Abstract
The genome of the bacterium Helicobacter pylori has evolved over the millennia since its migration out of Africa along with its human host approximately 60,000 years ago. Human migrations, after thousands of years of permanent settlement in those lands, resulted in seven prototypes of genetic populations of H. pylori with distinct geographical distributions. In all continents, present day isolates of H. pylori have molecular markers that reflect population migrations. The colonization of the Americas as well as the slave trade introduced European and African strains to the New World. The relationship between H. pylori genome and gastric cancer rates is linked to the presence of the cagA gene, but the knowledge on this subject is incomplete because other genes may be involved in certain populations. A new situation for Homo sapiens is the absence of H. pylori colonization in certain, mostly affluent, populations, apparently brought about by improved home sanitation and widespread use of antibiotics during the last decades. The disappearance of H. pylori from the human microbiota may be linked to emerging epidemics of esophageal adenocarcinoma, some allergic diseases such as asthma and some autoimmune disorders.
Keywords: Helicobacter pylori, Genome, Gastric carcinogenesis
INTRODUCTION
Helicobacter pylori is a Gram-negative spiral bacterium that colonizes the gastric mucosa of more than half of the world population and is the main recognized cause of gastritis, peptic ulcers, gastric adenocarcinoma and some types of gastric lymphoma.1-4 It has been estimated that human organisms consist of 1013 eukaryotic cells and 1014 microbial cells.5 H. pylori may be considered prominent member of the human microbiota and has accompanied constantly Homo sapiens in its complex migration history.6 H. pylori is usually acquired during childhood and is believed to be transmitted within families. The bacterium is well adapted to survive in the gastric mucus for decades, and most infected persons remain asymptomatic.
One of the characteristics of H. pylori is a great genetic diversity, and it is known that different strains may interact differently with their human host influencing the clinical outcome. The extensive genomic diversity among H. pylori isolates results from a high mutation rate and a frequent exchange of genetic material during infections with multiple H. pylori strains.7-11 H. pylori strains from different geographic areas show clear phylogeographic differentiation, and studies of the genetic variants serve as markers of human migrations. In addition, the worldwide geographic distribution of the different H. pylori populations seems to correlate the clinical outcomes. This review examines the role of H. pylori genomic variants in the biology and outcomes of the infection.
CARCINOGENIC POTENTIAL
Gastric cancer is the second leading cause of cancer-related death in the world.12 Based on epidemiologic evidence, the International Agency for Research on Cancer concluded in 1994 that the infection with H. pylori was a class I carcinogen for humans.13 It has been estimated that 5.5% of the total cases of cancer worldwide and more than 60% of gastric cancer cases are caused by this bacterial infection.14 Approximately one half of the gastric cancer cases occurs in East Asia, and the countries with the highest incidence rates are Korea, Mongolia, Japan, and China.15
H. pylori infection is a necessary but not a sufficient factor in gastric carcinogenesis. The hypothetical causal link between H. pylori infection and gastric cancer was challenged by Holcombe,16 making reference to several countries in Africa where the infection was practically universal but the gastric cancer rates were very low. He called this phenomenon the "African enigma." Similar trends have been observed in other populations around the world, and since gastric cancer is considered a multifactorial disease, factors such as diet and co-infections have been offered as potential explanations for the enigma.17-19
