Abstract
Our earlier findings established that cyclic AMP-dependent protein kinase functions in a signaling cascade that regulates mating and virulence of Cryptococcus neoformans var. grubii (serotype A). Mutants lacking the serotype A protein kinase A (PKA) catalytic subunit Pka1 are unable to mate, fail to produce melanin or capsule, and are avirulent in animal models, whereas mutants lacking the PKA regulatory subunit Pkr1 overproduce capsule and are hypervirulent. Because other mutations have been observed to confer different phenotypes in two diverged varieties of C. neoformans (grubii variety [serotype A] and neoformans variety [serotype D]), we analyzed the functions of the PKA genes in the serotype D neoformans variety. Surprisingly, the Pka1 catalytic subunit was not required for mating, haploid fruiting, or melanin or capsule production of serotype D strains. Here we identify a second PKA catalytic subunit gene, PKA2, that is present in both serotype A and D strains of C. neoformans. The divergent Pka2 catalytic subunit was found to regulate mating, haploid fruiting, and virulence factor production in serotype D strains. In contrast, Pka2 has no role in mating, melanin production, or capsule formation in serotype A strains. Our studies illustrate how different components of signaling pathways can be co-opted and functionally specialized during the evolution of related but distinct varieties or subspecies of a human fungal pathogen.
A highly conserved nutrient and G-protein-regulated cyclic AMP (cAMP) signaling cascade promotes mating and virulence in Cryptococcus neoformans (2, 22). The cAMP-dependent protein kinase (PKA) mediates most, if not all, of the physiological effects of the second messenger cAMP in fungi and other multicellular eukaryotes (reviewed in reference 60). In the inactive state, PKA exists as a holoenzyme comprised of two catalytic subunits and two regulatory subunits. Upon activation, the PKA catalytic subunits are released and phosphorylate target substrates that include metabolic enzymes and transcription factors (21, 22).
The cAMP-dependent protein kinases have undergone gene duplication and functional divergence during genome evolution. Multiple PKA isoforms are expressed in mammalian cells and have tissue-specific roles indicative of functional diversity (60). In Saccharomyces cerevisiae, the three PKA catalytic subunit isoforms Tpk1, Tpk2, and Tpk3 play redundant roles in yeast vegetative growth but have specialized roles in pseudohyphal filamentous growth. Tpk2 activates filamentation, whereas Tpk1 and Tpk3 repress filamentation (48, 51, 64). The plant fungal pathogen Ustilago maydis expresses two PKA catalytic subunit isoforms, Adr1 and Uka1 (24). Adr1 is a key regulator of filamentous growth, whereas Uka1 plays only a minor role. PKA also regulates morphogenesis and virulence in the plant pathogenic fungi Magnaporthe grisea and Cryphonectria parasitica and in the human pathogen Candida albicans (27, 43, 58).
The basidiomycete C. neoformans is an opportunistic human fungal pathogen that is the causative agent of cryptococcosis (reviewed in references 12 and 31). The prevalence of cryptococcal meningitis has increased worldwide as a result of human immunodeficiency virus infections, solid organ transplants, cytotoxic chemotherapy, and systemic corticosteroid use (42). C. neoformans serves as a model system for studies of fungal pathogenesis. Known virulence factors include an antiphagocytic capsule, the antioxidant melanin, the enzymes urease and phospholipase, and the ability to grow at 37°C (17, 18, 28, 36). C. neoformans has a defined sexual cycle in which compatible cells mate in response to peptide pheromones and nutritional signals to produce a dikaryotic mycelium, basidia, and basidiospores (33, 44, 55). α strains also differentiate by haploid fruiting involving filamentation and sporulation in response to nitrogen limitation, desiccation, and MFa pheromone (70, 71).
Based on the antigenicity of the capsular polysaccharide components, C. neoformans can be classified into four serotypes: A, B, C, and D (35). Serotype A strains are the most common clinical isolates in the world, representing 99% of isolates from AIDS patients (38). Although clinically less common, serotype D and AD hybrid strains are still important pathogens and can represent 10% or more of clinical isolates in some regions of the world such as Europe (6, 34).
Population genetic studies have shown that the A and D serotypes diverged ∼18 million years ago (25, 72). Serotype A strains are designated C. neoformans var. grubii, and serotype D strains are designated C. neoformans var. neoformans (26). This distinction into two different varieties or sibling species is further supported by molecular genetic studies. The Ste12α transcription factor is required for virulence in serotype D but not in serotype A (14, 15, 73). Conversely, the mitogen-activated protein kinase component Ste20α contributes to full virulence in serotype A but is dispensable for virulence in the congenic serotype D strains (69). Recent studies reveal that although serotype A and D strains can mate, unusual serotype AD diploid hybrids are produced that sporulate to produce largely inviable spores (39). Taken together, these findings reveal significant differences between serotypes A and D and suggest that these represent at least two varieties and possibly even distinct species.
It was previously demonstrated that cAMP-dependent protein kinase controls mating and virulence of C. neoformans serotype A strains (22). Here, we report the identification of a second PKA catalytic subunit (Pka2) and have taken a comparative genetics approach to elucidate the functions of the PKA catalytic subunits Pka1, Pka2, and the regulatory subunit Pkr1 in serotypes A and D. We demonstrate that Pka2 regulates mating and expression of virulence factors in serotype D, whereas Pka1 plays this role in serotype A. Additionally, we find that PKA is a key regulator of virulence in serotype A but not in serotype D, despite the essential role of PKA in virulence factor production in serotype D. These findings reveal that functional specialization of cAMP-dependent protein kinase catalytic subunits has occurred during the evolution of C. neoformans into the serotype A and D varieties such that the functions of the Pka1 and Pka2 subunits have been interchanged.
MATERIALS AND METHODS
C. neoformans strains and media.
All strains used in this study are listed in Table 1. Uracil auxotrophic strains were isolated by selecting spontaneous mutants resistant to 5-fluoroorotic acid as described previously (37). C. neoformans strains were grown on standard S. cerevisiae media (56). The selective medium for biolistic transformation (66), Niger seed medium (2), low-iron medium (LIM) (68), filament agar (FA) (71), V8 medium (2), and modified Eagle's medium (MEM) (28) were prepared as previously described. For cAMP rescue experiments, cAMP was added at a concentration of 10 mM to media. The pka1 mutant strains CDC36 (MATa pka1::ADE2 ade2) and CDC40 (MATα pka1::ADE2 ade2) were isolated as segregants from a cross of strains CDC25 (MATa pka1::ADE2 ura5 ade2) and JEC50 (MATα ade2). The pka2 serotype D mutant strains CDC99 (MATαpka2Δ::URA5 ura5) and CDC101 (MATa pka2Δ::URA5 ura5) strains were isolated from a cross of strains CDC89 (MATα pka2Δ::URA5 ura5 ade2) and JEC34 (MATa ura5). The pka1 pka2 double mutant serotype D strains CDC103 (MATα pka1::ADE2 pka2Δ::URA5) and CDC106 (MATa pka1::ADE2 pka2Δ::URA5) were constructed by crossing strains CDC89 and CDC25. The serotype D PKA2/pka2Δ diploid strain JKH15 was generated by mating strains CDC127 (MATα pka2Δ::URA5 ura5 ura3) and JEC34 (MATa PKA2 ura5 URA3) and selecting for diploids on minimal YNB medium (67% yeast nitrogen base without amino acids [Difco], 2% glucose, 20 g of Bacto agar [Difco]). The diploid or heterokaryon strains JKH16 and JKH20 were generated by mating JEC34 (MATa ura5 ADE2 LYS2) × JEC170 (MATα URA5 ade2 lys2) and CDC127 (MATα pka2Δ::URA5 ura5 ura3) × JKH19 (MATa pka2::ura5 ura5 URA3), respectively. The resulting prototrophic strains were selected on minimal YNB medium. The diploid or heterokaryon status of the strains was confirmed by using PCR primers specific for the MATa and MATα alleles of the STE20 gene, as described previously (39). The serotype D PKA2 reconstituted strain JKH21 was generated by biolistic transformation of strain JKH19 with plasmid pJH26 containing the wild-type serotype D PKA2 gene. Transformants were selected on synthetic medium lacking uracil and containing 1 M sorbitol. Genotype was confirmed both by Southern hybridization and expression analysis to confirm the presence of the wild-type transcript in the reconstituted strain.
TABLE 1.
