182 protein levels together with larger and more numerous GW bodies were observed in proliferating mammalian cells, as compared with quiescent cells (21). The size of GW bodies is different in phases of the cell cycle: small in early S phase and larger during late S and G2 (21). However, little is known about the role of mammalian GW182 in vivo.
Here, we investigate functions of GW182 in mouse development by generating a gene trap mutant mouse. Our data suggested that GW182 is required for miRNA-mediated gene suppression in the yolk sac endoderm. Disruption of GW182 leads to growth arrest and apoptosis of yolk sac endoderm that likely contribute to observed defects in hematopoiesis and embryonic lethality around day 9.5. Our findings provide the first in vivo evidence that GW182 is an important functional component in RISC for miRNA-mediated gene silencing, and selective regulation of miRNA activity plays a significant role in the proper development of yolk sac.
EXPERIMENTAL PROCEDURES
Mice and Animal Care
A mouse embryonic stem cell line (XE786, strain 129/Ola) containing a gene trap insertion into the fourth intron of the GW182 gene was purchased from the International Gene Trap Consortium. The gene trap vector pGT1Lxf contains a splice-acceptor sequence upstream of lacZ (β-galactosidase) gene and a neomycin resistance gene. XE786 embryonic stem cells were injected into C57BL/6 blastocysts to generate chimeric mice that were crossed to C57BL/6 to test germ line transmission. Two chimeric male mice that transmitted the GW182 gene trap allele in their germ line were bred to generate heterozygous (GW182gt/+) and homozygous (GW182gt/gt) mutant mice. For timed pregnancy, the mice were mated overnight, and the finding of the morning vaginal plug was considered as embryonic day 0.5 (E0.5). All of the animal studies were approved by the Institutional Animal Care and Use Committee at Boston University Medical Campus.
The insertion site of the gene trap vector was mapped by sequencing long range PCR products using primers located within LacZ and exon 4 of the GW182 gene (Fig. 1A and supplemental Fig. S1). For genotyping, two primers (P1 and P3) that flank the inserted cassette and a primer (P2) within LacZ were used (Fig. 1A). The sequences of these primers follow: P1, AGGGTCTTCTTATCCAGGTAGTGAT; P2, AATCAACTTTGGAGACATGCG GGC; and P3, AGG GCG ACACAGAGAAATCCTTGG.
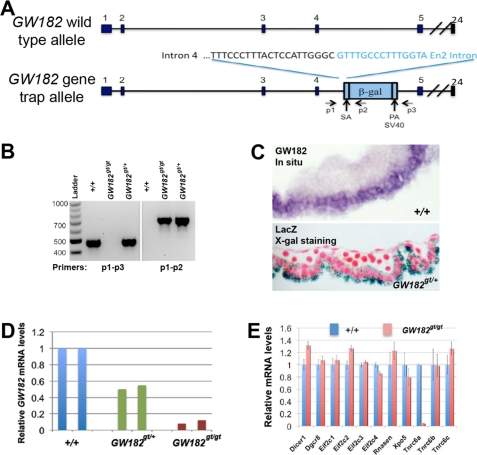
FIGURE 1.
Gene trap insertion selectively disrupts GW182 expression. A, gene trap insertion into the fourth intron of the GW182 locus. Nucleotides in black represent genomic sequences, whereas sequences from the gene trap vector are shown in blue. Locations of p1-p3 primers relative to the insertional cassette are shown. B, genotyping strategy to identify wild type (+/+) and gene trap (gt) alleles of GW182 by PCR. Primer pair p1 and p3 generates a 500-bp fragment from the wild type allele, whereas primer pair p1 and p2 produces a 750-bp fragment from the gene trap allele of GW182. C, expression of GW182 in the yolk sac endoderm detected by in situ hybridization and X-gal staining of GW182+/gt embryos at E9.5. D, dose-dependent reduction of GW182 mRNA levels in the yolk sac of heterozygous and homozygous gene trap mutant embryos at E9.5, as assayed by quantitative RT-PCR. E, mRNA array profiling showed that compared with the wild type controls at E9.5, the yolk sac of GW182gt/gt embryos exhibit selective disruption of GW182 expression without affecting mRNA levels of other genes involved in miRNA biogenesis.
miRNA and mRNA Array Analysis
Yolk sac of wild type and GW182gt/gt embryos at E9.5 was collected for total RNA isolation using TRIzol (Invitrogen). RNAs were purified according to the manufacturer's protocol before being subjected to Affymetrix® GeneChip® miRNA arrays and mouse gene 1.0 ST whole genome array (Affymetrix) for miRNA and mRNA expression profiling, respectively. The experiments were performed in triplicate. Differentially expressed miRNAs or mRNAs were identified using a two-sample t test (p < 0.05 considered significant). Array data were confirmed by using qRT-PCR for selected miRNAs and mRNAs. The Gene Set Enrichment Analysis program (Broad Institute) was used to identify targets of miRNAs that are enriched among genes significantly altered in the yolk sac of GW182gt/gt embryos. Specifically, we examined the enrichment of predicted targets among 3,543 differentially expressed genes for selected miRNAs including 15 miRNAs highly expressed in the yolk sac, 2 miRNAs expressed in the mesoderm compartment of yolk sac, and 15 miRNAs that are undetectable. The array data has been deposited into the GEO database in NCBI (GSE30244).
