Abstract
The persistence of an active subventricular zone neural stem cell niche in the adult mammalian forebrain supports its continued role in the production of new neurons and in generating cells to function in repair through adulthood. Unfortunately, with increasing age the niche begins to deteriorate, compromising these functions. The reasons for this decline are not clear. Studies are beginning to define the molecular and physiologic changes in the microenvironment of the aging subventricular zone niche. New revelations from aging studies will allow for a more thorough understanding of which reparative functions are lost in the aged brain, the progression of niche demise and the possibility for therauptic intervention to improve aging brain function.
Keywords: Aging, demyelinating lesions, neural stem cells, neurogenesis, Parkinson’s disease, ependymal damage, stroke, subventricular zone
Neurogenesis continues in primarily two regions of the adult mammalian brain. In the subgranular zone (SGZ) of the hippocampal dentate gyrus, adult neurogenesis is critical for processes involved in learning and memory [1–6], while neurogenesis in the subventricular zone (SVZ) generates new neurons destined for the olfactory bulb to function in fine olfaction discrimination [7–9]. With increasing age, neurogenesis declines, impacting its normal roles in neuron replacement and consequently its ability to function in repair mechanisms triggered by injury or disease. Relative to the young brain, the aged brain not only displays a more limited potential for recovery, but also has an enhanced susceptibility to degenerative diseases and injury. Thus, aging research must take into account many compounding issues that contribute to decline in a multitude of different ways. Due to cost-effectiveness and practicality, most studies examining adult neurogenesis have been conducted on young animals, resulting in a scarcity of data from aged animals. While this is beginning to change and more research is now being performed on aged animals, we can only anticipate that our understanding of age-related declines in brain functions and the potential for stem cell niche-based repair will increase and eventually provide sound advances in treating the aging brain. For this review, we will focus on the cellular and molecular changes associated with the aging SVZ stem cell niche and the effect aging has on SVZ functions.
Comparative Cytoarchitecture of Young versus Aged SVZ Neural Stem Cell Niche
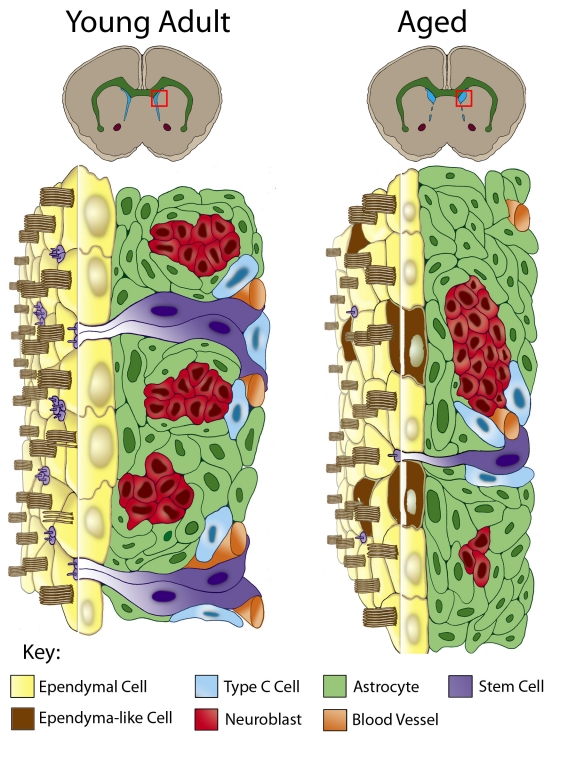
The cellular organization of the SVZ is critical to orchestrate events necessary for sustained stem cell niche output. The four cell types that comprise the SVZ and their organization within the niche were revealed based on ultrastructural characteristics observed using electron microscopy [10, 11]. The main cell types are astrocytes, transitory amplifying progenitors (Type C cells), neuroblasts and ependymal cells [10, 11] (Fig. 1). Of these, the neural stem cells (NSCs) have been identified as a slowly dividing subpopulation of SVZ astrocytes [12, 13] with an apical process contacting the ventricle and a basal process extending to underlying blood vessels [14–16].
Figure 1.
Comparative cytoarchitecture of the SVZ through aging. Due to age-related stenosis of the ventral lateral ventricle walls, only the dorsolateral SVZ remains proliferative and the dorsal ventricle (blue in upper coronal brain images) expands. The red box in each schematic represents the dorsolateral SVZ depicted in more detail below. The young adult SVZ is organized below an ependyma monolayer (yellow cells) and includes astrocytes (green cells), astrocytes with an apical and basal process spanning the SVZ (NSCs, purple cells), neuroblasts (red cells), Type C cells (blue cells) and basally located blood vessels (orange). In the aged SVZ there is a significant reduction of the SVZ, with fewer astrocytes possessing an apical process and fewer neuroblasts and Type C cells. Additionally, some SVZ astrocytes (brown cells) are found incorporated within the ependymal monolayer. These integrated astrocytes are derived from dividing astrocytes and take on ependyma-like characteristics.
This radial morphology is reminiscent of their predecessors, the radial glia [14, 15, 17, 18]. Type C cells are the intermediary progeny of SVZ NSCs and are typically found in close proximity to the basally located blood vessels [15, 16]. Type C cells give rise to highly migratory neuroblasts that organize into chains that transit the SVZ [19–21] and converge into the rostral migratory stream leading to the olfactory bulb. Once in the olfactory bulb, the neuroblasts defasiculate from the chains and migrate radially to their site of terminal differentiation [19–22].
