Abstract
Nitrous oxide (N2O) is a powerful atmospheric greenhouse gas and cause of ozone layer depletion. Global emissions continue to rise. More than two-thirds of these emissions arise from bacterial and fungal denitrification and nitrification processes in soils, largely as a result of the application of nitrogenous fertilizers. This article summarizes the outcomes of an interdisciplinary meeting, ‘Nitrous oxide (N2O) the forgotten greenhouse gas’, held at the Kavli Royal Society International Centre, from 23 to 24 May 2011. It provides an introduction and background to the nature of the problem, and summarizes the conclusions reached regarding the biological sources and sinks of N2O in oceans, soils and wastewaters, and discusses the genetic regulation and molecular details of the enzymes responsible. Techniques for providing global and local N2O budgets are discussed. The findings of the meeting are drawn together in a review of strategies for mitigating N2O emissions, under three headings, namely: (i) managing soil chemistry and microbiology, (ii) engineering crop plants to fix nitrogen, and (iii) sustainable agricultural intensification.
Keywords: nitrous oxide, denitrification, greenhouse gas, climate change, mitigating emissions
1. Introduction
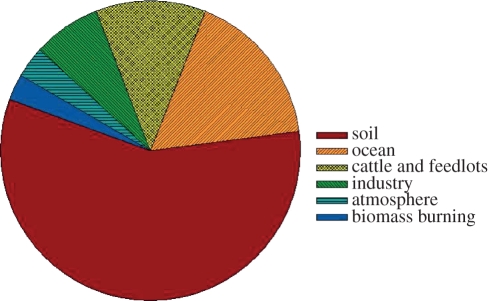
Nitrous oxide (N2O) is a colourless, non-toxic gas, commonly known as laughing gas. Since its discovery over 200 years ago, it has found use both as an anaesthetic and a fuel additive. However, in 1908, the invention of the Haber–Bosch process, allowing the abiological reduction of atmospheric nitrogen to ammonia (NH3; called nitrogen fixation), gave rise to the introduction of synthetic nitrogen-based fertilizers that has enabled dramatic increases in intensive farming. This, in turn, has led to increasing N2O emissions from the increased presence of reactive nitrogen in soil [1,2]. The deposition of nitrogen from motor vehicles, especially near busy roads, means that fossil fuels are also a major contributor to soil nitrogen levels [3]. The return of animal waste to soil and wastewater treatment further contribute to N2O emissions [4,5]. The cumulative effect over the past century has been an estimated approximately 20 per cent increase in atmospheric N2O concentration that is still increasing at a rate of 0.2–0.3% yr−1 [6]. More than two-thirds of these emissions come from bacterial and fungal respiratory processes in soils, broadly termed denitrification and nitrification [1,2]. Figure 1 illustrates the proportions of total global nitrous oxide emitted by various sources, including human activities.
Figure 1.
Proportions of total global nitrous oxide emitted by various sources and human activities. Adapted from data in the Contribution of Working Group III to the fourth assessment report of the intergovernmental panel on climate change, 2007. Eds B. Metz, O. R. Davidson, P .R. Bosch, R. Dave and L. A. Meyer. Cambridge, UK; New York, NY: Cambridge University Press.
N2O is a powerful greenhouse gas (GHG) with an atmospheric lifetime of 114 years [7]. Although N2O only accounts for around 0.03 per cent of total GHG emissions, it has an almost 300-fold greater potential for global warming effects, based on its radiative capacity, compared with that of carbon dioxide (CO2) [7]. Hence, when the impact of individual GHGs on global warming is expressed in terms of the Intergovernmental Panel on Climate Change approved unit of CO2 equivalents, N2O accounts for approximately 10 per cent of total emissions [6].
In the stratosphere, the main sink for N2O, ultraviolet photochemistry oxidizes NOx [8]. Today, N2O is a major cause of ozone layer depletion [9]. Since 1997, many of the non-biological emissions of N2O, for example, those associated with the transport industry, have been systematically lowered, whereas emissions from agriculture are essentially unchanged [7]. Although the 1997 Kyoto Protocol set emission limitations and reduction obligations, with respect to a basket of six gases, including N2O, on its signatories this Protocol expires in 2012. It is crucial that its successor is able to address fully the issue of soil-derived N2O emissions. Because of the ongoing decline of chlorofluorocarbons and the continuous increase of N2O in the atmosphere, the contributions of N2O to both the greenhouse effect and ozone depletion will be even more pronounced in the twenty-first century [9,10].
The availability of nitrogen (nitrate or ammonium) as well as phosphorus (phosphate) and potassium are crucial determinants of globally sustainable crop yields. There is widespread nitrogen and phosphate deficiency and thus potential yields are often not reached. This deficiency is particularly acute in the developing world where the need to apply nitrogen fertilizer or encourage biological nitrogen fixation will certainly increase. In these systems, the primary aim is food security, but with it will, undoubtedly, come yet further increases in N2O emissions. Thus, the environmental damage from the further intensification of agriculture will increase more rapidly unless means can be found to mitigate the emissions of biologically derived N2O [11].
Between 23 and 24 May 2011, a residential scientific meeting, entitled ‘Nitrous oxide (N2O) the forgotten greenhouse gas’, was held at the Kavli Royal Society International Centre, Chicheley Hall, Buckinghamshire, UK. The objective of this meeting was to bring together scientists from a wide range of disciplines, including biochemists, chemists, molecular biologists, geneticists, microbiologists, soil scientists, ecologists and environmental scientists to discuss four areas, namely: (i) biological sources of N2O emissions and the consequent problems; (ii) biological production and consumption of N2O; (iii) measuring and modelling N2O balances; finally (iv) strategies for mitigating N2O emissions. The papers published in this themed volume of Philosophical Transactions of the Royal Society B were presented and discussed at this meeting.
This paper provides an introduction and background to the nature of the problem of the biological sources of N2O, exploring the biological sources and sinks of N2O from different environments such as oceans, soils and wastewaters, and describes the genetic regulation and molecular details of the enzymes responsible. The techniques for measuring and assessing the amounts of N2O to provide global and local budgets are discussed. A summary is provided of the main conclusions reached by the papers in this issue. Finally, these findings are drawn together in a discussion of strategies to mitigate N2O release.
2. The nitrogen cycle
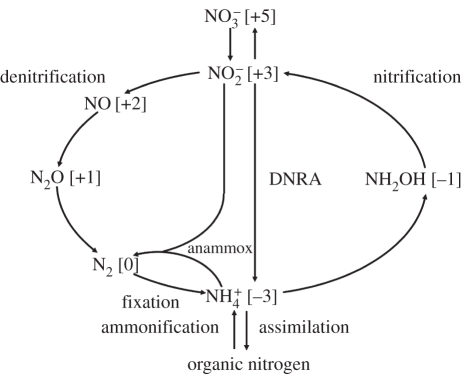
Nitrogen gas (N2), present at 78.08 per cent (v/v) in the atmosphere, possesses one of the most stable chemical linkages known, namely, a chemical triple bond that requires almost 103 kJ M−1 of energy to break into its component N atoms. The triple bond of N2 also has a very high-energy barrier towards breaking, necessitating the use of highly effective catalysts, or enzymes, to speed up the scission process. All biological organisms require nitrogen to synthesize amino acids, proteins, nucleic acids and many additional cofactors. The total nitrogen combined in biology originates from the atmosphere to where it is ultimately returned as the gas, N2. Figure 2 shows the best known, arguably, of all elemental cycles, the nitrogen (or N−) cycle. Nitrogen is driven through all its accessible redox states from the most strongly reduced state, as [NH3], in the −3 oxidation state, to the most highly oxidized state, nitrate ion, [NO3]−, in the +5 oxidation state. Various species with intermediate oxidation states are produced such as nitrite ion, [NO2]−, the gases nitric oxide, [NO] and nitrous oxide [N2O]. They arise through the actions of a number of biological processes the most prominent of which are termed nitrogen fixation, nitrification, dissimilatory nitrate reduction to ammonia (DNRA, or nitrate ammonification), anaerobic ammonia oxidation (anammox) and denitrification. Ammonium ion, [NH4]+, availability is the net result of immobilization, mineralization and nitrification [12]. Table 1 lists the enzymes and the genes that carry out the nitrogen cycle.