Strains
| Strain | Genotype | Source or reference |
|---|---|---|
| Serotype D strains | ||
| JEC20 | MATa | 44 |
| JEC21 | MATα | 44 |
| JEC34 | MATaura5 | J. C. Edman |
| JEC43 | MATα ura5 | J. C. Edman |
| JEC50 | MATα ade2 | J. C. Edman |
| JEC155 | MATα ura5 ade2 | J. C. Edman |
| JEC156 | MATaura5 ade2 | J. C. Edman |
| JEC170 | MATα ade2 lys2 | J. C. Edman |
| BAC21 | MATα gpa1::ADE2 | A. Alspaugh |
| BAC37 | MATα gpa1::ADE2 | A. Alspaugh |
| CDC25 | MATapka1::ADE2 ura5 ade2 | This study |
| CDC36 | MATapka1::ADE2 ade2 | This study |
| CDC40 | MATα pka1::ADE2 ade2 | This study |
| CDC43 | MATα pka1::ADE2 ade2 | This study |
| CDC68 | MATα pkr1Δ::URA5 ura5 | This study |
| CDC85 | MATα pka2Δ::URA5 ura5 | This study |
| CDC89 | MATα pka2Δ::URA5 ura5 ade2 | This study |
| CDC99 | MATα pka2Δ::URA5 ura5 | This study |
| CDC101 | MATapka2Δ::URA5 ura5 | This study |
| CDC103 | MATα pka1::ADE2 pka2Δ::URA5 ura5 ade2 | This study |
| CDC106 | MATapka1::ADE2 pka2Δ::URA5 ura5 ade2 | This study |
| CDC127 | MATα pka2Δ::URA5 ura5 ura3 (FOAr) | This study |
| JKH15 | MATα pka2Δ::URA5 ura5 ura3/MATaPKA2 ura5 URA3 (diploid) | This study |
| JKH16 | MATα URA5 ade2 lys2/MATaura5 ADE2 LYS2 (diploid) | This study |
| JKH19 | MATapka2Δ::ura5 ura5 (FOAr) | This study |
| JKH20 | MATα pka2Δ::URA5 ura5 ura3/MATapka2Δ::ura5 ura5 URA3 (dikaryon) | This study |
| JKH21 | MATapka2Δ::URA5 ura5 PKA2 | This study |
| Serotype A strains | ||
| H99 | MATα | 50 |
| F99 | MATα ura5 (FOAr) | 69 |
| CDC1 | MATα pka1::ADE2 ade2 | 22 |
| JKH4 | MATα pka2Δ::URA5 ura5 | This study |
| CHM3 | MATα lac1Δ::NAT | This study |
Isolation of the C. neoformans PKA1, PKA2, and PKR1 genes.
The PKA1 gene from serotype D was isolated by PCR with JEC20 genomic DNA as the template and the serotype A primers JOHE3104 (AAAGGATCCTAATGTTCCAAAAGGTGTCCG) and JOHE3098 (AAAGGATCCCTAAAACTCCACGAAGAAATG). The 1.9-kb PCR product was digested with BamHI and inserted into plasmid pUC19 to yield plasmid pCD18. The PKA1 sequence has been deposited in the National Center for Biotechnology Information (NCBI) GenBank database. The serotype D PKA2 gene was isolated by using the U. maydis Uka1 polypeptide sequence (24) to perform a BLASTp search of the Stanford C. neoformans genome sequence database (October 2000 release, 2.8× coverage; R. Hyman and R. Davis) (http://www-sequence.stanford.edu/group/C.neoformans/index.html). This yielded a 680-bp sequence from a second PKA catalytic subunit gene in the C. neoformans genome. Primer pairs based on this sequence were used for inverse PCR to obtain flanking sequences. Primers JOHE6074 (CTTCGATCCGCTGTCAAA) and JOHE6075 (GCCTAGAGAGGAAGGAAA) were used in an inverse PCR with SacI-digested and religated genomic DNA from the serotype D strain JEC21 as the template. Primers JOHE6077 (GTGGATACCGCTGGAAAT) and JOHE6078 (ATCGCAATATGGAGGGTG) were used in an inverse PCR with NheI-digested and religated genomic DNA from the serotype D strain JEC21 as the template. PCR products were cloned and sequenced, revealing sequence similarity to known PKA catalytic subunits. Based on the sequence generated by the inverse PCR strategy, the PCR primers JOHE6195 (TCCTTCACATGTCGATCC) and JOHE6079 (CACCCTCCATATTGCGAT) were utilized in a PCR with genomic DNA from the serotype D strain JEC21 as the template. The resulting 1.6-kb PCR product was used to screen a size-selected library of ∼4-kb ClaI fragments of JEC21 genomic DNA cloned in pBluescript. One positive clone (pCD49) was identified and sequenced. The serotype D PKA2 gene was independently isolated from a JEC21 bacterial artificial chromosome (BAC) library. An ∼4-kb EcoRI fragment containing the PKA2 gene was isolated and cloned into plasmid pJAF7, a transformation vector containing the URA5 selectable marker, to yield plasmid pJH26.
The serotype D PKR1 gene from JEC21 was isolated by using primers JOHE3245 (TTCCTCCACAGCTCTACTC) and JOHE3180 (CTGTTGGAGAAAATCAGGG). The 2.5-kb PCR product was cloned in the vector pCRIITopo (Invitrogen) to yield plasmid pCD28. The PKR1 sequence has been deposited in the NCBI GenBank database.
The PKA2 gene from the serotype A strain H99 was isolated by PCR with genomic DNA from the serotype A strain, H99, and the serotype D PKA2 primers JOHE6905 (GCTGATCAAGGTACATCC) and JOHE6515 (GCCTTGTTGTTTGCGAAG). An ∼2.5-kb product was amplified and cloned into the pCR2.1 vector (Invitrogen) to yield plasmid pCD80. The PKA2 serotype A sequence has been deposited in the NCBI GenBank database.
Disruption of the PKA1, PKR1, and PKA2 genes.
To disrupt the PKA1 gene in serotype D, a 2.5-kb BamHI fragment containing the ADE2 gene from plasmid pRCD28 (59) was inserted into a unique BglII site within the 1.9-kb fragment bearing the PKA1 gene in plasmid pCD18 to yield plasmid pCD24. To increase the efficiency of homologous recombination, the termini of a 5-kb BamHI fragment containing the pka1::ADE2 allele were treated with terminal transferase and ddATP. This fragment was then used to transform the ade2 ura5 auxotrophic strain JEC156 to adenine prototrophy by biolistic transformation with previously described methods (65). Stable transformants were selected on synthetic medium lacking adenine and containing uracil and 1 M sorbitol. pka1::ADE2 mutant strains were identified by PCR, confirmed by Southern hybridization, and obtained at a frequency of 6% (3 of 47).
The serotype D PKR1 and PKA2 genes were disrupted by PCR overlap (19). To construct the pkr1Δ::URA5 allele, in the first round, the 5′ end of the PKR1 gene was amplified with primers JOHE3245 and JOHE5951 (GGTCGAGCAACTTCGCTCCGTATTTTCGTCCTCTTC), the 3′ end of the gene was amplified with primers JOHE5952 (CCACCTCCTGGAGGCAAGCTCGGGCACTCCTGTATT) and JOHE3180, and the URA5 gene was amplified with primers JOHE5950 (GAAGAGGACGAAAATACGGAGCGAAGTTGCTCGACC) andJOHE5953 (AATACAGGAGTGCCCGAGCTTGCCTCCAGGAGGTGG).Primers JOHE3245 and JOHE3180 were then used in an overlap amplification with the first three products as templates to yield the 4.5-kb PCR product bearing the pkr1Δ::URA5 allele. The pkr1Δ::URA5 allele deletes 11 bp of the PKR1 gene and the URA5 insertion truncates the Pkr1 protein, resulting in the loss of both cAMP binding sites. The PCR product was enzymatically treated with terminal transferase and ddATP to dideoxyadenylate the termini and then directly introduced into the MATα ura5 serotype D strain JEC43 by biolistic transformation (65). Stable transformants were selected on synthetic medium lacking uracil and containing 1 M sorbitol. A mutation in the PKR1 gene was obtained at a frequency of ∼1% (1 of 96) and was confirmed by PCR and Southern hybridization.
To construct the serotype D pka2Δ::URA5 allele, in the first round, the 5′ end of the PKA2 gene was amplified with primers JOHE6195 (ATGGAGGGTGTTGTCGAA) and JOHE6148 (GGTCGAGCAACTTCGCTCCAAGAACGATAGACGCGA), the 3′ end of the gene was amplified with primers JOHE6145 (CCACCTCCTGGAGGCAAGGCAAGACCGTACATTCAC) and JOHE6079 (CACCCTCCATATTGCGAT), and the URA5 gene was amplified with primers JOHE6144 (TCGCGTCTATCGTTCTTGGAGCGAAGTTGCTCGACC) and JOHE6146 (GTGAATGTACGGTCTTGCCTTGCCTCCAGGAGGTGG). Primers JOHE6195 and JOHE6079 were then used in an overlap amplification with the first three products as templates to yield the 3-kb pka2Δ::URA5 allele. The 114-bp deletion of PKA2 in the pka2Δ::URA5 allele results in truncation of the Pka2 protein with subsequent loss of the kinase active site motif. The ddATP-blocked PCR product was then directly introduced into the ura5 serotype D strain JEC155 by biolistic transformation. Stable transformants were selected on synthetic medium lacking uracil and containing 1 M sorbitol. Mutants with a null PKA2 gene were obtained at a frequency of 3% (3 of 102). This was confirmed by Southern hybridization and PCR with primers JOHE6143 (TCGCGTCTATCGTTCTTG) and JOHE6147 (GTGAATGTACGGTCTTGC). The pka1 pka2 double mutants were isolated from progeny of a cross between the pka1 mutant strain CDC25 and the pka2 mutant strain CDC89.
