Abstract
Staphylococci cause bovine mastitis, with Staphylococcus aureus being responsible for the majority of the mastitis-based losses to the dairy industry (up to $2 billion/annum). Treatment is primarily with antibiotics, which are often ineffective and potentially contribute to resistance development. Bacteriophage endolysins (peptidoglycan hydrolases) present a promising source of alternative antimicrobials. Here we evaluated two fusion proteins consisting of the streptococcal λSA2 endolysin endopeptidase domain fused to staphylococcal cell wall binding domains from either lysostaphin (λSA2-E-Lyso-SH3b) or the staphylococcal phage K endolysin, LysK (λSA2-E-LysK-SH3b). We demonstrate killing of 16 different S. aureus mastitis isolates, including penicillin-resistant strains, by both constructs. At 100 μg/ml in processed cow milk, λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b reduced the S. aureus bacterial load by 3 and 1 log units within 3 h, respectively, compared to a buffer control. In contrast to λSA2-E-Lyso-SH3b, however, λSA2-E-LysK-SH3b permitted regrowth of the pathogen after 1 h. In a mouse model of mastitis, infusion of 25 μg of λSA2-E-Lyso-SH3b or λSA2-E-LysK-SH3b into mammary glands reduced S. aureus CFU by 0.63 or 0.81 log units, compared to >2 log for lysostaphin. Both chimeras were synergistic with lysostaphin against S. aureus in plate lysis checkerboard assays. When tested in combination in mice, λSA2-E-LysK-SH3b and lysostaphin (12.5 μg each/gland) caused a 3.36-log decrease in CFU. Furthermore, most protein treatments reduced gland wet weights and intramammary tumor necrosis factor alpha (TNF-α) concentrations, which serve as indicators of inflammation. Overall, our animal model results demonstrate the potential of fusion peptidoglycan hydrolases as antimicrobials for the treatment of S. aureus-induced mastitis.
INTRODUCTION
Bovine mastitis, an inflammation of the mammary gland caused mostly by bacterial infection, is the most widespread disease in dairy cattle (40, 47), resulting in the highest use of antibiotics in the dairy industry (27). It is also the most costly disease in animal agriculture, resulting in losses of up to $2 billion per year in the United States alone (37, 61), which corresponds to annual costs of approximately $200 per cow (39). Staphylococcus aureus is responsible for the largest share of intramammary infections in ruminants, accounting for up to 30% of clinical mastitis cases. S. aureus infections are also the most difficult to cure (48, 66). The efficacy of the vaccines that are currently available is limited (46, 66) (despite some promising developments in recent years [52]), and treatment is restricted to the use of broad-range antibiotics, such as tetracycline, penicillin, and pirlimycin (18, 24), usually administered through intramammary infusions (31, 33). Cure rates for antibiotic treatment are often lower than 15%, which is ascribed to poor penetration of the gland by the drugs and survival of S. aureus inside phagocytic or mammary gland epithelial cells (9, 56, 66). Moreover, the occurrence and spread of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), are of increasing concern not only in human clinics but also in farm animals, discouraging the use of broad-range antibiotics and warranting the need for novel antimicrobials for the treatment of S. aureus bovine mastitis (24, 42, 64).
Bacteriophage endolysins have received increasing attention as antimicrobial agents in recent years, particularly due to their high target cell specificity and because development of resistance against these enzymes is considered unlikely, due to the coevolution of phage and host and the fact that these enzymes cleave highly conserved bonds within the bacterial peptidoglycan (PG) (reviewed in references 6, 23, 29, and 44). Endolysins are produced at the end of the lytic multiplication cycle of the phage and gain access to the host cell wall from within, mostly through the pores in the cytoplasmic membrane created by phage-encoded holin proteins (67, 68). Degradation of the PG by the endolysin in combination with the bacterial cell's internal turgor pressure results in cell lysis and liberation of phage progeny. In the case of Gram-positive bacteria such as staphylococci, which lack an outer membrane, these proteins can also act as exolysins (i.e., lyse the bacteria from without), making them potential antimicrobials, with possible applications in biotechnology, medicine, food safety, and agriculture (28, 36, 44). Furthermore, application from without is expected to reduce the number of possible resistance mechanisms, as, e.g., active efflux from the bacterial cell or reduced membrane permeability would likely affect only agents attacking targets inside the cell (22).
Endolysins from a Gram-positive background show a modular architecture, consisting of at least one enzymatically active domain (EAD), which cleaves a specific bond in the PG, and at least one cell wall binding domain (CBD), which targets the enzyme to its substrate in the cell wall, often with high specificity (44). PG hydrolases specific for the same cell wall type but featuring different cut sites in the PG have been shown to act synergistically against their target cells when used in combination (3, 43). Moreover, the modular organization of phage endolysins allows modification and optimization of lytic and binding properties by rational design and molecular engineering, yielding chimeric enzymes (5, 15, 16, 20, 24, 60).
When the efficacy of antimicrobial agents for treatment of bovine mastitis is being assessed, in vitro experiments are of limited significance due to the complex interactions between pathogens, antimicrobials, and components of the host's immune response inside the mammary gland. On the other hand, high costs and complex management are associated with studies in cows. The mouse model of bovine mastitis, developed in the early 1970s (11), is a suitable and relatively inexpensive analysis as a prelude to a cow study (reviewed in references 9 and 48). Numerous studies have been carried out using this model to test the efficacy of various antimicrobial agents and stimulants of the immune response against S. aureus-induced mastitis (1, 7, 8, 12, 14, 19, 56). One of these agents is lysostaphin, a staphylococcal PG hydrolase that is similar to a bacteriophage endolysin with regard to its modular architecture and mechanism of action (57). Lysostaphin has been shown to treat or prevent mastitis caused by S. aureus both in mouse models and in cattle, when infused into the teat canal or expressed in mammary glands of transgenic animals (7, 39, 51, 66). A major drawback of using lysostaphin as a single therapeutic, however, is its susceptibility to resistance development, which is due to the fact that it cleaves within a variable part of the staphylococcal peptidoglycan, the pentaglycine bridge (57). This portion can be altered by the bacterium, as reflected by a number of characterized lysostaphin-resistant strains (17, 26, 34) and the identification of genes whose products have the enzymatic activity to perform this modification (13, 62).
To our knowledge, there are no studies to date reporting the use of bacteriophage endolysins or endolysin-derived antimicrobials in a mouse model of bovine mastitis. The enzymes λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b are chimeric lysins consisting of an endopeptidase domain of the streptococcal phage lysin λSA2 and SH3b CBDs from the staphylococcal phage lysin LysK and lysostaphin, respectively (5). The endopeptidase domain used for the fusions originates from the endolysin of the λSA2 prophage of Streptococcus agalactiae serotype V strain 2603 V/R (ATCC BAA-611), a human clinical isolate (54). The cut site of the λSA2 endopeptidase (54) is conserved in both streptococcal and staphylococcal PG, and the fusion to Staphylococcus-specific CBDs renders the enzymes highly active against S. aureus while maintaining considerable streptolytic activity (5). Here we evaluated the potential of λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b as antimicrobial agents against S. aureus-induced bovine mastitis using a mouse model.
