Abstract
Hsp90 is an essential and highly conserved modular molecular chaperone whose N and middle domains are separated by a disordered region termed the charged linker. Although its importance has been previously disregarded, because a minimal linker length is sufficient for Hsp90 activity, the evolutionary persistence of extensive charged linkers of divergent sequence in Hsp90 proteins of most eukaryotes remains unexplained. To examine this question further, we introduced human and plasmodium native and length-matched artificial linkers into yeast Hsp90. After evaluating ATPase activity and biophysical characteristics in vitro, and chaperone function in vivo, we conclude that linker sequence affects Hsp90 function, cochaperone interaction, and conformation. We propose that the charged linker, in addition to providing the flexibility necessary for Hsp90 domain rearrangements—likely its original purpose—has evolved in eukaryotes to serve as a rheostat for the Hsp90 chaperone machine.
Heat shock protein 90 (Hsp90) is an essential molecular chaperone in eukaryotes (1, 2). Hsp90 consists of three domains that are highly conserved from bacteria to mammals: an N-terminal ATP-binding domain, a middle client protein-binding domain, and a C-terminal dimerization domain (3, 4). In bacteria, N and middle domains of the Hsp90 paralog high-temperature protein G (HtpG) are separated by a short stretch of amino acids (5), whereas N and middle domains of eukaryotic Hsp90 proteins are linked by a much more extensive unstructured charged amino acid-rich region, termed the charged linker (4). Surprisingly, most of the 56-aa charged linker region of Saccharomyces cerevisiae Hsp90 (Hsp82) seems to be dispensable because, similar to HtpG, a minimal length is sufficient for chaperone activity (5–7). Importantly, the minimal linker in yeast Hsp90 need not be of a particular sequence (7). These data have given rise to the suggestion that the linker serves primarily if not solely to provide the flexibility necessary to facilitate the conformational rearrangement of N and middle domains upon ATP binding to Hsp90 (7–9). Although likely correct in the case of HtpG, this hypothesis does not satisfactorily explain the appearance and persistence in eukaryotic Hsp90 proteins of charged linkers characterized by extensive sequence divergence and length variability, nor does it account for the regulated posttranslational modification of specific amino acids within the linker of human Hsp90 (10, 11). Further, the substrate and ATP binding characteristics of isolated yeast Hsp90 N domains are significantly modified by appendage of the yeast Hsp90 charged linker sequence (12). Because the region is unstructured it has been necessary to remove much of it to obtain highly refractive crystals of yeast or human HSP90, making it impossible to visualize possible structural contributions of the charged linker to chaperone conformation.
To further examine the contribution of linker length and sequence to optimal Hsp90 activity in both yeast and mammalian cells, we chose to replace the S. cerevisiae charged linker (56-aa residues, Sc-CL) with the human (Homo sapiens) charged linker (63-aa residues, Hs-CL) to create a chimeric Hsp90 protein. Native yeast and human linkers were also replaced with the significantly longer Plasmodium falciparum Hsp90 linker (95-aa, Pf-CL). In addition, we replaced native yeast and human charged linkers with size-matched artificial (Gly-Ser-Ser- … repeats) linkers comprising 7 (GSS7), 56 (GSS56, corresponding to the length of the yeast charged linker), 63 (GSS63, corresponding to the length of the human charged linker), or 95 (GSS95, corresponding to the length of the P. falciparum charged linker) amino acids. Our data clearly show that linker sequence contributes to Hsp90 activity and affects Hsp90 conformation. In contrast, we found that, beyond a necessary minimum, linker length per se does not significantly influence chaperone function. Taken together, these findings suggest that the charged linker in eukaryotic Hsp90 serves two distinct purposes: besides providing the flexibility necessary for domain rearrangements, this region has evolved to act as a self-contained rheostat to modulate chaperone activity.
Results
Consequences of Charged Linker Swapping for Hsp90 Function in Yeast.