Figure 2.
The microbiological nitrogen cycle. Shown are the several microbial processes that respire or assimilate nitrogen (the oxidation states of N are given in parentheses). The name of each process is indicated. Nitrous oxide (N2O) is an intermediate in denitrification. The anammox reaction, used in wastewater treatment plants, is the catabolism between ammonia and nitrite to yield nitrogen gas, e.g. 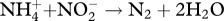 .
.
Table 1.
The genes and enzymes that carry out the bacterial nitrogen cycle.
| transformation | genes | encoding enzyme | references |
|---|---|---|---|
| N2 → NH3 | nifHDK | nitrogenase | [13] |
 |
narG | dissimilatory nitrate reductase | [14] |
 |
nirS, nirK | nitrite reductase haem cd1 and copper nitrite reductase | [15] |
| NO → N2O | norCB | nitric oxide reductase | [16,17] |
| N2O → N2 | nosZ | nitrous oxide reductase | [18–20] |
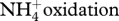 |
amo, hao | ammonia monooxygenase, hydroxylamine oxidoreductase | [21,22] |
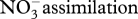 |
narB, nasA | assimilatory nitrate reductase | [23] |
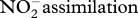 |
Nir | assimilatory nitrite reductase | [24] |
| NH3 assimilation | glnA | glutamine synthetase | [25] |
| organic N metabolism | ure | urease | [26] |
Atmospheric N2 is fixed into NH3 only by free-living and symbiotic bacteria and archaea (diazotrophs). Nitrogenase is the universal catalyst that breaks the triple bond to produce NH3. There are three known variants of the nitrogenase enzyme, all possessing complex, unique iron and sulphur clusters, one of which contains an additional metal ion, being molybdenum, iron or vanadium, in each variant. The ammonium ion can be oxidized to the nitrate ion [NO3]− in a three-step process called nitrification, the first of which is catalysed by the enzyme ammonia monooxygenase (AMO). [NO2]− and [NO3]− ions generated from nitrification may then be reduced either during DNRA or denitrification.
The main routes of N loss are by soil erosion, leaching, ammonia volatilization, ammonia oxidation and denitrification. Approximately, 62 per cent of total global N2O emissions is thought to be emitted from natural and agricultural soils (6 and 4.2 Tg N yr−1, respectively) [27,28] mainly owing to bacterial denitrification and ammonia oxidation, the first step in nitrification [2,29]. The other third of N2O emissions comes from the ocean via nitrification and denitrification [30]. Further anthropogenic sources of N2O include the production of nitric acid, power plants (fossil fuelled) and vehicle emissions [10]. These emissions are responsible for an 18 per cent increase of atmospheric N2O since the early 1900s [1] and are still increasing at a rate of 0.25 per cent per year [1,7].
Denitrification is the stepwise reduction of [NO3]− to N2 by four enzymes each generating intermediate products, namely, nitrite ion [NO2]−, NO and N2O. [NO3]− can also be reduced during nitrate ammonification to [NH4]+ via [NO2]−, with N2O being produced. Anammox is the process by which [NO2]− is reduced to N2 using [NH4]+ as an electron donor. The ability to denitrify is phylogenetically diverse, and can even be undertaken by microbes traditionally classified as belonging to a different functional group. For example, ammonia-oxidizing bacteria are also able to denitrify, reducing [NO2]− ion, sometimes referred to as nitrifier denitrification. N2O is also produced as a by-product during ammonia oxidation, the first step of nitrification. Two further major biological processes of nitrogen transformation are immobilization (or assimilation), the uptake of nitrogen by micro-organisms and its conversion to organic nitrogen, and mineralization or ammonification, the conversion of organic nitrogen to [NH4]+ [2,31].
While there are several enzymological pathways in fungi and bacteria that generate N2O, there is only one enzyme known that converts N2O to gaseous nitrogen, N2, namely, nitrous oxide reductase (N2OR) [2]. Failure of this enzyme to operate leads, for example, to the termination of the bacterial denitrification process at N2O rather than N2. This may be the key to the understanding of, and possible intervention in, the increased emissions of N2O as intensification of agriculture has tended to take place through the increased application of nitrogenous fertilizers [18].
3. Nitrous oxide emission factors
The upward trend in the atmospheric concentration of N2O over the 140 years between 1860 and 2000 from all sources has been well documented. Smith et al. [32] summarize the historical evidence and have now been able to account satisfactorily for the rises. They compare the amounts of new reactive N entering agricultural systems globally with the total emission of N2O, expressing the ratio of these two as an N2O emission factor (EF). This reactive N includes N newly fixed as synthetic fertilizer, and biologically fixed N, and also N mineralized from soil organic matter (SOM) when natural land is converted to agriculture [5] and NOx deposition. The historical upward trend observed in the atmospheric concentration of N2O can then be very closely matched with an overall EF close to 4 per cent. Thus, they have clearly shown that agriculture is the activity mainly responsible for the additional N2O emissions over the past century and a half. They also apply their methodology to analyse N2O emissions arising from biofuel production and reach the conclusion that, when rapeseed and maize (corn), which require nitrogenous fertilizer, are used to produce biodiesel and bioethanol, the N2O emitted could cause as much, or more, global warming as that avoided by replacement of the fossil fuel by biofuel. It is, therefore, important to avoid biofuel production based on crops with a high N demand but to use those that can be grown with little, or no, fertilizer N requirement such as willow and Miscanthus, the so-called ‘second generation’ biofuel crops.
Skiba et al. [33] argue that, especially in the agricultural sector, an EF can be too simplistic to reflect local variations in climate, ecosystems and management, and should not, therefore, be used to take account of the effects of any mitigation strategies. This paper examines deviations of observed N2O emissions from those calculated using the simple EF for all anthropogenic sources and strongly advocates the need to adopt specific EFs that reflect regional variability in climate, soil type and management. Although they can show how bottom-up emission inventories can be verified by top-down modelling they conclude that, in spite of the wealth of N2O emission measurements of the past 20 years, there are still not enough long-term datasets to provide the information needed to design EFs for different climate zones or soil types.
4. Biological production and consumption of nitrous oxide
(a). Enzymological aspects
N2O is produced by both fungi and certain classes of bacteria, including those living in soils and in the oceans, as part of their respiratory processes to generate energy. A recent study revealed that archaeal nitrification is dominating the N2O production in the ocean [34]. The possible contribution of the archaea to N2O production in terrestrial systems, however, is as yet unknown.
Shoun & Tanimoto [35] were the first to identify fungal (eukaryotes) denitrifying activities previously thought to be restricted only to bacteria (prokaryotes). Shoun et al. [36] review the fungal denitrification system. It comprises a copper-containing nitrite reductase (NirK) and a cytochrome P450 nitric oxide reductase (P450nor) that together reduces nitrite to N2O. The system is localized in mitochondria that are also able to function during anaerobic respiration. Some fungal systems use dissimilatory and assimilatory nitrate reductases to denitrify nitrate. Phylogenetic analysis of nirK genes showed that the fungal denitrifying system has the same ancestor as the bacterial counterpart, and thus probably originates from the proto-mitochondrion. Fungal denitrification is often accompanied by co-denitrification, in which a hybrid N2O species is formed upon the combination of the nitrogen atoms of nitrite with nitrogen donors such as amines and imines. The final product of fungal denitrification is N2O, because the enzyme N2OR is absent. Hence, fungal denitrification, under certain conditions, is expected to be a major source of N2O emissions. Shoun notes that acidification of environments, for example, by acid rain and excessive use of ammonia fertilizer, promote fungal activity resulting in further increases in N2O emissions. Prendergast-Miller et al. [37] have recently shown ectomycorrhizal fungal species possess the ability to produce N2O, suggesting that they may have a significant, but as yet unexplored, role in N2O production in forest ecosystems. Recent advances in isotopomer approaches promise the ability to be able to estimate the partition between fungal and bacterial N2O production in situ, and to allow estimates of the significance of fungal denitrification across a range of ecosystems [38].