MATERIALS AND METHODS
Plasmids, bacterial strains, and growth conditions.
Plasmids encoding the C-terminally 6×His-tagged chimeric proteins λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b in a pET21a backbone (EMD Biosciences, San Diego, CA) were described earlier (5), as well as the pET21a construct expressing mature lysostaphin (accession no. P10547.2) with a C-terminal 6×His tag (4). Overexpression of proteins was performed in Escherichia coli BL21(DE3) (Invitrogen, Carlsbad, CA) cultured at 37°C in modified Luria-Bertani (mLB) medium (15 g/liter tryptone, 8 g/liter yeast extract, 5 g/liter NaCl) (59) supplemented with 150 μg/ml ampicillin for plasmid selection and maintenance. Staphylococcal strains used include the mastitis strain S. aureus Newbould 305 (ATCC 29740) and a collection of 15 isolates from mastitic cows (a gift from Yasunori Tanji, Tokyo Institute of Technology, Yokohama, Japan) (63). Staphylococci were grown in tryptic soy broth (TSB) at 37°C.
Expression and purification of 6×His-tagged proteins.
Protein expression and purification were performed essentially as previously described (25), with the following modifications. Induced E. coli cultures were harvested, resuspended in 10 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 30% glycerol [pH 8.0]) per 1-liter culture, and sonicated on ice for 5 min (1-s pulses separated by 1-s rests). After removal of debris by centrifugation (9,000 × g for 30 min), 6×His-tagged proteins were purified from the cleared supernatant by immobilized metal ion affinity chromatography, using nickel-NTA Superflow resin (Qiagen, Valencia, CA). Purification columns were washed with 25 column volumes (CV) of lysis buffer supplemented with 0.1% Triton X-114 for removal of endotoxins (55), 40 CV of lysis buffer, and 15 CV of wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 30% glycerol [pH 8.0]). Target proteins were eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, 30% glycerol [pH 8.0]) in 500-μl fractions. Fractions with high protein concentrations were combined, filter sterilized (0.22 μm), and stored on ice. For animal experiments, proteins were transferred to Dulbecco's phosphate-buffered saline (DPBS; Thermo Scientific, Rockford, IL) supplemented with 1% glycerol via Zeba desalting columns (Thermo Scientific) and filter sterilized. Protein concentrations were measured spectrophotometrically using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE), and purities were determined via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Endotoxin concentrations were determined using a Limulus amoebocyte lysate (LAL) assay (Lonza, Walkersville, MD).
Plate lysis assay.
To test activity of chimeric lysins and lysostaphin against multiple mastitis strains, purified proteins (10, 1, and 0.1 μg in a volume of 10 μl) were spotted onto a freshly spread lawn of log-phase staphylococcal cells (optical density at 600 nm [OD600] = 0.4 to 0.6, diluted 1/4 immediately before plating) that had air dried for 15 min on gridded tryptic soy agar (TSA) plates. Buffer without protein was spotted as a control. The plates were air dried for another 10 min in a laminar flow hood and incubated overnight at 37°C. Cleared spots indicating cell lysis were scored within 24 h of plating the cells. Staphylococcal strains used for plate lysis are shown in Table 1. To determine minimum inhibitory amounts (MIAs) of lysins against S. aureus Newbould 305 in a plate lysis assay, 2-fold serial dilutions of each protein were spotted on a bacterial lawn as described above. The smallest amount of protein in a volume of 10 μl causing a lysis zone was defined as the protein's MIA.
Table 1.
Activities of λSA2-E-Lyso-SH3b, λSA2-E-LysK-SH3b, and lysostaphin against S. aureus mastitis isolates, as determined by plate lysis
| Strain | Source or reference | Susceptibilitya |
||
|---|---|---|---|---|
| λSA2-E-Lyso-SH3b | λSA2-E-LysK-SH3b | Lysostaphin | ||
| Newbould 305 | ATCC 29740 | +++ | +++ | +++ |
| SA001 | 63 | +++ | +++ | +++ |
| SA002 | 63 | +++ | +++ | +++ |
| SA003 | 63 | +++ | +++ | +++ |
| SA009 | 63 | +++ | +++ | +++ |
| SA019 | 63 | +++ | +++ | +++ |
| SA020 | 63 | +++ | +++ | +++ |
| SA021 | 63 | ++ | +++ | +++ |
| SA026 | 63 | ++ | +++ | +++ |
| SA028 | 63 | ++ | ++ | +++ |
| SA029 | 63 | +++ | +++ | +++ |
| SA031 | 63 | +++ | +++ | +++ |
| SA033 | 63 | ++ | ++ | +++ |
| SA047 | 63 | +++ | +++ | +++ |
| SA048 | 63 | +++ | +++ | +++ |
| SA049 | 63 | +++ | +++ | +++ |
Smallest amount of protein in a volume of 10 μl causing a lysis zone after overnight incubation: +++, 0.1 μg; ++, 1 μg. Scores represent averaged results from three separate experiments.
Activity in cow milk.
The activity of chimeric peptidoglycan hydrolases in cow milk was determined as described by Obeso et al. (49). Commercial whole-fat ultra-high-temperature-sterilized (UHT) milk (Parmalat) at 37°C was inoculated with either 2 × 103 CFU/ml or 2 × 106 CFU/ml of exponentially growing cells of S. aureus Newbould 305. Immediately after inoculation, purified lysins (100 μg/ml) or buffer (control) was added, and the milk samples were incubated at 37°C without shaking. Bacterial concentrations were determined by serial dilution plating on tryptic soy agar (TSA) of samples taken immediately before and after addition of enzymes and then at 1-h intervals for up to 3 h. The absence of bacteria in noninoculated milk was verified by direct plating on TSA. All experiments were repeated at least three times.
Mouse model of bovine mastitis.
