Abstract
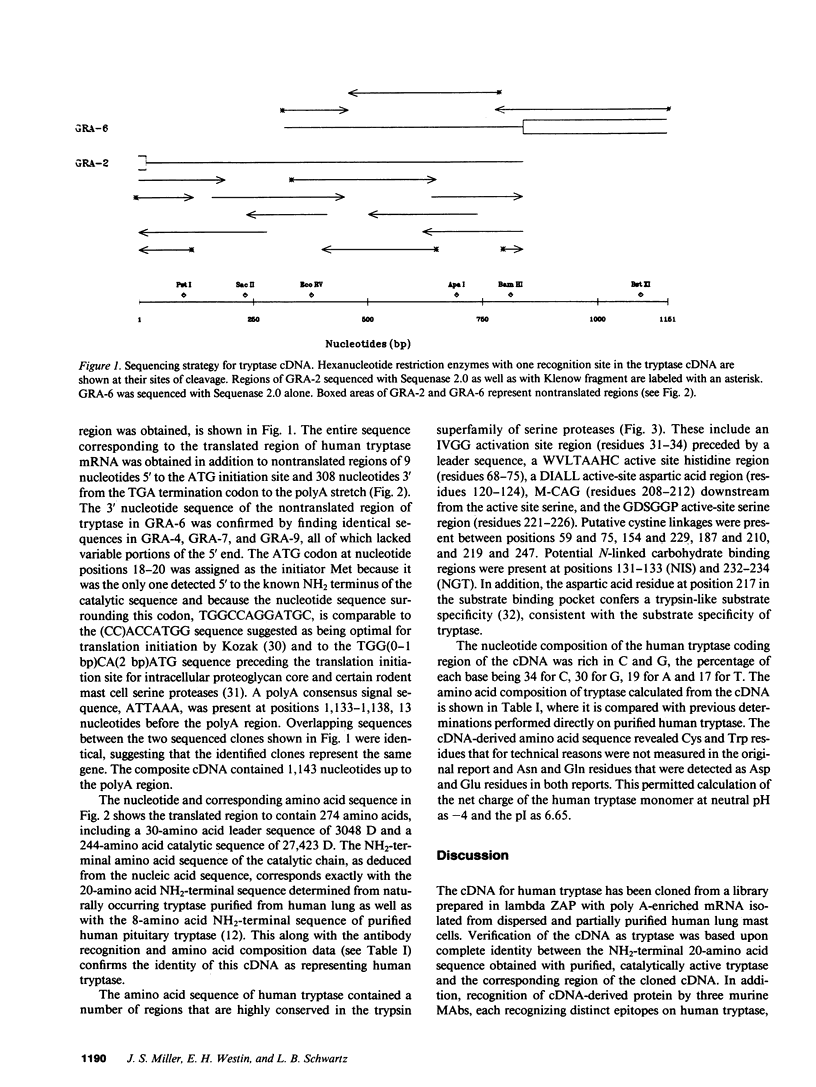
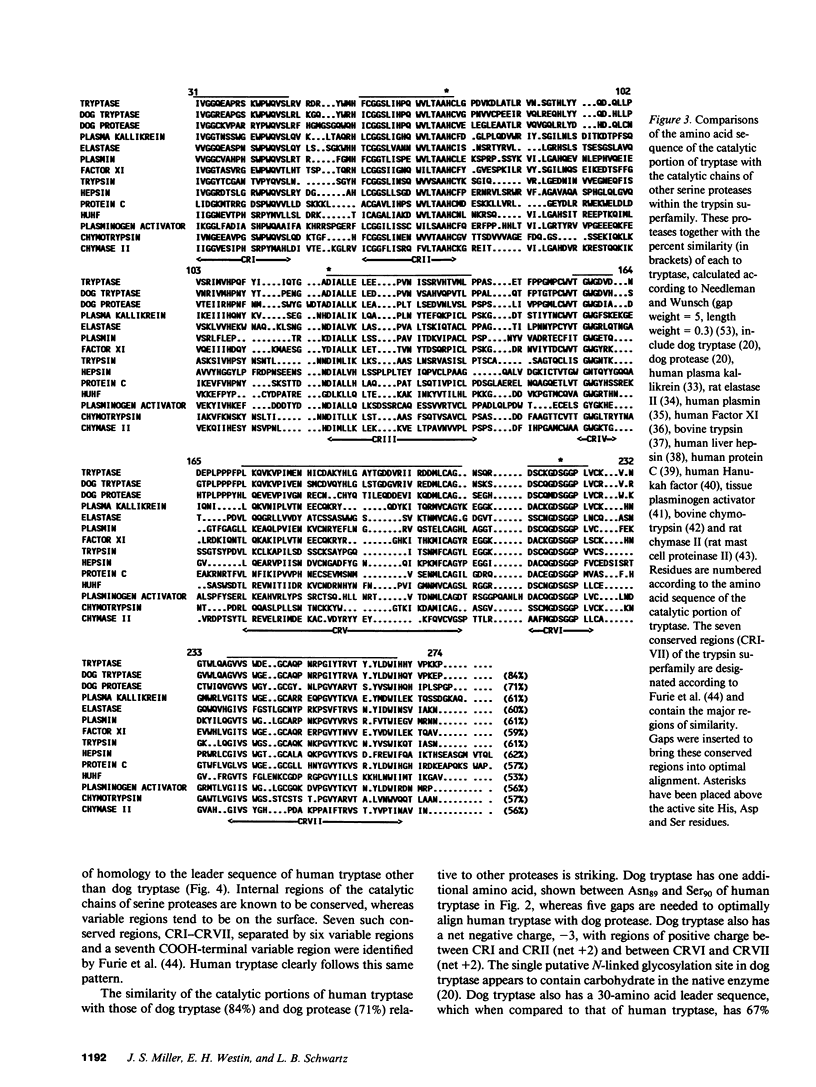
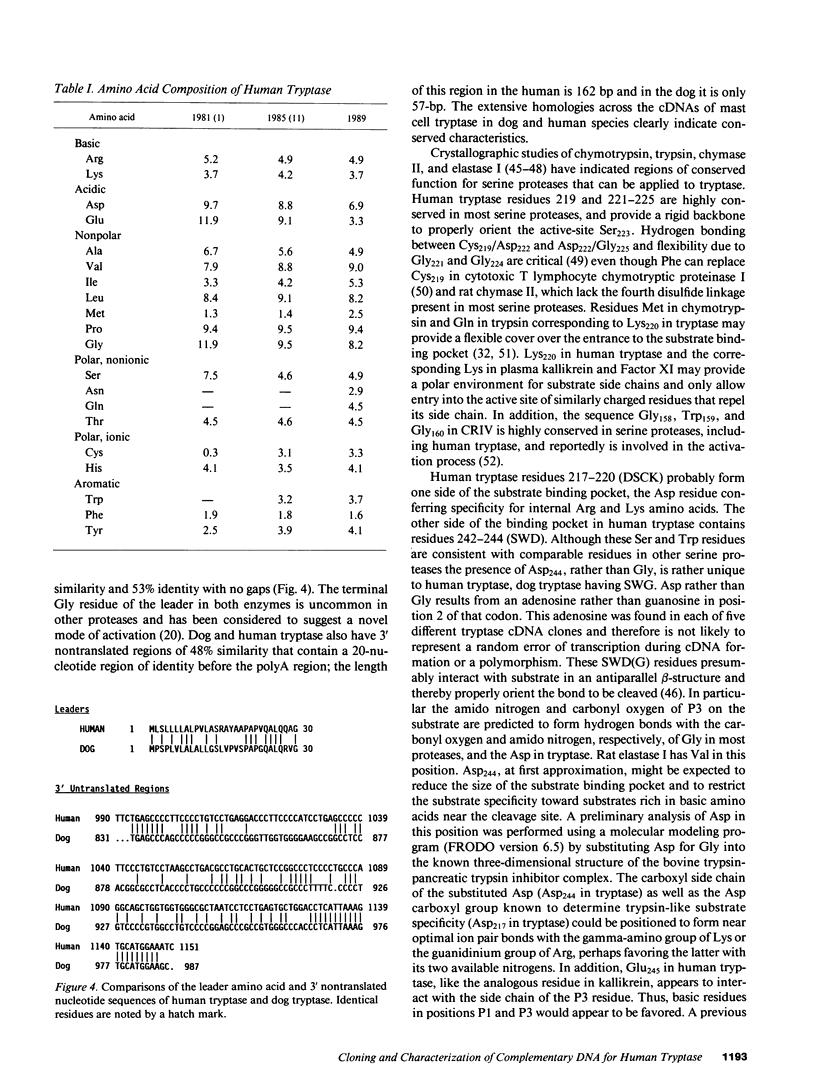
The amino acid sequence of human mast cell tryptase was determined from corresponding cDNA cloned from a lambda ZAP library made with mRNA derived from a human mast cell preparation. Tryptase is the major neutral protease present in human mast cells and serves as a specific marker of mast cells by immunohistologic techniques and as a specific indicator of mast cell activation when detected in biologic fluids. Based on nucleic acid sequence, human tryptase consists of a 244-amino acid catalytic portion of 27,423 D with two putative N-linked carbohydrate binding sites and a 30-amino acid leader sequence of 3,048 D. A His74, Asp120, Ser223 catalytic triad and four cystine groups were identified by analogy to other serine proteases. Regions of amino acid sequence that are highly conserved in serine proteases, in general, were conserved in tryptase. The catalytic portion of human tryptase had an 84% amino acid sequence similarity with that of dog tryptase; their leader sequences had a 67% similarity. Asp217 in the substrate binding pocket of human tryptase is consistent with a specificity for Arg and Lys residues at the site of cleavage (P1), whereas Glu245 is consistent with the known preference of human tryptase for substrates with Arg or Lys also at P3, analogous residues also being present in dog tryptase. Asp244, which is substituted for the Gly found in dog tryptase and in most serine proteases, is present in the putative substrate binding pocket and may confer additional substrate specificity on human tryptase for basic residues. Further studies now can be designed to elucidate these structure-function relationships.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter S. C., Metcalfe D. D., Bradford T. R., Schwartz L. B. Regulation of human mast cell tryptase. Effects of enzyme concentration, ionic strength and the structure and negative charge density of polysaccharides. Biochem J. 1987 Dec 15;248(3):821–827. doi: 10.1042/bj2480821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter S. C., Yates P., Margolius H. S., Schwartz L. B. Tryptase and kinin generation: tryptase from human mast cells does not activate human urinary prokallikrein. Int Arch Allergy Appl Immunol. 