The major contributor to the biological production of N2O in many environments is the respiratory NO reductase (NOR) found in denitrifying bacteria and in some ammonia-oxidizing organisms. Recently, the molecular structure of this enzyme, the bacterial nitric oxide reductase cNOR from Pseudomonas aeruginosa, has been solved by Shiro et al. [39]. Since 1971, the NO reduction activities of the bacterial membrane-bound NORs have been reported for many bacteria. Although there can be wide variations in the electron-donating moiety, the structures of the catalytic domains are invariant consisting of 12 transmembrane helices that bind one low-spin haem plus a high-spin haem that is adjacent to a non-haem iron centre, called FeB. The dinuclear pair (the haem iron and FeB) binds and activates two NO molecules forming the N–N bond of N2O. Shiro et al. [39] discuss a number of possible mechanisms for this reaction.
An intriguing evolutionary aspect of this study is confirmation of the long suspected close structural similarity between NOR and the main subunit of aerobic and micro-aerobic cytochrome oxidases (COX) that reduce oxygen to water in an energy conserving reaction that is tightly linked to the translocation of protons across a membrane. In this case, the high-spin haem is adjacent to a copper ion (CuB) that has replaced the FeB. These structural differences between cNOR and COX observed in the catalytic centre, and the delivery pathway of the catalytic protons, clearly reflect the functional differences between these respiratory enzymes. NOR, and hence N2O production, is thought to have preceded COX, and oxygen reduction, on the evolutionary timescale, consistent with the dramatic rise of oxygen in the Earth's atmosphere around 3.5 Ga (giga years ago) [40].
Another source of nitrous oxide is from nitrate-ammonifying (DNRA) bacteria [41]. It is now recognized that DNRA bacteria such as Salmonella and Escherichia coli can produce NO as a side product of nitrate metabolism. This endogenous NO can lead to de-repression of genes encoding systems that are concerned with the detoxification of NO and the repair of proteins potentially damaged by this cytotoxin. One regulator that mediates this de-repression is the NO-binding protein NsrR. In E. coli, NsrR regulates some 20 genes, including that for flavohaemoglobin (Hmp) which converts NO to N2O under anoxic conditions [42].
In contrast to the multiplicity of mechanisms by which N2O can be generated, only a single dominant sink for N2O is known, the respiratory N2O reductase (N2OR) typically found in denitrifying bacteria that reduce N2O to N2. N2OR is a homo-dimeric protein containing two structurally distinct copper cofactors per monomer that are crucial for activity, namely: CuZ and CuA [43]. These copper cofactors are inserted only into the apo-protein when it has been translocated from the cytoplasm to the periplasm [44]. Hence, severe copper depletion can lead to enzyme inactivation [45]. In N2OR itself, the catalytic state seems chemically fragile. For example, it loses activity if exposed even briefly to oxygen. The fragility of N2OR likely depends on the chemical nature of the Cu–S cluster of the catalytic centre. For many years, biochemists have known that CuZ can adopt different oxidation states and stabilities, as evidenced by changes in colour, that depend on the previous history of exposure of either the cell or the enzyme itself to oxygen. The paper from Dell'Acqua et al. [46] presents evidence on the N2OR purified from the marine organism Marinobacter hydrocarbonoclasticus that the catalytic centre, CuZ, can adopt different oxidation states. One form, CuZ*, [1Cu2+ : 3Cu+], is redox inert and, hence, enzymatically inactive. However, they have shown that it can be reactivated slowly by incubation for many minutes under non-physiological, highly reducing conditions. A so-called purple form in which the CuZ centre is in the oxidized, redox state [2Cu2+ : 2Cu+] is generated that can subsequently be reduced to the [1Cu2+ : 3Cu+] state. However, none of these redox states is a high-activity state. The high-activity state is reached only after complete reduction of the CuZ centre to an all-Cu(I) form [4Cu+]. However, very recent structural evidence [47] reveals a form of the enzyme that, unexpectedly, contains the CuZ cluster in the form [Cu4S2], whereas the previous X-ray structures of the low-activity state [1Cu2+ : 3Cu+, S] show the cluster to contain only one sulphide ion, [Cu4S]. One can speculate that the reductively reactivated, high-activity enzyme may well contain the [Cu4S2] cluster.
Within the cell, the maintenance of high-activity N2OR, or recovery of activity, say, after transient exposure to oxygen, is likely due to ancillary proteins that insert the copper cofactors into the apo-protein and are known to be required for N2OR activity. Thus, supply of sulphur and electrons is a requirement. In the nos gene cluster, there is a putative ABC transporter (possibly of sulphur), consisting of NosD, NosF and NosY [48]. In addition, the operon encodes a Cu chaperone, NosL. The membrane-bound regulator NosR, required for operon expression, appears to contain redox centres, including FeS clusters (perhaps for electron supply). Thus, the biosynthesis of N2OR and the maintenance of its reductase activity requires these ancillary proteins. These are all points of vulnerability that can lead to inactivation of N2OR and, hence, result in release of gaseous N2O. A clearer understanding of these processes, their regulation and operation will help define the optimal environmental conditions for maintenance of the activity of N2OR and hence the encouragement of the release of N2 rather than N2O.
(b). Microbiological aspects
Of the many factors that contribute to the emission of N2O from bacterial populations, one important determinant is the cellular abundance and another is the activities of the enzymes that produce and consume N2O [42]. Enzyme abundance is governed by expression of the corresponding genes of regulatory systems and signal transduction pathways that respond to intra- or extracellular signals. Because N2O is relatively inert at ambient temperature, and is not a potent toxin, micro-organisms can tolerate relatively high concentrations (millimolar). N2O does not, therefore, appear to be a signal that regulates the expression of any of the denitrification genes. From the point of view of mitigating N2O release from denitrification, the absence of regulation by N2O is a significant observation, because denitrifying populations do not apparently respond to N2O accumulation by making more of the N2OR. The expression of the genes encoding the enzymes that produce and consume N2O is regulated by environmental signals, typically oxygen and NO, acting through regulatory proteins, which, either directly or indirectly, control the frequency of transcription initiation. Because denitrification is an anaerobic respiration, it makes good physiological sense for denitrification genes to be upregulated by low oxygen concentrations. NO is an intermediate of the pathway, and is somewhat toxic. Regulation of denitrification gene expression by NO is therefore presumed to be a mechanism to coordinate NO production and consumption so as to avoid its accumulation to toxic levels.
Bakken et al. [49] nicely expand these points. For instance, in various mutants of Paracoccus denitrificans, the transcription of nosZ, that codes for N2OR, is equally effective with FnrP that responds to oxygen depletion or NNR, responding to NO. In P. denitrificans, N2OR is expressed much earlier than nitrite reductase (NIR) and NOR in response to low oxygen. Moreover, only a fraction of the cells are able to express NIR and NOR before all the oxygen has been depleted. In contrast, nearly 100 per cent of the cells appear to express N2OR, as judged from the rate of reduction of externally supplied N2O. The denitrification phenotype of P. denitrificans at pH 7 demonstrates highly efficient reduction of NOx all the way to N2, with only minor emissions of either NO or N2O. Bakken wryly observes that if the denitrifying communities of soils performed equally well, their contribution to emission of NO and N2O would be negligible. Although the performance of P. denitrificans appears to be exceptional, the soil bacterium Agrobacterium tumefaciens is unable to reduce N2O to N2 because it lacks nosZ. Indeed, strains which lack nosZ occur within many genera of denitrifying prokaryotes, and if organisms with such a truncated denitrification apparatus were to dominate in soils, it would lead to high N2O/(N2 + N2O) product ratios of denitrification. Bakken, therefore, proposes the term ‘denitrification regulatory phenotype’, that is a set of variables characterizing the organism's ability to perform a balanced and effective transition from oxic to anoxic respiration with only marginal emissions of intermediates. This rather detailed understanding of the bacterial nitrogen cycle to date has come from studies of Gram-negative bacteria but evidence is now appearing showing that Gram-positive bacteria, such as bacilli, can also carry out denitrification [50,51].