Quantitative Real Time PCR
For mRNAs, 1 μg of total RNA was subjected to reverse transcription using the SuperScript III first strand synthesis system (Invitrogen) followed by PCR using TaqMan Universal PCR Master Mix and TaqMan gene expression assays (Applied Biosystems). For miRNAs, 10 ng of total RNA was subjected to reverse transcription using the TaqMan microRNA reverse transcription kit (Applied Biosystems) followed by PCR using TaqMan microRNA assays (Applied Biosystems). Each experiment was performed in triplicate, and ribosomal protein L23a and U6 snRNA were selected for normalization of levels of mRNA or miRNA, respectively.
In Situ Hybridization
In situ hybridization to detect miRNA and GW182 and Tnrc6b mRNA expression was performed on 10-μm frozen sections of yolk sac of E9.5 and E10.5 mouse embryos using 5′ digoxigenin-labeled LNA probes (Exiqon) (22, 23) or digoxigenin-labeled antisense probe of GW182 and Tnrc6b (24).
Histology and Immunohistochemistry
Primary antibodies include an anti-Ki67 antibody (1:200, catalogue number 550609; B & D) and an anti-p21 antibody (1:200, catalogue number sc-6246; Santa Cruz Biotechnology). The formation of antigen-antibody complex was visualized using an ABC kit (Vector Laboratories, Burlingame, CA). TUNEL staining was performed to assess cell apoptosis using an apoptosis detection kit (catalogue number S7107; Chemicon). β-Galactosidase staining to detect lacZ expression from the GW182 gene trap allele was performed according to protocol described previously (25).
Western Blot
Cell lysates of the yolk sac of E9.5 embryos were prepared in denaturing gel loading buffer. The proteins were separated by 4–12% Bis-Tris gel followed by Western blot analysis. GAPDH was used as internal loading control for all samples. The antibodies used include mouse anti-p21 (1:1000, catalogue number sc-6246; Santa Cruz Biotechnology), rabbit anti-caspase 3 (1:1000, catalogue number 9662; Cell Signaling), and rabbit anti-GADPH (1:100,000; Abcam). The blot was detected by the Immune-star HRP Developer System (Bio-Rad) and photographed using a CCD camera (LAS-4000; Fuji).
RESULTS AND DISCUSSION
Gene Trap Insertion Disrupts GW182 Expression in Mouse Embryos
To investigate in vivo functions of GW182, we generated GW182 mutant mice using a gene trap embryonic stem cell line clone XE786, which possesses a trap insertional mutation in the fourth intron of the GW182 locus. We mapped the insertion site by long range PCR and DNA sequencing (Fig. 1A and supplemental Fig. S1, A and B). Using p1-p3 primers that flank the insertional cassette and p1-p2 primers that detect trap vector sequences, we distinguished the wild type GW182 allele from the gene trap (gt) allele (Fig. 1B). This insertion is predicted to result in a truncated GW182 protein with only 21 amino acids at the N terminus without any recognizable functional domains.
Examination of GW182 expression identified the yolk sac as a major site of expression in E9.5 embryos (Fig. 1C). In situ hybridization detected abundant GW182 mRNA selectively in the visceral endoderm, but much lower in the endothelium or hematopoietic cells (Fig. 1C). Consistently, X-gal staining of the yolk sac of E9.5 GW182gt/+ embryos showed that β-galactosidase, which is expressed from the insertional cassette, was expressed predominantly in the endoderm (Fig. 1C). Therefore, we chose the yolk sac to analyze changes in gene expression in GW182 heterozygous and homozygous gene trap mutant mice.
Gene trap insertion dose-dependently reduced GW182 mRNA expression, as analyzed by qRT-PCR using primers flanking the junction between exons 4 and 5 (Fig. 1D). Compared with wild type controls, only ∼10% GW182 mRNA was detected in GW182gt/gt embryos (Fig. 1D), indicating that GW182gt/gt embryos have an almost complete loss of GW182 expression. In contrast, the expression of all other genes involved in miRNA biogenesis, such as Dicer, Drosha, Dgcr8, and Agos, are not significantly altered in the GW182gt/gt yolk sac by mRNA array profiling (Fig. 1E). There is no significant compensatory increase in mRNA levels of Tnrc6b or Tnrc6c in the homozygous gene trap mutant yolk sac (Fig. 1E). Further, Northern blot analysis using a probe that detects LacZ coding sequences identified a single transcript in GW182gt/+ and GW182gt/gt mice, but not in wild type mice, and the size of this transcript (∼12 kb) is consistent with a predicted fused transcript of GW182 and gene trap cassette (supplemental Fig. S1C). Together, our results support that gene trap insertion specifically disrupts the expression of GW182 and that GW182gt/gt mice provide an ideal loss-of-function model to study roles of GW182 in vivo.