At its ventricular surface the SVZ is separated from the cerebral spinal fluid (CSF) of the lateral ventricles by a monolayer of multiciliated ependymal cells that is punctuated by small clusters of astrocytic processes that contact the CSF [14] (see Fig. 1). The apical process of the SVZ astrocyte contains a single primary cilium that extends into the CSF [12, 14]. Astrocytes with an apical process express neural stem cell markers (CD133, GFAP and Nestin), and markers of activated/dividing stem cells (ID1, Ki67 and phosphohistone H3) [14, 23], marking them as the NSCs. When viewed from the ventricular surface structural units (pinwheels), consisting of ependymal cells spiraling around several fine astrocytic processes, can be observed [14]. The cells of the pinwheels are anchored to each other via adherens junctions [14], reminiscent of the organization of stem cells and somatic cells in other niches, e.g., the bone marrow HSC niche and Drosophila ovariole and testis [24–27] (for reviews see [28, 29]). These regenerative units are critical for stem cell division, as one daughter will retain its position and stem cell identity via its adherens junctions and the other will generate a progenitor cell capable of moving away from the niche (for review see [30]).
In addition to the four main cell types, other cellular and acellular components influence the niche (Fig. 1). Blood vessels immediately subjacent to the SVZ run parallel to the direction of tangential neuroblast migration [31–33], and guide migratory neuroblasts via BDNF signaling [33]. Endothelial cells secretions also influence SVZ proliferation [15, 16, 34] and have been shown to home NSCs within the niche via SDF1/CXCR4 signaling [17]. In addition, a highly penetrative extracellular matrix (basal lamina) extends from the subjacent blood vessels to the basolateral surface of ependyma, forming intricate ‘fractones’ throughout the SVZ [35]. This basal lamina network has been shown to contact almost every cell in the SVZ making it an ideal conduit for the transport and sequestration of key signaling molecules that regulate the niche [35, 36].
During aging, many characteristics of the SVZ niche change (Fig. 1). One prominent feature associated with the aged SVZ niche of mice is ventral stenosis of the walls of the lateral ventricle, resulting in deterioration of most of the ventral SVZ [32]. This ‘zippering up’ of the ventral lateral ventricle walls thereby restricts the neurogenic region to only the dorsolateral region of the lateral ventricle [32]. As a consequence, or due to other age-related changes, the remaining ventricular cavity becomes enlarged; resulting in a thinning of the existing ependyma monolayer, and the need to maintain the integrity of the ventricle barrier. Quite surprisingly, we detected a large number of astrocytes incorporated within the ependyma of aged mice [32]. These incorporated astrocytes are cuboidal in shape, similar to the shape of normal ependymal cells. In addition, we observed that some integrated astrocytes had characteristics of ependymal cells, including multiple basal bodies of motile cilia (9+2 organization), expression of S100β and apical placement of mitochondria [32, 37]. We subsequently showed that the incorporated astrocytes originated from mitotically active astrocytes of the SVZ and over time took on characteristics of ependymal cells [37]. This phenomenon of what appears to be SVZ astrocyte-mediated repair to the ependyma could be reproduced in young adult mice when individual ependymal cells were released from the ventricle surface [37]. Together, these studies indicate the importance of maintaining the integrity of the ependymal boundary and the role the underlying SVZ has in this reparative function.
Compared to the rodent, the human SVZ has several unique characteristics. Work from several laboratories described distinct layers of the adult human SVZ [38–40]. Layer I consists of an ependymal monolayer lining the ventricular wall with some astrocytic processes contacting the ventricular wall [40–42]; however, the extent of this occurrence is not clear. Layer II, also known as the gap region, is unique in that it is rich in GFAP+ processes, with only some neuroblasts in the anterior regions [39, 40, 42]. Ependymal cells send basal processes into Layer II, perhaps making critical contacts with underlying basal lamina, as found in rodent. It is thought that Layer II may function as the corridor for neuroblast migration. However, only a modest RMS with limited numbers of neuroblasts extends to the olfactory bulb in humans [38–40, 42]. Layer III is the proliferative region of the human SVZ, with GFAP+/Ki67+ and CD133+ cells present (astrocytic ribbon) [39, 42]. Overall few neuroblasts are present in the human SVZ, compared to the rodent, and these are found mainly in Layer III. Surprisingly, some ependymal cells, which typically comprise the epithelial barrier of the ventricles, were reported found in small clusters (4–14 cells) in Layer III [40]. These cells had multiple motile cilia and were clustered together with their cilia at the center of the cluster [40]. It is unknown whether these structures provide any functional support to the region or are aberrant clusters (ependymomas).
The functional organization and significance of the human SVZ requires further investigation. Most reports have been from elderly postmortem tissue obtained hours after death and fixed for long periods of time (days). Characterization is often from pooled samples, combining young adult and elderly tissue in an effort to increase the sample size. Since age contributes to changes in the SVZ and its functions, data need to be separated for proper evaluation of age-related differences. Clearly the techniques for acquiring/processing human tissue need to be refined for better preservation of ultrastructure. Of potential benefit, some laboratories are now collecting intraoperative tissue whenever possible, instead of relying on postmortem tissue. Ultimately, the presence of the SVZ and RMS in human anterior brain tissue suggests its relevance and potential contribution to some aspect of normal brain function. Even if neurogenesis of olfactory bulb interneurons is not the main output/purpose of the human system, neural progenitors in the human SVZ likely contribute to other replacement/reparative functions.
