Abstract
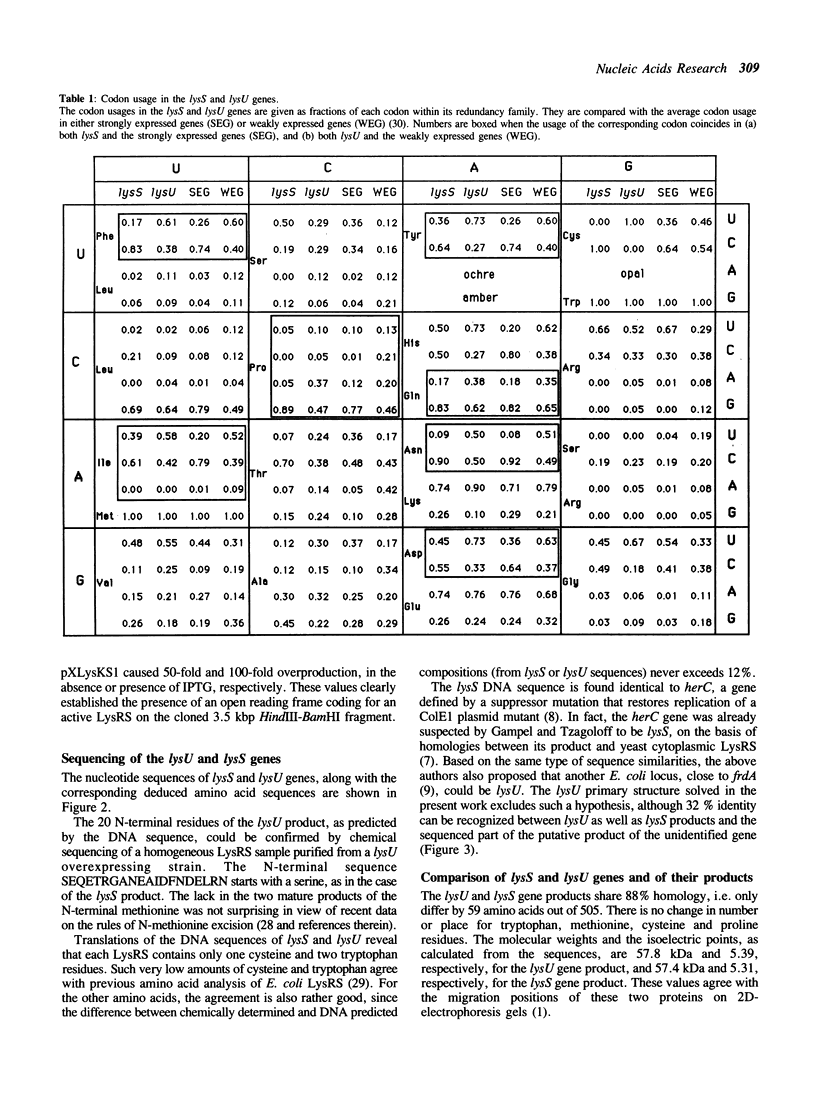
In Escherichia coli, two distinct lysyl-tRNA synthetase species are encoded by two genes: the constitutive lysS gene and the thermoinducible lysU gene. These two genes have been isolated and sequenced. Their nucleotide and deduced amino acid sequences show 79% and 88% identity, respectively. Codon usage analysis indicates the lysS product being more efficiently translated than the lysU one. In addition, the lysS sequence exactly coincides with the sequence of herC, a gene which is part of the prfB-herC operon. In contrast to the recent proposal of Gampel and Tzagoloff (1989, Proc. Natl. Acad. Sci. USA 86, 6023-6027), the lysU sequence is distinct from the open reading frame located adjacent to frdA, although large homologies are shared by these two genes.