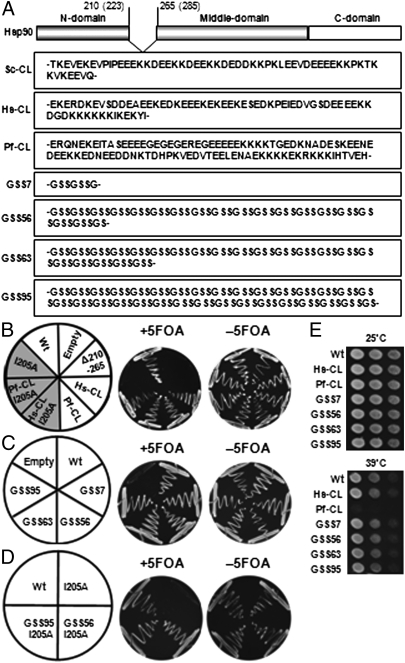
To distinguish the possible separate contributions of charged linker sequence and length diversity to Hsp90 function, we used yeast viability as a simple assay system to detect charged linker-dependent Hsp90 activity in vivo. We deleted the entire charged linker region from yHsp90 (Fig. 1A, yHsp90 Δ210–265). This deletion does not support yeast viability (“Δ210–265” in Fig. 1B). This construct was then complemented with several native Hsp90 charged linkers or with selected artificial linkers of varying length. Mutated or wild-type yHSP90 were then inserted into a single-copy plasmid with LEU2 as a selectable marker, and the plasmid was transformed into strain PP30 (pHSC82), in which chromosomal Hsp90 coding genes are deleted and an alternative wild-type HSP90 gene (HSC82) on a plasmid with the URA3 selectable maker is provided to maintain viability. When transformants are grown on plates containing 5-fluoroorotic acid (5-FOA), the URA3 plasmid is selected against and lost (cured), causing cells to rely solely on the transformed HSP90 gene to support growth. PP30 cells with wild-type yHsp90 expressed from a LEU2 single-copy plasmid were viable, whereas those containing only empty plasmid did not survive on 5-FOA plates (Fig. 1B). Using this assay, we were able to detect charged linker-dependent effects on Hsp90 activity in vivo. When yHsp90 Δ210–265 was complemented with either the charged linker from Homo sapiens HSP90α (yHsp90-Hs-CL) or the charged linker from P. falciparum Hsp90 (yHsp90-Pf-CL), yeast viability was maintained (Fig. 1B), although the rather extensive Pf-CL did not support optimal activity and was temperature sensitive (Fig. 1E). Hsp90 containing a short artificial uncharged linker comprising Gly-Ser-Ser-Gly-Ser-Ser-Gly was previously reported to support yeast growth (7). We used a similar approach to construct Hsp90 mutants in which the entire charged linker region was removed and replaced with Gly-Ser-Ser repeats of variable length. We introduced artificial linkers comparable to the lengths of the native linker in yeast, human, or plasmodium Hsp90 (yHsp90-GSS56, GSS63, or GSS95, respectively). In this way we were able to examine whether length of the native linker affects basic Hsp90 function (e.g., supporting yeast viability) independent of sequence. We confirmed that yeast expressing only yHsp90-GSS7 were viable (Fig. 1C). In addition, all of the Gly-Ser-Ser-repeat linker yHsp90 mutants, irrespective of their length, also supported yeast growth and were not temperature sensitive (Fig. 1 C and E). The lack of temperature sensitivity of yHsp90-GSS95 is in distinct contrast with the phenotype of yHsp90-Pf-CL (Fig. 1E), suggesting that the decreased robustness of yHsp90-Pf-CL is due to the sequence of the P. falciparum linker and not to its length. Importantly, although incorporation of the Pf-CL into yHsp90 significantly affected chaperone behavior in vivo, it did not affect the basic antiaggregation properties of the yHsp90 protein in vitro. Thus, yHsp90-Pf-CL and yHsp90-GSS95 proteins displayed comparable ability to prevent thermal denaturation of citrate synthase (SI Appendix, S1).
Fig. 1.
Functional consequences of yHsp90 charged linker swapping. (A) Schematic diagram of domain structure of Hsp90 displaying the charged linker sequence of yeast (Sc), human (Hs), and plasmodium (Pf) Hsp90. Also shown are the various GSS-repeat linker sequences used in this study. (B–D) S. cerevisiae strains (see text for description) were spread onto synthetic defined medium without leucine agar plates either with or without 5-FOA at 25 °C for 4 d. (E) Temperature sensitivity was assessed by growing the yeast stains on YPD plates at either 25 °C or 39 °C for 4 d.
At first glance, these data suggest that linker sequence does not affect eukaryotic Hsp90 activity. However, subsequent experiments suggest that the true role of the linker is more complex. We recently reported that a single mutation in the yHsp90 N domain (I205A) disrupted Hsp90 function and demonstrated lethality in yeast (“I205A” in Fig. 1B) (9). Truncation of the yHsp90 native linker restores viability to yeast expressing yHsp90-I205A (9). Here we show that substitution of the native yeast charged linker sequence with either full-length human or plasmodium charged linkers also restored viability to yeast expressing yHsp90-I205A (“Hs-CL I205A” and “Pf-CL I205A,” respectively, in Fig. 1B). These data suggest that the sequence of the native yeast linker and not merely its presence contributes to the lethality of the I205A mutation. To confirm the importance of linker sequence for yHsp90-I205A lethality, we made this mutation in yHsp90 expressing either GSS56 or GSS95. Both of these GSS artificial linkers also rescued I205A mutants (Fig. 1D).
Sequence, Not Length of the Charged Linker, Influences Hsp90 Chaperone Activity.