To evaluate the efficacy of purified, individually administered peptidoglycan hydrolases in a mouse model of bovine mastitis, female C57BL6/SJL mice were challenged intramammarily with S. aureus Newbould 305 as described earlier (65), followed by intramammary infusions of proteins or buffer as control. Two different procedures (A and B) were compared as described below. On the day of challenge, dams between days 7 and 15 of lactation were separated from their pups for 4 h. After that, pups were allowed to nurse for 1 h in order to deplete mammary glands of milk. Experimental animals were anesthetized by intraperitoneal injection of Avertin (375 μg/g body weight), and three of four glands used in the experiment (R3, R4, L3, L4) were infused with 102 CFU (procedure A) or 104 CFU (procedure B) of S. aureus in 50 μl of 0.4% trypan blue solution (Sigma, St. Louis, MO). To facilitate the inoculation, approximately 0.5 mm of the distal end of the teat was removed (snipped), and a 32-gauge luer-lock stub adapter (LSA-32; Access Technologies, Norfolk Medical, Inc., Skokie, IL) attached to a Hamilton syringe filled with the bacterial suspension was threaded approximately 4 mm into the teat canal. The fourth teat was snipped, but no infusion was performed on this gland. At 6 h (procedure A) or 30 min (procedure B) after the challenge, two of the challenged glands were infused with 25 μg of λSA2-E-Lyso-SH3b or λSA2-E-LysK-SH3b or lysostaphin in a volume of 50 μl of DPBS plus 1% glycerol, whereas the third challenged gland received 50 μl of buffer without protein. No infusion was performed on the fourth (snipped only) gland. Following infusions, Vetbond surgical glue (3M Animal Care Products, St. Paul, MN) was placed on all snipped teats to prevent leaking and cross-contamination, and mice were returned to their cages. Animals were euthanized 24 h after the bacterial challenge, and mammary glands were aseptically dissected and weighed. A portion of each gland was homogenized in DPBS (100 mg/ml) using a Polytron (Kinematica, Lucerne, Switzerland), and serial dilution plating on TSA was performed to determine intramammary bacterial concentrations. An aliquot of the homogenized gland was centrifuged (15,800 × g, 15 min, 5°C), and the supernatant was stored at −20°C until used for the tumor necrosis factor alpha (TNF-α) assay (see below).
To determine the effect of combined administration of λSA2-E-LysK-SH3b and lysostaphin, experiments were performed as delineated above, with the following modifications. All animals were challenged with 103 CFU per gland in four glands and treated 30 min after the challenge with buffer (control), λSA2-E-LysK-SH3b (25 μg/gland), lysostaphin (25 μg/gland), and a combination of λSA2-E-LysK-SH3b and lysostaphin (a mixture of 12.5 μg of each protein per gland). Every animal received all four treatments in four different glands in a randomized fashion. Mice were euthanized 18 h after the challenge. All animal experiments were conducted in accordance with a protocol approved by the Beltsville Agricultural Research Center Institutional Animal Care and Use Committee.
Determination of synergy in vitro.
A modified version of a classical checkerboard assay (32) using a plate lysis instead of a microdilution broth format was employed to examine synergistic effects between the chimeric peptidoglycan hydrolases and lysostaphin. The plate lysis format was chosen because the microdilution broth method described by Jones et al. (38) for determination of MICs failed to yield a clear MIC for either λSA2-E-Lyso-SH3b or λSA2-E-LysK-SH3b, making it difficult to set up checkerboard experiments with these proteins using the broth method. For the plate lysis checkerboard assays, twofold serial dilutions of both enzymes to be tested were prepared, mixed in two dimensions in a 96-well dish, and then spotted onto a freshly plated and air-dried lawn of S. aureus Newbould 305 on a gridded TSA plate as described above. The serial dilutions were prepared so that the largest amount spotted for each individual enzyme was its MIA. After overnight incubation, lysis zones were evaluated by densitometry using the software Alpha Imager (Alpha Innotech, San Leandro, CA). A photograph of the plate was taken, and for every square of the grid, the inverted integrated density value (IDV) of the circular spot area was calculated. From each value, a background value (corresponding to the area outside the spot) was subtracted. The lower of the two values corresponding to the MIAs of the two enzymes was defined as the cutoff value, and every spot on the plate yielding a value equal to or greater than the cutoff value was considered a lysis zone. For each lysis zone along the inhibitory line on the plate, the sum of the fractional inhibitory amounts (FIAs; corresponding to FICs in the microdilution broth method) of both proteins (ΣFIA = FIAA + FIAB) was calculated. According to Hall et al. (35), a ΣFIC of <0.5 indicates strong synergy. An isobologram was created as described by Loeffler and Fischetti (43) from the results of at least three experiments.
Determination of intramammary TNF-α concentration.
The Quantikine mouse TNF-α/TNFSF1A immunoassay (R&D Systems, Minneapolis, MN) was used for determination of TNF-α concentration in murine mammary glands. Seventy-five homogenized gland tissue samples from the animal experiments outlined above (8 to 15 samples per treatment) were randomly selected, and 50 μl of the cleared supernatants was used for the enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's instructions. Samples from glands not inoculated with bacteria were included as controls. A450 readings were taken in a Spectra Max 340 96-well plate reader (Molecular Devices, Sunnyvale, CA), wavelength correction was done by A540 readings, and TNF-α concentrations were determined using a standard curve.
Statistical analysis.
For the mouse experiments with individually administered enzymes using two different procedures, the variables log10 CFU and wet weight were analyzed as two-factor repeated-measures models using PROC MIXED (SAS Institute, Cary, NC) with mouse ID as the repeated factor. The unstructured covariance structure was used to model the within mouse correlation. For the experiments with λSA2-E-LysK-SH3b and lysostaphin tested in combination, one-factor repeated-measures models were employed. The compound symmetric structure was used for log10 CFU, and the autoregressive integrated moving average (ARIMA) (1) structure was used for wet weight. One probable outlier was excluded from the analysis. In case of the TNF-α experiment, a one-factor model was used. Two probable outliers were excluded from the analysis, and the variance grouping technique was used to correct for heterogeneous variances. The assumptions of all models used were checked and met. Means comparisons were done with Sidak adjusted P values so that the experiment-wise error was 0.05.
RESULTS
Chimeric lysins show activity against various mastitis causing S. aureus strains.
The chimeric lysins λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b were produced in E. coli and purified via their C-terminal 6×His tags, yielding >90% purity, as had been demonstrated previously (5) (data not shown). Both proteins as well as lysostaphin were tested in plate lysis assays against multiple S. aureus mastitis strains. These included the reference strain S. aureus Newbould 305 and a collection of 15 different isolates from mastitic cows in Japan (63). As shown in Table 1, all tested strains were susceptible to the lytic action of both enzymes, with 100 ng to 1 μg of protein causing visible lysis zones on the bacterial lawns. While the smallest amounts tested of both proteins were active against 12 of 16 strains, strains SA021 and SA026 required a larger amount of λSA2-E-Lyso-SH3b, and strains SA028 and SA033 were less sensitive to both enzymes. Lysostaphin exhibited strong lytic activity against all tested strains at 1 μg per spot.
Lytic activity in cow milk.