Independent from other factors that may modulate the risk of acquiring gastric cancer, the genotype of the infecting H. pylori strain is a determining factor. The carcinogenic effects of H. pylori infection have been linked to its virulence factors, mainly the cag pathogenicity island (cag PAI) and the vacuolating cytotoxin gene A (vacA).2,20,21 The cag PAI is a ~40 kb locus that contains 27 to 31 genes, hypothesized to have been acquired horizontally and integrated into the glutamate racemase gene.22 Present day isolates of H. pylori may or may not contain the cag PAI. The cytotoxin-associated gene A (cagA)23 is the most investigated gene of the cag PAI and the main recognized virulence factor. It encodes CagA, an oncoprotein that is injected into mammalian cells, undergoes phosphorylation by host cell kinases, and affects cytoskeletal and tissue structure, as well as cell proliferation.24,25 Several of the genes in the cag PAI encode for a type IV secretion system26 used for the injection of CagA into host cells. Infection with cagA-positive H. pylori strains is associated with high risk of peptic ulcers and gastric carcinoma.20,21,25 In East Asian countries, virtually all of the H. pylori isolates are cagA-positive, whereas in Western countries approximately 60% to 70% of isolates are cagA-positive.27 Mixed infections with cagA-positive and cagA-negative strains have been reported.28
CagA is known for the variability in its C-terminal region, which includes a Glu-Pro-Ile-Tyr-Ala (EPIYA) sequence that is present in variable numbers in CagA from different strains. On the basis of sequences flanking the EPIYA motifs, four distinct segments have been described: EPIYA-A, -B, -C and -D. EPIYA-A and EPIYA-B are in almost all CagA isolates. EPIYA-C is characteristic of CagA from H. pylori from Europe, North America, and Australia (thus named "Western CagA"). EPIYA-D is specific to CagA produced by H. pylori circulating in East Asian countries (termed "East Asian CagA"). Most CagA isolates contain three EPIYA segments (ABC or ABD), but the number can vary from one to seven.27 Studies have shown greater phosphorylation and oncogenic potential in East Asian CagA (due to the presence of the EPIYA-D segment) compared to Western CagA.29,30 Also, among Western CagA species, greater phosphorylation and oncogenic potential has been observed in those with a greater number of EPIYA-C motifs.27,31,32 Therefore, distinct CagA isoforms may contribute to the differences in gastric cancer rates between East Asian and Western countries.
Unlike the cag PAI, the gene vacA is present in virtually all H. pylori strains examined.33,34 It encodes VacA, a protein that may damage epithelial cells by inducing the formation of vacuoles. Strains vary considerably in production of cytotoxin activity, primarily due to variations in vacA gene structure. The regions of greatest diversity are localized near the 5' end of vacA (signal sequence, showing allele types s1a, s1b, s1c, or s2) and in the mid-region of vacA (alleles m1 or m2). H. pylori strains that possess vacA s1m1 genotypes are associated with increase in gastric epithelial cell injury and greater risk of peptic ulcer and gastric cancer compared with vacA s2m2 strains.35,36 vacA s1a predominates in Northern Europe; s1b in the Iberian peninsula and in Latin America; s1c is only found in South Eastern Asia.37 The relationship between vacA s1m1 alleles and gastric cancer is consistent with the distribution of vacA genotypes throughout the world. In regions where the prevalence rate gastric cancer is high, such as Colombia and Japan, most H. pylori strains contain type s1m1 alleles. Strains with vacA s2 alleles are more frequent in regions of the world with low rates of gastric cancer, such as Australia and North America. A third vacA polymorphic site, the intermediate (i) region was more recently identified, with variants i1 and i2. Strains type i1 were associated with higher risk of gastric adenocarcinoma.38
OUT OF AFRICA
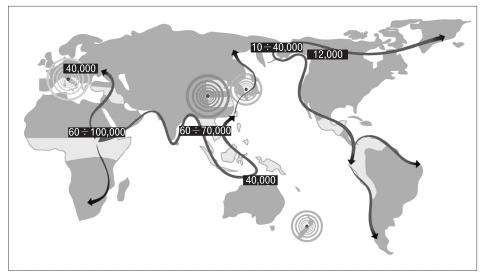
Increasing evidence supports the hypothesis that H. pylori was already established in human stomachs at least 100,000 years ago, before the human migration out of Africa about 60,000 years ago.26 Fig. 1, from Covacci et al.,26 displays the ancient human migrations and the approximate time of each migration wave. It has been hypothesized that after migration out of Africa, humans reached Asia by a southern coastal route, through India and then into South East Asia and Australasia.39 That route extended along the region known as Sundaland (including the Malay peninsula, Sumatra, Java, and Borneo), that was part of the Asian mainland as a result of low sea levels during the last ice age.40 Also due to the low sea levels, Australia, New Guinea, and Tasmania were connected in a continent called Sahul, separated from Sundaland by a few narrow deep-sea channels.41 The modern humans settled Europe about 30,000 to 40,000 years ago, probably entering via two routes: from Turkey along the Danube corridor into Eastern Europe, and along the Mediterranean coast. Finally, Homo sapiens and H. pylori crossed the Bering Strait together approximately 12,000 years ago and then continued their migration to the Americas.