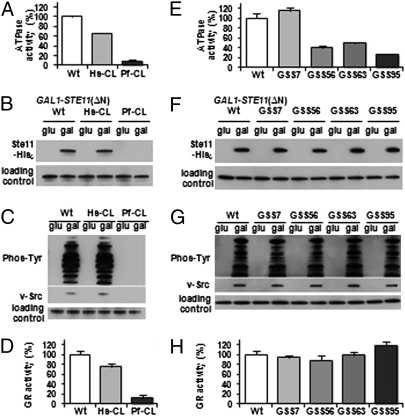
Because yHsp90-Pf-CL displayed temperature sensitivity in vivo, we assessed its chaperone activity, and that of yHsp90-Hs-CL for comparison, in greater detail. ATP binding and hydrolysis are necessary for Hsp90 function. As shown in Fig. 2A, the ATPase activity of yHsp90-Hs-CL was partially reduced (≈60% of wild type), and that of yHsp90-Pf-CL was nearly undetectable (<10% of wild type), whereas the ATP affinity of both proteins was not changed. We next examined whether charged linker swapping affected Hsp90-dependent chaperoning of client proteins. As shown in Fig. 2B, Ste11ΔN, an Hsp90 client kinase in yeast, was stably expressed in yHsp90 wild type and yHsp90-Hs-CL–expressing cells. However, Ste11ΔN was not detected in cells expressing yHsp90-Pf-CL. The oncogenic tyrosine kinase v-Src is a well-studied Hsp90-dependent client, whose ectopic expression in yeast is toxic. Galactose-induced expression of v-SRC under GAL1 promoter, and tyrosine kinase activity of v-Src protein (as measured by tyrosine phosphorylation of yeast total proteins) was supported by both yHsp90 wild type and yHsp90-Hs-CL, but not by yHsp90-Pf-CL (Fig. 2C). The steroid hormone receptor glucocorticoid receptor (GR) is also an Hsp90 client. Wild-type or charged linker Hsp90 mutants were transformed with a GR-expressing plasmid in yeast carrying a glucocorticoid-regulated LacZ reporter. As shown in Fig. 2D, GR activity was slightly reduced in cells expressing yHsp90-Hs-CL and significantly impaired in cells expressing yHsp90-Pf-CL. Consistent with the temperature sensitivity profile of these two linker mutants, the chaperone activity of yHsp90-Pf-CL is significantly impaired, even though it is sufficient to support yeast viability.
Fig. 2.
Sequence, not length, of charged linker dictates yHsp90 chaperone activity. (A and E) ATPase activities of wild-type and indicated mutant yHSP90 proteins. (B, C, F, G) Ste11ΔN (B and F) or v-Src (C and G) under control of a GAL1 promoter were transformed into cells expressing wild-type (Wt) or indicated mutant yHSP90 in the presence of glucose (glu) or galactose (gal). In vitro protein expression level and v-Src activity were detected by immunoblotting. GR activity was assessed in the same strains after transformation with two plasmids: one expressing GR and the other carrying a GR-responsive lacZ reporter. (D and H) Data are expressed as a percentage of GR activity observed in Wt cells and are depicted as the mean ± SD derived from three independent experiments.
We next examined the activity of our panel of GSS artificial linker yHsp90 mutants in these contexts. Only yHsp90-GSS7 displayed wild type ATPase activity [as previously reported (7)], whereas the ATPase activity of the three full-length artificial linker mutants (yHsp90-GSS56, -GSS63, and -GSS95) was reduced by a similar extent (20–40% of wild type) (Fig. 2E). Nevertheless, all of the artificial linker mutants efficiently and equivalently chaperoned Ste11ΔN, v-Src, and GR (Fig. 2 F–H). These data show that optimal chaperone function can be achieved by Hsp90 proteins whose linker length varies by more than 10-fold and that the reduced chaperone function of yHsp90-Pf-CL is not due solely to its significantly compromised ATPase activity.
Linker Sequence Is Important for Client Protein Interaction with Human Hsp90.
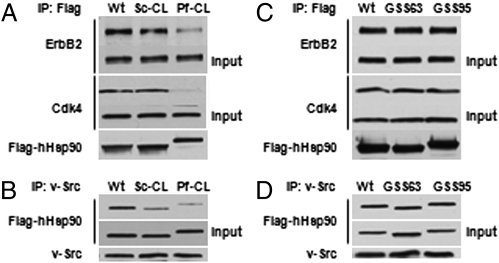
To address whether human Hsp90α (hHsp90) is similarly affected by charged linker swapping, we examined the impact of charged linker substitution on interaction of hHsp90 with several client proteins. We deleted the entire charged linker region from human Hsp90α (hHsp90 Δ223–285) and replaced it with either the charged linker from S. cerevisiae Hsp90 (Sc-CL) or with the charged linker from P. falciparum Hsp90 (Pf-CL) (Fig. 1A). We cotransfected COS7 cells with Flag-tagged hHsp90 wild type, Sc-CL, or Pf-CL plasmids, together with plasmid expressing ErbB2 tyrosine kinase, an Hsp90 client. As shown in Fig. 3A, immunoprecipitation with anti-Flag antibody detected ErbB2 interaction with hHsp90 wild type and with hHsp90-Sc-CL, but ErbB2 interaction with hHsp90-Pf-CL was greatly reduced. We obtained similar data for the endogenous Hsp90 client kinase Cdk4 (Fig. 3A). Association of the tyrosine kinase v-Src with hHsp90 was also sensitive to charged linker swapping. As shown in Fig. 3B, interaction with v-Src (stably expressed in NIH 3T3 cells) was somewhat reduced for hHsp90-Sc-CL and more noticeably reduced for hHsp90-Pf-CL mutants. However, in agreement with our yeast data, interaction of ErbB2, Cdk4, and v-Src with the artificial linker mutants hHsp90-GSS63 and hHsp90-GSS95 was indistinguishable from hHsp90 wild type (Fig. 3 C and D). The impact of charged linker substitution on Hsp90 chaperone activity in mammalian cells parallels the client protein interaction data described above. Using cystic fibrosis transmembrane conductance regulator maturation [a process highly dependent on Hsp90 and responsive to Hsp90 overexpression (13)] as an assay for Hsp90 chaperone activity in mammalian cells, we have observed that hHsp90-Sc-CL is as active as wild-type hHsp90, whereas hHsp90-Pf-CL chaperone activity is much reduced (SI Appendix, S2).