Full text
PDF
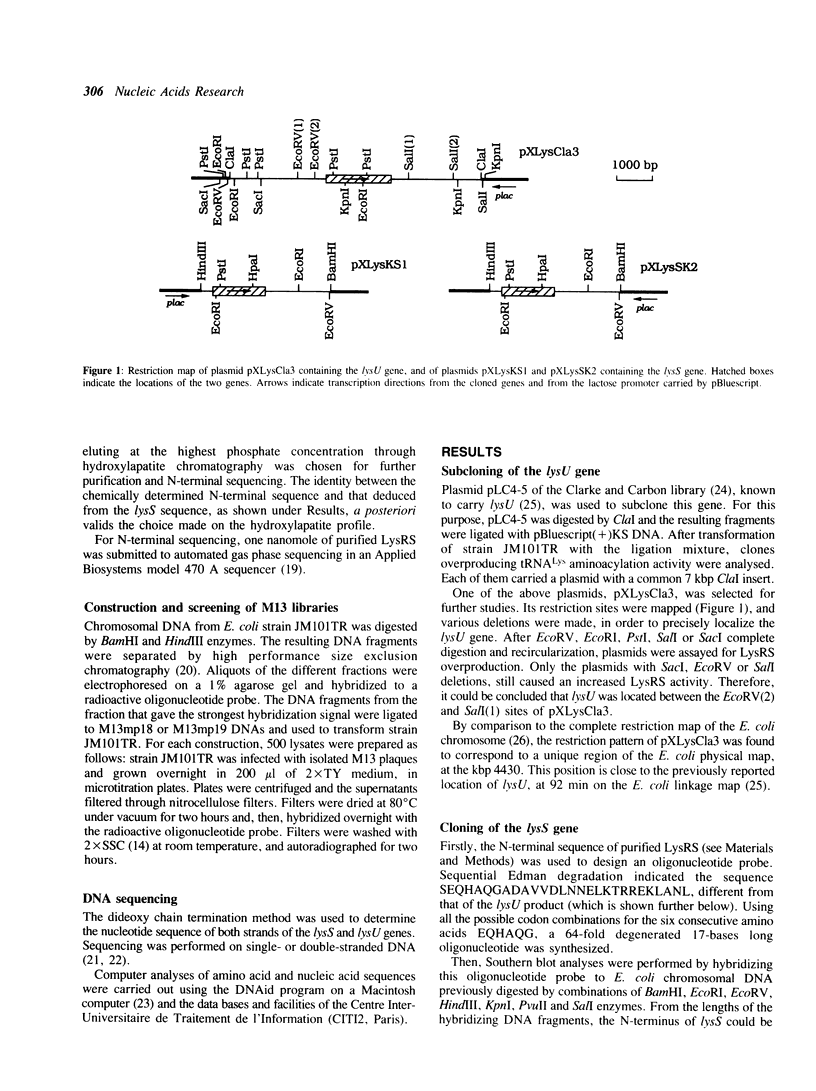





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvallet C., Hountondji C., Schmitter J. M. Analytical strategy for determination of active site sequences in aminoacyl-tRNA synthetases. J Chromatogr. 1988 Apr 22;438(2):347–357. doi: 10.1016/s0021-9673(00)90266-8. [DOI] [PubMed] [Google Scholar]
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J., Park C., Harayama S., Hazelbauer G. L. Structure of the Trg protein: Homologies with and differences from other sensory transducers of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3287–3291. doi: 10.1073/pnas.81.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Kendall K., Simon M. I. Structure of the serine chemoreceptor in Escherichia coli. Nature. 1983 Feb 17;301(5901):623–626. doi: 10.1038/301623a0. [DOI] [PubMed] [Google Scholar]
- Brevet A., Chen J., Lévêque F., Plateau P., Blanquet S. In vivo synthesis of adenylylated bis(5'-nucleosidyl) tetraphosphates (Ap4N) by Escherichia coli aminoacyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8275–8279. doi: 10.1073/pnas.86.21.8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buklad N. E., Sanborn D., Hirshfield I. N. Particular influence of leucine peptides on lysyl-transfer ribonucleic acid ligase formation in a mutant of Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1477–1478. doi: 10.1128/jb.116.3.1477-1478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier J., Sanchez R. Lysyl-tRNA synthetase from Escherichia coli K12. Chromatographic heterogeneity and the lysU-gene product. Biochem J. 1987 Nov 15;248(1):43–51. doi: 10.1042/bj2480043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. C., Hsieh J. C., Hui C. F., Tam M. F. Modified method for double stranded DNA sequencing and synthetic oligonucleotide purification. Nucleic Acids Res. 1988 Nov 11;16(21):10382–10382. doi: 10.1093/nar/16.21.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirakoglu B., Waller J. P. Do yeast aminoacyl-tRNA synthetases exist as soluble enzymes within the cytoplasm? Eur J Biochem. 1985 Jun 3;149(2):353–361. doi: 10.1111/j.1432-1033.1985.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cole S. T. Nucleotide sequence coding for the flavoprotein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Mar 1;122(3):479–484. doi: 10.1111/j.1432-1033.1982.tb06462.x. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardel F., Bensoussan P. DNAid: a Macintosh full screen editor featuring a built-in regular expression interpreter for the search of specific patterns in biological sequences using finite state automata. Comput Appl Biosci. 1988 Nov;4(4):483–486. doi: 10.1093/bioinformatics/4.4.483. [DOI] [PubMed] [Google Scholar]
- Emmerich R. V., Hirshfield I. N. Mapping of the constitutive lysyl-tRNA synthetase gene of Escherichia coli K-12. J Bacteriol. 1987 Nov;169(11):5311–5313. doi: 10.1128/jb.169.11.5311-5313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayat G., Mayaux J. F., Sacerdot C., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Escherichia coli phenylalanyl-tRNA synthetase operon region. Evidence for an attenuation mechanism. Identification of the gene for the ribosomal protein L20. J Mol Biol. 1983 Dec 15;171(3):239–261. doi: 10.1016/0022-2836(83)90092-x. [DOI] [PubMed] [Google Scholar]