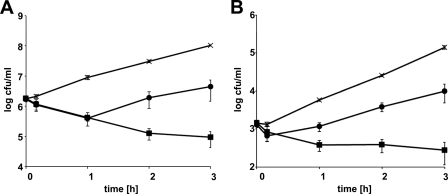
As a first approach to evaluate the suitability of λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b as antimicrobial agents against bovine mastitis-causing staphylococci, their lytic activity in cow milk against exponentially growing S. aureus cells was tested, using an in vitro assay described by Obeso et al. (49). Commercial ultra-high-temperature-processed whole milk at 37°C was inoculated with S. aureus Newbould 305 at two different inoculation levels (2 × 103 and 2 × 106 CFU/ml, mimicking bacterial concentrations at early and acute stages of mammary gland infection, respectively), followed by addition of enzymes at 100 μg/ml (Fig. 1). Both enzymes were demonstrated to be active in the milk, reducing bacterial numbers over the course of the experiment (3 h) compared to the buffer control for both high and low inoculation levels. However, whereas λSA2-E-LysK-SH3b merely caused a temporary decrease in viable counts, followed by resumed cell growth, λSA2-E-Lyso-SH3b continued to kill S. aureus cells until the end of the experiment. After 3 h, the difference in viable counts between the protein and control samples was approximately 3 log units for λSA2-E-Lyso-SH3b and between 1 and 1.5 log units for λSA2-E-LysK-SH3b.
Fig 1.
Effect of chimeric lysins on bacterial concentrations of S. aureus in whole cow milk at 37°C. Commercial UHT homogenized whole cow milk was inoculated with 2 × 106 (A) or 2 × 103 (B) CFU/ml of exponentially growing S. aureus Newbould 305, and λSA2-E-Lyso-SH3b (■) or λSA2-E-LysK-SH3b (●) at a concentration of 100 μg/ml, or buffer (×) as a control, was added after inoculation. Bacterial concentrations were determined by serial dilution plating of samples taken immediately before and after addition of enzyme or buffer and at 1-h intervals thereafter. Error bars represent standard errors of the means from at least three separate experiments.
Chimeric lysins reduce bacterial concentrations in a mouse model of mastitis.
Intramammary infection in murine mammary glands depleted of milk was initiated by infusing cells of the S. aureus mastitis strain Newbould 305 in a 0.4% trypan blue solution into the teat canal. Trypan blue had been shown in a previous study to be a suitable marker dye to confirm successful infusion of the mammary gland without eliciting a detectable immune response (65). Proteins used for treatment of the mice were purified using the Triton X-114 protocol for removal of bacterial endotoxins (55), resulting in endotoxin concentrations of <5 endotoxin units/ml (data not shown). Following inoculation, the infected glands were treated with 25 μg/gland of either λSA2-E-Lyso-SH3b, λSA2-E-LysK-SH3b, or lysostaphin, or with buffer as a control. Lysostaphin has been demonstrated to be a potent antimicrobial against intramammary S. aureus infection in a similar mouse model (7), and it was therefore used as a positive control in this study. Two procedures (A and B) were performed, with differences in the bacterial concentration used for inoculation and the time between inoculation and treatment, as shown in Table 2. At the time of mammary gland dissection (24 h after the challenge), the success of bacterial infusion and penetration of the glands was assessed by visualization of the trypan blue (see Fig. S1 in the supplemental material), and only glands successfully infected were used for further analysis. Mammary glands were analyzed for bacterial concentration and wet weight, the latter being an indicator of edema caused by the bacterium-induced inflammatory response (65). Statistical analysis revealed that there was no significant difference between procedure A (102 CFU, 6 h) and B (104 CFU, 30 min) in terms of the outcome of the experiment, regarding both bacterial numbers (P = 0.37) and wet weights (P = 0.24). In both cases, S. aureus numbers in the control (buffer-treated) glands at the time of harvest exceeded 106 CFU/mg, corresponding to approximately 109 CFU per average mammary gland (Table 2). Both chimeric lysins significantly (P < 0.05) reduced bacterial numbers in the mammary glands compared to the control (0.63 and 0.81 log units for λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b, respectively), but their effects were considerably weaker than that of lysostaphin (2.82-log unit reduction). A similar trend was observed for the effect of protein treatments on mammary gland wet weight. The mean wet weights for all treatments were lower than that of the buffer control; however, lysostaphin was the only treatment resulting in a statistically significant difference.
Table 2.
Mean intramammary concentrations of S. aureus Newbould 305 and mean wet weights of infected mammary glands receiving different treatmentsa
| Treatment | Log10 CFU/mg |
Wet wt (g) |
||||
|---|---|---|---|---|---|---|
| A | B | Mean | A | B | Mean | |
| Buffer | 6.14 (22) | 6.25 (21) | 6.19 ± 0.10 a | 1.73 (20) | 1.75 (19) | 1.74 ± 0.03 a |
| λSA2-E-Lyso-SH3b | 5.50 (7) | 5.63 (7) | 5.56 ± 0.22 b | 1.69 (7) | 1.72 (6) | 1.70 ± 0.03 ab |
| λSA2-E-LysK-SH3b | 5.23 (7) | 5.53 (7) | 5.38 ± 0.22 b | 1.69 (6) | 1.71 (7) | 1.70 ± 0.03 ab |
| Lysostaphin | 3.17 (7) | 3.57 (6) | 3.37 ± 0.23 c | 1.56 (6) | 1.65 (5) | 1.60 ± 0.03 b |
| S. aureus mean | 5.01 | 5.24 | 1.67 | 1.71 | ||
Glands were inoculated with 100 (procedure A) or 10,000 (procedure B) CFU/gland and treated with λSA2-E-Lyso-SH3b, λSA2-E-LysK-SH3b, lysostaphin, or buffer (control) after 6 h (A) or 30 min (B). The number of glands used for each treatment in the analysis is given in parentheses. Values followed by different letters are significantly different (P < 0.05).
Synergistic effect with λSA2 endopeptidase fusions and lysostaphin.
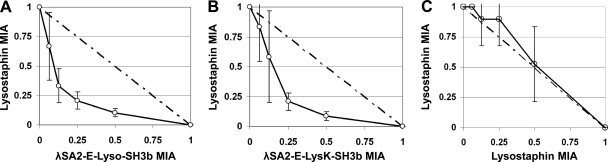
In order to identify a more robust mastitis treatment, the possibility of antimicrobial synergy between the λSA2 endopeptidase fusions and lysostaphin was explored. The staphylococcal peptidoglycan cut sites of lysostaphin and the λSA2 endopeptidase domain have been shown to be different (54, 58), giving rise to the potential for a synergistic effect when these proteins are applied simultaneously. To investigate this possibility, the checkerboard synergy testing method using a plate lysis format was employed. The minimum inhibitory amounts (MIAs) of lysostaphin, λSA2-E-Lyso-SH3b, and λSA2-E-LysK-SH3b against S. aureus Newbould 305 as determined by plate lysis were 0.042 ± 0.025 μg, 0.34 ± 0.13 μg, and 0.38 ± 0.18 μg, respectively. When checkerboard assays were performed using twofold serial dilutions of these MIAs (see Fig. S2 in the supplemental material), the resulting isobolograms yielded curves characteristic of antimicrobial synergy for the interactions of lysostaphin with either λSA2-E-Lyso-SH3b or λSA2-E-LysK-SH3b (Fig. 2A and B). As a control, lysostaphin was used in both dimensions of the checkerboard plate, yielding a curve expected of an additive effect (Fig. 2C). The calculated ΣFIAs were 0.42 ± 0.07 (lysostaphin with λSA2-E-Lyso-SH3b) and 0.46 ± 0.07 (lysostaphin with λSA2-E-LysK-SH3b), indicating a strong synergistic effect with both combinations.