5. Nitrous oxide emissions from soils
It is now well-recognized that microbial activity in soils is a major contributor to atmospheric loading of N2O. Clark et al. [52] have assessed the influence of different long-term fertilization and cultivation treatments in a 160 year-old field experiment, comparing the potential for denitrification with the size and diversity of the soil denitrifier communities. Denitrification potential was found to be much higher in soil from an area left to develop from arable into woodland than from a farmyard manure-fertilized arable treatment, which in turn was significantly higher than inorganic nitrogen-fertilized and unfertilized arable plots. These observations correlated with abundance of nirK but not nirS (dissimilatory nitrite reductase genes). Most genetic variation was seen in nirK where sequences resolved into separate groups according to soil treatment. They conclude that bacteria containing nirK are most likely responsible for the increased denitrification potential associated with nitrogen and organic carbon availability in this soil. Soil physicochemical properties (bulk density, pH, organic matter, organic C, N and C : N ratio) have an overriding influence on the potential denitrification activity resulting in increased N2O emissions in soils with high organic matter. Significantly, there were also structural differences in denitrifier communities in soils with high N and C contents. Thus, they possess proportionally fewer copies of the N2OR gene nosZ, so may be less able to close the nitrogen cycle by reducing N2O to N2. They also note that soil management (tillage) can lower GHG emissions.
Bakken et al. [49] report that, in model strains of P. denitrificans in pure cultures and in microbial communities extracted from soils, the N2O/(N2 + N2O) product ratio of denitrification is controlled by pH. The ratio increases with acidity. The effect is probably due primarily to interference with the assembly of the enzyme N2OR, rather than to the narrow pH range of the maximal activity of the enzyme. There have been many similar observations of pH effects on denitrification in soils, indicating a wide generality of the phenomenon [53–58]. These findings suggest that the continuing acidification of agricultural soils through excessive use of nitrogen fertilizers, as demonstrated for China [59], will enhance N2O emissions drastically. It is proposed that careful adjustment of pH in agricultural soils, say, by liming, should reduce N2O emissions from slightly acid soils. This needs to be tested rigorously in field trials.
Plants themselves have a strong influence on the microbial community of the rhizosphere, where most of the N2O generating activity occurs. The release of plant-derived low molecular weight organic compounds into the soil enhances heterotrophic activity, with denitrifiers and nitrate ammonifiers thought to compete for this carbon. Hence, N2O production and reduction rates are often positively correlated with total carbon or soluble organic carbon availability [60,61]. There is currently interest in understanding the physiological and genetic bases underpinning the influence of plant traits in regulating N2O emission, and the possibility that this could inform future breeding programmes to couple enhanced crop agronomic performance with environmental sustainability in terms of lowering net GHG emissions and increasing soil carbon stocks.
Denitrification enzymes require a variety of metal cofactors, including Mo, Fe, Cu and Zn. The absolute requirement of N2OR for Cu (and sulphur) for activity, as well as the absence of any parallel pathways that can reduce N2O, account for the critical role of this element in the success of this final step of denitrification. Many species of bacteria have scavenging systems, such as siderophores, excreted by cells to chelate Fe strongly in order to extract it from soils, or sequester it from the ocean, and to deliver Fe(II) to cell surface receptors for active uptake into the cell. Furthermore, Fe can also be stored within cells inside proteins, such as ferritins, for retrieval in times of external Fe stress (or to compartmentalize the Fe during dormancy to protect it from reacting with O2, thereby generating products potentially toxic to DNA). There are no such sequestering or storage systems yet known for copper in bacteria with the exception of some methanotrophic bacteria that excrete Cu-chelating compounds [62]. Hence, copper availability to the cell depends on the concentrations of Cu in the local external environment as well as on its state of chelation within soils. Zumft [2] first showed that by growing laboratory cultures of denitrifying bacteria in Cu-deficient media, high levels of N2O emissions occur compared with those in copper sufficient media, leading him to the conclusion that N2OR is a copper-dependent enzyme.
Copper in soil is found as the water-soluble cation Cu2+, but in reducing soils as the insoluble ion Cu+. Soil bacteria can take up Cu2+ or Cu+ either by energized or diffusive transport [63]. The biological availability of Cu in soils to crops is influenced by a number of factors: its chemical state, soil conditions (pH, redox, soil moisture, etc.), SOM, inputs (fertilizer, manure, animal feed, etc.), weather, crop type and maturity. Cu deficiency is often observed in alkaline soils. A negative correlation of Cu plant uptake and pH is seen in clay soils. Cu bioavailability is also lowered by adsorption of Cu on clay surfaces or, in soils with high organic matter such as humic acids, formation of metal–organic complexes [64].
However, free Cu2+ species can also be toxic to soil bacteria. Ore et al. [65] correlated copper toxicity in Nitrosomonas europaea to free ion metal activity in soil pore water; EC50 Cu2+ = 2 × 10−6 to 2 × 10−9 M. Two major uncertainties exist regarding the interaction of bacteria and free metal ions. First, not all soil bacteria have the same tolerance to free ion metals and microbial communities can adapt during long-term exposure, developing pollution-induced community tolerance; second, it is difficult to assess which bacterial cells are exposed to the free metal ions in the soil matrix. Thus, in Cu-limiting conditions, it was recently demonstrated that the bacterium P. denitrificans is able to acquire Cu from the soil matrix by excreting zinc coproporphyrin III in both aerobic and anaerobic environments [66].
Cu is also a required cofactor in [NO2]− reduction in some bacteria such as Achromobacter xylosoxidans. In a large-scale field study, Enwall et al. [67] found a positive relationship between soil Cu content and the abundance of nirK genes. Thus, Cu plays a key role in both NO2− and N2O reduction [68]. With approximately 40 per cent of Europe's arable soils being Cu deficient (less than 2 mg Cu kg−1), the potential for N2O mitigation (with a simultaneous crop yield increase) is high. Nevertheless, above certain concentrations metals in the soil can have adverse effects on soil nutrient cycling and soil food webs [69]. Investigating the trade-off between the effect of mineral micronutrients on N2O soil emissions and soil ecosystem functioning (nutrient cycling) is an important aspect with practical and environmental implications yet to be explored [70].
Plants and soil microbes compete for Cu uptake. Cu is a vital micronutrient to maximize crop yield and quality. Too little (less than 2 mg kg−1) or too much (greater than 30 mg kg−1) Cu in soils will result in adverse effects on plant growth. Cu supplements can be applied either as soil amendments or fertilizers (e.g. in the form of pig slurry or CuSO4) or foliar fertilizers (e.g. copper oxychloride) to the crops. Cu availability can also be controlled through changing SOM contents.
6. Nitrous oxide from oceans and in the atmosphere
Oceans are an important source of N2O. Freing et al. [71] present tracer data together with in situ measurements of N2O to estimate the concentration and production rates of biologically produced N2O in the ocean on a global scale. They estimate that oceanic N2O production is dominated by nitrification with a contribution of only approximately 7 per cent from denitrification, indicating that previously used approaches may have overestimated the contribution from denitrification. Continental shelf areas account for only a negligible fraction of the global production of N2O, whereas coastal zones such as estuaries probably contribute significantly to the total oceanic emissions of N2O because they are fertilized to an increasing degree by river run-off carrying a high load of organic nitrogen (eutrophication).
In the oceans, the estimated global annual subsurface N2O production ranges from 3.1 ± 0.9 to 3.4 ± 0.9 Tg N yr−1. The largest amount of subsurface N2O is produced in the upper 500 m of the water column. The oxygen minimum zones of the intermediate layers (between 300 and 700 m water depth) in various regions of the ocean are expanding and have been losing oxygen during the past 50 years. This could result in an expansion of the zones supporting denitrification, probably having an impact on the production and decomposition of N2O. Whether it would have a net positive or negative effect on N2O production remains unclear as the net behaviour of denitrification and its controlling mechanisms are not yet fully understood.
There is also evidence that the oceans are warming. As marine autotrophic and heterotrophic processes display sensitivities to temperature (to varying degrees), ocean warming might result in changes of the bacterial community structure and hence in changes of N2O production. Changes in ocean temperature also affect the solubility of N2O. Rising ocean temperature is likely to result in the N2O long-term storage capacity of the deep ocean being reduced. Oceanic N2O sources are thus likely to vary as ongoing changes of the ocean environment such as deoxygenation, warming and eutrophication occur.