The serotype A PKA2 gene was identified by using the serotype D PKA2 sequence in a BLASTn search of the Duke Bioinformatics website (December 2001 release; http://cneo.genetics.duke.edu/menu.html). The pka2Δ::URA5 disruption allele was engineered by using the PCR overlap technique. The 5′ end was amplified by using primers JOHE7873 (CACCATCAATCATCAAGC) and JOHE7875 (GGTCGAGCAACTTCGCTCCAAGGACGATAGACGCGA). The 3′ end of the gene was amplified using primers JOHE6145 (see above) and JOHE7876 (TGGTATTGAGGAGAGGTG). The URA5 gene was amplified with primers JOHE6146 (see above) and JOHE7874 (TCGCGTCTATCGTCCTTGGAGCGAAGTTGCTCGACC). Primers JOHE7873 and JOHE7876 were then used in an overlap amplification with the first three products to yield a 3-kb pka2Δ::URA5 allele. The ddATP-blocked PCR product was then directly introduced into the ura5 serotype A strain H99 FOAr (F99) by biolistic transformation. Transformants were selected on synthetic medium lacking uracil and containing 1 M sorbitol. Mutants with a null PKA2 gene were obtained at a frequency of ∼8% (2 or 24). Deletion mutants were confirmed by Southern hybridization and PCR. Primers used for distinguishing wild-type and pka2Δ::URA5 serotype A alleles were JOHE8192 (GGACCAGATCAATGTCTATA) and JOHE8193 (TTGTGAGAGAACGATCTCTG).
Identification of mutant alleles.
Genomic DNA was isolated as described previously (50). The DNA was digested with restriction enzymes, subjected to agarose gel electrophoresis, and analyzed by Southern blotting (54). The radiolabeled probe was synthesized with the Prime-It II DNA labeling kit (Stratagene) and [32P]dCTP (Amersham). The template for the serotype D PKA1 probe was a 1.6-kb BamHI cDNA fragment of the PKA1 gene, and the template for the serotype D PKR1 gene was a 1.6-kb BamHI cDNA fragment. Low-stringency hybridization to determine the presence of additional PKA catalytic subunit genes was carried out by hybridization at 50°C overnight in Church buffer (U.S. Biochemicals) followed by three washes in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.2% sodium dodecyl sulfate at room temperature for 15 min. The template for the serotype A PKA2 probe used in identifying pka2Δ::URA5 deletion strains was an ∼2.5-kb EcoRI fragment from plasmid pCD80 (described above). DNA was probed by using a nonradioactive alkaline phosphatase kit (Amersham) according to the manufacturer's instructions.
Expression analysis.
Each strain was inoculated into 5 ml of yeast extract-peptone-dextrose medium and grown overnight at 30°C. Five milliliters of yeast extract-peptone-dextrose medium in 125-ml flasks was inoculated with 200 μl of the overnight cultures and grown at 250 rpm and 30°C for 5 h prior to harvesting. RNA was isolated from the harvested cultures with Trizol (Gibco-BRL) by following the manufacturer's instructions. Fifteen micrograms (as indicated by spectrophotometric measurement) of RNA was separated on a denaturing gel and transferred to a nylon membrane. The resulting blots were probed with a 548-bp PKA2-specific PCR product and a 647-bp PKA1-specific PCR fragment. We note that a small transcript was observed in the pka1 mutants of both serotype A and serotype D.
Fusion assays.
Cell fusion efficiency was measured by mixing 2 × 106 cells of strains JEC34 and JEC170, JKH19 and JKH170, and JKH19 and CDC127. A 5-μl drop of the cell suspension was inoculated onto V8 medium (pH 7.0) and allowed to incubate for 24 h at 25°C in the dark. Four plates were prepared for each of the three cell mixes. The cells were then resuspended in 1 ml of double-distilled H2O, and 200 μl of the suspension (∼105 total cells) was plated onto YNB medium. The number of colonies on each plate was determined after 3 days (1).
Microscopy.
All images of fusion, mating, haploid fruiting, and confrontation assays were captured with a Nikon Eclipse E400 microscope equipped with a Nikon CoolPix digital camera. Differential interference microscopy and fluorescent images were taken with a Zeiss Axioskop 2 Plus fluorescent microscope equipped with an AxioCam MRM digital camera.
To visualize nuclei, diploid cells were grown in 10 ml of YNB liquid medium overnight at 37°C. The cells were then pelleted, washed twice with phosphate-buffered saline (PBS), and incubated for 15 min in cold 70% ethyl alcohol to fix the cells. The cells were again washed twice with PBS and permeabilized in PBS containing 1% Triton X-100. Following two more PBS washes, the cells were resuspended in 10 μl of PBS. One microliter of this suspension was mixed with 1 μl of 4′,6′-diamidino-2-phenylindole (DAPI) mix (2 μg of DAPI/ml, 1 mg of antifade/ml, and 45% glycerol). The cells were then observed with a 40× objective. The percentage of dikaryotic cells in each diploid was determined by randomly examining 300 DAPI-stained cells from each diploid and then counting the number of those cells that were dikaryotic.
Virulence assays.
Groups of 10 DBA mice were intranasally infected with 5 × 104 yeast cells of the isogenic serotype D wild-type strain (JEC21) and the pka1 (CDC40), pka2 (CDC99), pka1 pka2 (CDC103), and pkr1 (CDC68) mutant strains. Mice were anesthetized by intraperitoneal administration of phenobarbital and suspended by the incisors on a silk thread, and 50 μl of inocula was slowly dispensed directly into the nares. The mice were fed ad libitum and monitored with twice-daily inspections. Mice that appeared moribund or in pain were sacrificed by CO2 inhalation. Statistical analysis was performed by the Kruskal-Wallis test for significance (P values of ≤0.05 were considered significant).
Capsule assays.
To document capsule and cell size, each strain, except for the serotype D diploid strains, was inoculated into 5 ml of MEM or LIM containing an iron chelator and grown at 30°C for 5 days. Twenty cells from each culture were chosen at random, and the cell and capsule sizes were measured by using Axiovision 3.0 software (Carl Zeiss Microscopy). The serotype D diploid strains (JKH15, JKH16, and JKH20), the serotype D PKA2 reconstituted strains (JKH21, JKH22, and JKH23), and the appropriate control strains were inoculated onto plates of MEM and grown at 30°C for 5 days. Aliquots of all cultures were stained with India ink and viewed with a 100× objective.
Laccase assays.
To quantitate melanin production in serotype A, 108 cells of the wild-type strain (H99) and the pka1 (CDC1), pka2 (JKH4), and lac1 (CHM3) mutant strains were grown in 25 ml of l-DOPA medium (0.1% l-asparagine, 0.1% glucose, 0.3% KH2PO4, 0.01% l-DOPA, 0.025% MgSO4 · 7H2O [pH 5.6]) at 30°C and 250 rpm for 16 h. The cultures were then incubated at 25°C and 250 rpm for 24 h, then 1 ml of culture was centrifuged, and the supernatant was spectrophotometrically read for optical density at a wavelength of 475 nm.
Nucleotide sequence accession number.
The serotype D PKA1, serotype D PKR1, serotype D PKA2, and serotype A PKA2 sequences have been deposited in the NCBI GenBank database and given accession no. AF481770, AF481771, AF481772, and AY144605, respectively.
RESULTS
Identification of a second cAMP-dependent protein kinase catalytic subunit in C. neoformans.
S. cerevisiae and U. maydis both express multiple PKA catalytic subunits that have overlapping, redundant, or opposing functions. We sought to establish whether C. neoformans also expresses multiple PKA subunits and, if so, what function each subunit performs. It was previously demonstrated that the Pka1 catalytic subunit controls virulence factor production and is necessary for pathogenesis (22). Here we identified a second PKA catalytic subunit, Pka2, and analyzed the functions of Pka1 and Pka2 in both serotypes A and D.
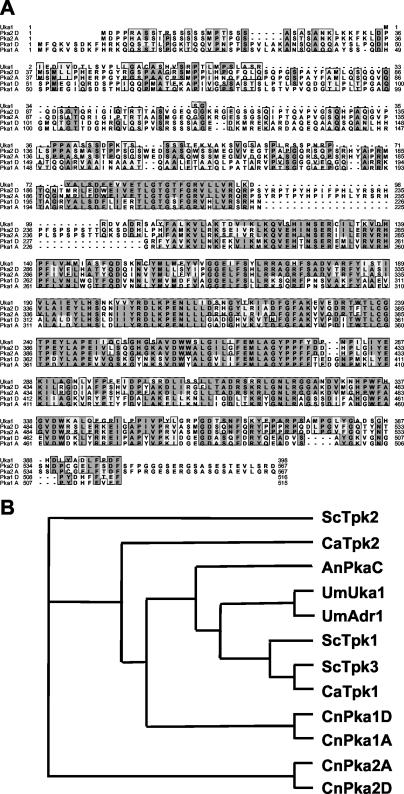
Sequences of both the PKA1 gene and a gene encoding a second PKA catalytic subunit homolog (PKA2) were identified in the Stanford C. neoformans genome sequence database (Hyman and Davis; http://www-sequence.stanford.edu/group/C.neoformans/index.html). The sequences for the PKA2 gene were found by a BLAST search of the database by using the cAMP-dependent protein kinase Uka1 protein sequence from U. maydis. The serotype A and D Pka1 and Pka2 protein sequences were aligned and compared to the Uka1 catalytic subunit (Fig. 1A). A phylogenetic tree (Fig. 1B) revealed that the Pka1 subunit was similar to the S. cerevisiae Tpk2 as well as the catalytic subunits from C. albicans, Aspergillus nidulans, and U. maydis, whereas the C. neoformans Pka2 subunit was much more distantly related to these other catalytic subunits. Inter- and intravarietal comparisons of the PKA catalytic subunits revealed that while the serotype A Pka1 and Pka2 proteins share 93% identity with their respective counterparts in serotype D, there is only 33 to 35% identity shared between the Pka1 and Pka2 subunits, either within or between varieties.