Fig. 3.
Charged linker sequence affects client protein interaction with hHSP90. COS7 cells (A and C) or v-Src–expressing NIH 3T3 cells (B and D) were transfected with wild-type or indicated mutant Flag-hHsp90 constructs together with ErbB2 (A and C). hHsp90-client protein complexes were immunoprecipitated (IP) using Flag antibody-conjugated agarose (A and C) or with v-Src antibody (B and D). Indicated coprecipitating proteins were detected by immunoblotting.
Linker Sequence, but Not Length, Is an Important Determinant of Hsp90 Solvent Accessibility and Protease Sensitivity.
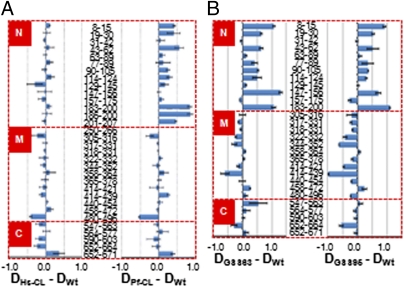
Hsp90 chaperone activity is regulated by dynamic conformational change coupled to ATP binding and hydrolysis. Our data show that both ATPase and chaperone activities are reduced in yHsp90-Hs-CL and yHsp90-Pf-CL mutants, suggesting that Hsp90 conformational equilibrium may be impacted by charged linker swapping. To address this possibility, we used amide hydrogen exchange mass spectrometry analysis (HX-MS) to analyze the conformational status in solution of these Hsp90 chimeric proteins. With this technique one is able to detect regions within full-length Hsp90 that contain slow and fast exchanging amide hydrogens, which in turn reflects dynamics of hydrogen bonds in secondary structure and solvent accessibility of distinct domains (8, 9). yHsp90 wild-type, Hs-CL, and Pf-CL proteins were incubated in D2O-containing buffer for 30 s and, after quenching of the exchange reaction, proteins were digested with pepsin to produce short peptide fragments. The rates of amide hydrogen-H/D-exchange for wild-type yHsp90 peptides were determined and subtracted from the rates of exchange for peptides derived from each yHsp90 mutant. Reproducibility of these experiments was high, and differences of more than 0.3 Da are statistically significant. As shown in Fig. 4A, we found markedly greater solvent accessibility (compared with wild type) of peptides encompassing the N domain of yHsp90-Pf-CL, with significant differences appearing in the N-terminal part of the nucleotide-binding domain (NBD; amino acids 8–15, 19–30, and 43–62) and in α-helices 8 and 9 (amino acids 183–207). The observed differences are similar in magnitude but opposite in direction compared with the influence of ATP on the yHsp90 NBD. The increased flexibility in region 8–30 is consistent with a reduced ability to N-terminally dimerize and, consequently, is also consistent with a lower ATPase activity. Likewise, the increased flexibility in helices 8 and 9 indicates a reduced ability of docking of the NBD and middle domain. In contrast, exchange rates of N-domain peptides prepared from yHsp90-Hs-CL were more similar to wild-type yHsp90.
Fig. 4.
Effect of charged linker swapping on Hsp90 deuteron incorporation kinetics. After incubation with D2O for 30 s and quenching the exchange reaction, proteins were digested with pepsin and analyzed by mass spectrometry. Deuteron incorporation into mutant yHSP90 proteins minus deuteron incorporation into wild-type yHSP90 protein is plotted for selected peptides derived from N, middle (M), and C domains of yHSP90. Error bars represent the SE derived from three independent experiments.
Interestingly, yHsp90-GSS95, the mutant with artificial linker of equivalent length to the P. falciparum linker, also displayed dramatically increased solvent accessibility of N-domain peptides (Fig. 4B). However, peptides derived from the middle domain of yHsp90-GSS95 were significantly more protected (compared with wild type) than were peptides from yHsp90-Pf-CL. The pattern for yHsp90-GSS63 (linker equivalent in length to Hs-CL) was qualitatively similar to that of the GSS95 mutant. These data show that increased solvent accessibility of the N domain cannot explain the reduced in vivo chaperone activity of yHsp90-Pf-CL. HX-MS analysis of yHsp90-GSS7, which displays normal ATPase activity (Fig. 2E) reveals little change from wild type in the N domain but shows increased protection of peptides derived from the middle domain. Middle-domain peptides are also more protected (compared with wild type) in yHsp90-GSS56 (linker length equivalent to Sc-CL), although this mutant is similar to GSS63 and GSS95 in also showing decreased protection in the N domain (SI Appendix, S3).