Fig 2.
Isobolograms of the plate lysis checkerboard synergy testing method showing synergy of λSA2-E-Lyso-SH3b with lysostaphin (A) and of λSA2-E-LysK-SH3b with lysostaphin (B) and the additive effect observed when lysostaphin was used on both axes of the plate (C). Lysis zones on checkerboard plates were evaluated by densitometry. For each square of the plate along the inhibitory line, protein amounts (as fractions of the enzymes' MIAs) were entered in an x/y plot. Error bars represent standard errors of the means from three to five independent experiments. The dashed line is the theoretical curve expected for an additive effect.
To determine if this synergy is effective in vivo, a combination of λSA2-E-LysK-SH3b and lysostaphin was tested in the mouse model described above (Table 3). λSA2-E-LysK-SH3b was chosen over λSA2-E-Lyso-SH3b for this experiment, as it tended to exhibit a slightly stronger effect in the mouse model (Table 2). In the combined treatment, each protein was administered at half the concentration of the individually applied proteins. As a minor modification of the mammary gland challenge protocol, the S. aureus concentration in the inoculum (103 CFU/gland) and the time between challenge and mammary gland dissection (18 h) were slightly different from those in the earlier experiments. However, bacterial CFU in the control glands reached comparable numbers after 18 h and 24 h, exceeding 106 CFU/mg, and similar effects were observed regarding reduction of bacterial numbers by λSA2-E-LysK-SH3b and lysostaphin administered individually (0.86- and 2.14-log reductions, respectively) compared to the control. The dual treatment reduced bacterial numbers by 3.36 log units compared to the control, which was a significantly (P < 0.05) greater effect than those of the individually applied proteins (Table 3). All protein-treated glands showed significantly lower wet weights than the control glands, suggesting decreased levels of inflammation (65), even though differences between the protein treatments were not statistically significant.
Table 3.
Mean intramammary concentrations of S. aureus 305 and mean wet weights of infected mammary glands treated with λSA2-E-LysK-SH3b and lysostaphin individually or in combinationa
| Treatment | No. of glands | Log10CFU/mg | Wet wt (g) |
|---|---|---|---|
| Buffer | 16 | 6.31 ± 0.21 a | 1.83 ± 0.03 a |
| λSA2-E-LysK-SH3b | 13 | 5.45 ± 0.23 b | 1.66 ± 0.03 b |
| Lysostaphin | 10 | 4.17 ± 0.25 c | 1.62 ± 0.04 b |
| λSA2-E-LysK-SH3b + lysostaphin | 8 | 2.95 ± 0.28 d | 1.68 ± 0.04 b |
Mammary glands were inoculated with 1,000 CFU/gland and then treated with λSA2-E-LysK-SH3b and lysostaphin either individually (25 μg/gland) or in combination (12.5 μg each/gland). Means followed by different letters are significantly different (P < 0.05).
Effect of lysin administration on TNF-α concentration in mammary gland tissue.
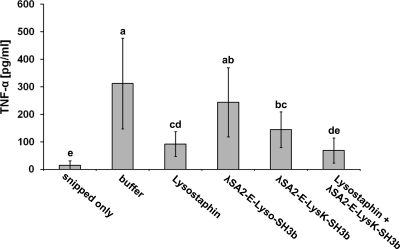
In addition to edema formation, which results in higher gland weights, the concentrations of certain proinflammatory cytokines, such as TNF-α, in mammary gland tissue can be used as indicators of inflammation (65). In order to determine the effects of different protein treatments on intramammary TNF-α concentrations, homogenized mammary gland samples from the mouse experiments in this study were randomly selected, and TNF-α concentrations were determined by an ELISA (Fig. 3). Glands challenged with S. aureus and treated with buffer alone showed a >20-fold increase in TNF-α compared to glands that had not been infused with bacteria (i.e., glands that were snipped only). Despite high variability observed for some treatments, all protein treatment sample means except those for λSA2-E-Lyso-SH3b were significantly (P < 0.05) lower than the mean for the buffer-treated control glands. Furthermore, the effects of different protein treatments on TNF-α concentrations showed a trend similar to that of the effects on bacterial concentrations (Tables 2 and 3), with λSA2-E-Lyso-SH3b being least and the combination of lysostaphin and λSA2-E-LysK-SH3b being most effective. The combination treatment did not significantly differ from the lysostaphin treatment; however, it was also the only treatment resulting in TNF-α concentrations not significantly different from those in glands not infused with bacteria (snipped only).
Fig 3.
Effect of protein treatments on intramammary TNF-α concentration. “Snipped only” represents samples from glands that were snipped but not inoculated with bacteria. “Buffer” indicates glands that were inoculated with bacteria and later received DPBS without lysin. The remaining treatments consisted of inoculation of glands with bacteria, followed by administration of lysins in DPBS. Protein concentrations were 25 μg/gland for individually administered proteins (lysostaphin, λSA2-E-Lyso-SH3b, and λSA2-E-LysK-SH3b) and 12.5 μg/gland for each protein in the combination treatment (lysostaphin + λSA2-E-LysK-SH3b). Error bars represent standard deviations from 8 to 15 samples per treatment. Treatment means with different letters are significantly different (P < 0.05).
DISCUSSION
The chimeric fusion proteins λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b each consist of the same streptococcal EAD and a unique staphylococcal CBD. Both chimeras were previously demonstrated to kill mastitis-causing streptococci as well as lab strains of S. aureus in several in vitro assays (5). Here we showed that both chimeric enzymes are active against 16 of 16 previously characterized bovine mastitis isolates in plate lysis assays and against one of these (Newbould 305) in cow milk and a mouse model of mastitis. Both constructs demonstrated antimicrobial synergy with lysostaphin in the checkerboard assay, and when they were tested in the mouse model, a more robust eradication of S. aureus was achieved when both lysostaphin and the λSA2-E-LysK-SH3b fusion were added simultaneously.