Disruption of GW182 Results in Embryonic Lethality Associated with Defects in Yolk Sac Endoderm and Impaired Hematopoiesis
GW182gt/+ mice are viable and fertile and appear normal. Therefore, we bred these heterozygous mice to generate GW182gt/gt mice. To assess whether GW182 is essential for embryonic survival, we analyzed progeny from heterozygous mating at various stages. At E9.5, GW182gt/gt embryos were comparable with heterozygous littermates in size and were identified at the expected Mendelian ratio (Fig. 2A and supplemental Table S1). In addition, we did not observe significant differences in cell apoptosis (supplemental Fig. S2) or general morphology of tissues, including heart, somite, pharyngeal arches, and brain, between GW182gt/gt embryos and controls at E9.5 (Fig. 2A). However, at E10.5, although GW182gt/gt embryos accounted for ∼25% of progenies (supplemental Table S1), approximately half of them were dead and partially degenerated (Fig. 2A). At later stages such as E12.5 and postnatal week 4, less than 5% of progenies were homozygous mutants (supplemental Table S1). These observations indicate that GW182 is required for embryonic survival after E9.5.
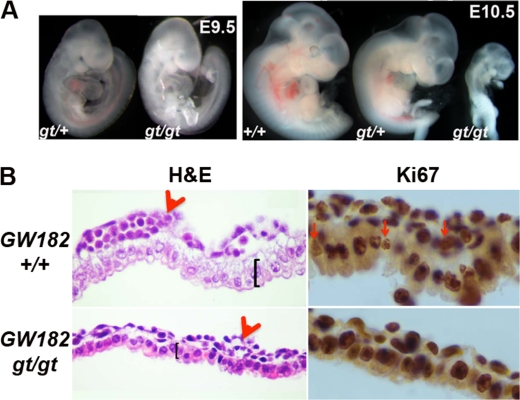
FIGURE 2.
Gene trap disruption of GW182 leads to embryonic lethality and defects in the yolk sac. A, comparison of control embryos and GW182gt/gt embryos at E9.5 and E10.5. B, H&E staining and Ki67 immunolabeling of the yolk sac identified impaired hematopoiesis, abnormal endoderm, and reduced cell proliferation in GW182gt/gt embryos at E9.5.
Upon closer examination, GW182gt/gt embryos at E9.5 appear paler with fewer erythrocytes than heterozygous or wild type embryos (Fig. 2A), suggesting impaired hematopoiesis. At E9.5, the major site for hematopoiesis is the yolk sac where GW182 is abundantly expressed in the endoderm (Fig. 1C). This connection prompted us to assess changes in the yolk sac of GW182gt/gt embryos. Indeed, compared with wild type control, GW182gt/gt embryos have a thinner endoderm layer with condensed nuclei, as shown by hemotoxylin and eosin staining (Fig. 2B). The number of ki67+ mitotic cells is also significantly reduced in both the endoderm and mesoderm of the yolk sac in GW182gt/gt embryos (Fig. 2B). In addition, blood islands in the yolk sac of GW182gt/gt embryos are smaller with fewer hematopoietic cells than wild type controls (Fig. 2B). Further, differentiation of erythroid progenitors is delayed based on abnormalities of cell morphology (supplemental Fig. S3, A and B). In addition, mRNA levels of hemoglobin genes are significantly reduced in GW182gt/gt embryos (supplemental Fig. S3C). Based on our observation that GW182 is expressed in the yolk sac endoderm rather than mesoderm where hematopoietic progenitors reside, impaired hematopoiesis in GW182gt/gt embryos is likely secondary to the defects in the endoderm but may be a primary cause of embryonic lethality at E10.5.
Disruption of GW182 Results in Derepression of miRNA Targets in Yolk Sac without Alteration of miRNA Expression
To investigate roles of GW182 in the yolk sac endoderm, we assessed changes in both miRNA and mRNA expression in the yolk sac of E9.5 GW182gt/gt embryos using microarrays (Affymetrix). Of 207 miRNAs that are detectable in wild type yolk sac samples, 50 miRNAs are highly expressed (Table 1). These include: 1) miR-17/20/93/106 family members sharing the same seed sequence, together with miR-19 and miR-25/92 transcribed from three individual loci; 2) miR-290–5p/292–5p/292–3p/291b-5p/293 cluster; 3) 11 miRNAs that are transcribed from an imprinted Dlk1-Dio3 region; and 4) miR-466b-3p/467a/467c/669c cluster transcribed from an intron of Smfbt gene, a polycomb group repressor abundantly expressed in yolk sac (Table 1). Compared with miRNA expression in controls, the GW182gt/gt yolk sac exhibited an almost identical expression levels, apart from five miRNAs with mild changes in expression (Fig. 3A). In contrast, mRNA profiling showed significant changes in expression of 3,543 genes in GW182gt/gt yolk sac, including 1,652 genes that are down-regulated and 1,891 genes up-regulated. The results of these arrays suggested that GW182 is dispensable for miRNA biogenesis or stability of mature miRNA, consistent with previous findings based on in vitro assays (26). However, disruption of GW182 alters mRNA expression in vivo, suggesting a role for GW182 in miRNA-mediated regulation in mRNA expression.