1987;83(3):321–324. doi: 10.1159/000234315. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham S., Stevens R. L., Nicodemus C. F., Gartner M. C., Austen K. F., Weis J. H. Molecular cloning of a cDNA that encodes the peptide core of a mouse mast cell secretory granule proteoglycan and comparison with the analogous rat and human cDNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3763–3767. doi: 10.1073/pnas.86.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M., Henderson R., Steitz T. A. I. Serine proteinases. The structure of alpha-chymotrypsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):67–76. doi: 10.1098/rstb.1970.0009. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Castells M., Schwartz L. B. Tryptase levels in nasal-lavage fluid as an indicator of the immediate allergic response. J Allergy Clin Immunol. 1988 Sep;82(3 Pt 1):348–355. doi: 10.1016/0091-6749(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Fujikawa K., McMullen B. A., Davie E. W. Human plasma prekallikrein, a zymogen to a serine protease that contains four tandem repeats. Biochemistry. 1986 May 6;25(9):2410–2417. doi: 10.1021/bi00357a017. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Silverton E. W., Davies D. R. Refined crystal structure of gamma-chymotrypsin at 1.9 A resolution. Comparison with other pancreatic serine proteases. J Mol Biol. 1981 Jun 5;148(4):449–479. doi: 10.1016/0022-2836(81)90186-8. [DOI] [PubMed] [Google Scholar]
- Craig S. S., DeBlois G., Schwartz L. B. Mast cells in human keloid, small intestine, and lung by an immunoperoxidase technique using a murine monoclonal antibody against tryptase. Am J Pathol. 1986 Sep;124(3):427–435. [PMC free article] [PubMed] [Google Scholar]
- Cromlish J. A., Seidah N. G., Marcinkiewicz M., Hamelin J., Johnson D. A., Chrétien M. Human pituitary tryptase: molecular forms, NH2-terminal sequence, immunocytochemical localization, and specificity with prohormone and fluorogenic substrates. J Biol Chem. 1987 Jan 25;262(3):1363–1373. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlhammer H., Bode W., Huber R. Crystal structure of bovine trypsinogen at 1-8 A resolution. II. Crystallographic refinement, refined crystal structure and comparison with bovine trypsin. J Mol Biol. 1977 Apr 25;111(4):415–438. doi: 10.1016/s0022-2836(77)80062-4. [DOI] [PubMed] [Google Scholar]