Activity in milk or a milk-like environment is an essential requirement for an anti-mastitis drug administered through the teat canal. Even if treatment is performed on glands depleted of milk or at dry-off, inhibition by residual milk components may potentially impair the efficacy of any antimastitis agent. The λSA2 endolysin is highly active in cow milk against streptococcal cells (M. Schmelcher, unpublished data), rendering the fusion proteins λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b (which target both streptococcal and staphylococcal cells in vitro) promising candidates to target S. aureus in milk. Although conclusions as to the efficacy of a protein antimicrobial inside a mammary gland should not be drawn from the results of ex vivo milk experiments, we employed cow milk assays as a standardized and quick way to screen for candidate antimastitis enzymes. Commercially heat-treated sterile cow milk was used in an effort to reduce the complexity of the flora in the milk; however, it should be noted that the activity of the lysins observed in this setting is potentially different from the activity in raw cow milk, as S. aureus has been reported to aggregate and associate with fat globules in raw milk, and this association is eliminated by pasteurization, i.e., heat treatment (41, 50). Even though both chimeric proteins diminished bacterial numbers in milk compared to a no-enzyme control, cells exposed to λSA2-E-LysK-SH3b as opposed to λSA2-E-Lyso-SH3b resumed growth during the experiment independent of the inoculation level. This clear difference in efficacy between the two enzymes was unexpected, as they differ only in approximately 50% of the amino acids in their 63-residue SH3b CBD and have been shown to exhibit very similar (less than a twofold difference) activities in vitro (5) (Table 1). While the duration of the milk assays (3 h) is likely too short to expect selection for and outgrowth of a subpopulation of resistant S. aureus cells, possible explanations for the rapid return of the S. aureus growth might include reduced affinity of the LysK SH3b domain for the staphylococcal cell wall due to changes in the cell envelope resulting from growth in milk and a more rapid inactivation of λSA2-E-LysK-SH3b in milk, e.g., through binding to milk components, as has been suggested for both bacteriophage endolysins and bacteriocins (10, 24, 53). While Gram-positive bacteriophage endolysins such as LysK usually remain tightly bound to cell wall fragments after host cell lysis in order to prevent diffusion and consequently exolysis of intact host cells not yet infected by the phage (45), a bacteriocin such as lysostaphin relies on diffusion through the growth medium to accomplish exolysis of target cells and may therefore have evolved to exhibit a lesser degree of interaction with components of the growth environment (e.g., milk).
The effects of lysostaphin on intramammary S. aureus numbers were similar for the different experimental conditions tested here and consistent with what was reported previously by Bramley and Foster (7), who also found a 2- to 3-log10 reduction in CFU after infusion of lysostaphin. Also, for λSA2-E-Lyso-SH3b and λSA2-E-LysK-SH3b, the outcome of the experiment seems to be largely independent of the initial inoculum and the time between challenge and treatment. The reason for this may lie in the previously reported independence of the infection level from the concentration of the initial inoculum (the maximal infection with a resulting bacterial burden of 108 to 1010 CFU/gland is usually achieved within 24 h, even with inocula lower than 100 CFU/gland [9]) and the fact that the infection in mice proceeds very fast compared to that in cows due to the lower number of resident phagocytes in the murine gland (2), which could potentially make the time between challenge and treatment a less crucial factor. However, it should be noted that conclusions as to how each individual experimental parameter (e.g., time to lysin administration or amount of S. aureus inoculum) affects the outcome of the experiment are difficult to infer from these studies, because these two parameters were modified simultaneously in each procedure.
Synergy between peptidoglycan hydrolases featuring different cut sites in the PG is not a new phenomenon (3, 43), and therefore the in vitro synergistic effects shown here between the chimeric lysins (which cleave the d-Gln-l-Lys bond in the stem peptide) (54) and lysostaphin (which cleaves glycyl-glycine bonds in the interpeptide bridge) were not unexpected. As suggested for the enzymes Pal and Cpl-1 (43), these positive interactions could be due to enhanced destructive effects when two different bonds are targeted simultaneously within the three-dimensional structure of the PG, or they could be due to increased accessibility of one cut site after cleavage of the other. While the checkerboard assay is a commonly used method to demonstrate synergy between two antimicrobial agents, the novel plate lysis format employed here, which involved spotting proteins on a freshly plated lawn of bacteria, may be a practical method for other cases where no clear MIC in liquid broth can be obtained.
λSA2-E-LysK-SH3b was chosen to be tested in vivo in combination with lysostaphin due to its slightly higher reduction of bacterial numbers and wet weights compared to λSA2-E-Lyso-SH3b when applied individually. However, the higher activity of λSA2-E-Lyso-SH3b in cow milk suggests that this protein may be better suited for administration in a bovine mammary gland. The combination of λSA2-E-LysK-SH3b and lysostaphin was significantly more effective in reducing S. aureus numbers than any of the individual protein treatments, with intramammary TNF-α concentrations showing the same trend. Combined application of antimicrobial agents not only takes advantage of synergistic effects but also is expected to reduce the chance of resistant strain development, as two simultaneous mutations are likely required to render a cell resistant to both agents (3, 28). Furthermore, combinations of antimicrobials have been shown to act synergistically even against strains highly resistant to one of the components (21). In addition to mixtures of two different PG hydrolases as investigated in this work, other classes of antimicrobials, such as antibiotics (21) and bacteriocins (30), have also been reported to exhibit synergy when combined with endolysins, and such combinations may therefore hold promise for treatment of infections such as bovine mastitis. Our chimeric enzymes showed activity against all mastitis isolates tested. When a subset of these 16 strains was tested for antibiotic susceptibility, two of six isolates showed increased resistance against penicillin G (30-fold and 500-fold higher MICs for SA021 and SA026, respectively, than for a reference strain) (41), whereas both strains were susceptible to our lysins at levels comparable to those of the antibiotic-sensitive strains (Table 1). Differences in susceptibility between the tested strains can possibly be explained by strain-specific differences in surface structures (e.g., capsules, teichoic acids, and other cell wall polymers), which may affect accessibility of cut sites or binding ligands of the enzymes. The parental λSA2 endolysin has previously been shown to exhibit only marginal activity in plate lysis against staphylococci (5, 25), whereas the staphylococcal phage lysin LysK has been demonstrated in our lab to be active against all 16 mastitis isolates described here at concentrations similar to those of the chimeric enzymes. However, in contrast to λSA2, LysK shows only weak activity in cow milk (unpublished data). These findings disqualify both parental phage lysins from being used as antimicrobials against S. aureus-induced bovine mastitis.
A mouse model of S. aureus-induced bovine mastitis can certainly not replace experiments in cows, as both mammary glands and milk differ significantly between cattle and mice. However, the consequences of bacterial infection regarding infiltration of polymorphonuclear leukocytes, tissue damage, and interactions between pathogens and host cells have been demonstrated to be similar in both animals (reviewed in references 9 and 48). Therefore, the mouse model can give valuable insight into the effects of antimicrobials on pathogens in a representative complex growth environment, constituting an inexpensive tool for primary evaluation of potential antimastitis agents. This is the first study evaluating the use of chimeric phage endolysin-derived antimicrobials in a mouse model of intramammary S. aureus infection. Although our results suggest that the effects of the individually administered chimeric enzymes may not be strong enough to prevent or cure acute bovine staphylococcal mastitis infections, certainly the mouse model results with the combination of λSA2-E-LysK-SH3b and lysostaphin support the testing of this combination in cows for the ability to cure or decrease the severity of such infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by NIH grant 1RO1AI075077-01A1, NRI grant 2007-35204-18395, and U.S. State Department funds (all awards to D.M.D.).