TABLE 1.
miRNAs that are highly expressed in yolk sac of E9.5 embryos
miRNAs that share the same seed sequence are highlighted with bold type. ES, embryonic stem cell.
| miRNAs | Signal intensity of array | Chromosomal location |
|---|---|---|
| miRNAs from miR-17–92 clusters | ||
| mmu-miR-17_st | 53,668 | 14:115442893–115442976 (+) |
| mmu-miR-18a_st | 9,035 | 14:115443073–115443168 (+) |
| mmu-miR-20a_st | 33,302 | 14:115443379–115443485 (+) |
| mmu-miR-19b_st | 11,566 | 14:115443527–115443613 (+) |
| mmu-miR-92a_st | 14,154 | 14:115443649–115443728 (+) |
| mmu-miR-93_st | 9,980 | 5:138606751–138606838 (−) |
| mmu-miR-106b_st | 6,750 | 5:138606965–138607046 (−) |
| mmu-miR-20b_st | 3,703 | X:50095290–50095369 (−) |
| mmu-miR-106a_st | 14,217 | X:50095680–50095744 (−) |
| ES-enriched miRNAs from the same cluster | ||
| mmu-miR-290–5p_st | 49,621 | 7:3218627–3218709 (+) |
| mmu-miR-292–3p_st | 55,769 | 7:3219190–3219271 (+) |
| mmu-miR-292–5p_st | 17,935 | 7:3219190–3219271 (+) |
| mmu-miR-293_st | 51,770 | 7:3220344–3220423 (+) |
| miRNAs from imprinted Dlk1-Dio3 region | ||
| mmu-miR-665_st | 4,870 | 12:110824524–110824617 (+) |
| mmu-miR-431_st | 7,934 | 12:110828657–110828747 (+) |
| mmu-miR-433_st | 6,057 | 12:110829925–110830048 (+) |
| mmu-miR-127_st | 35,477 | 12:110831056–110831125 (+) |
| mmu-miR-379_st | 13,643 | 12:110947270–110947335 (+) |
| mmu-miR-494_st | 9,217 | 12:110953528–110953612 (+) |
| mmu-miR-376b_st | 4,240 | 12:110961668–110961749 (+) |
| mmu-miR-382_st | 5,357 | 12:110971981–110972056 (+) |
| mmu-miR-134_st | 7,051 | 12:110972349–110972419 (+) |
| mmu-miR-541_st | 35,658 | 12:110980619–110980708 (+) |
| mmu-miR-409–3p_st | 16,673 | 12:110981368–110981446 (+) |
| miRNAs from intron of Smfbt | ||
| mmu-miR-467c_st | 4,033 | 2:10395558–10395654 (+) |
| mmu-miR-466b-3p_st | 3,183 | 2:10395846–10395927 (+) |
| mmu-miR-467a_st | 23,477 | 2:10397973–10398045 (+) |
| mmu-miR-669c_st | 8,510 | 2:10430923–10431031 (+) |
| miRNAs from intron of Smc4 | ||
| mmu-miR-15b_st | 2,861 | 3:68813694–68813757 (+) |
| mmu-miR-16_st | 33,558 | 3:68813824–68813918 (+) |
| Other miRNAs | ||
| mmu-miR-103_st | 12,760 | 11:35595898–35595983 (+) |
| mmu-miR-107_st | 9,382 | 19:34895177–34895263 (−) |
| mmu-miR-130b_st | 3,821 | 16:17124154–17124235 (−) |
| mmu-miR-130a_st | 6,022 | 2:84581272–84581335 (−) |
| mmu-miR-200c_st | 14,221 | 6:124668340–124668408 (−) |
| mmu-miR-126–3p_st | 8,895 | 2:26446877–26446949 (+) |
| mmu-miR-709_st | 372,600 | 8:86609998–86610085 (+) |
| mmu-miR-23a_st | 2,953 | 8:86732417–86732491 (+) |
| mmu-miR-191_st | 11,217 | 9:108470650–108470723 (+) |
| mmu-miR-805_st | 3,934 | MT:16115–16209 (−) |
| mmu-miR-185_st | 2,970 | 16:18327494–18327558 (−) |
| mmu-miR-690_st | 62,347 | 16:28600021–28600129 (−) |
| mmu-miR-1195_st | 15,164 | 17:71209818–71209940 (−) |
| mmu-miR-26a_st | 21,528 | 10:126432586–126432669 (+) |
| mmu-miR-22_st | 5,738 | 11:75277218–75277312 (+) |
| mmu-miR-685_st | 4,898 | 14:51427159–51427267 (−) |
| mmu-miR-351_st | 3,680 | X:50406432–50406530 (−) |
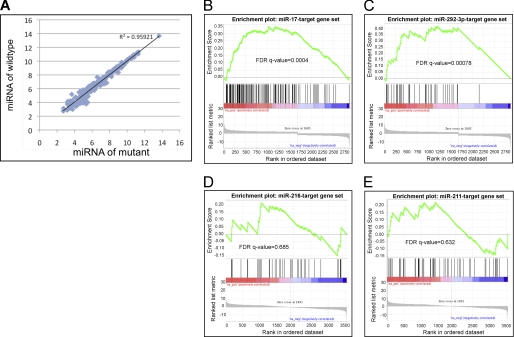
FIGURE 3.