Age-Related Changes in SVZ Neurogenesis
Multiple reports, using label-retention strategies and immunohistochemistry, demonstrate an age-related decline in neurogenesis of about 50% in elderly mice compared to young adult mice [8, 32, 43, 44]. The consequence of reduced neurogenesis can be observed in the OB where fewer newly generated interneurons are found in aged mice [8]. This decline is associated with a functional change in odor discrimination. In studies using odor cues in a food aversion experiment, aged mice could detect two discrete odors (similar to young adult mice), but could not discriminate between two very similar odors (fine odor detection), unlike their young counterparts [8].
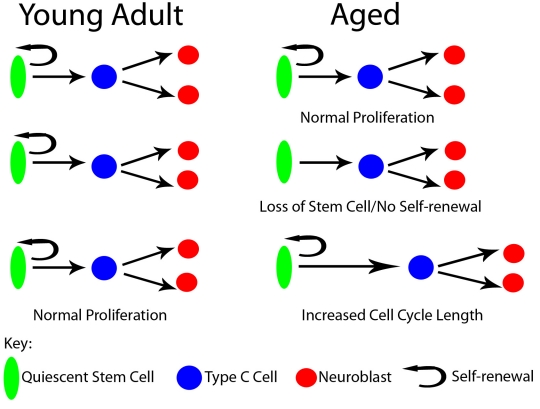
While an age-related decline in SVZ neurogenesis is observed and linked to functional declines in olfaction, the reasons for these declines are not clearly defined. Aging cells have been shown to accumulate DNA damage from numerous insults, affecting proliferation (see [45] for review, Fig. 2). In such events the cell cycle may be lengthened to accommodate DNA repair, resulting in slower niche output. When one group administered a single dose of two different thymidine analogues 12.5 hours apart, almost a 50% reduction of cells incorporating both analogues was observed in the aged SVZ compared to young adult SVZ [44]. However, cell cycle length was not fully examined to determine if altering the interval between analogues in aged mice would generate a different percentage of cells incorporating both analogues, thereby verifying a lengthening of the cell cycle. In addition to a potential lengthening of the cell cycle, it is possible that proliferating cells exit the cell cycle completely and stop dividing altogether, a process leading to cellular senescence. By combining Ki67 immunostaining and BrdU label retention, we were able show that by mid-age (1 year old) there was a 1.5 fold increase in the number of progenitors that exited the cell cycle [32]. Ahlenius et al. [43] used a senescence cell staining kit to observe a two-fold increase in senescent cells within neurospheres generated from elderly SVZ tissue. These data indicate significant alterations in cycling progenitor cells can lead to a decline in the proliferative output of the aged SVZ. However, it is important to note that all previous studies have been performed on global cell populations and lack cell-specific markers to determine which cells are being influenced. To date, the age-related decrease in neurogenesis was shown to result from a decrease in the proliferative fraction of multiple SVZ cell types-proliferating NSCs decreased 38% and proliferating neuroblasts decreased by 58% [43]. Future studies are needed to determine the mechanism in which each cell type is altered, since different processes of aging may differentially affect each cell population.
Figure 2.
Proliferation in the young, adult SVZ via the stem cell generates another quiescent stem cell and neural progeny through a transitory amplifying cell. In the aged SVZ proliferation is reduced due to loss of stem cell numbers, inability to self-renew or increases in cell cycle length.
Cells that remain proliferative require telomerase activity to maintain telomere length, preventing replicative cellular senescence [46–49]. Interestingly, mTERT (the enzyme responsible for elongating telomeres) expression and telomerase activity are reduced in an age-dependant manner in the SVZ [50, 51]. Telomerase deficient mice have decreased levels of SVZ neurogenesis [51, 52]; however, the fraction of proliferative neuroblasts did not change, suggesting that NSCs are affected by decreased telomerase activity but the proliferative capacity of their neural progeny is not.
Researchers are beginning to connect the underlying pathways activated by DNA damage and leading to decreased proliferation. Upon sensing DNA damage, cyclin dependent kinase (Cdk) inhibitors postpone cell cycle re-entry. Expression of two Cdk inhibitors, p16INK4a and p21KIP1 [53, 54], are increased in the SVZ in an age-dependant manner [43, 55]. Knocking out p16INK4a increases neurogenesis in aged mice; however, the increase in neurogenesis does not reach the same level found in young adult mice. Also, since upregulating p16INK4a in young adult mice does not significantly decrease proliferation [55], other factors likely contribute to the age-related decline.
If damage to the cell becomes too great to maintain its stem cell identity, NSCs may exit the cell Cycle or undergo cell death, foregoing self-renewal and effectively diminishing the stem cell pool (see Fig. 2). The neurosphere assay, in which high concentrations of growth factors activate otherwise quiescent NSCs, has been used frequently to evaluate relative numbers of NSCs. Based on this assay, a loss of NSCs throughout aging would result in declines in the number of neurospheres generated. Multiple laboratories have consistently shown that elderly tissue generates 22–78% fewer neurospheres [8, 43, 55, 56]. However, in one study where cells were FACS sorted for nestin-GFP prior to neurosphere generation, an identical number of neurospheres were generated from young adult and elderly SVZ tissue [43]. This suggests that while there are fewer NSCs in aged mice, the remaining NSCs have a similar capacity for neurosphere generation as young adult NSCs.