FIG. 1.
Two C. neoformans genes encode PKA catalytic subunits. (A) PKA catalytic subunits identified in C. neoformans were aligned with the Uka1 protein from the related basidiomycete fungal pathogen U. maydis with the CLUSTALW feature of MacVector (Oxford Molecular). Conserved residues are boxed, with identities shown in bold letters and darkly shaded boxes, and similarities are indicated by lightly shaded boxes. (B) The evolutionary relationships between the PKA catalytic subunits of C. neoformans, U. maydis, S. cerevisiae, A. nidulans, and C. albicans were compared by using the neighbor-joining function of the ClustalX program (62). A phylogenetic tree was then constructed with the TreeView program (46). The proteins are positioned on the tree according to sequence similarity. Evolutionary dissimilarity is proportional to the horizontal distance joining any two proteins on the tree.
cAMP-dependent protein kinase is not essential in C. neoformans.
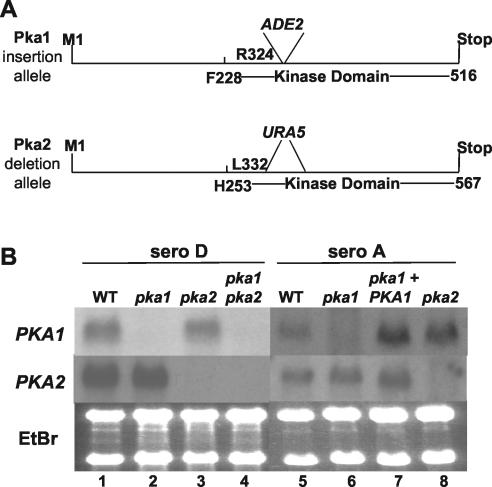
To determine the biological functions of PKA in serotype D, the PKA1 gene was disrupted by inserting the ADE2 gene into the kinase domain at the same site at which the serotype A PKA1 gene was previously disrupted. A large region of the kinase domain of the PKA2 gene was deleted and replaced with a URA5 cassette by PCR overlap (Fig. 2A). These mutations truncate the Pka1 and Pka2 proteins to remove most of the catalytic region to result in a complete loss of enzymatic activity. The pka1::ADE2 and pka2Δ::URA5 alleles were introduced into recipient strains MATa ura5 ade2 (JEC156) and MATα ura5 ade2 (JEC155), respectively, by biolistic transformation and selection of ADE+ or URA+ isolates. pka1 and pka2 mutants in which gene replacement without ectopic integration had occurred were identified by PCR and Southern blotting (data not shown). pka1 pka2 double mutants were readily isolated from a cross between compatible pka1 and pka2 mutant strains, and the pka1 and pka2 single mutants and the pka1 pka2 double mutant strains exhibited no overt growth defects. Northern blot analysis confirmed that both the PKA1 and PKA2 genes are expressed and that the pka1 and pka2 mutations lead to a loss in expression of the wild-type messages (Fig. 2B, lanes 1 to 4). In the pka1 disruption strains, a small aberrant transcript was observed (data not shown). To determine whether the transcript affected the observed pka1 mutant phenotypes, the PKA1 open reading frame was completely deleted in both serotypes A and D. Phenotypic studies revealed that the pka1 deletion strains were indistinguishable from the original disruption strains with regard to melanin and capsule production and the ability to mate. These data show that the melanin, capsule, and mating phenotypes observed in the original pka1 insertional mutants were not caused by a dominant-negative effect resulting from the small transcript.
FIG. 2.
PKA1 and PKA2 are not expressed in pka1 and pka2 mutant strains. (A) Identical regions of the PKA1 and PKA2 genes were disrupted (PKA1) or deleted (PKA2). In the PKA1 gene, the ADE2 gene was inserted at the region of DNA representing amino acid 325 in both serotypes A and D. In the PKA2 gene, the URA5 gene was used to replace the region representing amino acids 332 to 376 in both serotypes A and D. (B) pka1 and pka2 mutants lack their respective wild-type transcripts. PCR fragments generated from regions of the PKA1 and PKA2 genes downstream of the inserted ADE2 or URA5 genes, respectively, were used to probe RNA blots. RNA was generated from serotype D wild-type (WT) (JEC21), pka1 (CDC43), pka2 (CDC85), and pka1 pka2 (CDC106) strains and from serotype A wild-type (H99), pka1 (CDC1), pka1 plus PKA1 (CDC13), and pka2 (JKH4) mutant strains. Ethidium bromide-stained rRNA served as a loading control.
Extensive searches in the Stanford (13× coverage) and The Institute for Genomic Research (9.5× coverage; http://www.TIGR.org/tdb/e2k1/cna1/) serotype D databases and the Whitehead Institute and Duke University serotype A database (11× coverage) did not reveal any additional PKA catalytic subunits. In summary, these findings demonstrate that the cAMP-dependent protein kinase is dispensable for viability in C. neoformans.
Pka2 is required for virulence factor production in serotype D.
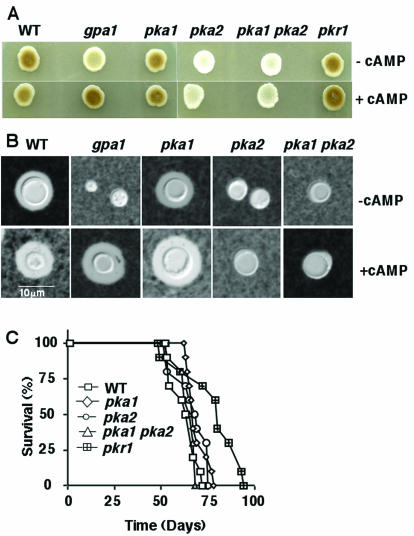
C. neoformans elaborates two specialized virulence factors, capsule and melanin, which both contribute to virulence. Because signaling by cAMP and Pka1 promotes melanin and capsule production in C. neoformans serotype A strains (22), we tested whether PKA plays a similar role in serotype D. In response to carbon source limitation, C. neoformans produces the enzyme laccase, which oxidizes diphenolic substrates to produce melanin as a protective antioxidant shell in the cell wall (45, 52, 74). To assay melanin production, colonies were cultured on Niger seed agar. As shown in Fig. 3A, the serotype D pka2 mutant exhibited a severe defect in melanin production, whereas the pka1 mutant produced melanin to the same extent as the wild type. A pka1 pka2 double mutant displayed a melanin defect similar to the pka2 single mutant strain. cAMP restored melanin production in a gpa1 mutant (as previously observed) but not in the pka2 mutant (Fig. 3A), consistent with the model that Gpa1, a heterotrimeric G protein Gα subunit, regulates cAMP production, whereas Pka2 is downstream of the site of cAMP activation. These findings demonstrate that the Pka2 catalytic subunit, and not the Pka1 subunit, promotes melanin production in serotype D strains.
FIG. 3.
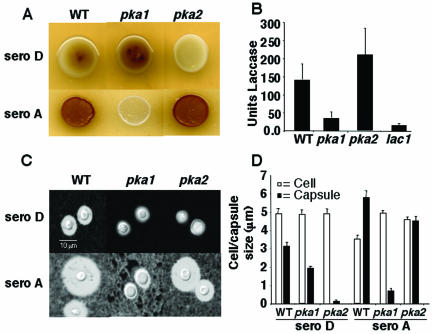
The Pka2 catalytic subunit controls virulence factor production, but not virulence, in serotype D. (A) Melanin production was assessed by growing cells of serotype D wild-type (WT) (JEC21), gpa1 (BAC21), pka1 (CDC40), pka2 (CDC99), pka1 pka2 (CDC103), and pkr1 (CDC68) mutant strains on Niger seed (Guizotia abyssinica) and incubating them for 3 days at 30°C. (B) Capsule production was assessed in wild-type (JEC21), gpa1 (BAC37), pka1 (CDC40), pka2 (CDC99), and pka1 pka2 (CDC106) mutant strains. Cells were grown in LIM in the presence or absence of cAMP at 30°C for 5 days, and capsules were detected with India ink. Magnification, ×1,000. (C) Groups of 10 DBA mice were infected with 5 × 104 yeast cells of wild-type, pka1, pka1 pka2, and pkr1 mutant strains by inhalation. The percent survival was plotted over 100 days. The average survival of animals infected with the wild-type strain was 61.6 days, compared to 68 days for the pka1 mutant (P = 0.1), 65.6 days for the pka2 mutant (P = 0.21), and 62.4 days for the pka1 pka2 mutant (P = 0.88).