- Ferrara P., Duchange N., Zakin M. M., Cohen G. N. Internal homologies in the two aspartokinase-homoserine dehydrogenases of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1984 May;81(10):3019–3023. doi: 10.1073/pnas.81.10.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Grosjean H. On codon usage. Nature. 1979 Jan 25;277(5694):328–328. doi: 10.1038/277328a0. [DOI] [PubMed] [Google Scholar]
- Freedman R., Gibson B., Donovan D., Biemann K., Eisenbeis S., Parker J., Schimmel P. Primary structure of histidine-tRNA synthetase and characterization of hisS transcripts. J Biol Chem. 1985 Aug 25;260(18):10063–10068. [PubMed] [Google Scholar]
- Gampel A., Tzagoloff A. Homology of aspartyl- and lysyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6023–6027. doi: 10.1073/pnas.86.16.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hirel P. H., Lévêque F., Mellot P., Dardel F., Panvert M., Mechulam Y., Fayat G. Genetic engineering of methionyl-tRNA synthetase: in vitro regeneration of an active synthetase by proteolytic cleavage of a methionyl-tRNA synthetase--beta-galactosidase chimeric protein. Biochimie. 1988 Jun;70(6):773–782. doi: 10.1016/0300-9084(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Bloch P. L., Van Bogelen R. A., Neidhardt F. C. Multiple forms of lysyl-transfer ribonucleic acid synthetase in Escherichia coli. J Bacteriol. 1981 Apr;146(1):345–351. doi: 10.1128/jb.146.1.345-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Tenreiro R., Vanbogelen R. A., Neidhardt F. C. Escherichia coli K-12 lysyl-tRNA synthetase mutant with a novel reversion pattern. J Bacteriol. 1984 May;158(2):615–620. doi: 10.1128/jb.158.2.615-620.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Yeh F. M. An in vivo effect of the metabolites L-alanine and glycyl-L-leucine on the properties of the lysyl-tRNA synthetase from Escherichia coli K-12. II. Kinetic evidence. Biochim Biophys Acta. 1976 Jul 2;435(3):306–314. doi: 10.1016/0005-2787(76)90111-8. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Jacobo-Molina A., Peterson R., Yang D. C. cDNA sequence, predicted primary structure, and evolving amphiphilic helix of human aspartyl-tRNA synthetase. J Biol Chem. 1989 Oct 5;264(28):16608–16612. [PubMed] [Google Scholar]
- Kawakami K., Jönsson Y. H., Björk G. R., Ikeda H., Nakamura Y. Chromosomal location and structure of the operon encoding peptide-chain-release factor 2 of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5620–5624. doi: 10.1073/pnas.85.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner T. J., Myers A. M., Lee S., Tzagoloff A. Isolation and characterization of the yeast gene coding for the alpha subunit of mitochondrial phenylalanyl-tRNA synthetase. J Biol Chem. 1987 Mar 15;262(8):3690–3696. [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lorber B., Kern D., Giegé R., Ebel J. P. Covalent attachment of aspartic acid to yeast aspartyl-tRNA synthetase induced by the enzyme. FEBS Lett. 1982 Sep 6;146(1):59–64. doi: 10.1016/0014-5793(82)80705-9. [DOI] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Control of primer formation for ColE1 plasmid replication: conformational change of the primer transcript. Cell. 1986 Jan 17;44(1):125–136. doi: 10.1016/0092-8674(86)90491-5. [DOI] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Effects of point mutations on formation and structure of the RNA primer for ColE1 DNA replication. Cell. 1984 Feb;36(2):513–522. doi: 10.1016/0092-8674(84)90244-7. [DOI] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Abnormal induction of heat shock proteins in an Escherichia coli mutant deficient in adenosylmethionine synthetase activity. J Bacteriol. 1988 Apr;170(4):1582–1588. doi: 10.1128/jb.170.4.1582-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mirande M., Waller J. P. Molecular cloning and primary structure of cDNA encoding the catalytic domain of rat liver aspartyl-tRNA synthetase. J Biol Chem. 1989 Jan 15;264(2):842–847. [PubMed] [Google Scholar]