N2O concentrations in the atmosphere are rising steadily with consequences not only for global warming but also for ozone destruction. The paper by Portmann et al. [72] reports the effects of N2O, together with other gases CO2, CH4 and halocarbons, on stratospheric ozone levels over the past 100 years and predicts its future evolution using a chemical model of the stratosphere. This model and the underlying chemistry are set out in their paper. It is concluded that, as halocarbons return toward pre-industrial levels, N2O and CO2 are likely to play the dominant roles in ozone depletion. They show, however, that there are nonlinear interactions between these gases that preclude the unambiguous separation of their effects on ozone. For example, the chemical destruction of O3 by N2O is buffered by the thermal effects of CO2 in the middle stratosphere by approximately 20 per cent. Nonetheless, it is clear that N2O is expected to be the largest ozone-destroying compound in the foreseeable future. Hence, successful mitigation of release of anthropogenic N2O provides a more important opportunity for reduction in future ozone depletion than any of the remaining uncontrolled halocarbon emissions.
7. Nitrous oxide emissions from wastewater treatment
An excellent example of the type of local analyses that can be applied to a single source of N2O emission is provided by the paper from Law et al. [73] on wastewater treatment plants. Despite its relatively small contribution to the overall global GHG emissions, N2O emissions from biological nutrient removal wastewater treatment plants can be very significant in terms of the contributions to their overall carbon footprint. N2O emissions vary substantially depending on the design and operation of the plants, and on the flow and characteristics of wastewater. Such variations indicate that N2O may be mitigated through engineering proper process design and operation. Preliminary strategies remain to be verified through full-scale applications. Law et al. note that in most wastewater treatment plants in contrast, for example, to soils where denitrification is often the primary source of N2O, autotrophic NH3 oxidation makes a relatively greater contribution than heterotrophic denitrification.
8. Strategies for mitigating nitrous oxide emissions
Evidence presented in this volume and elsewhere makes clear the damaging effects on climate of atmospheric N2O. Therefore, strategies to ameliorate N2O emission arising from intensive agricultural practices should be developed in order to decrease current levels of N2O emissions and to forestall further rises predicted to occur as usage of nitrogenous fertilizer increases across the globe. Strategies that might be adopted arise from three quite different approaches: first, by managing soil chemistry and microbiology to ensure that bacterial denitrification runs to completion, generating N2 instead of N2O; second, by reducing dependence on fertilizers through engineering crop plants, for example to fix nitrogen themselves in order to sustain growth and yield, or by capitalizing on C–N interactions in the rhizosphere; third, by promoting sustainable agricultural intensification, that is, producing more output from the same area of land while reducing the negative environmental impacts. We consider each of these strategies in turn.
(a). Managing soil chemistry and microbiology
It seems unlikely that it will ever be possible to develop farming practices that completely eliminate N2O emissions from soil denitrifiers in agriculture. The ability to denitrify is phylogenetically diverse, and recent developments in techniques for quantifying N2O production from denitrification show its occurrence to be more widespread than previously thought. However, it should be possible to mitigate N2O emissions by using our understanding of the enzymology and microbiology of denitrification to design protocols to manipulate soil chemistry and physics and, thereby, the physiology of denitrifying bacteria to ensure that the reduction of N2O to N2 is, as far as possible, unconstrained.
Much evidence has been presented in the papers in this volume, and elsewhere, that it is the failure of the enzyme N2OR to operate that curtails the denitrification process at N2O rather running on to N2. Two key factors that can cause this are low soil concentrations of Cu available to the bacterium and soil pH values below 7. Cu availability will depend not only on the absolute Cu concentration in the soil but also on the presence of competing chemical chelators, such as humic acids. Hence, there is the possibility of using SOM management, copper application or liming as primary controls of copper availability and pH values. Recent work investigated the effect of O2 on 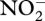 -dependent denitrification and the emission of NO, N2O and N2 in cultures of soil extracted bacteria [74]. There was evidence that N2OR can be temporarily inactivated by sudden exposure to even low levels of O2, whereas the other enzymes of denitrification continue to function. In soils themselves, N2O–N2 ratios are higher as the soil pore O2 concentration increases. This may, in part, reflect a greater contribution of ammonia-oxidizing bacteria to N2O emission, but could also arise from the sensitivity of N2OR to O2. It will be difficult in soils to show in vivo enzyme inactivation.
-dependent denitrification and the emission of NO, N2O and N2 in cultures of soil extracted bacteria [74]. There was evidence that N2OR can be temporarily inactivated by sudden exposure to even low levels of O2, whereas the other enzymes of denitrification continue to function. In soils themselves, N2O–N2 ratios are higher as the soil pore O2 concentration increases. This may, in part, reflect a greater contribution of ammonia-oxidizing bacteria to N2O emission, but could also arise from the sensitivity of N2OR to O2. It will be difficult in soils to show in vivo enzyme inactivation.
A full list of factors known to influence the ratio of N2 to N2O during denitrification include [NO3]− and C availability, partial pressure of O2, water-holding capacity, Cu availability, as well as soil pH. The set of management options by which soil conditions might be manipulated either to lower emission of N2O, or to increase its reduction to N2 would include liming, manure addition, biochar or zeolite addition, minimal tillage, integrated fertilizer residue management, crop residue addition, as well as controlled release fertilizer, nitrification inhibitors, plant trait, plant breeding. Results reported by Bakken et al. [49] do indeed suggest that mitigation of N2O emissions by increasing the pH of soils is currently a most promising management option. The pervasive effect of pH on the product stoichiometry of denitrification lies within the pH range 5–7, that of most agricultural soils. A recent paper points out the importance of assessing emissions according to the unit of product [75]. It shows very clearly the rapid increase in N2O emissions when N fertilizer is added in excess of crop requirements. By considering agronomic conditions optimizing rather than minimizing nitrogen fertilizer application rates, N2O emissions are reduced. A fuller discussion of all these aspects is given by Richardson et al. [18] which also contains descriptions of various management practices.
It may also be possible through plant breeding to manipulate denitrification through inputs into the plant rhizosphere, thereby changing the composition of plant-derived carbon flow or nitrogen uptake demand, or through crop spacing, tillage or integrated inorganic fertilizer, residue and SOM management. Breeding for plant release of biological nitrification inhibitors that block the AMO and hydroxylamine oxidoreductase pathways in ammonia-oxidizing bacteria promises to allow manipulation of soil nitrogen concentrations, and hence the soil denitrification potential. However, the effects on N2O production are unknown. Such opportunities for managing N2O emissions need to be considered in the light of effects on soil carbon levels and chemistry, not only because of the other key GHGs, CO2 and CH4, but also because of the important balance between fertilizer application increasing carbon sequestration through greater biomass production versus the undesirable alternative consequence of increased N2O emission.
A key step in the future will be whether we can use technical advances in geochemistry and environmental biochemistry to monitor a wide set of parameters, both of the soil and the bacterial processes, in field studies so that we can take an ecosystems biology approach to allow identification, and ranking, of the various factors that regulate N2O production and consumption. We note the recent development of field-deployable instruments capable of measuring nitrous oxide isotopic ratios, based on the principle of laser cavity ring down spectroscopy (CRDS) [76] that can measure continuously in real time the abundance of isotopically labelled 14N15N16O and 15N14N16O relative to 14N14N16O in N2O. Unlike mass spectrometry, this technique can distinguish between the two isotopomers 14N15N16O and 15N14N16O. The nitrogen isotopic site preference, the difference between the isotope ratios of the central and terminal nitrogen atom, can distinguish between N2O produced via the hydroxylamine oxidation pathway and that of nitrate reduction as well as between fungal and bacterial N2O production.
Central to the development of appropriate mitigation practices is addressing the challenge of spatial scale. N2O production impacts us at different spatial scales, from cellular production to the landscape, and to the global impact of climate change, and feedbacks within and between these scales. The challenge we face is in understanding phenomena of global magnitude that have their foundations at the microscale, and to formulate appropriate management practices for mitigation that are informed by regulation at the microscale. Recent efforts have demonstrated links between microbial gene expression, environmental parameters and N2O-genic processes at the microcosm scale, but there is still much progress to be made when relating this to processes at the macroscale, exacerbated by the high-spatial heterogeneity of N2O emission [77]. The scaling up, or even scaling down, of N2O producing processes in the plant–soil–microbe system is essential to inform policymakers of the environmental factors driving climate change that can be targeted for management, and may help reduce model uncertainty, which is vital for accurate prediction of emissions and for the formulation of appropriate mitigation strategies. We have invested much effort into examining the drivers of microbial activity at the rhizosphere to plot scales, but there is still uncertainty over whether this regulation is still relevant at the landscape scale, how we can extrapolate between scales, and whether the drivers of N2O production/reduction that can be targeted for management vary depending on the spatial scale being considered. To address this will require integration of molecular, microbiology, physiology, physics, biogeochemistry and mathematical modelling approaches.