The Pka2 catalytic subunit was also found to regulate capsule production. Capsule is induced in vivo by host conditions that can be mimicked in vitro by growing cells in the presence of 5% CO2-HCO3− or iron-limiting conditions (28, 67). The capsule protects fungal cells from phagocytosis and can be detected by microscopic examination of cells suspended in India ink. Yeast cells were grown in liquid LIM containing an iron chelator for 5 days at 30°C to induce capsule formation. In contrast to the wild-type and pka1 mutant strains, which produce capsule, the gpa1, pka2, and pka1 pka2 mutants were hypocapsular (Fig. 3B). cAMP restored capsule production in the gpa1 mutant but not in the pka2 mutant. In summary, the Pka2 catalytic subunit regulates capsule and melanin production, and the Pka1 subunit does not.
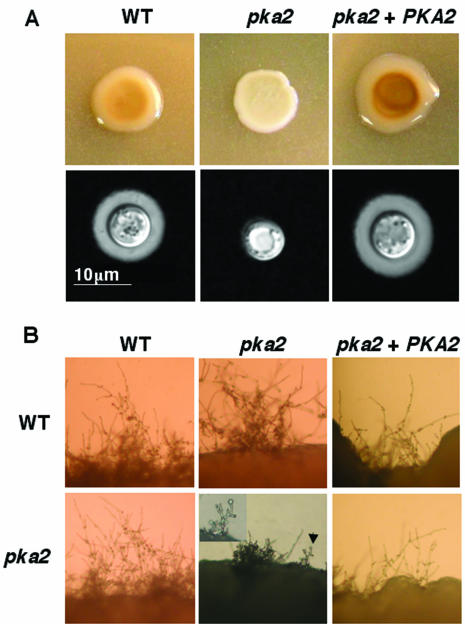
To verify that Pka2 is responsible for the observed phenotypes in serotype D, we isolated a reconstituted pka2 plus PKA2 strain. The genotype of the strain was verified by Southern hybridization, and the presence of wild-type transcripts and transcript levels were determined by RNA analysis (data not shown). Melanin and capsule production in the reconstituted strain were comparable to those of the wild-type strain (Fig. 4A). The mating defect observed in the bilateral pka2 × pka2 mating (see below) was also complemented in the reconstituted strain (Fig. 4B).
FIG. 4.
A serotype D wild-type (WT) PKA2 gene restores mating and capsule and melanin production in a pka2 mutant. (A) Serotype D wild-type (JEC21) and pka2 (JKH19) mutant strains and the pka2 plus PKA2 reconstituted strain (JKH21) were grown on Niger seed agar medium and incubated for 3 days at 30°C (upper panel). The same strains were also grown on a MEM plate for 3 days at 30°C, and capsule production was detected with India ink (lower panel). Magnification, ×1,000. (B) Serotype D wild-type (JEC34), pka2 (JKH19), and the pka2 plus PKA2 reconstituted (JKH21) strains were cocultured with either a wild-type (JEC170) or pka2 (CDC127) strain on V8 agar medium for 3 days at room temperature, and edges of the mating mixture were photographed. Magnification, ×100. In the pka2 × pka2 mating, an arrow points to a basidium produced on a short filament. The indicated basidium is shown at higher magnification (×200) in the insert.
PKA is not required for virulence of C. neoformans serotype D strains.
We next tested whether Pka2 is required for virulence of C. neoformans in a murine model of cryptococcosis. Mice were infected by inhalation with 5 × 104 cells of the wild type, the pka1 and pka2 single mutants, the pka1 pka2 double mutant, and the pkr1 mutant strain, and animal survival was monitored over a period of 100 days (Fig. 3C). In this model, the cryptococcus infection initiates in the lungs and hematogenously disseminates to the brain and other tissues (spleen and kidney), and the majority of animals develop severe hydrocephalus and die from central nervous system infection. The average survival of mice infected with the wild-type strain was 61.6 days compared to 68 days for the pka1 mutant (P = 0.1), 65.6 days for the pka2 mutant (P = 0.21), and 62.4 days for the pka1 pka2 mutant (P = 0.88) (Fig. 3C). Thus, no significant differences were found in survival rates of mice infected with the mutants in comparison to those of mice infected with the wild-type strain. Therefore, we conclude that neither Pka1 nor Pka2 is essential for virulence in the murine intranasal model of cryptococcosis in the serotype D strains of C. neoformans, even though Pka2 contributes to capsule and melanin production. Additionally, virulence of the pkr1 mutant was not significantly different from that of a wild-type strain (P = 0.08) (Fig. 3C), indicating that Pkr1 also does not regulate virulence in serotype D strains under the conditions tested.
Pka2 is not necessary for virulence factor production in serotype A.
Previous work has shown that, in contrast to serotype D, the Pka1 catalytic subunit is essential for virulence factor production in serotype A (22). Therefore, we determined what role, if any, the serotype A Pka2 catalytic subunit plays in these processes. To address this, we deleted the same region of the serotype A PKA2 gene as was deleted in serotype D (Fig. 2A). Northern blots confirmed that the serotype A pka1 and pka2 mutants lacked the wild-type PKA1 and PKA2 transcripts, respectively (Fig. 2B). We examined capsule and melanin formation in the resulting pka2Δ::URA5 mutant strain. Melanin production was examined by growth on Niger seed agar at 30°C (serotype D) or 37°C (serotype A) (Fig. 5A). Quantitation of melanin production in serotype A strains confirmed that the pka2 strain produced melanin at levels similar to those of the wild type, whereas the pka1 mutant produced little or no melanin, similar to a lac1 mutant that is deficient in laccase production (Fig. 5B).
FIG. 5.
Functions of Pka1 and Pka2 in capsule and melanin biosynthesis are reversed in serotype A and D. (A) Serotype D wild-type (WT) (JEC21), pka1 (CDC43), and pka2 (CDC85) mutant strains and serotype A wild-type (H99), pka1 (CDC1), and pka2 (JKH4) mutant strains were grown on Niger seed agar and incubated for 3 days at 30°C (sero D) or 37°C (sero A). Melanin production was not observed in the serotype D pka2 strain or the serotype A pka1 strain but was produced in the wild type and produced at slightly elevated levels in the serotype D pka1 and serotype A pka2 strains. (B) Melanin production in serotype A strains was quantitated by growing 108 cells of either wild-type (H99), pka1 (CDC1), pka2 (JKH4), or lac1 (CHM3) mutant strains at 250 rpm and 30°C for 16 h in l-DOPA medium prior to transferring cultures to 250 rpm and 25°C for 24 h. Spectrophotometric readings of the culture supernatants were taken as the optical density at 475 nm: 1 U of laccase = optical density at 475 nm/0.001 (74). Each bar represents an average of the results from four independent replicates with the standard errors of the means indicated by error bars. (C) Serotype D wild-type (JEC21), pka1 (CDC43), and pka2 (CDC85) mutant strains and serotype A wild-type (H99), pka1 (CDC1), and pka2 (JKH4) mutant strains were inoculated in 5 ml of MEM (28), incubated at 30°C for 5 days, and examined with India ink. Magnification, ×1,000. (D) Twenty cells from the cultures used for panel B were measured for cell (diameter) and capsule (radius) size. Each bar represents the average of 20 measurements.
Capsule production by the serotype A and D wild-type strains and the pka1 and pka2 mutant strains was examined following incubation in MEM (28). Cell and capsule size of serotype A and serotype D pka1 and pka2 mutants were quantitated by measuring both cell diameter and capsule radius. In contrast to the severe hypocapsular phenotype observed in serotype D pka2 mutants, serotype A pka2 mutants produced wild-type levels of capsule and the serotype A pka1 mutants exhibited a clear defect, as previously reported (22). Thus, the functions of the Pka1 and Pka2 catalytic subunits have been reversed during the divergence of serotypes A and D.
Pka2 is required for mating and haploid fruiting in serotype D.