- Foster D. C., Yoshitake S., Davie E. W. The nucleotide sequence of the gene for human protein C. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4673–4677. doi: 10.1073/pnas.82.14.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Chung D. W., Hendrickson L. E., Davie E. W. Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochemistry. 1986 May 6;25(9):2417–2424. doi: 10.1021/bi00357a018. [DOI] [PubMed] [Google Scholar]
- Furie B., Bing D. H., Feldmann R. J., Robison D. J., Burnier J. P., Furie B. C. Computer-generated models of blood coagulation factor Xa, factor IXa, and thrombin based upon structural homology with other serine proteases. J Biol Chem. 1982 Apr 10;257(7):3875–3882. [PubMed] [Google Scholar]
- Gershenfeld H. K., Hershberger R. J., Shows T. B., Weissman I. L. Cloning and chromosomal assignment of a human cDNA encoding a T cell- and natural killer cell-specific trypsin-like serine protease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1184–1188. doi: 10.1073/pnas.85.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Homologies in serine proteinases. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):77–87. doi: 10.1098/rstb.1970.0010. [DOI] [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krieger M., Kay L. M., Stroud R. M. Structure and specific binding of trypsin: comparison of inhibited derivatives and a model for substrate binding. J Mol Biol. 1974 Feb 25;83(2):209–230. doi: 10.1016/0022-2836(74)90388-x. [DOI] [PubMed] [Google Scholar]
- Leytus S. P., Loeb K. R., Hagen F. S., Kurachi K., Davie E. W. A novel trypsin-like serine protease (hepsin) with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry. 1988 Feb 9;27(3):1067–1074. doi: 10.1021/bi00403a032. [DOI] [PubMed] [Google Scholar]
- Lobe C. G., Finlay B. B., Paranchych W., Paetkau V. H., Bleackley R. C. Novel serine proteases encoded by two cytotoxic T lymphocyte-specific genes. Science. 1986 May 16;232(4752):858–861. doi: 10.1126/science.3518058. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Quinto C., Swain W., Pictet R. L., Nikovits W., Rutter W. J. Primary structure of two distinct rat pancreatic preproelastases determined by sequence analysis of the complete cloned messenger ribonucleic acid sequences. Biochemistry. 1982 Mar 16;21(6):1453–1463. doi: 10.1021/bi00535a053. [DOI] [PubMed] [Google Scholar]
