Abstract
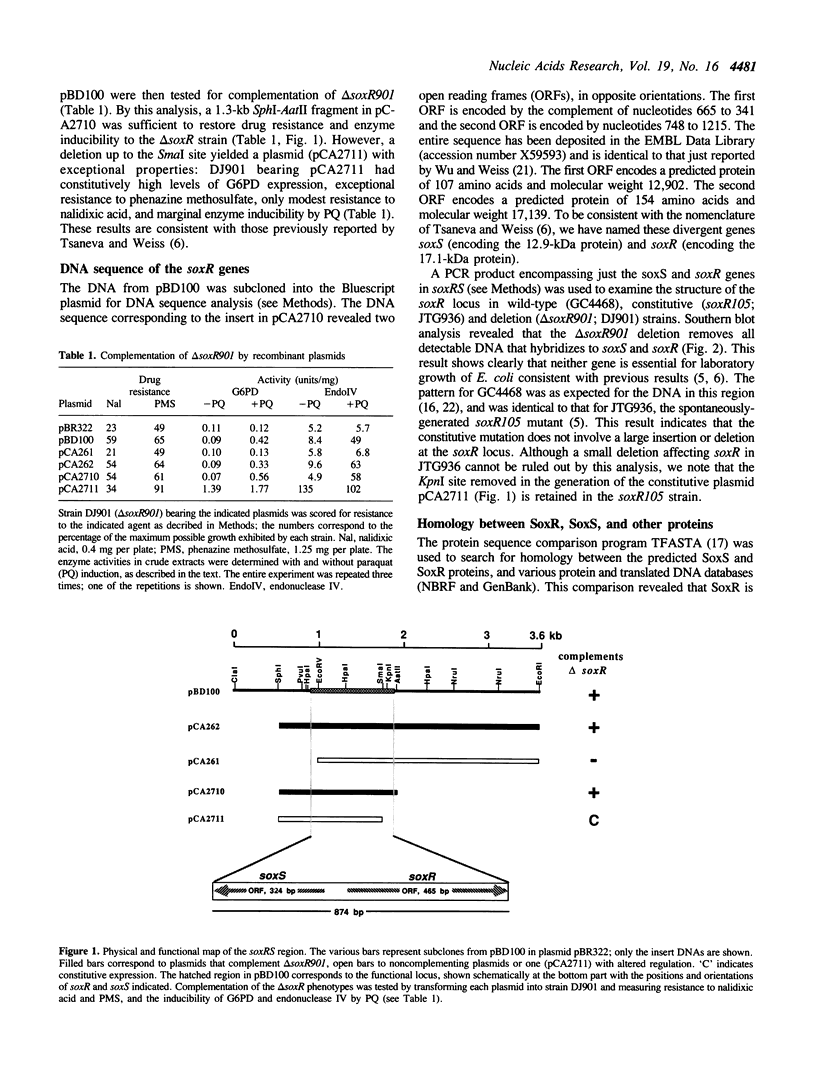
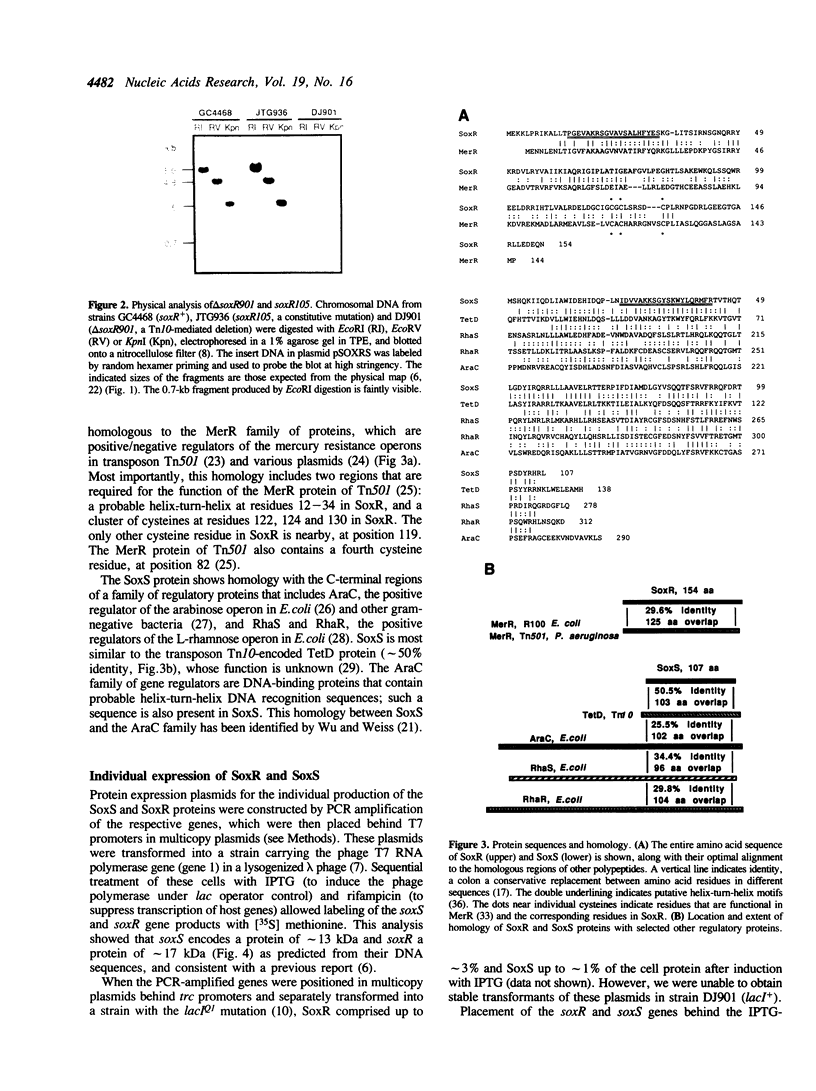
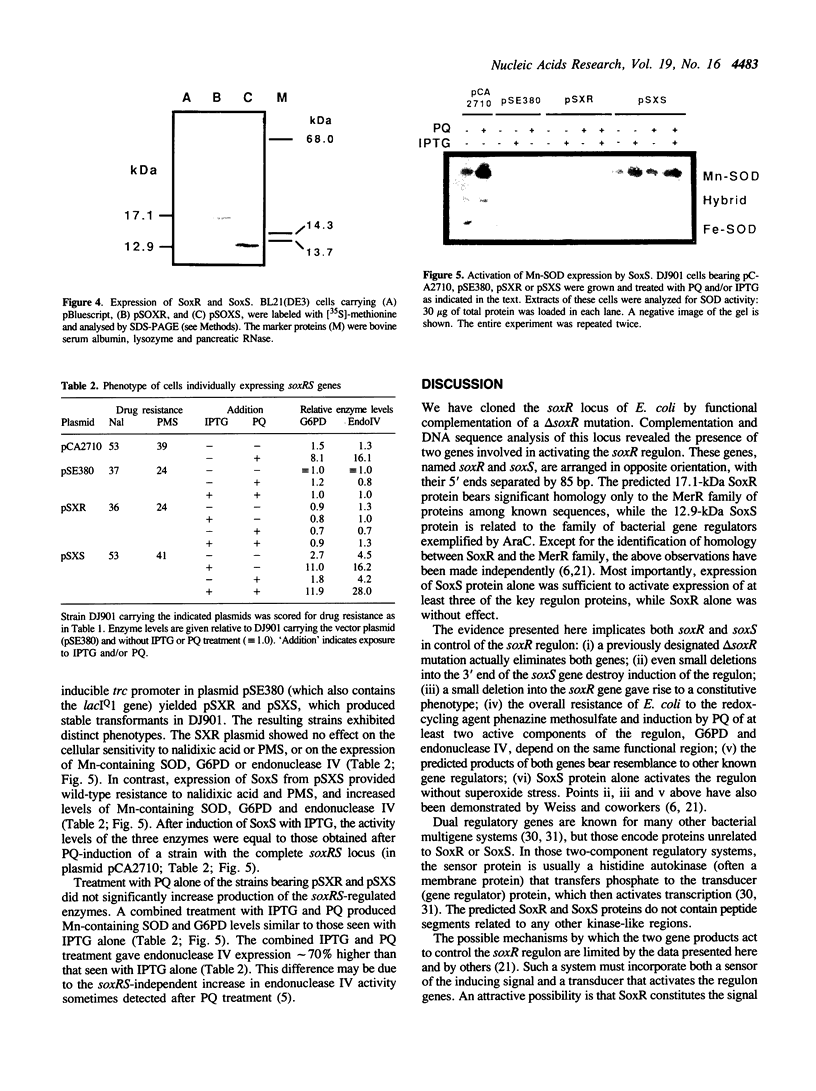
The soxR locus of Escherichia coli K12 mediates transcriptional activation of a complex oxidative stress regulon in response to superoxide-generating (redox-cycling) agents. We have cloned the soxR locus, which is positioned near the uvrA gene at 92.2 min on the genetic map, by monitoring complementation of a delta soxR mutation. Subclones from the soxR region in the delta soxR strain simultaneously restored cellular resistance to the redox-cycling agent phenazine methosulfate and inducibility of at least two of the regulon proteins, glucose-6-phosphate dehydrogenase and endonuclease IV, by paraquat, another redox-cycling agent. DNA sequence analysis revealed the presence of two genes involved in activating the soxR regulon. These genes, named soxR and soxS, are arranged divergently with their 5' ends separated by only 85 bp. The predicted 12.9-kDa SoxS protein is related to the AraC family of one-component gene regulators, but corresponds only to the putative DNA-binding regions of these proteins. The 17.1-kDa SoxR protein bears significant homology only to the MerR family of proteins including a predicted DNA-binding helix-turn-helix and a cluster of cysteine residues positioned similarly to those that regulate the activity of MerR in response to Hg2+. This suggests that SoxR could be a metal-binding gene regulator that acts as the intracellular sensor for superoxide. SoxS is evidently the proximal activator of the regulon genes: antibiotic resistance and high-level expression of at least three of the regulon proteins was effected in vivo by the individual expression of SoxS, but not of SoxR, whether or not the cells were exposed to paraquat. These data, together with the recently reported paraquat-inducibility of the soxS gene (Wu, I., and Weiss, B. (1990) J. Bacteriol. 