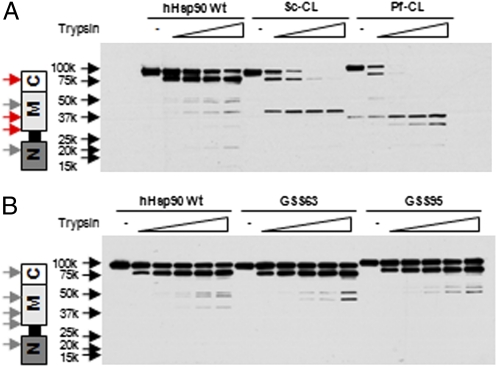
We further investigated the effect of charged linker substitution on the conformational status of hHsp90 by visualizing trypsin sensitivity. Previous work has identified several trypsin cleavage sites in hHsp90, and the proteolytic fingerprint is in agreement with the chaperone's domain structure (14, 15). We transfected COS7 cells with Flag-tagged hHsp90 wild-type, Sc-CL, or Pf-CL expression plasmids, and we immunoprecipitated Hsp90 with anti-Flag antibody. After a high-salt wash to remove interacting proteins, we exposed the precipitated hHsp90 to increasing concentrations of trypsin. We used an antibody recognizing the N domain of hHsp90 to generate the proteolytic fingerprint. As shown in Fig. 5A, we detected an hHsp90 fingerprint that is consistent with previous reports (14), with a predominant cleavage site in the C-terminal dimerization domain (amino acids 614–619) and additional cleavage sites in the middle domain (amino acid 399) and at the boundary between the charged linker and middle domain (amino acid 288). Replacement of the native linker with either Sc-CL or Pf-CL resulted in increased trypsin sensitivity, although replacement with the P. falciparum linker had the greatest impact. In contrast, replacement of the native human linker with either GSS63 or GSS95 produced a proteolytic fingerprint for hHsp90 that was essentially identical to that of the wild-type protein (Fig. 5B). We confirmed that linker swapping affected the trypsin sensitivity of yHsp90 in a similar fashion. Replacement of the native yeast linker with Pf-CL dramatically increased trypsin sensitivity, whereas replacement with the equivalent length GSS95 produced a proteolytic fingerprint that was identical to that of the wild-type protein (SI Appendix, S4).
Fig. 5.
Charged linker swapping affects protease sensitivity of human and yeast Hsp90. COS7 cells were transfected with indicated Flag-hHsp90 constructs. (A and B) Cells were lysed, and proteins were immunoprecipitated by Flag antibody-conjugated agarose. Immunoprecipitates were digested with TPCK-treated trypsin and detected by immunoblotting with N-terminal antibody to hHsp90. Left: Molecular mass (kDa) and schematic diagram of domain structure of Hsp90. Red arrows indicate major trypsin cleavage sites in hHsp90 or yHsp90; gray arrows indicate minor cleavage sites.
Overexpression of Yeast Aha1 Restores Chaperone Function of yHsp90 Pf-CL.
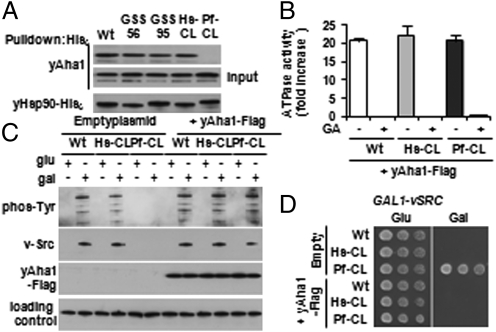
Although incorporation of the longer GSS synthetic linkers uniformly reduced the ATPase activity of yHsp90 (Fig. 2A), the chaperone activity of these proteins in cells was indistinguishable from that of wild-type yHsp90. This suggests that one or more cochaperones present in yeast are able to overcome the reduced ATPase activity displayed by these yHsp90 mutants in vitro. Aha1 is a unique cochaperone that binds simultaneously to both N and middle domains of Hsp90 and, by facilitating a rate-limiting conformational change, significantly stimulates Hsp90 ATPase activity (16, 17). Because the GSS Hsp90 mutants seem to function normally in yeast and even yHsp90-Pf-CL supports yeast viability at low temperature, even though its native ATPase activity is <10% that of wild type, we hypothesized that these mutants are activated in vivo by yeast Aha1 (yAha1). We first examined endogenous Aha1 coprecipitation with His-tagged yHsp90 wild-type and various mutant proteins (Fig. 6A). Although yHsp90 wild-type, Hs-CL, GSS56, and GSS95 proteins pulled down equivalent amounts of endogenous yAha1, consistent with their equivalent in vivo activity, no yAha1 was detectable in pull-downs of yHsp90-Pf-CL. We also queried the impact of charged linker swapping on interaction of yHsp90 and hHsp90 with other cochaperones, including Sti1Hop, Cdc37p50, and Sba1p23 (SI Appendix, S5). Aha1 was the only cochaperone whose interaction with Hsp90 was uniformly and consistently abrogated by the presence of the P. falciparum linker in both yeast and human backgrounds.