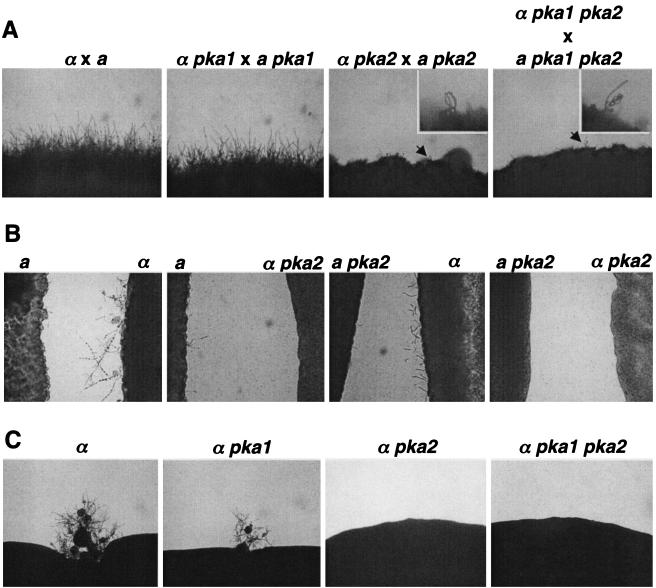
The role of the cAMP-dependent protein kinase in mating in serotype D strains was investigated. pka1 and pka2 single mutants and pka1 pka2 double mutant cells were capable of mating with a wild-type strain of the opposite mating type (data not shown). Next, bilateral mutant crosses were performed in which MATα pka1 mutant cells were cocultured with MATa pka1 cells and MATα pka2 mutant cells were incubated with MATa pka2 cells on V8 mating medium. In the pka2 × pka2 bilateral mutant mating, following coincubation for 2 days, we observed the production of short mating filaments with long chains of basidiospores (Fig. 6A). After several more days, the basidial heads and basidiospores were overgrown by vegetative growth. This is in contrast to wild-type and pka1 × pka1 matings that formed abundant filaments and basidiospores (Fig. 6A). pka1 pka2 × pka1 pka2 bilateral matings were morphologically similar to the pka2 × pka2 mutant crosses (Fig. 6A). These observations indicate that Pka2 is required in at least one partner for wild-type mating in C. neoformans serotype D, whereas Pka1 is not.
FIG. 6.
The Pka2 catalytic subunit promotes mating, pheromone production, and haploid fruiting. (A) To assess mating, MATα wild-type (JEC21), pka1 (CDC43), pka2 (CDC85), and pka1 pka2 (CDC103) mutant strains were cocultured with a corresponding MATa partner on V8 agar medium for 4 days at room temperature, and the edges of the mating mixture were photographed. Magnification, ×100. An arrow points to chains of basidiospores produced on short hyphae and a basidium observed in the bilateral mating between pka2 mutant strains. The indicated basidium is shown at a higher magnification (×200) in the inserts. (B) MATa cells of serotype D wild-type and pka2 mutant strains were confronted with either a MATα wild-type or a MATα pka2 mutant partner on FA at room temperature in the absence of light for 2 days. Morphological responses to pheromone were photographed. Magnification, ×400. (C) To assess haploid fruiting, the MATα wild-type, pka1, pka2, and pka1 pka2 mutant strains were grown on FA in the dark for 16 days at room temperature, and edges of the growth patch were photographed. Magnification, ×400.
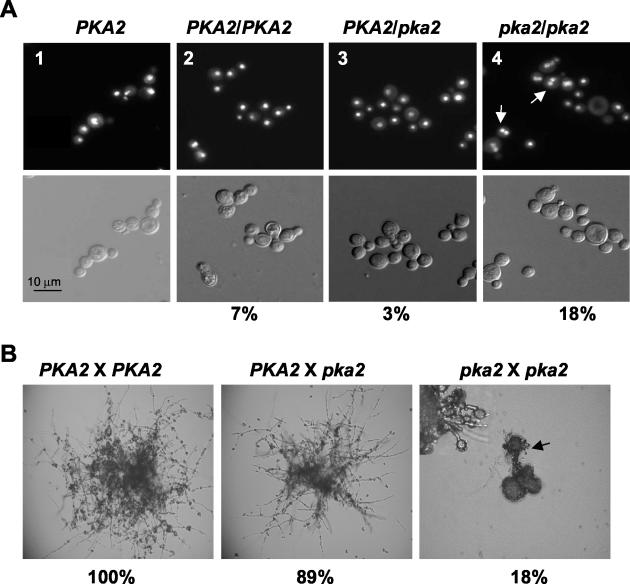
We next tested whether the pka2 mutation results in a cell fusion defect. To address this, cell fusion assays were performed in which equal numbers of cells from two marked strains (wild type × wild type, wild type × pka2, or pka2 × pka2) were cocultured on V8 medium (pH 7.0) for 24 h prior to resuspension in water and plating on YNB medium. After 3 days of incubation at 25°C, the number of colonies on each plate were counted. The relative fusion efficiency between PKA2 × PKA2, PKA2 × pka2, and pka2 × pka2 crosses was determined and calculated by normalization to the PKA2 × PKA2 fusion. The absolute fusion efficiency of the wild-type mating, based on the 4 replica plates, was ∼1.2% (499 colonies/4 × 105 total cells plate). This is similar to previously reported fusion efficiencies for C. neoformans wild-type strains (20). Figure 7B shows that in contrast to the colonies produced by the PKA2 × PKA2 or the PKA2 × pka2 fusion products, the colonies resulting from the pka2 × pka2 cross were morphologically defective, producing basidia and basidiospores from very short filaments, and the fusion efficiency was reduced by ∼5-fold (Fig. 7B).
FIG. 7.
Pka2 is involved in both nuclear and cell fusion. (A) Nuclear fusion was assessed by engineering homozygous PKA2/PKA2 (panel 2), heterozygous PKA2/pka2Δ (panel 3), or homozygous pka2Δ/pka2Δ diploid or dikaryotic strains (panel 4). Cells were grown in YNB medium overnight at 37°C prior to fixation and staining with DAPI (top panel). White arrows in panel 4 indicate examples of dikaryotic cells. Cells from a wild-type haploid strain (JEC34) are shown in panel 1. Differential interference microscopy images of the same cells are shown in the bottom panel. Cells were photographed at a magnification of ×400. The percentages indicate the number of dikaryotic cells that were found in a total of 300 cells that were examined per diploid strain. (B) Cell fusion efficiency was examined between wild-type (JEC34) and wild-type (JEC170) strains, wild-type (JEC170) and pka2Δ (JKH19) strains, or pka2Δ (JKH19) and pka2Δ (CDC127) strains. Equal numbers of cells were cocultured on V8 medium (pH 7.0) overnight. Cells were then transferred to YNB medium, grown at 25°C for 3 days on YNB agar medium, and photographed. Magnification, ×100. The number of fusion products on each plate were also counted. Four replica plates were generated for each fusion. The arrow points to a basidium produced by the pka2 × pka2 fusion product (insert). Magnification, ×200. The percentage of cell fusion was normalized to the wild-type × wild-type colony number and is listed as a percentage below the fusion product images.
To determine whether the pka2 mutation results in a nuclear fusion defect that is responsible for the observed aberrant mating in a bilateral cross, we utilized strains with selectable markers to create a homozygous wild-type diploid (PKA2/PKA2), a presumptive homozygous pka2 mutant diploid (pka2/pka2), and a heterozygous PKA2/pka2 diploid. In addition to observing thermal dimorphism (filamentation at 25°C and yeast cells at 37°C) that is characteristic of diploid strains (57), we used MATa- and MATα-specific PCR primers to confirm that both mating types were present in the resulting strains (data not shown). DAPI staining of the diploid cells revealed that while only a small number of the homozygous PKA2/PKA2 diploid cells and the heterozygous PKA2/pka2 diploid cells were dikaryotic (7 and 3%, respectively), 18% of the cells from the homozygous pka2 × pka2 mutant fusion product were dikaryotic (Fig. 7A, compare panels 2 and 3 with panel 4). Examination of the parental pka2Δ cells revealed that these cells were uninucleate. These data suggest that PKA2 promotes both nuclear fusion and filamentation.
Next, we addressed whether a loss of pheromone production or an inability to respond to pheromones contributed to the mating defect observed in a pka2 bilateral mutant cross. Cells of pka1 and pka2 single mutants and pka1 pka2 double mutants were confronted with either wild-type cells or the corresponding mutant strain of the opposite mating type on FA medium in the dark at room temperature. Figure 6B shows the results of the confrontation assays with the pka2 mutant strains. FA medium provides nitrogen starvation and desiccation signals that support production and response to pheromones. In the normal response to pheromones, MATα strains produce abundant conjugation tubes after 2 days (data not shown) and initiate haploid fruiting upon longer incubation (Fig. 6C). MATa cells produce fewer conjugation tubes and dramatically enlarge in response to pheromones. We found that pka1 mutants responded normally (data not shown), whereas pka2 mutants exhibited greatly reduced filamentation in a bilateral confrontation. An attenuation in pheromone response was also observed in wild-type cells confronted with pka2 mutants. These data suggest that Pka2 is essential for pheromone response and is partially required for efficient pheromone production, whereas Pka1 is not. MATα cells produce hyphal filaments and basidiospores in response to nitrogen starvation and desiccation through a process known as monokaryotic or haploid fruiting. As shown in Fig. 6C, wild-type and isogenic pka1 mutants undergo haploid fruiting, whereas pka2 mutants do not.
DISCUSSION
A highly conserved Gα protein-cAMP-PKA signaling pathway controls differentiation and virulence of serotype A pathogenic isolates of C. neoformans (2, 3, 21). In this report we show that components of the PKA pathway have different functions in serotype A and D strains of C. neoformans. In serotype A, the Pka1 catalytic subunit is required for melanin and capsule production and virulence while the Pka2 subunit is dispensable for these processes (Fig. 5). In contrast, serotype D strains do not require Pka1 to regulate capsule and melanin production, but instead, Pka2 has assumed this regulatory role. The addition of exogenous cAMP did not restore melanin production and capsule formation in pka2 mutant strains, indicating that Pka2 is the target of cAMP signaling (Fig. 3A and B). Thus, although different PKA catalytic subunits play distinct roles in serotype A and D strains, the function of the PKA pathway is conserved with respect to virulence factor production. Surprisingly, PKA is not required for virulence in serotype D, whereas PKA plays a prominent role in virulence in serotype A (Fig. 3C) (22).