173, 2864-2871), indicate that SoxR and SoxS may constitute a novel type of two-component regulatory system in which the two proteins act sequentially to activate transcription of the various regulon genes in response to superoxide stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Albright L. M., Huala E., Ausubel F. M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. doi: 10.1146/annurev.ge.23.120189.001523. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Braus G., Argast M., Beck C. F. Identification of additional genes on transposon Tn10: tetC and tetD. J Bacteriol. 1984 Nov;160(2):504–509. doi: 10.1128/jb.160.2.504-509.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991 Jan 25;266(3):1478–1483. [PubMed] [Google Scholar]
- Greenberg J. T., Chou J. H., Monach P. A., Demple B. Activation of oxidative stress genes by mutations at the soxQ/cfxB/marA locus of Escherichia coli. J Bacteriol. 1991 Jul;173(14):4433–4439. doi: 10.1128/jb.173.14.4433-4439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Ballard B. T., Walsh C. T. The MerR metalloregulatory protein binds mercuric ion as a tricoordinate, metal-bridged dimer. Science. 1990 Feb 23;247(4945):946–948. doi: 10.1126/science.2305262. [DOI] [PubMed] [Google Scholar]
- Kao S. M., Hassan H. M. Biochemical characterization of a paraquat-tolerant mutant of Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10478–10481. [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lei S. P., Lin H. C., Heffernan L., Wilcox G. araB Gene and nucleotide sequence of the araC gene of Erwinia carotovora. J Bacteriol. 1985 Nov;164(2):717–722. doi: 10.1128/jb.164.2.717-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. D., Demple B. Analysis of class II (hydrolytic) and class I (beta-lyase) apurinic/apyrimidinic endonucleases with a synthetic DNA substrate. Nucleic Acids Res. 1990 Sep 11;18(17):5069–5075. doi: 10.1093/nar/18.17.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. D., Johnson A. W., Demple B. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. J Biol Chem. 1988 Jun 15;263(17):8066–8071. [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Fritzinger D. C., Pridmore R. D., Barnes W. M., Haberstroh L., Silver S. Mercuric ion-resistance operons of plasmid R100 and transposon Tn501: the beginning of the operon including the regulatory region and the first two structural genes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5975–5979. doi: 10.1073/pnas.81.19.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Chu L., Silver S., Misra T. K. Mercury operon regulation by the merR gene of the organomercurial resistance system of plasmid pDU1358. J Bacteriol. 1989 Aug;171(8):4241–4247. doi: 10.1128/jb.171.8.4241-4247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Park S. J., Summers A. O. Genetic analysis of transcriptional activation and repression in the Tn21 mer operon. J Bacteriol. 1989 Jul;171(7):4009–4018. doi: 10.1128/jb.171.7.4009-4018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner C. M., Schleif R. Is the amino acid but not the nucleotide sequence of the Escherichia coli araC gene conserved? J Mol Biol. 1982 Feb 5;154(4):649–652. doi: 10.1016/s0022-2836(82)80020-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin J. F., Schleif R. F. Positive regulation of the Escherichia coli L-rhamnose operon is mediated by the products of tandemly repeated regulatory genes. J Mol Biol. 1987 Aug 20;196(4):789–799. doi: 10.1016/0022-2836(87)90405-0. [DOI] [PubMed] [Google Scholar]
- Tsaneva I. R., Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990 Aug;172(8):4197–4205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup L. K., Kogoma T. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J Bacteriol. 1989 Mar;171(3):1476–1484. doi: 10.1128/jb.171.3.1476-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon of Escherichia coli. J Bacteriol. 1991 May;173(9):2864–2871. doi: 10.1128/jb.173.9.2864-2871.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]