We are grateful to Yasunori Tanji for the gift of strains and to Juli Foster-Frey for excellent technical assistance. Mention of a trade name, proprietary product or vendor does not constitute a guarantee or warranty of the product by USDA or imply its approval to the exclusion of other suitable products or vendors.
Footnotes
Published ahead of print 27 January 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Anderson JC, Craven N. 1984. Assessment in the mouse of cefoperazone as a treatment for mastitis. Vet. Rec. 114:607–612 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JC, Heneghan DJ. 1979. Extrapolation from experimental chronic staphylococcal mastitis in mice to experimental infections in cattle. Br. Vet. J. 135:527–535 [DOI] [PubMed] [Google Scholar]
- 3.Becker SC, Foster-Frey J, Donovan DM. 2008. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol. Lett. 287:185–191 [DOI] [PubMed] [Google Scholar]
- 4.Becker SC, Foster-Frey J, Powell A, Kerr D, Donovan DM. 2011. Lysostaphin: molecular changes that preserve staphylolytic activity, p 18–22. In Mendez-Vilas A. (ed), Proceedings of the International Conference on Antimicrobial Research. World Scientific Publishing Co., Singapore [Google Scholar]
- 5.Becker SC, Foster-Frey J, Stodola AJ, Anacker D, Donovan DM. 2009. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 443:32–41 [DOI] [PubMed] [Google Scholar]
- 6.Borysowski J, Weber-Dabrowska B, Gorski A. 2006. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. (Maywood) 231:366–377 [DOI] [PubMed] [Google Scholar]
- 7.Bramley AJ, Foster R. 1990. Effects of lysostaphin on Staphylococcus aureus infections of the mouse mammary gland. Res. Vet. Sci. 49:120–121 [PubMed] [Google Scholar]
- 8.Brouillette E, Grondin G, Lefebvre C, Talbot BG, Malouin F. 2004. Mouse mastitis model of infection for antimicrobial compound efficacy studies against intracellular and extracellular forms of Staphylococcus aureus. Vet. Microbiol. 101:253–262 [DOI] [PubMed] [Google Scholar]
- 9.Brouillette E, Malouin F. 2005. The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect. 7:560–568 [DOI] [PubMed] [Google Scholar]
- 10.Celia LK, Nelson D, Kerr DE. 2008. Characterization of a bacteriophage lysin (Ply700) from Streptococcus uberis. Vet. Microbiol. 130:107–117 [DOI] [PubMed] [Google Scholar]
- 11.Chandler RL. 1970. Experimental bacterial mastitis in the mouse. J. Med. Microbiol. 3:273–282 [DOI] [PubMed] [Google Scholar]
- 12.Chandler RL. 1971. Studies on experimental mouse mastitis relative to the assessment of pharmaceutical substances. J. Comp. Pathol. 81:507–514 [DOI] [PubMed] [Google Scholar]
- 13.Climo MW, Ehlert K, Archer GL. 2001. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craven N, Williams MR, Anderson JC. 1982. Enhanced killing of penicillin-treated S. aureus by host defences: effects of amoxycillin, cloxacillin and nafcillin in vitro and in experimental mastitis. Comp. Immunol. Microbiol. Infect. Dis. 5:447–456 [DOI] [PubMed] [Google Scholar]
- 15.Croux C, Ronda C, López R, García JL. 1993. Interchange of functional domains switches enzyme specificity: construction of a chimeric pneumococcal-clostridial cell wall lytic enzyme. Mol. Microbiol. 9:1019–1025 [DOI] [PubMed] [Google Scholar]
- 16.Daniel A, et al. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:1603–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeHart HP, Heath HE, Heath LS, LeBlanc PA, Sloan GL. 1995. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl. Environ. Microbiol. 61:1475–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deluyker HA, Van Oye SN, Boucher JF. 2005. Factors affecting cure and somatic cell count after pirlimycin treatment of subclinical mastitis in lactating cows. J. Dairy Sci. 88:604–614 [DOI] [PubMed] [Google Scholar]
- 19.Diarra MS, et al. 2003. Lactoferrin against Staphylococcus aureus mastitis. Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. 95:33–42 [DOI] [PubMed] [Google Scholar]
- 20.Díaz E, López R, García JL. 1990. Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc. Natl. Acad. Sci. U. S. A. 87:8125–8129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djurkovic S, Loeffler JM, Fischetti VA. 2005. Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme Cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. Antimicrob. Agents Chemother. 49:1225–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donovan DM, et al. 2009. Peptidoglycan hydrolase enzyme fusions for treating multi-drug resistant pathogens. Biotech Int. 21:6–10 [Google Scholar]
- 23.Donovan DM. 2007. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat. Biotechnol. 1:113–122 [DOI] [PubMed] [Google Scholar]
- 24.Donovan DM, et al. 2006. Peptidoglycan hydrolase fusions maintain their parental specificities. Appl. Environ. Microbiol. 72:2988–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donovan DM, Foster-Frey J. 2008. LambdaSa2 prophage endolysin requires Cpl-7-binding domains and amidase-5 domain for antimicrobial lysis of streptococci. FEMS Microbiol. Lett. 287:22–33 [DOI] [PubMed] [Google Scholar]
- 26.Ehlert K, Schröder W, Labischinski H. 1997. Specificities of FemA and FemB for different glycine residues: FemB cannot substitute for FemA in staphylococcal peptidoglycan pentaglycine side chain formation. J. Bacteriol. 179:7573–7576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erskine RJ, Walker RD, Bolin CA, Bartlett PC, White DG. 2002. Trends in antibacterial susceptibility of mastitis pathogens during a seven-year period. J. Dairy Sci. 85:1111–1118 [DOI] [PubMed] [Google Scholar]
- 28.Fischetti VA. 2005. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 13:491–496 [DOI] [PubMed] [Google Scholar]
- 29.Fischetti VA. 2010. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 300:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García P, Martínez B, Rodríguez L, Rodríguez A. 2010. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. J. Food Microbiol. 141:151–155 [DOI] [PubMed] [Google Scholar]
- 31.Gehring R, Smith GW. 2006. An overview of factors affecting the disposition of intramammary preparations used to treat bovine mastitis. J. Vet. Pharmacol. Ther. 29:237–241 [DOI] [PubMed] [Google Scholar]
- 32.Graham S, Coote PJ. 2007. Potent, synergistic inhibition of Staphylococcus aureus upon exposure to a combination of the endopeptidase lysostaphin and the cationic peptide ranalexin. J. Antimicrob. Chemother. 59:759–762 [DOI] [PubMed] [Google Scholar]