Fig. 1.
World map indicating human migrations (arrows) and time range in years since the migrations happened. H. pylori accompanied man during the migrations and the four major H. pylori populations (as known in 1999: Europe, Northern Asia, Southern Asia, and New Zealand) are represented by concentric circles of different colors. Light green areas indicate the locations where development of agriculture and animal breeding started, resulting in expansion of human populations (From Covacci A, et al. Science 1999;284:1328-1333, with permission). 26
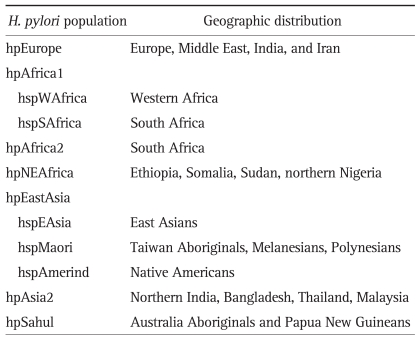
During the last decade, genotyping of H. pylori strains obtained from multiple human populations around the world has demonstrated that the genetic diversity of this bacterium reflects human migrations and subsequent geographic and ethnic separation between human groups. Analyses of genetic diversity of H. pylori are more frequently carried out by comparing sequences of housekeeping or virulence associated genes. Some authors have suggested that genotyping of H. pylori may be superior to human genetic markers to distinguish geographically related populations.42 Analysis of multilocus sequence typing (MLST) of housekeeping genes has shown to be a robust and consistent test to study ancestry and evolution of H. pylori populations. Based MLST of seven housekeeping genes (atpA, efp, mutY, ppa, trpC, ureI, yphC) and one virulence-associated gene (vacA) of 370 strains isolated from 27 human populations, Falush et al.43 identified four main clusters of H. pylori populations with distinct geographical distribution identified as: 1) hpEurope, 2) hpAfrica1, 3) hpAfrica2, and 4) hpEastAsia. Further analysis split hpEastAsia into hspAmerind, hspEAsia and hspMaori subpopulations. hpAfrica1 was subdivided into hspWAfrica and hspSAfrica.43 More recent studies identified other main clusters: hpNEAfrica, hpAsia2, and hpSahul (Table 1).6,41
Table 1.
EVOLUTION OF ASIAN H. pylori STRAINS
It could be speculated that the H. pylori genome from the ancient Asian strains were less carcinogenic than the present more carcinogenic Asian and European strains. This change may be reflected in the elevated gastric cancer rates observed in Korea and Japan, where the H. pylori strains possess CagA with EPIYA-D motifs, linked to higher phosphorylation and higher cancer risk. Although the genotype of the ancient Asian strains is not known, it is generally assumed that that their virulence and carcinogenic potential was very low.44 Censini et al.22 linked the hypothetical emergence of virulent variants from less virulent variants to the acquisition into the H. pylori genome of the cag PAI. Present day evidence suggests that the secular evolution of the ancient Asian strains differed in Asia and the Americas. In both continents the cag PAI was inserted into the H. pylori genome, but the CagA protein in East Asian strains has an EPIYA-D motif and the Western version has instead one or more EPIYA-C motifs. Recent evidence supports a possible evolutionary pathway for the EPIYA-D segment by sequence rearrangement within the cagA gene.45 This work, by Furuta et al.,45 supports that the left half of the EPIYA-D segment characteristic of East Asian CagA was derived from Western-type EPIYA, and suggests that Amerind-type EPIYA could have been the intermediate, through rearrangements of specific sequences within the gene.