Fig. 6.
Overexpression of Aha1 rescues deficient chaperone activity of yHsp90 charged linker mutants. (A) Yeast cells expressing indicated His-yHSP90 constructs were lysed, and proteins were precipitated by Ni-NTA; associating endogenous Aha1 was detected by immunoblotting. (B) ATPase activities of purified wild-type and indicated mutant yHsp90 proteins were determined in the presence of 20 μM Aha1 protein. Geldanamycin (GA, 10 μM) was added to confirm HSP90 as the sole source of ATP hydrolysis in the assay. Data are expressed as fold activation (compared with samples with no Aha1 added) and are depicted as the mean ± SD derived from three independent experiments. (C and D) v-Src was transformed into cells that did or did not express exogenous Aha1 (as indicated) and also expressed either wild-type (Wt) or indicated mutant yHsp90 constructs. (C) v-Src protein expression and activity were detected by immunoblotting; (D) v-Src activity was also monitored by its negative effect on cell growth.
Next, we looked at the ability of excess Aha1 to stimulate Hsp90 ATPase activity in vitro. Using a 20-fold molar excess of purified yAha1 protein, we found that the ATPase activity of purified yHsp90-Pf-CL was stimulated to a similar degree (≈20-fold), as were yHsp90-Hs-CL and yHsp90 wild-type proteins (Fig. 6B). We examined the sensitivity of wild-type yHsp90 and yHsp90-Pf-CL proteins to a wide range of purified yAha1 concentrations and observed an approximate twofold difference in ATPase stimulation over the range of yAha1 concentrations tested (SI Appendix, S6). These data suggested that overexpression of yAha1 might restore full chaperone activity to yHsp90-Pf-CL in vivo. To test this hypothesis, we overexpressed Flag-tagged yAha1 in the context of yHsp90 wild type, Hs-CL, and Pf-CL, and we reexamined Hsp90 chaperone activity (Fig. 6 C and D). Although overexpression of Aha1 did not affect the v-Src activity in yHsp90 wild type and Hs-CL–expressing cells, v-Src protein expression and kinase activity were restored to normal levels by yAha1 overexpression in yeast harboring yHsp90-Pf-CL (Fig. 6C). Restoration of v-Src expression and activity was confirmed by Src-dependent loss of viability in all yeast cells overexpressing yAha1, including those with yHsp90-Pf-CL (which in the absence of excess yAha1 remained viable; Fig. 6D). Similar results were observed for active Ste11ΔN and GR upon yAha1 overexpression (SI Appendix, S7). Further, yAha1 overexpression corrected the temperature sensitivity of yeast harboring yHsp90-Pf-CL (SI Appendix, S8A). Of interest, Aha1 overexpression was unable to rescue the lethality of yHsp90-I205A (SI Appendix, S8B), suggesting that mutation in this distal portion of the N domain disrupts Hsp90 architecture in a manner that is independent of Aha1. This hypothesis is consistent with our observation that inclusion of the plasmodium linker sequence in yHsp90, which negatively affects endogenous Aha1 interaction, nevertheless restores viability to yeast expressing yHsp90-I205A (Fig. 1B).
Discussion
We previously identified an N-domain point mutation of yeast Hsp90 (I205A) that was lethal but could be rescued by significant truncation of the native yeast charged linker region (9). In the present study, we investigated whether linker sequence, not simply its presence, might contribute to the lethality of the I205A mutation. To our surprise, yeast Hsp90 harboring this otherwise lethal mutation but containing full-length human or P. falciparum charged linker sequences in place of the native linker sequence were fully viable, suggesting that a sequence-dependent interaction (or lack thereof) of I205A with the yeast linker was critical for the observed lethality. This hypothesis is further supported by our data showing that replacement of the yeast linker with an artificial (uncharged) linker of various lengths also reversed the lethality of this mutation.
These unexpected findings prompted us to explore in detail the role of the charged linker in Hsp90 function. Although the linker region is divergent in sequence, its negative charge contributes significantly to the net charge of eukaryotic Hsp90 proteins (SI Appendix, S9). In yeast and human Hsp90 proteins, the native linkers contribute a negative net charge of −8 and −12, respectively. The plasmodium linker increases the net negative charge of yeast and human Hsp90 by −19. Thus, the temperature sensitivity and impaired chaperone activity of yeast Hsp90 containing the plasmodium linker could be a consequence of the significant change in net charge. Alternatively, spatial clustering of charged residues throughout the linker may be important. Although technically difficult to verify, this hypothesis is supported by the fact that phosphorylation of specific serine (uncharged) residues in the linker of mammalian Hsp90 increases the local negative charge and affects chaperone activity (18). Lysine acetylation sites have also been identified in the linker of mammalian Hsp90 (http://www.phosphosite.org). Because acetylation neutralizes the positive charge of lysine, this posttranslational modification also alters the local net charge of the linker.