- 33.Gruet P, Maincent P, Berthelot X, Kaltsatos V. 2001. Bovine mastitis and intramammary drug delivery: review and perspectives. Adv. Drug Deliv. Rev. 50:245–259 [DOI] [PubMed] [Google Scholar]
- 34.Gründling A, Missiakas DM, Schneewind O. 2006. Staphylococcus aureus mutants with increased lysostaphin resistance. J. Bacteriol. 188:6286–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall MJ, Middleton RF, Westmacott D. 1983. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 11:427–433 [DOI] [PubMed] [Google Scholar]
- 36.Hermoso JA, García JL, García P. 2007. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr. Opin. Microbiol. 10:461–472 [DOI] [PubMed] [Google Scholar]
- 37.Jasper DE, et al. 1982. Bovine mastitis research needs, funding and sources of support, p 182–193. Proceedings of the National Mastitis Council, 21st Annual Meeting. [Google Scholar]
- 38.Jones RN, Barry AL, Gavan TL, Washington JA., II 1985. Susceptibility tests: microdilution and macrodilution broth procedures, p 972–977. In Balows A, Hausler JWJ, Shadomy HJ. (ed), Manual of clinical microbiology. American Society for Microbiology, Washington, DC [Google Scholar]
- 39.Kerr DE, et al. 2001. Lysostaphin expression in mammary glands confers protection against staphylococcal infection in transgenic mice. Nat. Biotechnol. 19:66–70 [DOI] [PubMed] [Google Scholar]
- 40.Kossaibati MA, Hovi M, Esslemont RJ. 1998. Incidence of clinical mastitis in dairy herds in England. Vet. Rec. 143:649–653 [DOI] [PubMed] [Google Scholar]
- 41.Kuang Y, Jia H, Miyanaga K, Tanji Y. 2009. Effect of milk on antibacterial activity of tetracycline against Escherichia coli and Staphylococcus aureus isolated from bovine mastitis. Appl. Microbiol. Biotechnol. 84:135–142 [DOI] [PubMed] [Google Scholar]
- 42.Lee JH. 2003. Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl. Environ. Microbiol. 69:6489–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeffler JM, Fischetti VA. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47:375–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loessner MJ. 2005. Bacteriophage endolysins—current state of research and applications. Curr. Opin. Microbiol. 8:480–487 [DOI] [PubMed] [Google Scholar]
- 45.Loessner MJ, Kramer K, Ebel F, Scherer S. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44:335–349 [DOI] [PubMed] [Google Scholar]
- 46.Middleton JR. 2008. Staphylococcus aureus antigens and challenges in vaccine development. Expert Rev. Vaccines 7:805–815 [DOI] [PubMed] [Google Scholar]
- 47.Myllys V, et al. 1998. Bovine mastitis in Finland in 1988 and 1995—changes in prevalence and antimicrobial resistance. Acta Vet. Scand. 39:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Notebaert S, Meyer E. 2006. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet. Quart. 28:2–13 [DOI] [PubMed] [Google Scholar]
- 49.Obeso JM, Martínez B, Rodríguez A, GarcíA P. 2008. Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 128:212–218 [DOI] [PubMed] [Google Scholar]
- 50.O'Flaherty S, Coffey A, Meaney WJ, Fitzgerald GF, Ross RP. 2005. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 41:274–279 [DOI] [PubMed] [Google Scholar]
- 51.Oldham ER, Daley MJ. 1991. Lysostaphin: use of a recombinant bactericidal enzyme as a mastitis therapeutic. J. Dairy Sci. 74:4175–4182 [DOI] [PubMed] [Google Scholar]
- 52.Pereira UP, Oliveira DG, Mesquita LR, Costa GM, Pereira LJ. 2011. Efficacy of Staphylococcus aureus vaccines for bovine mastitis: a systematic review. Vet. Microbiol. 148:117–124 [DOI] [PubMed] [Google Scholar]
- 53.Pol IE, Mastwujk HC, Slump RA, Popa ME, Smid EJ. 2001. Influence of food matrix on inactivation of Bacillus cereus by combinations of nisin, pulsed electric field treatment, and carvacrol. J. Food Prot. 64:1012–1018 [DOI] [PubMed] [Google Scholar]
- 54.Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR. 2007. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl. Environ. Microbiol. 73:7150–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reichelt P, Schwarz C, Donzeau M. 2006. Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr. Purif. 46:483–488 [DOI] [PubMed] [Google Scholar]
- 56.Sanchez MS, Ford CW, Yancey RJ., Jr 1994. Effect of tumor necrosis factor-alpha, interleukin-1 beta, and antibiotics on the killing of intracellular Staphylococcus aureus. J. Dairy Sci. 77:1251–1258 [DOI] [PubMed] [Google Scholar]
- 57.Schindler CA, Schuhardt VT. 1964. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc. Natl. Acad. Sci. U. S. A. 51:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schindler CA, Schuhardt VT. 1965. Purification and properties of lysostaphin—a lytic agent for Staphylococcus aureus. Biochim. Biophys. Acta 97:242–250 [DOI] [PubMed] [Google Scholar]
- 59.Schmelcher M, et al. 2010. Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl. Environ. Microbiol. 76:5745–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmelcher M, Tchang VS, Loessner MJ. 2011. Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity. Microb. Biotechnol. 4:651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sordillo LM, Streicher KL. 2002. Mammary gland immunity and mastitis susceptibility. J. Mammary Gland. Biol. Neoplasia 7:135–146 [DOI] [PubMed] [Google Scholar]
- 62.Sugai M, et al. 1997. epr, which encodes glycylglycine endopeptidase resistance, is homologous to femAB and affects serine content of peptidoglycan cross bridges in Staphylococcus capitis and Staphylococcus aureus. J. Bacteriol. 179:4311–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Synnott AJ, et al. 2009. Isolation from sewage influent and characterization of novel Staphylococcus aureus bacteriophages with wide host ranges and potent lytic capabilities. Appl. Environ. Microbiol. 75:4483–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vanderhaeghen W, Hermans K, Haesebrouck F, Butaye P. 2010. Methicillin-resistant Staphylococcus aureus (MRSA) in food production animals. Epidemiol. Infect. 138:606–625 [DOI] [PubMed] [Google Scholar]
- 65.Wall R, et al. 2009. Enhanced host immune recognition of mastitis causing Escherichia coli in CD-14 transgenic mice. Anim. Biotechnol. 20:1–14 [DOI] [PubMed] [Google Scholar]
- 66.Wall RJ, et al. 2005. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat. Biotechnol. 23:445–451 [DOI] [PubMed] [Google Scholar]
- 67.Young I, Wang I, Roof WD. 2000. Phages will out: strategies of host cell lysis. Trends Microbiol. 8:120–128 [DOI] [PubMed] [Google Scholar]
- 68.Young R, Bläsi U. 1995. Holins: form and function in bacteriophage lysis. FEMS Microbiol. Rev. 17:191–205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.