While in vitro analysis of NSCs can be informative, in vivo quantification would allow for analysis of changes in the NSC pool within the context of the aged niche. The use of NSC molecular markers and label-retention assays support a decrease in the NSC pool and additional studies have revealed a 50% decrease in the NSC markers Nestin and Sox2 [43]. Yet these methods are imprecise, as most markers are also expressed by non-NSCs in the SVZ and label-retention analysis could also be explained by changes in cell cycling, as previously described. As an alternative means to examine the NSC pool, our lab used whole mount preparations of the lateral ventricle wall to quantify the total number of astrocyte processes contacting the ventricle throughout aging, working on the assumption that this population of astrocytes is the NSC [14, 17, 23]. In mice aged from 3 months to 2 years, we observed a consistent decline in the number of astrocytic processes (NSCs) contacting the ventricle (Fig. 3, BAS & JCC, manuscript in prep), supporting an age-associated loss of NSCs. Key to understanding the loss to the NSC pool is the need to address what is happening to these cells. Cell attrition can be from cell death or the inability of the NSC to maintain self-renewal capability with each division cycle (see Fig. 2). As previously described, ependymal repair increases with aging. What is not known is whether this form of repair requires the NSC to symmetrically divide to generate two ependyma repair cells, thereby effectively depleting the NSC pool. Similarly, do other types of injury or disease also deplete the NSC pool? Alternatively, it is possible that an NSC is only capable of a certain numbers of divisions. All of these questions require answers before we can fully understand the reason for the demise of the SVZ niche with aging.
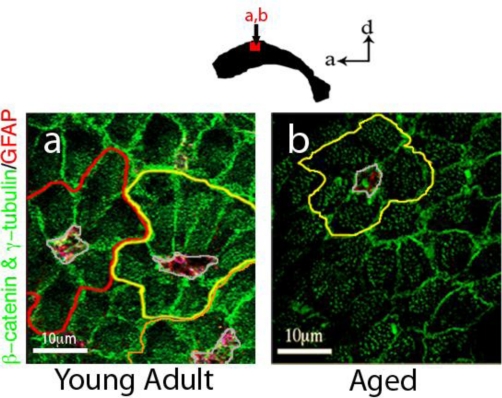
Figure 3.
Apical processes of SVZ astrocytes that contact the ventricle decline with age. a, In young adult (3-month old) mice, many pinwheels (outlined in red, yellow and orange) and their core of astrocytic processes (outlined in grey) can be detected. b, In elderly (2-year old) mice, there are significantly fewer intact pinwheel structures (only one is found in this section). In addition, only one or two astrocytic processes are found at the core, versus 3–5 in young mice. Images were taken using a Leica TCS SP2 confocal microscope. β-catenin defines cell boundaries of astrocytes and ependymal cells, γ-tubulin labels basal bodies (multiple found in ependymal cells and a single basal body of the primary cilium found in astrocytes) and GFAP marks astrocytes.
SVZ’s Capacity for Repair in Injury and Disease
The SVZ has been shown to change from its steady state production of new olfactory bulb neurons to heightened proliferation when injury/disease occurs in neighboring brain tissue. Changes typically involve newly generated cells migrating away from their prescribed RMS pathway to sites associated with the injury or disease. Here, we will discuss investigations into the impact several injuries, typically associated with aging (stroke, Parkinson’s disease, demyelination and ependymal damage), have on SVZ functions and the extent of invoked repair. Other reviews cover the role of transplanted stem cells in neural degenerative diseases, so this topic will not be covered here.
Stroke
Stroke is caused by blockage of a cerebral artery. This results in focal ischemia and the loss of neurons and glial cells, often causing sensory or cognitive impairments. Data from many groups now show that ischemic or hypoxic conditions increase neurogenesis in the SVZ and redirect newly generated neuroblasts to sites of injury in the striatum [57–64]. Early studies using rat models of focal stroke generated by transient middle cerebral artery occlusion showed significant increases in SVZ neurogenesis and migration of chains of neuroblasts to the peri-infarct striatum [57, 58, 63, 64]. In several reports, differentiation to medium-sized spiny neurons was observed, but survival of newly generated neurons in the peri-infarct region was extremely low [57, 63]. Arvidsson et al. reported only a 0.2% replacement of lost neurons by newly generated SVZ neurons 6 weeks post-infarct [57].
Similar findings have been reported in humans. While the cytoarchitectural arrangement of the human SVZ is different from the rodent [38, 40, 42] (see above Comparative Cytoarchitecture of Young versus Aging SVZ Neural Stem Cell Niche), the cell types are essentially similar and as in the rodent model there is an increase in proliferating cells within the SVZ following stroke. One group identified the proliferating SVZ cells as astrocytes and reported an increase in Ki67+ cells in the astrocytic ribbon layer [62]. An associated increase in the width of the gap layer was also seen, with increased numbers of neuroblasts within this region. This finding would appear to suggest that the gap layer functions in the transport of newly generated neuroblasts [62]. However, the number of neuroblasts found in the penumbra of the lesion varied from rare to very low numbers [58, 59, 61, 62], indicating that additional support and an increased supply of new cells to the injured area is required for significant and substantial repair and replacement following stroke. Since cell damage and loss from stroke is well documented and pre-stroke conditions are not restored following a stroke, interventions that take advantage of enhancing trophic and neuroprotective support to the existing SVZ or the use of transplanted cells to provide cell replacement and/or molecular support are clearly needed to bypass the current limitations of the brain’s reparative functions.
New studies investigating neuroprotective measures capable of reducing neuronal destruction following stroke in aged humans show G-CSF, a cytokine, acts to reduce infarct size and improve functional outcome after experimental stroke in young rats [65, 66]. Recently, similar studies were performed in aged rats [67] and increases in animal survival and transient functional improvements (motor: running inclined plane; cognitive: radial-arm maze) were detected. While increased numbers of BrdU+ cells were detected in the SVZ, no increase in new neurons in the peri-infracted area was detected, nor was a reduction in infarct size detected. Additional studies are required to determine how G-CSF acts to promote motor and cognitive improvements and survival. Further examination of the role of SVZ proliferation and neurogenesis in stroke or under hypoxic conditions can be found in several recent reviews [68–70].