(b). Engineering crop plants
A recent review discusses the feasibility of, and assesses the way forward in, reducing dependence on fertilizers through engineering crop plants to fix nitrogen themselves in order to sustain growth and yield [78]. This paper drew on a meeting convened by the Bill and Melinda Gates Foundation. Three approaches were considered. The first is the development of root nodule symbioses in cereals. Legumes and actinorhizal (non-legume) plants have evolved productive nitrogen-fixing symbioses with rhizobial and Frankia bacteria, respectively. The main steps required to make symbiotic nitrogen-fixing cereals include engineering bacteria to recognize and infect a host cereal root cell, and having the plant subsequently establish a low-oxygen environment such as a root nodule. The second approach discussed was the application, as fertilizers, of nitrogen-fixing endophytic bacteria that form nodule-independent associations with cereal crops. Although commercial biofertilizers containing such bacteria are available, it is unclear whether the enhancement of plant growth is the result of nitrogen fixation or of bacterial molecules that act as plant growth hormones. Nevertheless, biofertilizers represent an existing, and the only currently available, technology. The third method considered was the introduction of the nitrogenase enzyme system into a plant organelle. To achieve this, the complete biosynthetic pathway of the several components of the nitrogenase enzyme must be engineered into cereals and targeted to a low-oxygen compartment within the plant. In a related approach, a recent paper has reported expression of the Nos operon proteins from Pseudomonas stutzeri in transgenic plants to assemble N2OR, the objective being to bestow on plants the ability to reduce N2O to N2 themselves. Both the single-gene transformants (nosZ) and the multi-gene transformants (nosFLZDY) produced active recombinant N2OR. Enzymatic activity was detected using the methyl viologen-linked enzyme assay, showing that extracts from both types of transgenic plants exhibited N2O-reducing activity [79].
All these approaches are challenging but the rewards would be great. It has been claimed that, if the coupling of nitrogen supply and carbon metabolism could be achieved, excess nitrogen would not be lost to the environment, thereby resulting in lower N2O emissions.
(c). Sustainable agricultural intensification
Agriculture contributes a disproportionate amount of GHGs with high impact on warming, notably about 47 per cent and 58 per cent of total CH4 and N2O emissions, respectively. Of all global land area, 14 per cent is used for food production, which ties up a vast amount of carbon. Changes in agricultural practices that affect this store could have a considerable effect on global warming.
Sustainable agricultural intensification is defined as producing more output from the same area of land while reducing the negative environmental impacts and at the same time increasing contributions to natural capital and the flow of environmental services [80,81]. A sustainable production system would thus exhibit most of the following attributes:
— using crop varieties and livestock breeds with a high ratio of productivity to use of externally derived inputs;
— avoiding the unnecessary use of external inputs;
— harnessing agro-ecological processes such as nutrient cycling, biological nitrogen fixation, allelopathy, predation and parasitism;
— minimizing use of technologies or practices that have adverse impacts on the environment and human health;
— making productive use of human capital in the form of knowledge and capacity to adapt and innovate, and social capital to resolve common landscape-scale problems; and
— quantifying and minimizing the impacts of system management on externalities such as GHG emissions, clean water availability, carbon sequestration, conservation of biodiversity, and dispersal of pests, pathogens and weeds.
In terms of technologies, therefore, productive and sustainable agricultural systems make the best of both crop varieties and livestock breeds and their agro-ecological and agronomic management. The pioneering rice breeder, Peter Jennings, who led early advancements in high-yielding rice varieties during the first green revolution, has argued for an ‘agronomic revolution’: Pretty states ‘It is now widely recognized that rice yield gaps result from agronomic failings, and that future yield increases depend heavily on this science. Agronomy's time has come to lift farm productivity out of stagnancy’ [81]. Agronomy refers to the management of crops and livestock in their specific circumstances, and matches with the emergence of the term agro-ecology to indicate that there is a need to invest in science and practice that gives farmers a combination of the best possible seeds and breeds and their management in local ecological contexts.
This suggests that sustainable intensification will very often involve more complex mixes of domesticated plant and animal species and associated management techniques, requiring greater skills and knowledge by farmers. To increase production efficiently and sustainably, farmers need to understand under what conditions agricultural inputs (seeds, fertilizers and pesticides) can either complement or contradict biological processes and ecosystem services that inherently support agriculture. In all cases, farmers need to see for themselves that added complexity and increased efforts can result in substantial net benefits to productivity, but they need also to be assured that increasing production actually leads to increases in income. Too many successful efforts in raising production yields have ended in failure when farmers were unable to market the increased outputs. Understanding how to access rural credit, or how to develop warehouse receipt systems and, especially, how to sell any increased output, become as important as learning how to maximize input efficiencies or build fertile soils.
9. Conclusions
Despite decades of research on N2O emissions, few mitigation options have been proposed and even fewer trialled. A key target should be to improve the product stoichiometry of denitrification (N2/N2O) in agro-ecosystems. The understanding now reached of the genetics, microbiology, enzymology and chemistry allows trials in the field to be designed. The availability of mobile monitoring systems, such as MS and CRDS, together with isotopic spiking, and coupling to molecular ecology approaches provide the means to diagnose, distinguish and quantify the pathways operating and, hence, to allow a description of the fate of applied N to be reached. This should enable the exploration of different management options to ascertain their effectiveness. Systematic studies of complex interactions in such eco-systems that are contributing globally to the release of the potent GHG N2O are now feasible. They should be providing prescriptions for the minimization of N2O emissions from soils under a wide variety of circumstances.
Acknowledgements
We thank the following colleagues L. Bakken, H. W. Bange, K. Goulding, P. Hirsch, N. Le Brun, I. Moura, R. Portmann, U. Skiba, K. Smith and S. Spiro for helpful discussions.