PKA promotes melanin and capsule production in serotype D, but the pka2 mutant strains still produce some melanin and capsule and are thus hypocapsular and not acapsular (Fig. 5). Melanin contributes to but is not essential for virulence, and even albino strains lacking laccase are capable of lethal infection (53). The difference in virulence between serotype A strain H99, in which PKA controls virulence, and the serotype D strain JEC21, in which PKA does not control virulence, are dramatic. Nintety-five percent of all C. neoformans infections are caused by serotype A strains (12). Furthermore, it has been reported that frequently those infections that have been attributed to serotype D strains are likely the result of serotype AD hybrids (8). Differences in capsule and melanin content and production may contribute to the observed differences in virulence.
Pka2 is required for both mating and haploid fruiting in serotype D strains, whereas Pka1 is not required for either process in serotype D but is essential for mating in serotype A (Fig. 6A and C) (22). The Pka2 requirement in mating includes morphological changes involved in pheromone sensing, cell and nuclear fusion, and also possibly a role in promoting pheromone production and hyphal maturation (4, 69). The PKA pathway may, for example, promote expression of elements of the pheromone response pathway during nutritional limitation. Thus, it appears that the cAMP signaling pathway regulates filamentation in this fungus in parallel with the mitogen-activated protein kinase cascade, reminiscent of the regulation of pseudohyphal growth in S. cerevisiae (32, 40, 41).
Our findings are compatible with at least two models to explain the roles of the divergent PKA subunits. In one model, the two PKA subunits act differentially on the same targets, whereas in the second model, they act on different targets. PKA targets in other systems include Hgl1 and Prf1 in U. maydis (23, 29), Flo8 and Sfl1 in S. cerevisiae (16, 48, 49, 51), and Rst2 in Schizosaccharomyces pombe (30). Studies are in progress to identify PKA targets in C. neoformans to test these models.
Divergence of PKA function is also observed in other fungi. In the yeast S. cerevisiae, the regulatory and catalytic subunits of PKA are encoded by the BCY1 and TPK1, TPK2, and TPK3 genes, respectively (63, 64). PKA regulates growth, metabolism, stress responses, entry into stationary phase, and pseudohyphal differentiation (reviewed in references 47 and 61). The three PKA isoforms have overlapping functions in growth, stress, and stationary phase responses. In pseudohyphal differentiation, the three isoforms have opposing roles and Tpk2 activates while Tpk1 and Tpk3 repress pseudohyphal growth (48, 51). Tpk2 activates pseudohyphal growth by inducing expression of the cell wall flocculin gene FLO11. Tpk2 activates the Flo8 transcription factor, which positively regulates expression of FLO11, and represses Sfl1, a negative regulator of FLO11 (48, 51). Furthermore, genome-wide transcriptional profiling revealed that Tpk2 negatively controls iron uptake genes and positively controls genes involved in trehalose degradation and water homeostasis (51). Functional specialization of PKA catalytic subunits in pseudohyphal differentiation is attributable to subtle differences in the conserved catalytic regions and not to differences in gene regulation or structural differences in the more divergent amino-terminal regions of these proteins (48). Additionally, functional diversity of PKA isoforms may be due to differences in subcellular localization. Inactivation of all three isoforms of the PKA catalytic subunit in S. cerevisiae results in loss of cell viability, indicating that the three PKA isoforms are functionally redundant for growth (64). In contrast, C. neoformans pka1 pka2 mutants are viable, indicating that PKA is not essential for growth in C. neoformans. In accord with this model, adenylyl cyclase mutants are also viable in C. neoformans but inviable in S. cerevisiae (3, 9, 13).
In the human fungal pathogen C. albicans, PKA regulates the serum-induced yeast-hypha dimorphic transition (5, 10, 11). Ability to undergo the dimorphic transition has been linked to virulence and is regulated by the two PKA catalytic subunit isoforms Tpk1 and Tpk2 (7, 58). The C. albicans Tpk1 and Tpk2 proteins have redundant functions in growth and stress responses but exhibit functional differences in morphogenesis depending on environmental conditions (7). Tpk1 is required for filamentation on solid inducing media, whereas Tpk2 is required for filamentation in liquid inducing media. The functional specialization of PKA isoforms in the filamentation response is mediated by the catalytic domains, whereas agar invasion by yeast cells is mediated by the unique N-terminal domain of Tpk2. It is not yet known what structural features contribute to the functional specialization of the PKA isoforms in serotype A and D strains of C. neoformans, but domain exchange experiments can be performed to address this question.
Acknowledgments
We thank Cristl Arndt for technical assistance; Andy Alspaugh, Toshiaki Harashima, and Xuewen Pan for scientific discussions; Jill Blankenship for assistance with genetic crosses and basidiospore dissection; Chris Martin and Marisol de Jesus-Berrios for the laccase mutant strain; James Fraser for the pJAF7 transformation vector; and Connie Nichols, John Perfect, and Andy Alspaugh for comments on the manuscript.
This work was supported by NIAID R01 grants AI39115 and AI42159 (J.H.) and P01 award AI44975 from the NIAID to the Duke University Mycology Research Unit. G.C. is a Burroughs Wellcome New Investigator in Molecular Pathogenic Mycology. J.H. is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an Associate Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Alspaugh, J. A., J. R. Perfect, and J. Heitman. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 11:3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alspaugh, J. A., R. Pukkila-Worley, T. Harashima, L. M. Cavallo, D. Funnell, G. M. Cox, J. R. Perfect, J. W. Kronstad, and J. Heitman. 2002. Adenylyl cyclase functions downstream of the Gα protein GPA1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayad-Durieux, Y., P. Knechtle, S. Goff, F. Dietrich, and P. Philippsen. 2000. A PAK-like protein kinase is required for maturation of young hyphae and septation in the filamentous ascomycete Ashbya gossypii. J. Cell Sci. 113:4563-4575. [DOI] [PubMed] [Google Scholar]
- 5.Bahn, Y. S., and P. Sundstrom. 2001. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J. Bacteriol. 183:3211-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett, J. E., K. J. Kwon-Chung, and D. H. Howard. 1977. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am. J. Epidemiol. 105:582-586. [DOI] [PubMed] [Google Scholar]
- 7.Bockmuhl, D. P., and J. F. Ernst. 2001. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics 157:1523-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boekhout, T., B. Theelen, M. Diaz, J. W. Fell, W. C. J. Hop, E. C. A. Abeln, F. Dromer, and W. Meyer. 2001. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology 147:891-907. [DOI] [PubMed] [Google Scholar]
- 9.Boutelet, F., A. Petitjean, and F. Hilger. 1985. Yeast cdc35 mutants are defective in adenylate cyclase and are allelic with cyr1 mutants while CAS1, a new gene, is involved in the regulation of adenylate cyclase. EMBO J. 4:2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 11.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 13.Casperson, G. F., N. Walker, and H. R. Bourne. 1985. Isolation of the gene encoding adenylate cyclase in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 82:5060-5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, Y. C., L. A. Penoyer, and K. J. Kwon-Chung. 2001. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc. Natl. Acad. Sci. USA 98:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, Y. C., B. L. Wickes, G. F. Miller, L. A. Penoyer, and K. J. Kwon-Chung. 2000. Cryptococcus neoformans STE12α regulates virulence but is not essential for mating. J. Exp. Med. 191:871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conlan, R. S., and D. Tzamarias. 2001. Sfl1 functions via the co-repressor Ssn6-Tup1 and the cAMP-dependent protein kinase Tpk2. J. Mol. Biol. 309:1007-1015. [DOI] [PubMed] [Google Scholar]
- 17.Cox, G. M., H. C. McDade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Casadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 18.Cox, G. M., J. Mukherjee, G. T. Cole, A. Casadevall, and J. R. Perfect. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson, R. C., J. R. Blankenship, P. R. Kraus, M. d. Berrios, C. M. Hull, C. D'Souza, P. Wang, and J. Heitman. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607-2615. [DOI] [PubMed] [Google Scholar]
- 20.Dong, H., and W. Courchesne. 1998. A novel quantitative mating assay for the fungal pathogen Cryptococcus neoformans provides insight into signalling pathways responding to nutrients and temperature. Microbiology 144:1691-1697. [DOI] [PubMed] [Google Scholar]
- 21.D'Souza, C., and J. Heitman. 2001. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25:349-364. [DOI] [PubMed] [Google Scholar]
- 22.D'Souza, C. A., J. A. Alspaugh, C. Yue, T. Harashima, G. M. Cox, J. R. Perfect, and J. Heitman. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21:3179-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durrenberger, F., R. D. Laidlaw, and J. W. Kronstad. 2001. The hgl1 gene is required for dimorphism and teliospore formation in the fungal pathogen Ustilago maydis. Mol. Microbiol. 41:337-348. [DOI] [PubMed] [Google Scholar]