The nature of the charged linker affects Hsp90 ATPase activity. The charged linker motif has been shown previously to exert a large impact on Hsp90 N-domain conformation (12). We explored this further using amide hydrogen exchange analysis to map dynamic changes in solvent accessibility of the N, middle, and C domains of yeast Hsp90 containing different linkers. We found that whereas the human linker caused minimal structural disruption, an uncharged linker of equivalent length or longer dramatically increased solvent accessibility of peptides throughout the N domain. These changes are opposite of those caused by ATP binding to the N domain and are consistent with the reduced ATPase activities of all GSS yHsp90 proteins excepting yHsp90-GSS7, whose ATPase activity is equivalent to wild-type yHsp90. Importantly, amide hydrogen exchange analysis of yHsp90-GSS7 revealed little impact on N-domain solvent accessibility. Despite the negative impact of the full-length GSS linkers on yHsp90 ATPase activity, none of these mutants were temperature sensitive, and all allowed for the normal chaperoning of the Hsp90 clients Ste11ΔN, v-Src, and GR.
Inclusion of the plasmodium linker also disrupted yHsp90 N-domain structure and reduced ATPase activity to ≈10% that of wild type, but this mutant was temperature sensitive and was unable to chaperone any client protein examined. Likewise, in mammalian cells human Hsp90 containing the plasmodium linker interacted poorly with its clients ErbB2, Cdk4, and v-Src, whereas the interaction of human Hsp90 containing the GSS95 linker was indistinguishable from wild type. It is certainly possible, albeit unlikely, that the small difference in (reduced) ATPase activity between the GSS Hsp90 mutants and Hsp90-Pf-CL (Fig. 2 A and E) is responsible for these proteins’ disparate chaperone activities in vivo. However, we believe it to be more likely that the divergent chaperone activity of these proteins is a direct consequence of the reduced ability of Hsp90-Pf-CL to interact with the endogenous cochaperone Aha1, which potently stimulates Hsp90 ATPase activity (16). Because the ATPase activity of yHsp90-Pf-CL is enhanced, albeit less efficiently, by yAha1 in vitro, we suspected that overexpression of yAha1 in yeast might restore full chaperone activity to yHsp90-Pf-CL in vivo, and that is what we observed. These data suggest that the net or localized charge of the linker sequence (or an aspect of the sequence that is independent of charge), but not its length, affects the efficiency of Aha1 binding to Hsp90. Because Aha1 associates simultaneously with both N and middle domains of Hsp90 (16, 17), our findings suggest that linker sequence may influence the proper positioning of Aha1 interaction motifs in each domain, although this hypothesis remains to be investigated in further detail (a preliminary model and summary of these results is shown in SI Appendix, S10).
Further, our data support the additional conclusion that native in vitro ATPase activity is a less reliable predictor of Hsp90 chaperone function in vivo than is ability to interact with Aha1 in cells (or to be stimulated by the cochaperone in vitro), further emphasizing the importance of Aha1 for optimal eukaryotic Hsp90 function.
We have provided evidence for the importance of linker sequence in modulating eukaryotic Hsp90 activity in vivo. Our data strongly suggest that, besides providing the necessary flexibility to correctly orient the various Hsp90 domains to permit ATP hydrolysis (for which only a very short linker length is required), the charged linker has undergone an expansion in size and a gain of function in eukaryotes that provides unique regulatory sites able to modulate Hsp90 chaperone activity in a client-, cochaperone-, and perhaps also in an environment-specific manner.
Materials and Methods
Strains and Plasmids.
We used the S. cerevisiae strain PP30 (a, trp1-289, leu2-3,112, his3-200, ura3-52, ad2-101oc, lys2-801 AM, hsc82KANMX4, hsp-82KanMX4) as the host strain for expression of HSP82 mutant alleles, as previously described (9). We introduced charged linker mutations into the HSP82 gene of the plasmid pHSP82, which we transformed into PP30 yeast. To generate yeast expressing only HSP82 or its mutants, cells were cultured in medium without leucine but containing 0.1% 5-FOA. The pcDNA3-Flag-Hsp90α plasmids were constructed as described previously (9).
Growth Conditions.
Yeast cells were grown on yeast extract peptone dextrose (YPD; 2% Bacto peptone, 1% yeast extract, 2% glucose) or on YPGal (YPD with 2% galactose, 20 mgl−1 adenine). Selective growth was on dropout 2% glucose medium supplemented with appropriate amino acids. COS7 cells and NIH 3T3-v-SRC cells were maintained at 37 °C and 5% CO2 in DMEM containing 1 mM sodium pyruvate and 10% FBS. FuGene6 (Roche) or Lipofectamine2000 (Invitrogen) were used to transiently transfect COS7 cells or NIH 3T3-Src cells, respectively.