References
- 1.Lassey K., Harvey M. 2007. Nitrous oxide: the serious side of laughing gas. Water Atmos. 15, 1–10 [Google Scholar]
- 2.Zumft W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61, 533–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NERC. 2005. Global nitrogen enrichment (GANE): reports and key findings. See www.nerc.ac.uk/research/programmes/gane/results.asp (accessed 15 December 2011)
- 4.Kimochi Y., Inamori Y., Mizuochi M., Xu K.-Q., Matsumura M. 1998. Nitrogen removal and N2O emission in a full-scale domestic wastewater treatment plant with intermittent aeration. J. Biosci. Bioeng. 86, 202–206 10.1016/s0922-338x(98)80114-1 (doi:10.1016/s0922-338x(98)80114-1) [DOI] [Google Scholar]
- 5.Davidson E. A. 2009. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2, 659–662 10.1038/ngeo608 (doi:10.1038/ngeo608) [DOI] [Google Scholar]
- 6.Bates B., Kundzewicz Z., Wu S., Palutikof J. 2008. Climate change and water. Technical Paper of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC [Google Scholar]
- 7.IPCC 2007. Working group I: the physical science basis. In IPCC fourth assessment report: climate change 2007 (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miler H. L.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 8.Crutzen P., Oppenheimer M. 2008. Learning about ozone depletion. Clim. Change 89, 143–154 10.1007/s10584-008-9400-6 (doi:10.1007/s10584-008-9400-6) [DOI] [Google Scholar]
- 9.Ravishankara A. R., Daniel J. S., Portmann R. W. 2009. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125 10.1126/science.1176985 (doi:10.1126/science.1176985) [DOI] [PubMed] [Google Scholar]
- 10.IPCC 2001. Atmospheric chemistry and greenhouse gases. In Climate change 2001: the scientific basis. Contribution of Working Group I to the third assessment report of the intergovernmental panel on climate change (eds Houghton J., Ding Y., Griggs D., Noguer M., van der Linden P., Dai X., Maskell K., Johnson C. A.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 11.The Royal Society 2009. Reaping the benefits: science and the sustainable intensification of global agriculture. London, UK: The Royal Society [Google Scholar]
- 12.Bothe H., Ferguson S. J., Newton W. E. 2006. Biology of the nitrogen cycle. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 13.Day D. A., Poole P. S., Tyerman S. D., Rosendahl L. 2001. Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell. Mol. Life Sci. 58, 61–71 10.1007/pl00000778 (doi:10.1007/pl00000778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertero M. G., Rothery R. A., Palak M., Hou C., Lim D., Blasco F., Weiner J. H., Strynadka N. C. 2003. Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nat. Struct. Biol. 10, 681–687 10.1038/nsb969 (doi:10.1038/nsb969) [DOI] [PubMed] [Google Scholar]
- 15.Adman E. T. 1995. A taste of copper. Nat. Struct. Biol. 2, 929–931 10.1038/nsb1195-929 (doi:10.1038/nsb1195-929) [DOI] [PubMed] [Google Scholar]
- 16.Hino T., Matsumoto Y., Nagano S., Sugimoto H., Fukumori Y., Murata T., Iwata S., Shiro Y. 2010. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 330, 1666–1670 10.1126/science.1195591 (doi:10.1126/science.1195591) [DOI] [PubMed] [Google Scholar]
- 17.Watmough N. J., Field S. J., Hughes R. J. L., Richardson D. J. 2009. The bacterial respiratory nitric oxide reductase. Biochem. Soc. Trans. 37, 200–300 10.1042/BST0370200 (doi:10.1042/BST0370200) [DOI] [PubMed] [Google Scholar]
- 18.Richardson D., Felgate H., Watmough N., Thomson A., Baggs E. 2009. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle: could enzymic regulation hold the key? Trends Biotechnol. 27, 388–397 10.1016/j.tibtech.2009.03.009 (doi:10.1016/j.tibtech.2009.03.009) [DOI] [PubMed] [Google Scholar]
- 19.Scala D. J., Kerkhof L. J. 1998. Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol. Lett. 162, 61–68 10.1016/s0378-1097(98)00103-7 (doi:10.1016/s0378-1097(98)00103-7) [DOI] [PubMed] [Google Scholar]
- 20.Brown K., Djinovic-Carugo K., Haltia T., Cabrito I., Saraste M., Moura J. G., Moura I., Tegoni M., Cambillau C. 2000. Revisiting the catalytic CuZ cluster of nitrous oxide (N2O) reductase. J. Biol. Chem. 275, 41 133–41 136 10.1074/jbc.M008617200 (doi:10.1074/jbc.M008617200) [DOI] [PubMed] [Google Scholar]
- 21.Rotthauwe J. H., Witzel K. P., Liesack W. 1997. The ammonia monooxygenase structural gene amoa as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63, 4704–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arp D. J., Sayavedra-Soto L. A., Hommes N. G. 2002. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch. Microbiol. 178, 250–255 10.1007/s00203-002-0452-0 (doi:10.1007/s00203-002-0452-0) [DOI] [PubMed] [Google Scholar]
- 23.Allen A. E., Booth M. G., Frischer M. E., Verity P. G., Zehr J. P., Zani S. 2001. Diversity and detection of nitrate assimilation genes in marine bacteria. Appl. Environ. Microbiol. 67, 5343–5348 10.1128/AEM.67.11.5343-5348.2001 (doi:10.1128/AEM.67.11.5343-5348.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohashi Y., Shi W., Takatani N., Aichi M., Maeda S. I., Watanabe S., Yoshikawa H., Omata T. 2011. Regulation of nitrate assimilation in cyanobacteria. J. Exp. Bot. 62, 1411–1424 10.1093/jxb/erq427 (doi:10.1093/jxb/erq427) [DOI] [PubMed] [Google Scholar]
- 25.Nolden L., Farwick M., Krämer R., Burkovski A. 2001. Glutamine synthetases of Corynebacterium glutamicum: transcriptional control and regulation of activity. FEMS Microbiol. Lett. 201, 91–98 10.1016/s0378-1097(01)00244-0 (doi:10.1016/s0378-1097(01)00244-0) [DOI] [PubMed] [Google Scholar]
- 26.Ciurli S., Marzadori C., Benini S., Deiana S., Gessa C. 1996. Urease from the soil bacterium Bacillus pasteurii: immobilization on Ca-polygalacturonate. Soil Biol. Biochem. 28, 811–817 10.1016/0038-0717(96)00020-x (doi:10.1016/0038-0717(96)00020-x) [DOI] [Google Scholar]
- 27.Skiba U., Smith K. A. 2000. The control of nitrous oxide emissions from agricultural and natural soils. Chemosphere Glob. Change Sci. 2, 379–386 10.1016/S1465-9972(00)00016-7 (doi:10.1016/S1465-9972(00)00016-7) [DOI] [Google Scholar]
- 28.Smith P., et al. 2008. Greenhouse gas mitigation in agriculture. Phil. Trans. R. Soc. B 363, 789–813 10.1098/rstb.2007.2184 (doi:10.1098/rstb.2007.2184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okereke G. U. 1993. Growth yield of denitrifiers using nitrous oxide as a terminal electron acceptor. World J. Microbiol. Biotechnol. 9, 59–62 10.1007/bf00656518 (doi:10.1007/bf00656518) [DOI] [PubMed] [Google Scholar]
- 30.Bange H. W., Freing A., Kock A., Löscher C. R. 2010. Marine pathways to nitrous oxide (N2O). In Nitrous oxide and climate change (ed. Smith K.), pp. 36–62 London, UK: Earthscan [Google Scholar]
- 31.Brussaard L., et al. 1997. Biodiversity and ecosystem functioning in soil. Ambio 26, 563–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith K. A., Mosier A. R., Crutzen P. J., Winiwarter W. 2012. The role of N2O derived from crop-based biofuels, and from agriculture in general, in Earth's climate. Phil. Trans. R. Soc. B 367, 1169–1174 10.1098/rstb.2011.0313 (doi:10.1098/rstb.2011.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skiba U., et al. 2012. UK emissions of the greenhouse gas nitrous oxide. Phil. Trans. R. Soc. B 367, 1175–1185 10.1098/rstb.2011.0356 (doi:10.1098/rstb.2011.0356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santoro A. E., Buchwald C., McIlvin M. R., Casciotti K. L. 2011. Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333, 1282–1285 10.1126/science.1208239 (doi:10.1126/science.1208239) [DOI] [PubMed] [Google Scholar]
- 35.Shoun H., Tanimoto T. 1991. Denitrification by the fungus Fusarium oxysporum and involvement of cytochrome P450 in the respiratory nitrite reduction. J. Biol. Chem. 266, 11 078–11 082 [PubMed] [Google Scholar]