Parkinson’s Disease
Dopamine (DA), a neurotransmitter that controls many diverse functions within the adult brain such as movement, reward and cognition, has also been implicated in regulating SVZ proliferation in the adult brain. The majority of DA neurons reside in either the Substantia Nigra pars compacta (SNpc) where they project to the dorsal striatum and control voluntary movements – the population primarily lost in Parkinson’s disease – or the ventral tegmental area (VTA), known to innervate the nucleus accumbens and contribute to mood, emotion and addiction. DA neurons from both the SNpc and VTA have been reported to target the region around the SVZ [71]. In addition, neuronal progenitors in the adult rodent SVZ express DA receptors and dopaminergic afferents form synapse-like structures with SVZ progenitor cells [72]. Specifically, Type C cells were found to express primarily D2-like (D2, D3, and D4) dopamine receptors [72], while migratory neuroblasts expressed receptors from both D1-like (D1 and D5) and D2-like families. These findings remind us of dopamine’s role in embryonic neurogenesis, where the nigrostriatal pathway supplies DA to the lateral ganglionic eminence (LGE) beginning at E13 and DA levels remain high in the developing neostriatum and LGE from E13 onwards – throughout the period of neostriatal neurogenesis. Both D1-like and D2-like DA receptors were found on progenitor cells of the LGE. D1-like receptor activation reduced G1/S-phase entry, whereas D2-like receptors promoted G1/S-phase entry [73]. It therefore seems reasonable to hypothesize that the regulatory roles of the major DA receptor families in the adult SVZ would parallel those observed in the embryonic LGE [73, 74]. To assess the role of DA on SVZ functions, DA neuron ablation studies have been performed. Loss of nigrostriatal dopaminergic innervation following administration of the dopaminergic neurotoxins 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, targets SNpc A9 DA neurons) [72] or 6- hydroxydopamine (6-OHDA, lesions to both SNpc and VTA DA neurons at the ventral midbrain bundle) [75, 76] resulted in decreased proliferation in the rodent SVZ, a finding that was also replicated in non-human primates [77]. SVZ proliferation was then restored with select agonists of D2-like receptors [72, 75, 77, 78]. Limited studies in humans who have suffered from Parkinson’s disease also revealed a 30% decrease in dividing cells of the SVZ and a reduction in nestin+ cells in the olfactory bulbs [72, 79]. One mechanism of action put forward is DA-mediated release of EGF in the SVZ [79]. EGFR+ cells have been detected in the human SVZ and DA was found to stimulate EGF release via the PKC pathway resulting in selective expansion of EGFR+ progenitor cells. EGFR+ cells were significantly decreased in postmortem Parkinson’s disease brains compared to age-matched controls [79]. These findings support an indirect role for DA in EGF release that then influences SVZ neurogenesis and suggest a conserved pathway across all mammalian species.
New genetic mouse models of Parkinson’s disease may provide more appropriate models of Parkinson’s disease and how loss of DA affects SVZ functions. Current chemical models result in acute lesioning of DA neurons and rapid loss of DA, this contrasts the rather gradual loss of DA supply experienced in Parkinson’s disease. While olfactory dysfunction is observed in PD patients, those patients that also experienced cognitive impairments (PD with dementia) showed an increased loss of proliferative cells in the dentate gyrus of the hippocampus, indicating that both neurogenic niches are affected. Since, as pointed out above (Comparative Cytoarchitecture of Young versus Aged SVZ Neural Stem Cell Niche), the role of human SVZ neurogenesis in olfaction appears to be much less robust than in rodents, the loss of dopaminergic innervation and consequential loss of DA supply to the SVZ may result in distinct symptoms in humans versus rodents. Further studies are needed to evaluate the consequence of decreased neurogenesis in Parkinsonian patients.
Demyelinating Lesions
While the primary role of the SVZ is to produce neuroblasts, the adult SVZ has also been found to generate both non-myelinating NG2+ oligodendrocyte progenitor cells (OPCs) and mature myelinating oligodendrocytes as part of its normal function. However, the number of oligodendrocytes produced is relatively small. Following a demyelinating lesion (lysolecithin-induced) in the corpus callosum, oligodendrogenesis is significantly upregulated (up to a 4-fold increase) as progenitor cells from the SVZ now contribute new OPCs or mature oligodendrocytes to the lesion [80–82]. This capacity to contribute to remyelinating repair has not been fully evaluated and it is not clear whether the generation of new oligodendrocytes results in functional remyelination (see [83]). To help explain the lineage change in SVZ NSC-generated progeny, a new report revealed that demyelination via lysolecithin injection upregulated chordin in the SVZ and promoted a glial fate in DCX-positive progenitors that then target the lesion site in the corpus callosum [84]. Minipump delivery of chordin significantly increased the percentage of progenitors expressing NG2 and Olig2, and the number of CC1 and CNP-expressing mature oligodendrocytes, compared to saline infusions [84]. Thus, chordin may enable the degree of lineage plasticity necessary to generate additional oligodendrocytes following demyelinating injury or disease.
It is perhaps noteworthy that age-related myelin breakdown and the increased vulnerability of oligodendrocytes with age have been proposed as part of a developmental model for cognitive decline and Alzheimer’s disease [85]. However, it seems unlikely that SVZ supplied OPCs would be either sufficient or capable of the level of myelination repair that would be required in the aged and elderly brain. Clearly some intervention to enhance this potential reparative function is required.