GW182 is required for miRNA-mediated gene suppression but not for miRNA biogenesis or stability. A, miRNA array analysis revealed that the levels of miRNAs in the yolk sac of GW182gt/gt embryos at E9.5 were not significantly altered. B and C, predicted targets of miR-17 and miR-292–3p, two miRNAs that are expressed in the yolk sac endoderm, are significantly enriched among genes up-regulated in the yolk sac of GW182gt/gt embryos. D and E, targets of miR-216 and miR-211, two miRNAs that are not expressed in the yolk sac endoderm, are not enriched in the up-regulated gene list. FDR, false discovery rate.
To assess connections between miRNAs and GW182-regulated mRNAs, we evaluated whether gene targets of yolk sac-enriched miRNAs (Table 2, Group I) are over-represented among genes up-regulated in GW182gt/gt yolk sac, compared with targets of miRNAs that are selectively expressed in yolk sac mesoderm (Table 2, Group II, and Fig. 4C) and those that are undetectable in the yolk sac (Table 2, Group III). We selected more than 10 miRNAs in both Groups I and III after ranking based on signal intensity on the array (as a measurement of relative expression level), tissue specificity of expression, conservation across species, and defined seed sequences. Analysis using the Gene Set Enrichment Analysis (Broad Institute) program showed that predicted targets of 9 of 14 miRNAs within Group I were significantly over-represented in list of genes up-regulated in GW182gt/gt yolk sac (Table 2 and Fig. 3, B and C, and supplemental Fig. S4), and none was over-represented among the down-regulated genes. On the top of the list of over-represented miRNA targets are predicted target genes of miR-17/106 and miR-292–3p. Predicted targets of the other five miRNAs within Group I are not enriched in either up-regulated or down-regulated genes in GW182gt/gt yolk sac. One possibility is that these miRNAs are not selectively expressed in yolk sac endoderm where GW182 is highly expressed (Fig. 1C). In contrast to miRNAs in Group I, none of the predicted targets of two miRNAs in Group II are differentially expressed in GW182gt/gt yolk sac (Table 2). 14 of 15 miRNAs within Group III do not have target genes enriched in either up- or down-regulated lists except for miR-217 (Table 2 and Fig. 3, D and E, and supplemental Fig. S5). It is unknown why targets of miR-217 are enriched in genes up-regulated in GW182gt/gt yolk sac, because miR-217 shares little sequence similarity with other miRNAs in miRBase. In summary, comparison of target gene enrichment among these three groups of miRNAs provides strong evidences that GW182 is required for miRNA-mediated gene suppression in developing yolk sac endoderm.
TABLE 2.
Targets of miRNAs significantly up-regulated in GW182 mutant yolk sac
The targets of miRNA over-represented in genes up-regulated in the GW182 mutant yolk sac are highlighted with bold type.
| Targets of miRNAs | Size | FDR q-val |
|---|---|---|
| Group I: miRNAs highly expressed in yolk sac | ||
| MIR-17 targets | 171 | 0.00E+00 |
| MIR-292–3P targets | 55 | 0.00E+00 |
| MIR-106 targets | 112 | 4.31E-04 |
| MIR-25 targets | 115 | 1.46E-03 |
| MIR-19 targets | 146 | 6.26E-03 |
| MIR-16 targets | 141 | 5.56E-03 |
| MIR-200BC targets | 134 | 6.50E-03 |
| MIR-103 targets | 68 | 5.78E-03 |
| MIR-26 targets | 87 | 8.95E-03 |
| miR-709 targets | 55 | 5.15E-02 |
| MIR-290–5P targets | 30 | 1.04E-01 |
| miR-292–5P targets | 30 | 1.07E-01 |
| MIR-690 targets | 54 | 1.96E-01 |
| MIR-409–3P targets | 15 | 3.62E-01 |
| Group II: miRNAs expressed in the mesoderm of yolk sac | ||
| miR-126–3p targets | 0 | |
| miR-451 targets | 0 | |
| Group III: miRNAs not expressed in yolk sac | ||
| MIR-217 targets | 25 | 5.64E-03 |
| MIR-144 targets | 89 | 1.10E-02 |
| MIR-101 targets | 75 | 1.36E-02 |
| MIR-1 targets | 79 | 2.47E-02 |
| MIR-196 targets | 37 | 2.51E-02 |
| MIR-218 targets | 90 | 2.68E-02 |
| MIR-137 targets | 119 | 3.49E-02 |
| MIR-33 targets | 32 | 3.80E-02 |
| MIR-135 targets | 80 | 3.88E-02 |
| MIR-494 targets | 48 | 4.08E-02 |
| MIR-10 targets | 28 | 9.28E-02 |
| MIR-224 targets | 24 | 1.12E-01 |
| MIR-122 targets | 20 | 3.05E-01 |
| MIR-211 targets | 54 | 6.32E-01 |
| miR-216 targets | 40 | 6.85E-01 |
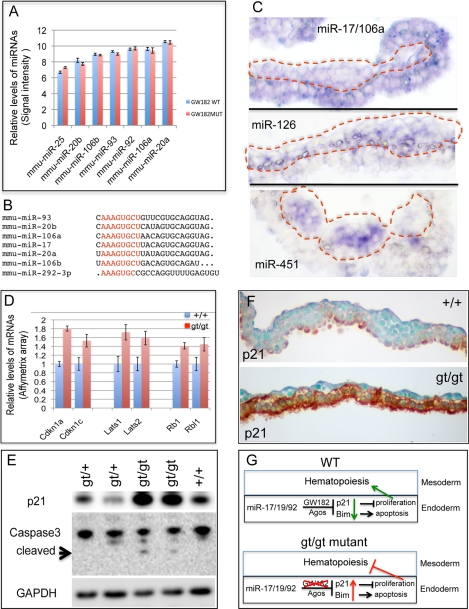
FIGURE 4.