In Vivo v-Src Activation.
v-SRC is under control of the GAL1 promoter. Its induction and activation were analyzed as described previously (16), with the exception that yeast cells were grown on 2% glucose to repress the GAL1 promoter. Cells were also spotted onto YPD or YPGal-2% agar.
Immunoprecipitation and Immunoblotting.
These experiments were performed as previously described (9). Cells were lysed in buffer containing 20 mM Hepes, 100 mM NaCl, 1 mM MgCl2, 0.1% Nonidet P-40, 20 mM Na2MoO4, phosphatase, and protease inhibitors (Roche). Immunoprecipitated proteins were detected by immunoblotting with respective antibodies.
Hsp90 ATPase Activity.
ATPase activities were measured using a method described previously (19, 20). This is an enzyme-coupled assay that typically uses 2 μM yeast Hsp90 and 20 μM human Hsp90. All activities are shown as averages of three separate measurements.
Amide Hydrogen Exchange.
Methods for performing amide hydrogen exchange experiments were similar to those described previously (21). Amide hydrogen exchange was initiated by a 20-fold dilution of 200 pmol wild-type or variant Hsp82 into D2O containing 25 mM Hepes (pH 7.4), 50 mM KCl, and 5 mM MgCl2 at 30 °C. After incubation the exchange reaction was quenched, and samples were directly injected into an HPLC-MS setup as described previously. Data acquisition and analysis was performed as previously described (21).
Supplementary Material
Acknowledgments
This research was supported in part by funds from the Intramural Research Program of the National Cancer Institute. S.T. is partially supported by a fellowship from the Japanese Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114414109/-/DCSupplemental.
References
- 1.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 2.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 4.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 5.Shiau AK, Harris SF, Southworth DR, Agard DA. Structural analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127:329–340. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Louvion JF, Warth R, Picard D. Two eukaryote-specific regions of Hsp82 are dispensable for its viability and signal transduction functions in yeast. Proc Natl Acad Sci USA. 1996;93:13937–13942. doi: 10.1073/pnas.93.24.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hainzl O, Lapina MC, Buchner J, Richter K. The charged linker region is an important regulator of Hsp90 function. J Biol Chem. 2009;284:22559–22567. doi: 10.1074/jbc.M109.031658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graf C, Stankiewicz M, Kramer G, Mayer MP. Spatially and kinetically resolved changes in the conformational dynamics of the Hsp90 chaperone machine. EMBO J. 2009;28:602–613. doi: 10.1038/emboj.2008.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumi S, et al. Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat Struct Mol Biol. 2009;16:1141–1147. doi: 10.1038/nsmb.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurokawa M, Zhao C, Reya T, Kornbluth S. Inhibition of apoptosome formation by suppression of Hsp90beta phosphorylation in tyrosine kinase-induced leukemias. Mol Cell Biol. 2008;28:5494–5506. doi: 10.1128/MCB.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogiso H, et al. Phosphorylation analysis of 90 kDa heat shock protein within the cytosolic arylhydrocarbon receptor complex. Biochemistry. 2004;43:15510–15519. doi: 10.1021/bi048736m. [DOI] [PubMed] [Google Scholar]
- 12.Scheibel T, et al. The charged region of Hsp90 modulates the function of the N-terminal domain. Proc Natl Acad Sci USA. 1999;96:1297–1302. doi: 10.1073/pnas.96.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollapour M, et al. Threonine 22 phosphorylation attenuates Hsp90 interaction with cochaperones and affects its chaperone activity. Mol Cell. 2011;41:672–681. doi: 10.1016/j.molcel.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemoto T, Sato N, Iwanari H, Yamashita H, Takagi T. Domain structures and immunogenic regions of the 90-kDa heat-shock protein (HSP90). Probing with a library of anti-HSP90 monoclonal antibodies and limited proteolysis. J Biol Chem. 1997;272:26179–26187. doi: 10.1074/jbc.272.42.26179. [DOI] [PubMed] [Google Scholar]
- 15.Hartson SD, et al. p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules. Biochemistry. 2000;39:7631–7644. doi: 10.1021/bi000315r. [DOI] [PubMed] [Google Scholar]
- 16.Panaretou B, et al. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol Cell. 2002;10:1307–1318. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 17.Retzlaff M, et al. Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol Cell. 2010;37:344–354. doi: 10.1016/j.molcel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Wayne N, Bolon DN. Charge-rich regions modulate the anti-aggregation activity of Hsp90. J Mol Biol. 2010;401:931–939. doi: 10.1016/j.jmb.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prodromou C, et al. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 1999;18:754–762. doi: 10.1093/emboj/18.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panaretou B, et al. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rist W, Graf C, Bukau B, Mayer MP. Amide hydrogen exchange reveals conformational changes in hsp70 chaperones important for allosteric regulation. J Biol Chem. 2006;281:16493–16501. doi: 10.1074/jbc.M600847200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.