The value of H. pylori genome as a marker of human migrations has been well illustrated in studies carried out in Ladakh, an isolated location in the trans-Himalayan region of Northern India.42 Two separate groups with independent social and religious characteristics inhabit Ladakh: Buddhists, primarily of Mongoloid Tibetan descent, and Muslims who migrated from Pakistan in the 14th century. The classical human genetic markers were insufficient to distinguish the two Ladakh communities. But H. pylori sequence analyses clearly showed the differences between Buddhist and Muslim groups.42 In India, a study using MLST of seven housekeeping genes found that most H. pylori isolates share significant homology with hpEurope strains, and the cag PAI revealed European ancestry as well.46 A recent study of H. pylori strains from Cambodia,47 analyzing the same seven housekeeping genes classified them in two groups, hpEurope and hspEAsia, and provided evidence supporting three ancient human migrations: 1) from India, introducing HpEurope into Southeast Asia, 2) from China, carrying hspEAsia, and 3) from Southern China into Thailand carrying hpAsia2. Their findings also support 2 recent migrations within the last 200 years: 1) from Chinese to Thailand and Malaysia spreading hspEAsia strains and 2) from Indians to Malaysia carrying hpAsia2 and hpEurope.47 A study of H. pylori isolates from Malaysia classified them as hpEastAsia, hpAsia2 or hpEurope, and revealed a new subpopulation, hspIndia, within hpAsia2.48
In Oceania, there is evidence of two ancient migrations: one reached New Guinea and Australia, and a second, more recent, extended through Melanesia and from there to the Polynesian islands. These migrations were accompanied by two distinct H. pylori populations, hpSahul and hspMaori, respectively.41 After the European settling, the hpEurope predominates.49
THE AMERICAS PRE- AND POST-COLUMBUS
It is thought that when humans traveled across the Bering Strait from Asia to North America, they were carrying H. pylori, and therefore, their descendants today should be carrying H. pylori strains with Asian genotypes. Consistent with this hypothesis, multiple studies including Native North American and South American H. pylori strains have found evidence of genetic similarity with Asian strains.43,50-53 The genetic diversity of the original settlers of the Americas has significantly increased mainly through the genetic flow of Europeans and Africans over the last 500 years. This is a fact extensively reflected in the H. pylori populations.
1. The colonization of the Americas
The Spanish Crown sponsored three trips of Columbus, starting in 1492, to what they thought was a new route to the "Indias." But instead of India they found the new American continent, at that time unknown to the Europeans or the Asians. The Spanish settlers brought not only political and social changes; they also transformed most Amerindian into mestizo populations (mixture of Amerindian and European ancestry). At present, the Amerindian component varies greatly, in proportion to the density of the original native population. In Mexico and Guatemala, where the density of the original population is greater, the Amerindian component is greater than in areas such as Southern Brazil and Central Colombia, with lower density of original native populations.54,55 In general, mestizos in Latin America are mostly colonized by hpEurope strains and the original hpAmerind strains seem to be restricted to a minority of isolated Amerindian populations. As mentioned above, the H. pylori genome variants reflect the human demographic evolution.
2. The slave trade
H. pylori infection is ubiquitous in Africa, but gastric ulcers and gastric cancer are rare events.56 In the 16th century Portuguese merchants started exporting slaves from Africa to the Americas. African slaves had been brought to Europe for centuries, mostly from the Muslim dominated north coast. They were well educated and had a tendency to rebellion, which limited the trade. Slavery was also a traditional practice within some indigenous African societies.57 Various African kings operated several forms of slavery. When the foreign traders came with a demand for labor, West Africans swiftly developed an organized trade.57 The exportation of African slaves to the Americas lasted about four centuries, reaching the largest numbers when the British, Spanish, French and Dutch traders joined the business. It is estimated that about 11 million slaves were forcefully recruited for the Atlantic trade. At least one million died during the voyage or soon after, mainly from malnutrition and diseases encountered during the forced marches and in slave camps. Most slaves came from West Africa and included multitude of ethnic groups representing north, central and southern West Africa.58 The identification of ancestry specific markers has allowed investigators to build reasonable hypotheses about the predominant H. pylori genotypes in the Americas before and after the Spanish expeditions to the New World.49,53 The original predominant H. pylori strains were apparently hspAmerind, a subpopulation derived from hpEastAsia. After Columbus and the following Atlantic slave trade, such strains are rare and there is a predominance of hpEurope in mestizos and hspWAfrica in African Americans and mulattoes (mixture of African and European ancestry).