Ependymal cell replacement
In the aged SVZ, we have reported that dividing SVZ astrocytes integrate within the ependymal cell monolayer and take on antigenic and morphologic characteristics of ependymal cells [32, 37]. BrdU+ astrocytes fully integrated within the ependyma exhibit adherens junctions with neighboring ependymal cells and over time show combined characteristics of astrocytes (GFAP+) and ependymal cells (GFAP+/s100β+, multiple basal bodies of cilia, apical mitochondria). Insertion of astrocytes into the ependyma appears to be an age-associated form of regenerative repair likely to be triggered by wear-and-tear to the ependymal surface and/or expansion of the lateral ventricle due to age-related hydrocephalus. A similar integration of dividing astrocytes within the lateral ventricle wall is not seen in young, 3 month old mice. However, injection of neuraminidase into the lateral ventricle to cleave sialic acid of sialoglycoproteins and thereby release ependymal cells from the ventricle lining allowed us to demonstrate that ependymal cell replacement by dividing astrocytes occurs on a ‘per need’ basis [37].
In the aged brain the role of ependymogenesis appears to be maintenance of the ciliated epithelial lining, which separates the CSF from the SVZ. It is important to note that maintenance of this border will only occur above an active SVZ. We did not observe any BrdU+ astrocytes within the ependyma of the medial wall of the lateral ventricle, the 3rd ventricle or the cerebral aquaduct [37]. SVZ astrocyte-mediated generation of ependymal-like cells reminds us of the origin of ependymal cells from radial glia origin of ependymal cells in late embryonic/early postnatal development [86]. Therefore, it is in keeping with the radial glial origin of adult neural stem cells, the SVZ astrocytes, that they too can generate new ependymal cells in the adult brain. Instead of ependymal cell replacement, we found that severe damage to the ependyma, as observed when high doses of neuraminidase are injected into the ventricle, resulted in a glial scar at the ventricle surface. Thus, following either modest loss or severe loss of ependymal cells, it is important to stress the need to maintain a barrier to the CSF. In the case of modest loss, ependymal-like cells are generated for replacement, while in the case of severe loss an immediate repair must occur, resulting in a glial scar. It is unclear whether the newly generated ependymal cells are fully functional; however, we have found that many do possess basal bodies of multiple cilia.
It is now well established that mature ependymal cells do not reenter the cell cycle; no dividing ependymal cells have been found in either young adult or elderly mice [12, 86–89]. Notch signaling is reported to be required for maintaining ependymal cells in a state of quiescence and it has been reported that inhibition of the Notch pathway is sufficient to allow ependymal cells to reenter the cell cycle resulting in the production of primarily new olfactory bulb neurons [90]. Interestingly, following MCAO-induced stroke some ependymal cells were found to enter the cell cycle presumably via Notch inactivation. In this case primarily astrocytes, and some neuroblasts that died before differentiating into mature neurons, were generated [90]. It is important to note, especially in light of the above-mentioned importance of an ependymal cell boundary, that although forebrain ependymal cells generated both neurons and glial cells, they failed to self-renew. This not only distinguishes them from stem cells, but also indicates that in generating astrocytes and some neurons following stroke-associated injury, ependymal cells relinquish their role in providing an important boundary.
Summary
In all of the above disease/injury models it is critical to determine whether the SVZ niche response is a result of the pathology itself or is a reparative function initiated to resolve or compensate for a particular damage. Since injury and disease are often associated with inflammation and angiogenesis, it is important to consider whether these processes are just presenting the proliferation and migration factors that entice the germinal stem cell niche to produce more progenitors capable of responding to migration signals. It is still unclear whether the adult SVZ stem cell niche is truly capable of providing real and significant compensation in the event of injury/disease or whether during the process of aging multiple injuries may activate ‘repair’ mechanisms, but with the effect ultimately to diminish the stem cell niche’s functional capacity. Importantly, many of these ‘repair’ mechanisms do not lead to functional recovery of the injured tissue. Thus, it is critical to ask whether these brain injuries are in part responsible for the decline in SVZ neurogenesis seen in aging. It is, however, likely that modulation via molecular intervention may provide some benefit, but functional restoration will have to be verified. While new studies are likely to provide the way forward, we must also come to a better understanding of the molecular mechanisms that sustain the adult neural stem cell niche and its raison d’être.
Molecular Mechanisms Regulating the SVZ Niche
As we discussed in Age-Related Changes in SVZ Neurogenesis above, neurogenesis is significantly reduced in the aging brain. Whether this is due to loss of niche integrity, depletion of stem cell populations, induction of stem cell senescence or defects in cell-cell signaling mechanisms is still unclear. Most likely it is a combination of all of these factors that contribute to the demise of the aging adult SVZ niche. While the roles of various growth factors and neurotransmitters in supporting the young adult SVZ have been extensively reviewed elsewhere [7, 91–94], examination of changes in key molecular signaling pathways and their effects on the aging SVZ have been minimal. Currently, several molecular pathways stand out as potential regulators of stem cell quiescence and neurogenesis. Here, we will discuss those that are the most noteworthy in the regulating the SVZ stem cell niche and that may be compromised in the aged brain.