- Mirande M., Waller J. P. The yeast lysyl-tRNA synthetase gene. Evidence for general amino acid control of its expression and domain structure of the encoded protein. J Biol Chem. 1988 Dec 5;263(34):18443–18451. [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Moore P. A., Jayme D. W., Oxender D. L. A role for aminoacyl-tRNA synthetases in the regulation of amino acid transport in mammalian cell lines. J Biol Chem. 1977 Nov 10;252(21):7427–7430. [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Plateau P., Gueron M., Blanquet S. Determination of dinucleoside 5', 5"'-P1, P4- tetraphosphates by 31P and 1H NMR spectroscopy. Biochimie. 1981 Nov-Dec;63(11-12):827–830. doi: 10.1016/s0300-9084(82)80267-8. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Royal N. J., Neuman de Vegvar H., Herlihy W. C., Biemann K., Schimmel P. Primary structure of a large aminoacyl-tRNA synthetase. Science. 1981 Sep 25;213(4515):1497–1501. doi: 10.1126/science.7025207. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Yogeeswaran G., Zamecnik P. C., Remy P. Covalent modification of phenylalanyl-tRNA synthetase with phenylalanine during the amino acid activation reaction catalyzed by the enzyme. J Biol Chem. 1985 Aug 15;260(17):9509–9512. [PubMed] [Google Scholar]
- Rymo L., Lundvik L., Lagerkvist U. Subunit structure and binding properties of three amino acid transfer ribonucleic acid ligases. J Biol Chem. 1972 Jun 25;247(12):3888–3897. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanni A., Mirande M., Ebel J. P., Boulanger Y., Waller J. P., Fasiolo F. Structure and expression of the genes encoding the alpha and beta subunits of yeast phenylalanyl-tRNA synthetase. J Biol Chem. 1988 Oct 25;263(30):15407–15415. [PubMed] [Google Scholar]
- Schmitter J. M., Mechulam Y., Fayat G., Anselme M. Rapid purification of DNA fragments by high-performance size-exclusion chromatography. J Chromatogr. 1986 Jun 13;378(2):462–466. doi: 10.1016/s0378-4347(00)80743-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellami M., Fasiolo F., Dirheimer G., Ebel J. P., Gangloff J. Nucleotide sequence of the gene coding for yeast cytoplasmic aspartyl-tRNA synthetase (APS); mapping of the 5' and 3' termini of AspRS mRNA. Nucleic Acids Res. 1986 Feb 25;14(4):1657–1666. doi: 10.1093/nar/14.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet F., Cunin R., Jacobs A., Piette J., Gigot D., Lauwereys M., Piérard A., Glansdorff N. Evolutionary divergence of genes for ornithine and aspartate carbamoyl-transferases--complete sequence and mode of regulation of the Escherichia coli argF gene; comparison of argF with argI and pyrB. Nucleic Acids Res. 1984 Aug 10;12(15):6277–6289. doi: 10.1093/nar/12.15.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Vaughn V., Neidhardt F. C. Gene for heat-inducible lysyl-tRNA synthetase (lysU) maps near cadA in Escherichia coli. J Bacteriol. 1983 Feb;153(2):1066–1068. doi: 10.1128/jb.153.2.1066-1068.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Miyada C. G. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 1987;152:432–442. doi: 10.1016/0076-6879(87)52050-x. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]