In South America, H. pylori strains from Amerindians in the Venezuelan Amazon were compared to strains from a mestizo population from Caracas by examining three independent highly polymorphic H. pylori genetic loci. Amerindian strains were found to have genotypes related to East Asia, while the mestizo population harbored strains with Western patterns.52
Recent studies including H. pylori isolates from Mexican indigenous groups show unique polymorphisms of the cagA and vacA genes revealing Asian, European and African sequences.50 Concatenated analysis of housekeeping genes showed that although many isolates clustered within the European groups, some strains clustered in a position intermediate between East Asian and European groups. They represent evidence supporting that Amerindian strains are being displaced by European strains. This novel Amerindian group had been previously found in isolates from indigenous populations in Colombia (Huitotos), Venezuela (Piaroas), and Peru (Shimaa).50,51,59-61 However, mestizos from the Americas (of mixed Asian and European ancestry) harbor HpEurope strains.51 The low diversity of Amerindian strains may be linked to their apparent tendency to disappear when colonizing non-Amerindian hosts.
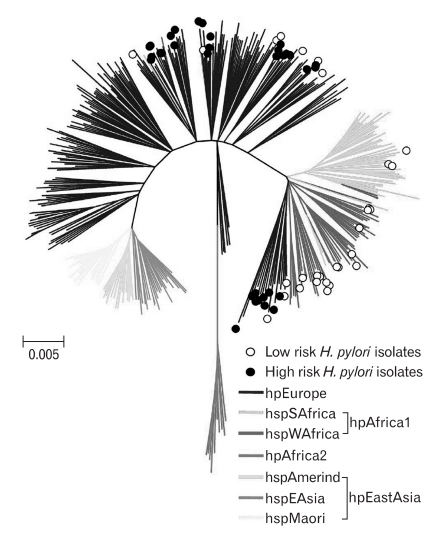
In Colombia, inhabitants of the high-altitude Andes Mountains have very high incidence rates of gastric cancer compared to inhabitants of the Pacific coast. Both populations have similarly high prevalence of H. pylori infection. People living in the mountains are mostly mestizos (of Amerindian descent with European admixture), whereas people living on the Pacific coast are predominantly mulattos (mixed African and European ancestry). The prevalence of cagA+ vacA s1m1 H. pylori strains in the high risk region is only slightly lower than in the low-risk region.62 Using MLST of seven housekeeping genes, we analyzed cagA+ vacA s1m1 strains from both regions of Colombia and compared them to 380 reference strains.63 All strains of the high risk region (Andes Mountains) were classified as hpEurope (n=35), while among strains from the Pacific coast, 34.5% (n=10) were classified as hpEurope, and the remaining 65.5% (n=19) were classified as hpAfrica1 (3 hspSAfrica, 16 hspWAfrica) (Fig. 2). In this study, strains of European ancestry were associated with more advanced precancerous gastric lesions and greater oxidative damage in gastric mucosa than strains of African ancestry.63 Known differences in gastric cancer rates may, at least partially, be explained by H. pylori genotypes: high rates in mestizos colonized with more virulent hpEurope strains, as documented in the Colombian Andes Mountains, and low rates in African descendents colonized with the less virulent hspAfrica1 strains, as documented in dwellers of the Colombian Pacific coast.
Fig. 2.
Phylogeography of H. pylori. Neighbor-joining tree of 64 cagA+ vacA s1m1 Colombian H. pylori isolates from the high-risk (black circles) and low-risk (open circles) regions, along with 380 reference strains that were previously classified into distinct ancestral haplogroups. Branches are drawn to scale to represent evolutionary distance. The colors of the branches represent the classification of strains into distinct populations (From de Sablet T, et al. Gut 2011;60:1189-1195, with permission).63
In the United States, African Americans have gastric cancer rates approximately twice those of Caucasians.64 This phenomenon is probably not related to H. pylori genotypes but more related to the prevalence of H. pylori infection in these two ethnic groups, approximately double in African Americans than in Caucasians.65 Gastric cancer rates in U.S. African Americans are much lower than those of mestizos in Latin America.
SECULAR (CENTURIES OLD) TRENDS OF GASTRIC CANCER: TWO EPIDEMICS?