Up-regulation of key cell cycle regulators and apoptosis in the yolk sac of GW182gt/gt embryos at E9.5. A, highly expressed in yolk sac, levels of miR-17/106 family members are not altered in GW182gt/gt mutant. B, miRNAs highly expressed in yolk sac with the same seed sequence. C, miR-17/106 is expressed in both endoderm and mesoderm of yolk sac with selectively enriched expression in endoderm; expression of mir-126–3p is mainly in the mesoderm, whereas miR-451 is in hematopoietic cells of blood island. D, mRNA levels of targets of miR-17/106/93 and miR-25/92, such as p21, p57, Lats1/2, and Rb, are significantly increased in GW182gt/gt yolk sac, as assayed by Affymetrix mRNA array (p < 0.05 for all these genes). E, Western blot detected a significant increase in P21 and cleaved caspase 3 levels in the yolk sac of GW182gt/gt embryos at E9.5. F, increased P21 in the yolk sac endoderm of GW182gt/gt embryos was detected by immunostaining. G, model of GW182 function in miRNA-mediated gene silencing in the yolk sac.
Derepression of Key Cell Cycle and Apoptosis Regulators Is Associated with Growth Arrest and Apoptosis of Yolk Sac Endoderm of GW182gt/gt Embryos
Highly expressed in embryonic stem cells and in the early embryos, miRNAs from miR-17/20/93/106 clusters are known to play a significant role in cell proliferation by targeting critical regulators of cell cycle such as Cdkn1a (p21), Cdkn1c (P27), Lats1, Lats2, Rb1, and Rbl (27–30). In addition, it is known that miRNAs from the same cluster promote cell survival by targeting regulators of apoptosis, such as Bim (BCL2-like 11, an apoptosis facilitator) and Pten (28, 29, 31). In our miRNA array data (Table 1), miRNAs from miR-17/20/93/106 clusters are highly expressed in yolk sac, and their levels are not altered in GW182gt/gt mutant (Figs. 3A and 4A). Seven miRNAs highly expressed in yolk sac share the same seed sequence (Fig. 4B). These array data were confirmed by qRT-PCR for selected miRNAs (supplemental Fig. S6). By in situ hybridization, we found that expression of miR-17/106 selectively enriched in yolk sac endoderm, as compared with miR-126 or miR-451 preferentially expressed in yolk sac vasculature and hematopoietic cells, respectively (Fig. 4C). Interestingly, p21, p57, Rb1, Rbl1 (p107), Lats1, and Lats2 are among a large number of predicted targets of miRNAs from miR-17/20/93/106 clusters and miR-292–3p that are significantly derepressed in GW182gt/gt yolk sac compared with wild type at E9.5 (Fig. 4D). In addition, we found a significant increase of apoptosis-associated genes in GW182gt/gt yolk sac, such as Bim (FC>1.41, p < 0.02) and Pten (FC>1.21, p < 0.05), known targets of miR-17/20/93/106 and miR-19 and miR-25/92 from the same clusters. The increased levels of some of these mRNAs were confirmed by qRT-PCR analysis (supplemental Fig. S6). Consistently, Western blot assays showed that the p21 protein level was dramatically elevated in GW182gt/gt yolk sac associated with the appearance of cleaved Caspase-3, an indicator of apoptosis (Fig. 4E). Further, increased p21 is detected predominantly in the yolk sac endoderm of GW182gt/gt embryos (Fig. 4F) by immunohistochemical staining. Together, our results strongly suggest that GW182 is required for miRNAs from miR-17/20/93/106 clusters to repress the expression of key cell cycle and apoptosis regulators such as p21, Bim, and Pten in control of cell survival and growth of yolk sac endoderm.
Conclusions
This study identified an important role of GW182 in miRNA pathway in the development of yolk sac. Loss of GW182 leads to defective miRNA function and results in increased expression of miRNA target genes involved in regulating cell cycle and apoptosis. These include p21 and Bim targeted by miRNAs from miR-17/19/92 clusters that are highly expressed in the yolk sac endoderm (Fig. 4G). Although up-regulation of p21, p57, Rb1, Rbl1, Lats1, and Lats2 blocks the endodermal cell proliferation, increased levels of Bim and Pten likely induce apoptosis through mitochondrial pathway. Defects in yolk sac endoderm in GW182gt/gt embryos likely contribute to the abnormal hematopoiesis and early embryonic lethality (Fig. 4G), because yolk sac endoderm play a critical role in supporting hematopoiesis in the mesoderm (Fig. 4G).