Notch activity is fundamental in maintaining embryonic NSCs in an undifferentiated state by suppressing proneural gene expression and supporting progenitor survival [95–98]. The canonical pathway, in which Notch targets Hes gene activation, is critical for embryonic NSCs [99] and inhibition of intracellular signaling of all Notch receptors (via inactivation of Rbpj) leads to a decrease in cell proliferation, as actively dividing neural stem cells prematurely differentiate into postmitotic neurons. In the adult SVZ, Imayoshi et al. found that inhibition of intracellular signaling by all Notch receptors resulted in transient increases in transit-amplifying type C proliferation and subsequent increases in neurogenesis. However, as a consequence, this ultimately resulted in depletion of all neural stem cells and neurogenesis was eventually lost. These results suggest that Notch signaling inhibits transition from slowly dividing NSCs to type C cells and is necessary to maintain the adult stem cell niche. In the aged brain, the use of Hes5::GFP reporter mice revealed that signaling levels in the dentate gyrus did not change with aging, indicating that Notch activity was still present and defined the quiescent NSCs population [100]. Therefore, the age-related deficits in neurogenesis observed in the aging SGZ are likely due to other signaling molecules. It is not currently known how Notch activity is affected in the elderly SVZ.
Maintenance of adult NSCs [101–105] and the migration of SVZ neuroblasts [102] have been attributed in part to the action of hedgehog (Hh). Through conditional removal of Smoothered (Smo), the effector of Hh signaling, it was shown that Hh signaling, while not required for telencephalic progenitor proliferation from E12.5 until birth, is required during the first two weeks of postnatal development, as removal results in the rapid depletion of neural progenitors [102, 104]. What is not clear from these studies is whether Hh signaling is required for the initial establishment of the postnatal stem cell niche or for its long-term maintenance during adulthood. Balordi et al [101], using tamoxifen-inducible Cre (NestinCreERT2) lines to remove Smo function in the adult niche, found an impaired ability for SVZ astrocytes to replenish the SVZ niche following antimitotic treatment to kill rapidly dividing Type C cells and neuroblasts. Hh signaling is via the primary cilia and requires Kif3a, a subunit necessary for intraflagellar transport and primary cilia assembly [106]. Interestingly, while it was found that Hh signaling is particularly important for the expansion and establishment of germinal centers away from the ventricular zone (e.g., the SGZ), removal of Kif3a in the SVZ resulted in reduced proliferation, but not as dramatic as that found in the SGZ.
Intracerebroventricular infusion of EGF induced dramatic proliferation and migration of SVZ progenitors through epidermal growth factor receptor (EGFR) signaling [107–111]. When SVZ NSCs were specifically tracked it was found that they invaded most neighboring brain regions, e.g., striatum, septum, corpus callosum and fimbria-fornix. The cells generated were mainly NG2+ progenitors and premyelinating and myelinating oligodendrocytes [110]. A demyelinating lesion further increased the number SVZ progenitors that migrated and differentiated into oligodendrocytes [109, 110, 112–114]. Infusion of TGF-α (an EGFR ligand) also resulted in a dramatic enlargement of the SVZ, but resulted in little migration. When TFG- α was infused into a dopamine-depleted striatum large numbers of SVZ progenitors migrated into the striatum [115]; however, these progenitors did not differentiate into dopaminergic neurons.
Multiple members of the EGF family, including TGF-a and amphiregulin, are produced by the choroids plexus and would be capable of modulating EGF receptor (EGFR) signaling [109, 116, 117]. Not surprisingly there are lower levels of EGFR in aged brain [8], most likely due to a decrease in Type C cells, and decreased signaling through EGFR has been detected in elderly mice [8, 44]. Intracerebro-ventricular infusion of heparin binding EGF (HB-EGF) (and also FGF-2) into the aged (23–25 month) mouse brain has been reported to restore SVZ proliferation to levels found in the young adult [118] suggesting the NSCs in the elderly brain are not receiving adequate signals from the niche to maintain proliferation and neurogenesis. However, the long-term consequence of over-stimulating the remaining NSCs in the elderly brain is not known. Does this act to further exhaust the aged NSC pool? Ultimately, both the ability to stimulate and revitalize the SVZ niche without compromising its future capacities has significant implications for regenerative medicine.
Future Directions for Aging Research
As we have pointed out above, it is not clear how NSCs are affected by the process of aging or conversely the extent to which the demise of the SVZ stem cell niche contributes to the aging of the brain. Ultimately, to address the needs of the aged NSC niche, an understanding of the key factors (molecular and cytoarchitectural) leading to its decline will provide the best strategies to help restore characteristics found in young niche. Many signaling pathways appear to be affected throughout the course of aging, but it is important to note that molecular mechanisms targeting the NSCs alone may not necessarily be enough to restore the aged niche. A functional niche would also need to support the production of future neural and non-neural progeny and offer the capacity for controlled migration of progeny away from the niche.