In epidemiologic terms, an epidemic is defined as an unexpected increase in the frequency of a disease. Chronologically, epidemics display a bell-shaped curve: an ascending portion, a plateau and descending portion. Sonnenberg66,67 has analyzed time trends of mortality from peptic ulcer and gastric cancer in several countries in terms of their birth cohort pattern. The rates rose in generations born during the 18th century until the mid-19th century and then have declined in all subsequent generations. He links the decline of mortality rates to the decreasing prevalence of H. pylori infection in the general population. But, in his words, the sudden increase in mortality for these H. pylori related a disease within a relatively short time during the 19th century remains an enigma. It might be that the enigma is related to the secular evolution of the H. pylori genome, with the acquisition of the cag PAI, which occurred both in Asia and the West.44 Sonnenberg's graphs also show that the steady decline in cancer rates stops for cohorts born around the third decade of the twentieth century, for which increasing rates are observed. This "second" epidemic is driven by younger persons and has previously been detected in Spain and the United States.68,69 The causes of this apparently incipient epidemic are unknown. There is no evidence suggesting that a recent evolution of the H. pylori genome has taken place. The generalized abuse of antacid medications is a phenomenon that preceded this new epidemic, but a causal link to the new epidemic has not been established.
EPILOGUE
The co-evolution of Homo sapiens and H. pylori throughout millennia may be coming to an end, especially in affluent societies in which marked improvements in home and food sanitation have taken place and antibiotics may be abused to treat minor or to "prevent" infections. The prevalence of the infection has been declining steadily, especially in younger individuals. This results in cohorts that have never been infected with H. pylori. In most subjects, H. pylori is commensal, not related to disease. It is hypothesized that the loss of the natural balance between the two species may result in disease and the total absence of H. pylori for life has been linked to new epidemics being observed at the present time. One such epidemic is the adenocarcinoma of the lower esophagus, linked to reflux esophagitis and Barrett's esophagus. These may reflect relatively excessive gastric acid secretion, which could be linked to the absence of H. pylori infection. Other diseases apparently increasing in frequency lately are allergic disease such as asthma and autoimmune diseases. It has been proposed that these new epidemics may be related to the so-called hygiene hypothesis.70 Exposure to H. pylori and other infections during childhood may drive a Th1 (pro-inflammatory) immune response that may set a pattern of response to later antigenic stimuli. In the absence of infections, antigenic stimuli after the childhood years may lead to Th2 (allergic) immune responses, linked to asthma. This hypothesis has been recently supported in an animal model.71
ACKNOWLEDGEMENTS
This work was supported by the grant P01-CA28842 from the U.S. National Cancer Institute.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 3.Piazuelo MB, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010;24:853–869. doi: 10.1016/j.idc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 5.Blaser MJ. Theodore E. Woodward Award: global warming and the human stomach: microecology follows macroecology. Trans Am Clin Climatol Assoc. 2005;116:65–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel DA, Salama N, Krishna U, et al. Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc Natl Acad Sci U S A. 2001;98:14625–14630. doi: 10.1073/pnas.251551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennemann L, Didelot X, Aebischer T, et al. Helicobacter pylori genome evolution during human infection. Proc Natl Acad Sci U S A. 2011;108:5033–5038. doi: 10.1073/pnas.1018444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kersulyte D, Chalkauskas H, Berg DE. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol Microbiol. 1999;31:31–43. doi: 10.1046/j.1365-2958.1999.01140.x. [DOI] [PubMed] [Google Scholar]
- 10.Salama N, Guillemin K, McDaniel TK, Sherlock G, Tompkins L, Falkow S. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc Natl Acad Sci U S A. 2000;97:14668–14673. doi: 10.1073/pnas.97.26.14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suerbaum S, Josenhans C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol. 2007;5:441–452. doi: 10.1038/nrmicro1658. [DOI] [PubMed] [Google Scholar]