It is unclear how endoderm defects in GW182 mutant affect the hematopoiesis of mesoderm. Evidence of low GW182 expression in mesoderm and of the fact that levels of targets of miR-126 and miR-451 are not altered in GW182gt/gt mutant suggests the lack of a direct role of GW182 in the mesoderm of yolk sac. Indeed, the levels of endothelial specific genes, such as Pecam1, Kdr, and Tie1, are slightly increased in mutant yolk sac, suggesting that the vasculogenesis is preserved in GW182gt/gt mutant (supplemental Fig. S7). Disruption of GW182 in endoderm also did not alter the mRNA levels of Indian Hedgehog and VEGF (32, 33), signal molecules known to be produced by endoderm and playing significant role in hematopoiesis. We do observe a 1.8-fold increase of both BMP4 and BMPR2 expression and a 1.3-fold increase of Smad5 level (supplemental Fig. S7), suggesting an increased BMP signaling activity in GW182 mutant yolk sac. BMP4 is specifically expressed in the mesodermal compartment of yolk sac (34); therefore up-regulation of BMP4 might be due to the primary defects in endoderm.
Comparison of the phenotypes of GW182gt/gt embryos to those of mouse mutants of miRNA biosynthetic genes or individual miRNAs suggested GW182 as an important regulator of miRNA function rather than miRNA expression or stability. GW182gt/gt embryos survive at E9.5 without significant defects in embryonic growth or miRNA levels. In contrast, the loss of Dicer or Ago2 affects miRNA expression, and their mutants die before E9.5 with significant growth retardation (35–37). The milder phenotypes of GW182gt/gt embryos compared with Dicer−/− and Ago2−/− embryos are likely caused by the redundant roles of other two GW182 family members in yolk sac and in other structures of embryo and/or residual 10% of GW182 expression and function in homozygous gene trap mutants. Indeed, we found the enriched expression of both Tnrc6b and Tnrc6c (supplemental Fig. S8) in the yolk sac endoderm. Although GW182gt/gt embryos exhibit improved survival and morphogenesis compared with Dicer−/− and Ago2−/− embryos, they die earlier than individual miRNA mutant mice, likely because of roles of GW182 in controlling functions of multiple miRNAs. For example, deletion of miR-17–92 cluster and its two paralogs, miR-106a-363 and miR-106b-25, resulted in embryonic lethality at E15 (28, 38). GW182gt/gt embryos and these miRNA mutants all exhibit apoptosis, likely because of their shared cell cycle and apoptotic target genes. However, GW182 likely is required for functions of other highly expressed miRNAs in the yolk sac such as miR-292–3p, miR-293, miR-291a-5p, and miR-291b-5p, which are known to target p21 and to be required for proper embryonic stem cell G1/S phase transition (27).
Although a large majority of GW182gt/gt embryos die, 5% of them survive into adulthood and show mild growth defects. Live GW182gt/gt mice exhibit similarly reduced GW182 mRNA expression in multiple tissues. We speculate that genetic background contributes to observed variability in survival of GW182gt/gt embryos, because all of the embryos analyzed in this study were of a mixed genetic background.
Supplementary Material
Acknowledgments
We are all grateful for the comments and suggestions provided by Alan Fine, Arjun Guha, Felicia Chen, Katrina Steiling, Avrum Spira, and Lindsey Madden.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 HL47049 and R01 HL081800.
mRNA array data can be accessed through the NCBI GEO Database under NCBI accession number GSE30244.

This article contains supplemental Table S1 and Figs. S1–S8.
- miRNA
- microRNA
- RISC
- RNA-induced silencing complex
- En
- embryonic day n
- X-gal
- 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside.
REFERENCES
- 1. Ambros V. (2001) microRNAs. Tiny regulators with great potential. Cell 107, 823–826 [DOI] [PubMed] [Google Scholar]
- 2. Kim V. N., Han J., Siomi M. C. (2009) Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 [DOI] [PubMed] [Google Scholar]
- 3. Bartel D. P. (2004) MicroRNAs. Genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 4. Czech B., Hannon G. J. (2011) Small RNA sorting. Matchmaking for Argonautes. Nat. Rev. Genet. 12, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eystathioy T., Chan E. K., Tenenbaum S. A., Keene J. D., Griffith K., Fritzler M. J. (2002) A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell 13, 1338–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baillat D., Shiekhattar R. (2009) Functional dissection of the human TNRC6 (GW182-related) family of proteins. Mol. Cell Biol. 29, 4144–4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J., Rivas F. V., Wohlschlegel J., Yates J. R., 3rd, Parker R., Hannon G. J. (2005) A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7, 1261–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sen G. L., Blau H. M. (2005) Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7, 633–636 [DOI] [PubMed] [Google Scholar]
- 9. Eulalio A., Tritschler F., Izaurralde E. (2009) The GW182 protein family in animal cells. New insights into domains required for miRNA-mediated gene silencing. RNA 15, 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jakymiw A., Lian S., Eystathioy T., Li S., Satoh M., Hamel J. C., Fritzler M. J., Chan E. K. (2005) Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 7, 1267–1274 [DOI] [PubMed] [Google Scholar]