- Malinowski D. P., Sadler J. E., Davie E. W. Characterization of a complementary deoxyribonucleic acid coding for human and bovine plasminogen. Biochemistry. 1984 Aug 28;23(18):4243–4250. doi: 10.1021/bi00313a035. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Percy C., Young R. A. Gene isolation by screening lambda gt11 libraries with antibodies. Methods Enzymol. 1987;152:458–469. doi: 10.1016/0076-6879(87)52054-7. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Pfeffer D. Direct sequencing of denatured plasmid DNA. Methods Enzymol. 1987;152:556–562. doi: 10.1016/0076-6879(87)52061-4. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington S. J., Woodbury R. G., Reynolds R. A., Matthews B. W., Neurath H. The structure of rat mast cell protease II at 1.9-A resolution. Biochemistry. 1988 Oct 18;27(21):8097–8105. doi: 10.1021/bi00421a019. [DOI] [PubMed] [Google Scholar]
- Sawyer L., Shotton D. M., Campbell J. W., Wendell P. L., Muirhead H., Watson H. C. The atomic structure of crystalline porcine pancreatic elastase at 2.5 A resolution: comparisons with the structure of alpha-chymotrypsin. J Mol Biol. 1978 Jan 15;118(2):137–208. doi: 10.1016/0022-2836(78)90412-6. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Atkins P. C., Bradford T. R., Fleekop P., Shalit M., Zweiman B. Release of tryptase together with histamine during the immediate cutaneous response to allergen. J Allergy Clin Immunol. 1987 Dec;80(6):850–855. doi: 10.1016/s0091-6749(87)80276-2. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R., Littman B. H., Wintroub B. U. The fibrinogenolytic activity of purified tryptase from human lung mast cells. J Immunol. 1985 Oct;135(4):2762–2767. [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986 Jun 5;261(16):7372–7379. [PubMed] [Google Scholar]
- Schwartz L. B., Irani A. M., Roller K., Castells M. C., Schechter N. M. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987 Apr 15;138(8):2611–2615. [PubMed] [Google Scholar]
- Schwartz L. B., Kawahara M. S., Hugli T. E., Vik D., Fearon D. T., Austen K. F. Generation of C3a anaphylatoxin from human C3 by human mast cell tryptase. J Immunol. 1983 Apr;130(4):1891–1895. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Austen K. F. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981 Nov 25;256(22):11939–11943. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Seldin D., Austen K. F. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981 Apr;126(4):1290–1294. [PubMed] [Google Scholar]