- 24.Dürrenberger, F., K. Wong, and J. W. Kronstad. 1998. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. USA 95:5684-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan, M., B. P. Currie, R. R. Gutell, M. A. Ragan, and A. Casadevall. 1994. The 16S-like, 5.8S and 23S-like rRNAs of the two varieties of Cryptococcus neoformans: sequence, secondary structure, phylogenetic analysis and restriction fragment polymorphisms. J. Med. Vet. Mycol. 32:163-180. [DOI] [PubMed] [Google Scholar]
- 26.Franzot, S. P., I. F. Salkin, and A. Casadevall. 1999. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. J. Clin. Microbiol. 37:838-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao, S., and D. L. Nuss. 1996. Distinct roles for two G protein α subunits in fungal virulence, morphology, and reproduction revealed by targeted gene disruption. Proc. Natl. Acad. Sci. USA 93:14122-14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granger, D. L., J. R. Perfect, and D. T. Durack. 1985. Virulence of Cryptococcus neoformans: regulation of capsule synthesis by carbon dioxide. J. Clin. Investig. 76:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann, H. A., R. Kahmann, and M. Bolker. 1996. The pheromone response factor coordinates filamentous growth and pathogenicity in Ustilago maydis. EMBO J. 15:1632-1641. [PMC free article] [PubMed] [Google Scholar]
- 30.Higuchi, T., Y. Watanabe, and M. Yamamoto. 2002. Protein kinase A regulates sexual development and gluconeogenesis through phosphorylation of the Zn finger transcriptional activator Rst2p in fission yeast. Mol. Cell. Biol. 22:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hull, C. M., and J. Heitman. 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36:557-615. [DOI] [PubMed] [Google Scholar]
- 32.Kubler, E., H. U. Mosch, S. Rupp, and M. P. Lisanti. 1997. Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 272:20321-20323. [DOI] [PubMed] [Google Scholar]
- 33.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821-833. [PubMed] [Google Scholar]
- 34.Kwon-Chung, K. J., and J. E. Bennett. 1984. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am. J. Epidemiol. 120:123-130. [DOI] [PubMed] [Google Scholar]
- 35.Kwon-Chung, K. J., J. E. Bennett, and J. C. Rhodes. 1982. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie Leeuwenhoek 48:25-38. [DOI] [PubMed] [Google Scholar]
- 36.Kwon-Chung, K. J., and J. C. Rhodes. 1986. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect. Immun. 51:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon-Chung, K. J., A. Varma, J. C. Edman, and J. E. Bennett. 1992. Selection of ura5 and ura3 mutants from the two varieties of Cryptococcus neoformans on 5-fluoroorotic acid medium. J. Med. Vet. Mycol. 30:61-69. [PubMed] [Google Scholar]
- 38.Kwon-Chung, K. J., A. Varma, and D. H. Howard. 1990. Ecology of Cryptococcus neoformans and prevalence of its two varieties in AIDS and non-AIDS associated Cryptococcosis, p. 103-113. In H. Vanden Bossche, D. W. R. Mackenzie, G. Cauwenbergh, J. V. Cutsem, E. Drouhet, and B. Dupont (ed.), Mycoses in AIDS patients. Plenum Press, New York, N.Y.
- 39.Lengeler, K. B., G. M. Cox, and J. Heitman. 2001. Serotype AD strains of Cryptococcus neoformans are diploid or aneuploid and are heterozygous at the mating-type locus. Infect. Immun. 69:115-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, H., C. A. Styles, and G. R. Fink. 1993. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science 262:1741-1744. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz, M. C., and J. Heitman. 1997. Yeast pseudohyphal growth is regulated by GPA2, a G protein α homolog. EMBO J. 16:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell, T. K., and R. A. Dean. 1995. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell 7:1869-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, T. D. E., and J. C. Edman. 1993. The α-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 13:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurudeen, T. A., and D. G. Ahearn. 1979. Regulation of melanin production by Cryptococcus neoformans. J. Clin. Microbiol. 10:724-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 47.Pan, X., T. Harashima, and J. Heitman. 2000. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 3:567-572. [DOI] [PubMed] [Google Scholar]
- 48.Pan, X., and J. Heitman. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan, X., and J. Heitman. 2002. Protein kinase A operates a molecular switch that governs yeast pseudohyphal growth. Mol. Cell. Biol. 22:3981-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perfect, J. R., N. Ketabchi, G. M. Cox, C. W. Ingram, and C. L. Beiser. 1993. Karyotyping of Cryptococcus neoformans as an epidemiological tool. J. Clin. Microbiol. 31:3305-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson, L. S., and G. R. Fink. 1998. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 95:13783-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosas, A. L., J. D. Nosanchuk, M. Feldmesser, G. M. Cox, H. C. McDade, and A. Casadevall. 2000. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect. Immun. 68:2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salas, S. D., J. E. Bennett, K. J. Kwon-Chung, J. R. Perfect, and P. R. Williamson. 1996. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 184:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Shen, W.-C., R. C. Davidson, G. M. Cox, and J. Heitman. 2002. Pheromones stimulate mating and differentiation via paracrine and autocrine signaling in Cryptococcus neoformans. Eukaryot. Cell 1:366-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherman, F. 1991. Getting started with yeast, p. 3-21. In C. Guthrie and G. R. Fink (ed.), Methods in enzymology, vol. 194. Academic Press, Inc., San Diego, Calif. [DOI] [PubMed]
- 57.Sia, R. A., K. B. Lengeler, and J. Heitman. 2000. Diploid strains of the pathogenic basidiomycete Cryptococcus neoformans are thermally dimorphic. Fungal Genet. Biol. 29:153-163. [DOI] [PubMed] [Google Scholar]
- 58.Sonneborn, A., D. P. Bockmuhl, M. Gerads, K. Kurpanek, D. Sanglard, and J. F. Ernst. 2000. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol. Microbiol. 35:386-396. [DOI] [PubMed] [Google Scholar]
- 59.Sudarshan, S., R. C. Davidson, J. Heitman, and J. A. Alspaugh. 1999. Molecular analysis of the Cryptococcus neoformans ADE2 gene, a selectable marker for transformation and gene disruption. Fungal Genet. Biol. 27:36-48. [DOI] [PubMed] [Google Scholar]
- 60.Taylor, S. S., J. A. Buechler, and W. Yonemoto. 1990. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 59:971-1005. [DOI] [PubMed] [Google Scholar]
- 61.Thevelein, J. M., L. Cauwenberg, S. Colombo, J. H. D. Winde, M. Donation, F. Dumortier, L. Kraakman, K. Lemaire, P. Ma, D. Nauwelaers, F. Rolland, A. Teunissen, P. V. Dijck, M. Versele, S. Wera, and J. Winderickx. 2000. Nutrient-induced signal transduction through the protein kinase A pathway and its role in the control of metabolism, stress resistance, and growth in yeast. Enzyme Microb. Technol. 26:819-825. [DOI] [PubMed] [Google Scholar]
- 62.Thompson, J. D., F. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toda, T., S. Cameron, P. Sass, M. Zoller, J. D. Scott, B. McMullen, M. Hurwitz, E. G. Krebs, and M. Wigler. 1987. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 7:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toda, T., S. Cameron, P. Sass, M. Zoller, and M. Wigler. 1987. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50:277-287. [DOI] [PubMed] [Google Scholar]
- 65.Toffaletti, D. L., and J. R. Perfect. 1994. Biolistic DNA delivery for Cryptococcus neoformans transformation, p. 303-308. In B. Maresca and G. S. Kobayashi (ed.), Molecular biology of pathogenic fungal: a laboratory manual. Telos Press, New York, N.Y.
- 66.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vartivarian, S. E., E. J. Anaissie, R. E. Cowart, H. A. Sprigg, M. J. Tingler, and E. S. Jacobson. 1993. Regulation of cryptococcal capsular polysaccharide by iron. J. Infect. Dis. 167:186-190. [DOI] [PubMed] [Google Scholar]
- 68.Vartivarian, S. E., R. E. Cowart, E. J. Anaissie, T. Tashiro, and H. A. Sprigg. 1995. Iron acquisition by Cryptococcus neoformans. J. Med. Vet. Mycol. 33:151-156. [PubMed] [Google Scholar]
- 69.Wang, P., C. B. Nichols, K. B. Lengeler, M. E. Cardenas, G. M. Cox, J. R. Perfect, and J. Heitman. 2002. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1:257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, P., J. R. Perfect, and J. Heitman. 2000. The G-protein β subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu, J., R. J. Vilgalys, and T. G. Mitchell. 2000. Multiple gene genealogies reveal dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans. Mol. Ecol. 9:1471-1481. [DOI] [PubMed] [Google Scholar]
- 73.Yue, C., L. M. Cavallo, J. A. Alspaugh, P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 1999. The STE12α homolog is required for haploid filamentation but largely dispensable for mating and virulence in Cryptococcus neoformans. Genetics 153:1601-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu, X., J. Gibbons, J. Garcia-Rivera, J. Casadevall, and P. R. Williamson. 2001. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect. Immun. 69:5589-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]