REFERENCES
- [1].Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- [7].Conover JC, Notti RQ. The neural stem cell niche. Cell Tissue Res. 2008;331:211–224. doi: 10.1007/s00441-007-0503-6. [DOI] [PubMed] [Google Scholar]
- [8].Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- [10].Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of hte subventricular germinal zone in the adult mammalian brain. J Neuroscience. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [12].Doetsch R, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- [13].Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- [19].Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- [21].Luskins MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- [22].Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- [23].Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5:515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boyle M, Wong C, Rocha M, Jones DL. Neural differentiation, NCAM-mediated adhesion, and gap junctional communication in neuroectoderm. A study in vitro. Cell Stem Cell. 2007;1:470–478. doi: 10.1083/jcb.106.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Keane RW, Mehta PP, Rose B, Honig LS, Loewenstein WR, Rutishauser U. J Cell Biol. 1988;106:1307–1319. doi: 10.1083/jcb.106.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- [27].Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, Macdonald HR, Trumpp A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- [29].Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- [31].Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr, Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. doi: 10.1002/cne.21090. [DOI] [PubMed] [Google Scholar]
- [32].Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- [33].Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- [35].Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- [36].Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- [37].Luo J, Shook BA, Daniels SB, Conover JC. Subventricular zone-mediated ependyma repair in the adult mammalian brain. J Neurosci. 2008;28:3804–3813. doi: 10.1523/JNEUROSCI.0224-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- [39].Kam M, Curtis MA, McGlashan SR, Connor B, Nannmark U, Faull RL. The cellular composition and morphological organization of the rostral migratory stream in the adult human brain. J Chem Neuroanat. 2009;37:196–205. doi: 10.1016/j.jchemneu.2008.12.009. [DOI] [PubMed] [Google Scholar]
- [40].Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- [41].Roelofs RF, Fischer DF, Houtman SH, Sluijs JA, Van Haren W, Van Leeuwen FW, Hol EM. Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. Glia. 2005;52:289–300. doi: 10.1002/glia.20243. [DOI] [PubMed] [Google Scholar]
- [42].Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- [43].Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-a null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jacobs JJ, de Lange T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol. 2004;14:2302–2308. doi: 10.1016/j.cub.2004.12.025. [DOI] [PubMed] [Google Scholar]
- [48].Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- [49].Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- [50].Caporaso GL, Lim DA, Alvarez-Buylla A, Chao MV. Telomerase activity in the subventricular zone of adult mice. Mol Cell Neurosci. 2003;23:693–702. doi: 10.1016/s1044-7431(03)00103-9. [DOI] [PubMed] [Google Scholar]
- [51].Ferron SR, Marques-Torrejon MA, Mira H, Flores I, Taylor K, Blasco MA, Farinas I. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J Neurosci. 2009;29:14394–14407. doi: 10.1523/JNEUROSCI.3836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ferron S, Mira H, Franco S, Cano-Jaimez M, Bellmunt E, Ramirez C, Farinas I, Blasco MA. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development. 2004;131:4059–4070. doi: 10.1242/dev.01215. [DOI] [PubMed] [Google Scholar]
- [53].Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- [54].Li X, Tang X, Jablonska B, Aguirre A, Gallo V, Luskin MB. p27(KIP1) regulates neurogenesis in the rostral migratory stream and olfactory bulb of the postnatal mouse. J Neurosci. 2009;29:2902–2914. doi: 10.1523/JNEUROSCI.4051-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- [58].Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- [61].Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, Martinez-Ramirez S, Jimenez-Xarrie E, Marin R, Marti-Vilalta JL, Garcia-Verdugo JM. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PubMed] [Google Scholar]
- [63].Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- [64].Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50:602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- [65].Minnerup J, Heidrich J, Wellmann J, Rogalewski A, Schneider A, Schabitz WR. Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia. Stroke. 2008;39:1855–1861. doi: 10.1161/STROKEAHA.107.506816. [DOI] [PubMed] [Google Scholar]
- [66].Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- [67].Popa-Wagner A, Stocker K, Balseanu AT, Rogalewski A, Diederich K, Minnerup J, Margaritescu C, Schabitz WR. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke. 2010;41:1027–1031. doi: 10.1161/STROKEAHA.109.575621. [DOI] [PubMed] [Google Scholar]
- [68].Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63:155–164. doi: 10.1016/j.neures.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [69].Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, Bresolin N, Comi GP, Corti S. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66:757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55:345–352. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [72].Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- [73].Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Popolo M, McCarthy DM, Bhide PG. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26:229–244. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- [76].Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, Kuhn G, Winkler J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol. 2006;197:113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- [77].Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91:1292–1301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- [79].O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- [82].Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB. Proc Natl Acad Sci U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- [84].Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- [86].Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [88].Bruni JE, Del Bigio MR, Clattenburg RE. Ependyma: normal and pathological. Brain Res. 1985;356:1–19. doi: 10.1016/0165-0173(85)90016-5. [DOI] [PubMed] [Google Scholar]
- [89].Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisen J. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- [91].Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- [92].Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- [93].Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- [94].Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- [95].Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- [96].Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- [97].Lutolf S, Radtke F, Aguet M, Suter U, Taylor V. Notch1 is required for neuronal and glial differentiation in the cerebellum. Development. 2002;129:373–385. doi: 10.1242/dev.129.2.373. [DOI] [PubMed] [Google Scholar]
- [98].Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- [99].Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006–1022. doi: 10.1111/j.1460-9568.2007.05370.x. [DOI] [PubMed] [Google Scholar]
- [100].Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- [101].Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J Neurosci. 2007;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- [104].Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- [105].Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- [107].Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- [109].Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. pidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- [113].Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25:11092–11106. doi: 10.1523/JNEUROSCI.2981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cantarella C, Cayre M, Magalon K, Durbec P. Intranasal HB-EGF administration favors adult SVZ cell mobilization to demyelinated lesions in mouse corpus callosum. Dev Neurobiol. 2008;68:223–236. doi: 10.1002/dneu.20588. [DOI] [PubMed] [Google Scholar]
- [115].Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson’s disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci. 2004;24:8924–8931. doi: 10.1523/JNEUROSCI.2344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Falk A, Frisen J. Amphiregulin is a mitogen for adult neural stem cells. J Neurosci Res. 2002;69:757–762. doi: 10.1002/jnr.10410. [DOI] [PubMed] [Google Scholar]
- [117].Kornblum HI, Hussain RJ, Bronstein JM, Gall CM, Lee DC, Seroogy KB. Prenatal ontogeny of the epidermal growth factor receptor and its ligand, transforming growth factor alpha, in the rat brain. J Comp Neurol. 1997;380:243–261. doi: 10.1002/(sici)1096-9861(19970407)380:2<243::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [118].Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]