- 12.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer (IARC) Schistosomes, liver flukes and Helicobacter pylori. Volume 61. Lyon: IARC Press; 1994. Monographs on the evaluation of carcinogenic risks to human; pp. 177–240. [PMC free article] [PubMed] [Google Scholar]
- 14.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008. Cancer Incidence and Mortality Worldwide in 2008 [Internet] Lyon: International Agency for Research on Cancer; 2010. [cited 2011 Nov 22]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 16.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correa P, Malcom G, Schmidt B, et al. Review article: antioxidant micronutrients and gastric cancer. Aliment Pharmacol Ther. 1998;12(Suppl 1):73–82. doi: 10.1111/j.1365-2036.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 18.Fox JG, Wang TC, Nagler-Anderson C. The African enigma: the parasite’s perspective. Gut. 2001;49:156–157. doi: 10.1136/gut.49.1.156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whary MT, Sundina N, Bravo LE, et al. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005;14:1464–1469. doi: 10.1158/1055-9965.EPI-05-0095. [DOI] [PubMed] [Google Scholar]
- 20.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 21.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239–248. doi: 10.1007/s00535-009-0014-1. [DOI] [PubMed] [Google Scholar]
- 25.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 27.Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer Sci. 2011;102:36–43. doi: 10.1111/j.1349-7006.2010.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figura N, Vindigni C, Covacci A, et al. cagA positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: relevance to histological damage. Gut. 1998;42:772–778. doi: 10.1136/gut.42.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashi H, Tsutsumi R, Fujita A, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci U S A. 2002;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura M, Ohnishi N, Tanaka S, Yanagiya K, Hatakeyama M. Differential oncogenic potential of geographically distinct Helicobacter pylori CagA isoforms in mice. Int J Cancer. 2009;125:2497–2504. doi: 10.1002/ijc.24740. [DOI] [PubMed] [Google Scholar]
- 31.Naito M, Yamazaki T, Tsutsumi R, et al. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology. 2006;130:1181–1190. doi: 10.1053/j.gastro.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 32.Sicinschi LA, Correa P, Peek RM, et al. CagA C-terminal variations in Helicobacter pylori strains from Colombian patients with gastric precancerous lesions. Clin Microbiol Infect. 2010;16:369–378. doi: 10.1111/j.1469-0691.2009.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 34.Cover TL, Tummuru MK, Cao P, Thompson SA, Blaser MJ. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 35.Atherton JC, Peek RM, Jr, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 36.Miehlke S, Kirsch C, Agha-Amiri K, et al. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322–327. [PubMed] [Google Scholar]
- 37.van Doorn LJ, Figueiredo C, Mégraud F, et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116:823–830. doi: 10.1016/s0016-5085(99)70065-x. [DOI] [PubMed] [Google Scholar]
- 38.Rhead JL, Letley DP, Mohammadi M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 39.Macaulay V, Hill C, Achilli A, et al. Single, rapid coastal settlement of Asia revealed by analysis of complete mitochondrial genomes. Science. 2005;308:1034–1036. doi: 10.1126/science.1109792. [DOI] [PubMed] [Google Scholar]
- 40.Pope KO, Terrell JE. Environmental setting of human migrations in the circum-Pacific region. J Biogeogr. 2008;35:1–21. [Google Scholar]
- 41.Moodley Y, Linz B, Yamaoka Y, et al. The peopling of the Pacific from a bacterial perspective. Science. 2009;323:527–530. doi: 10.1126/science.1166083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirth T, Wang X, Linz B, et al. Distinguishing human ethnic groups by means of sequences from Helicobacter pylori: lessons from Ladakh. Proc Natl Acad Sci U S A. 2004;101:4746–4751. doi: 10.1073/pnas.0306629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falush D, Wirth T, Linz B, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 44.Correa P. Gastric cancer: two epidemics? Dig Dis Sci. 2011;56:1585–1586. doi: 10.1007/s10620-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 45.Furuta Y, Yahara K, Hatakeyama M, Kobayashi I. Evolution of cagA oncogene of Helicobacter pylori through recombination. PLoS One. 2011;6:e23499. doi: 10.1371/journal.pone.0023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devi SM, Ahmed I, Francalacci P, et al. Ancestral European roots of Helicobacter pylori in India. BMC Genomics. 