- 11. Zipprich J. T., Bhattacharyya S., Mathys H., Filipowicz W. (2009) Importance of the C-terminal domain of the human GW182 protein TNRC6C for translational repression. RNA 15, 781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lazzaretti D., Tournier I., Izaurralde E. (2009) The C-terminal domains of human TNRC6A, TNRC6B, and TNRC6C silence bound transcripts independently of Argonaute proteins. RNA 15, 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun J. E., Huntzinger E., Fauser M., Izaurralde E. (2011) GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell 44, 120–133 [DOI] [PubMed] [Google Scholar]
- 14. Chekulaeva M., Mathys H., Zipprich J. T., Attig J., Colic M., Parker R., Filipowicz W. (2011) miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 18, 1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fabian M. R., Cieplak M. K., Frank F., Morita M., Green J., Srikumar T., Nagar B., Yamamoto T., Raught B., Duchaine T. F., Sonenberg N. (2011) miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 18, 1211–1217 [DOI] [PubMed] [Google Scholar]
- 16. Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. (2006) mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 20, 1885–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behm-Ansmant I., Rehwinkel J., Izaurralde E. (2006) MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb. Symp. Quant Biol. 71, 523–530 [DOI] [PubMed] [Google Scholar]
- 18. Ding L., Spencer A., Morita K., Han M. (2005) The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell 19, 437–447 [DOI] [PubMed] [Google Scholar]
- 19. Ding L., Han M. (2007) GW182 family proteins are crucial for microRNA-mediated gene silencing. Trends Cell Biol. 17, 411–416 [DOI] [PubMed] [Google Scholar]
- 20. Schneider M. D., Najand N., Chaker S., Pare J. M., Haskins J., Hughes S. C., Hobman T. C., Locke J., Simmonds A. J. (2006) Gawky is a component of cytoplasmic mRNA processing bodies required for early Drosophila development. J. Cell Biol. 174, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lian S., Jakymiw A., Eystathioy T., Hamel J. C., Fritzler M. J., Chan E. K. (2006) GW bodies, microRNAs and the cell cycle. Cell Cycle 5, 242–245 [DOI] [PubMed] [Google Scholar]
- 22. Obernosterer G., Martinez J., Alenius M. (2007) Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat. Protoc. 2, 1508–1514 [DOI] [PubMed] [Google Scholar]
- 23. Cushing L., Kuang P. P., Qian J., Shao F., Wu J., Little F., Thannickal V. J., Cardoso W. V., Lü J. (2011) miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 45, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsao P. N., Vasconcelos M., Izvolsky K. I., Qian J., Lu J., Cardoso W. V. (2009) Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malpel S., Mendelsohn C., Cardoso W. V. (2000) Regulation of retinoic acid signaling during lung morphogenesis. Development 127, 3057–3067 [DOI] [PubMed] [Google Scholar]
- 26. Eulalio A., Helms S., Fritzsch C., Fauser M., Izaurralde E. (2009) A C-terminal silencing domain in GW182 is essential for miRNA function. RNA 15, 1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y., Baskerville S., Shenoy A., Babiarz J. E., Baehner L., Blelloch R. (2008) Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat. Genet. 40, 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ventura A., Young A. G., Winslow M. M., Lintault L., Meissner A., Erkeland S. J., Newman J., Bronson R. T., Crowley D., Stone J. R., Jaenisch R., Sharp P. A., Jacks T. (2008) Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132, 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olive V., Jiang I., He L. (2010) mir-17–92, a cluster of miRNAs in the midst of the cancer network. Int. J. Biochem. Cell Biol. 42, 1348–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He L., Thomson J. M., Hemann M. T., Hernando-Monge E., Mu D., Goodson S., Powers S., Cordon-Cardo C., Lowe S. W., Hannon G. J., Hammond S. M. (2005) A microRNA polycistron as a potential human oncogene. Nature 435, 828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olive V., Bennett M. J., Walker J. C., Ma C., Jiang I., Cordon-Cardo C., Li Q. J., Lowe S. W., Hannon G. J., He L. (2009) miR-19 is a key oncogenic component of mir-17–92. Genes Dev. 23, 2839–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baron M. H. (2003) Embryonic origins of mammalian hematopoiesis. Exp. Hematol 31, 1160–1169 [DOI] [PubMed] [Google Scholar]
- 33. Damert A., Miquerol L., Gertsenstein M., Risau W., Nagy A. (2002) Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development 129, 1881–1892 [DOI] [PubMed] [Google Scholar]
- 34. Astorga J., Carlsson P. (2007) Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development 134, 3753–3761 [DOI] [PubMed] [Google Scholar]
- 35. Bernstein E., Kim S. Y., Carmell M. A., Murchison E. P., Alcorn H., Li M. Z., Mills A. A., Elledge S. J., Anderson K. V., Hannon G. J. (2003) Dicer is essential for mouse development. Nat. Genet. 35, 215–217 [DOI] [PubMed] [Google Scholar]
- 36. Liu J., Carmell M. A., Rivas F. V., Marsden C. G., Thomson J. M., Song J. J., Hammond S. M., Joshua-Tor L., Hannon G. J. (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 [DOI] [PubMed] [Google Scholar]
- 37. Spruce T., Pernaute B., Di-Gregorio A., Cobb B. S., Merkenschlager M., Manzanares M., Rodriguez T. A. (2010) An early developmental role for miRNAs in the maintenance of extraembryonic stem cells in the mouse embryo. Dev. Cell 19, 207–219 [DOI] [PubMed] [Google Scholar]
- 38. Mendell J. T. (2008) miRiad roles for the miR-17–92 cluster in development and disease. Cell 133, 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.