- Schwartz L. B., Metcalfe D. D., Miller J. S., Earl H., Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987 Jun 25;316(26):1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B. Monoclonal antibodies against human mast cell tryptase demonstrate shared antigenic sites on subunits of tryptase and selective localization of the enzyme to mast cells. J Immunol. 1985 Jan;134(1):526–531. [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Sekizawa K., Caughey G. H., Lazarus S. C., Gold W. M., Nadel J. A. Mast cell tryptase causes airway smooth muscle hyperresponsiveness in dogs. J Clin Invest. 1989 Jan;83(1):175–179. doi: 10.1172/JCI113855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Hougland M. W., Johnson D. A. Human lung tryptase. Purification and characterization. J Biol Chem. 1984 Sep 10;259(17):11046–11051. [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]
- Tanaka T., McRae B. J., Cho K., Cook R., Fraki J. E., Johnson D. A., Powers J. C. Mammalian tissue trypsin-like enzymes. Comparative reactivities of human skin tryptase, human lung tryptase, and bovine trypsin with peptide 4-nitroanilide and thioester substrates. J Biol Chem. 1983 Nov 25;258(22):13552–13557. [PubMed] [Google Scholar]
- Toneguzzo F., Glynn S., Levi E., Mjolsness S., Hayday A. Use of a chemically modified T7 DNA polymerase for manual and automated sequencing of supercoiled DNA. Biotechniques. 1988 May;6(5):460–469. [PubMed] [Google Scholar]
- Vanderslice P., Craik C. S., Nadel J. A., Caughey G. H. Molecular cloning of dog mast cell tryptase and a related protease: structural evidence of a unique mode of serine protease activation. Biochemistry. 1989 May 16;28(10):4148–4155. doi: 10.1021/bi00436a004. [DOI] [PubMed] [Google Scholar]
- Wenzel S. E., Fowler A. A., 3rd, Schwartz L. B. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988 May;137(5):1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Katunuma N., Kobayashi K., Titani K., Neurath H., Anderson W. F., Matthews B. W. Covalent structure of a group-specific protease from rat small intestine. Appendix: crystallographic data for a group specific protease from rat intestine. Biochemistry. 1978 Mar 7;17(5):811–819. doi: 10.1021/bi00598a010. [DOI] [PubMed] [Google Scholar]