2007;8:184. doi: 10.1186/1471-2164-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breurec S, Guillard B, Hem S, et al. Evolutionary history of Helicobacter pylori sequences reflect past human migrations in South-east Asia. PLoS One. 2011;6:e22058. doi: 10.1371/journal.pone.0022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tay CY, Mitchell H, Dong Q, Goh KL, Dawes IW, Lan R. Population structure of Helicobacter pylori among ethnic groups in Malaysia: recent acquisition of the bacterium by the Malay population. BMC Microbiol. 2009;9:126. doi: 10.1186/1471-2180-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077–1083. doi: 10.2169/internalmedicine.47.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camorlinga-Ponce M, Perez-Perez G, Gonzalez-Valencia G, et al. Helicobacter pylori genotyping from American indigenous groups shows novel Amerindian vacA and cagA alleles and Asian, African and European admixture. PLoS One. 2011;6:e27212. doi: 10.1371/journal.pone.0027212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domínguez-Bello MG, Pérez ME, Bortolini MC, et al. Amerindian Helicobacter pylori strains go extinct, as european strains expand their host range. PLoS One. 2008;3:e3307. doi: 10.1371/journal.pone.0003307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghose C, Perez-Perez GI, Dominguez-Bello MG, Pride DT, Bravi CM, Blaser MJ. East Asian genotypes of Helicobacter pylori strains in Amerindians provide evidence for its ancient human carriage. Proc Natl Acad Sci U S A. 2002;99:15107–15111. doi: 10.1073/pnas.242574599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaoka Y, Orito E, Mizokami M, et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180–184. doi: 10.1016/s0014-5793(02)02617-0. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Lewis CM, Jakobsson M, et al. Genetic variation and population structure in native Americans. PLoS Genet. 2007;3:e185. doi: 10.1371/journal.pgen.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Ray N, Rojas W, et al. Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet. 2008;4:e1000037. doi: 10.1371/journal.pgen.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segal I, Ally R, Mitchell H. Helicobacter pylori: an African perspective. QJM. 2001;94:561–565. doi: 10.1093/qjmed/94.10.561. [DOI] [PubMed] [Google Scholar]
- 57.Fage JD. Slavery and the slave trade in the context of West African history. J Afr Hist. 1969;10:393–404. [Google Scholar]
- 58.Salas A, Richards M, Lareu MV, et al. The African diaspora: mitochondrial DNA and the Atlantic slave trade. Am J Hum Genet. 2004;74:454–465. doi: 10.1086/382194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kersulyte D, Kalia A, Gilman RH, et al. Helicobacter pylori from Peruvian amerindians: traces of human migrations in strains from remote Amazon, and genome sequence of an Amerind strain. PLoS One. 2010;5:e15076. doi: 10.1371/journal.pone.0015076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mane SP, Dominguez-Bello MG, Blaser MJ, et al. Host-interactive genes in Amerindian Helicobacter pylori diverge from their Old World homologs and mediate inflammatory responses. J Bacteriol. 2010;192:3078–3092. doi: 10.1128/JB.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki M, Kiga K, Kersulyte D, et al. Attenuated CagA oncoprotein in Helicobacter pylori from Amerindians in Peruvian Amazon. J Biol Chem. 2011;286:29964–29972. doi: 10.1074/jbc.M111.263715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bravo LE, van Doom LJ, Realpe JL, Correa P. Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma? Am J Gastroenterol. 2002;97:2839–2842. doi: 10.1111/j.1572-0241.2002.07031.x. [DOI] [PubMed] [Google Scholar]
- 63.de Sablet T, Piazuelo MB, Shaffer CL, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189–1195. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howlader N, Noone AM, Krapcho M, et al. Surveillasnce Epidemiology and End Results (SEER) Cancer Statistics Review, 1975-2008 [Internet] Bethesda: National Cancer Institute; 2011. [cited 2011 Nov 22]. Available from: http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 65.Epplein M, Signorello LB, Zheng W, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev. 2011;20:826–834. doi: 10.1158/1055-9965.EPI-10-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sonnenberg A. Differences in the birth-cohort patterns of gastric cancer and peptic ulcer. Gut. 2010;59:736–743. doi: 10.1136/gut.2009.195008. [DOI] [PubMed] [Google Scholar]
- 67.Sonnenberg A. Time trends of mortality from gastric cancer in Europe. Dig Dis Sci. 2011;56:1112–1118. doi: 10.1007/s10620-010-1553-2. [DOI] [PubMed] [Google Scholar]
- 68.Aragonés N, Pollán M, López-Abente G, et al. Time trend and age-period-cohort effects on gastric cancer incidence in Zaragoza and Navarre, Spain. J Epidemiol Community Health. 1997;51:412–417. doi: 10.1136/jech.51.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson WF, Camargo MC, Fraumeni JF, Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. Jama. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]