- 36.Shoun H., Fushinobu S., Jiang L., Kim S.-W., Wakagi T. 2012. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Phil. Trans. R. Soc. B 367, 1186–1194 10.1098/rstb.2011.0335 (doi:10.1098/rstb.2011.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prendergast-Miller M. T., Baggs E. M., Johnson D. 2011. Nitrous oxide production by the ectomycorrhizal fungi Paxillus involutus and Tylospora fibrillosa. FEMS Microbiol. Lett. 316, 31–35 10.1111/j.1574-6968.2010.02187.x (doi:10.1111/j.1574-6968.2010.02187.x) [DOI] [PubMed] [Google Scholar]
- 38.Sutka R. L., Adams G. C., Ostrom N. E., Ostrom P. H. 2008. Isotopologue fractionation during N2O production by fungal denitrification. Rapid Commun. Mass Spectrom. 22, 3989–3996 10.1002/rcm.3820 (doi:10.1002/rcm.3820) [DOI] [PubMed] [Google Scholar]
- 39.Shiro Y., Sugimoto H., Tosha T., Nagano S., Hino T. 2012. Structural basis for nitrous oxide generation by bacterial nitric oxide reductases. Phil. Trans. R. Soc. B 367, 1195–1203 10.1098/rstb.2011.0310 (doi:10.1098/rstb.2011.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ananyev G. M., Zaltsman L., Vasko C., Dismukes G. C. 2001. The inorganic biochemistry of photosynthetic oxygen evolution/water oxidation. Biochim. Biophys. Acta Bioenerg. 1503, 52–68 10.1016/s0005-2728(00)00215-2 (doi:10.1016/s0005-2728(00)00215-2) [DOI] [PubMed] [Google Scholar]
- 41.Stremińska M. A., Felgate H., Rowley G., Richardson D. J., Baggs E. M. In press Nitrous oxide production in soil isolates of nitrate-ammonifying bacteria. Environ. Microbiol. Rep. (doi:10.1111/j.1758-2229.2011.00302.x) [DOI] [PubMed] [Google Scholar]
- 42.Spiro S. 2012. Nitrous oxide production and consumption: regulation of gene expression by gas-sensitive transcription factors. Phil. Trans. R. Soc. B 367, 1213–1225 10.1098/rstb.2011.0309 (doi:10.1098/rstb.2011.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown K., Tegoni M., Prudencio M., Pereira A. S., Besson S., Moura J. J., Moura I., Cambillau C. 2000. A novel type of catalytic copper cluster in nitrous oxide reductase. Nat. Struct. Mol. Biol. 7, 191–195 10.1038/73288 (doi:10.1038/73288) [DOI] [PubMed] [Google Scholar]
- 44.Simon J., Einsle O., Kroneck P. M. H., Zumft W. G. 2004. The unprecedented nos gene cluster of Wolinella succinogenes encodes a novel respiratory electron transfer pathway to cytochrome c nitrous oxide reductase. FEBS Lett. 569, 7–12 10.1016/j.febslet.2004.05.060 (doi:10.1016/j.febslet.2004.05.060) [DOI] [PubMed] [Google Scholar]
- 45.Zumft W. G., Matsubara T. 1982. A novel kind of multi-copper protein as terminal oxidoreductase of nitrous oxide respiration in Pseudomonas perfectomarinus. FEBS Lett. 148, 107–112 10.1016/0014-5793(82)81253-2 (doi:10.1016/0014-5793(82)81253-2) [DOI] [Google Scholar]
- 46.Dell'Acqua S., Pauleta S. R., Moura J. J. G., Moura I. 2012. Biochemical characterization of the purple form of Marinobacter hydrocarbonoclasticus nitrous oxide reductase. Phil. Trans. R. Soc. B 367, 1204–1212 10.1098/rstb.2011.0311 (doi:10.1098/rstb.2011.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pomowski A., Zumft W. G., Kroneck P. M. H., Einsle O. 2011. N2O binding at a [4Cu:2S] copper-sulphur cluster in nitrous oxide reductase. Nature 477, 234–237 10.1038/nature10332 (doi:10.1038/nature10332) [DOI] [PubMed] [Google Scholar]
- 48.Honisch U., Zumft W. G. 2003. Operon structure and regulation of the nos gene region of Pseudomonas stutzeri, encoding an ABC-Type ATPase for maturation of nitrous oxide reductase. J. Bacteriol. 185, 1895–1902 10.1128/jb.185.6.1895-1902.2003 (doi:10.1128/jb.185.6.1895-1902.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakken L. R., Bergaust L., Liu B., Frostegård Å. 2012. Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Phil. Trans. R. Soc. B 367, 1226–1234 10.1098/rstb.2011.0321 (doi:10.1098/rstb.2011.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verbaendert I., Boon N., De Vos P., Heylen K. 2011. Denitrification is a common feature among members of the genus Bacillus. Syst. Appl. Microbiol. 34, 385–391 10.1016/j.syapm.2011.02.003 (doi:10.1016/j.syapm.2011.02.003) [DOI] [PubMed] [Google Scholar]
- 51.Jones C. M., Welsh A., Throbäck I. N., Dörsch P., Bakken L. R., Hallin S. 2011. Phenotypic and genotypic heterogeneity among closely related soil-borne N2- and N2O-producing Bacillus isolates harboring the nosZ gene. FEMS Microbiol. Ecol. 76, 541–552 10.1111/j.1574-6941.2011.01071.x (doi:10.1111/j.1574-6941.2011.01071.x) [DOI] [PubMed] [Google Scholar]
- 52.Clark I. M., Buchkina N., Jhurreea D., Goulding K. W. T., Hirsch P. R. 2012. Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the Broadbalk Wheat Experiment. Phil. Trans. R. Soc. B 367, 1235–1244 10.1098/rstb.2011.0314 (doi:10.1098/rstb.2011.0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kučera I., Matyášek R., Dadák V. 1986. The influence of pH on the kinetics of dissimilatory nitrite reduction in Paracoccus denitrificans. Biochim. Biophys. Acta Bioenerg. 848, 1–7 10.1016/0005-2728(86)90153-2 (doi:10.1016/0005-2728(86)90153-2) [DOI] [Google Scholar]
- 54.Baumann B., van der Meer J. R., Snozzi M., Zehnder A. J. B. 1997. Inhibition of denitrification activity but not of mRNA induction in Paracoccus denitrificans by nitrite at a suboptimal pH. Antonie van Leeuwenhoek 72, 183–189 10.1023/a:1000342125891 (doi:10.1023/a:1000342125891) [DOI] [PubMed] [Google Scholar]
- 55.Bergaust L., Mao Y., Bakken L. R., Frostegård Å. 2010. Denitrification response patterns during the transition to anoxic respiration and post-transcriptional effects of suboptimal pH on nitrogen oxide reductase in Paracoccus denitrificans. Appl. Environ. Microbiol. 76, 6387–6396 10.1128/aem.00608-10 (doi:10.1128/aem.00608-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ŠImek M., Cooper J. E. 2002. The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 53, 345–354 10.1046/j.1365-2389.2002.00461.x (doi:10.1046/j.1365-2389.2002.00461.x) [DOI] [Google Scholar]
- 57.Šimek M., Jíšová L., Hopkins D. W. 2002. What is the so-called optimum pH for denitrification in soil? Soil. Biol. Biochem. 34, 1227–1234 10.1016/s0038-0717(02)00059-7 (doi:10.1016/s0038-0717(02)00059-7) [DOI] [Google Scholar]
- 58.Thomsen J. K., Geest T., Cox R. P. 1994. Mass spectrometric studies of the effect of pH on the accumulation of intermediates in denitrification by Paracoccus denitrificans. Appl. Environ. Microbiol. 60, 536–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo J. H., et al. 2010. Significant acidification in major Chinese croplands. Science 327, 1008–1010 10.1126/science.1182570 (doi:10.1126/science.1182570) [DOI] [PubMed] [Google Scholar]
- 60.Philippot L., Hallin S., Börjesson G., Baggs E. M. 2009. Biochemical cycling in the rhizosphere having an impact on global change. Plant Soil 321, 61–81 10.1007/s11104-008-9796-9 (doi:10.1007/s11104-008-9796-9) [DOI] [Google Scholar]
- 61.Morley N., Baggs E. M. 2010. Carbon and oxygen controls on N2O and N2 production during nitrate reduction. Soil Biol. Biochem. 42, 1864–1871 10.1016/j.soilbio.2010.07.008 (doi:10.1016/j.soilbio.2010.07.008) [DOI] [Google Scholar]
- 62.Kim H. J., Graham D. W., DiSpirito A. A., Alterman M. A., Galeva N., Larive C. K., Asunskis D., Sherwood P. M. A. 2004. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 305, 1612–1615 10.1126/science.1098322 (doi:10.1126/science.1098322) [DOI] [PubMed] [Google Scholar]
- 63.Andreazza R., Okeke B., Pieniz S., Brandelli A., Lambais M., Camargo F. 2010. Bioreduction of Cu(II) by cell-free copper reductase from a copper resistant Pseudomonas sp. NA. Biol. Trace Elem. Res. 143, 1–11 10.1007/s12011-010-8899-3 (doi:10.1007/s12011-010-8899-3) [DOI] [PubMed] [Google Scholar]
- 64.Alloway B. J. (ed.) 2008. Micronutrient deficiencies in global crop production. Heidelberg, Germany: Springer [Google Scholar]
- 65.Ore S., Mertens J., Brandt K. K., Smolders E. 2010. Copper toxicity to bioluminescent Nitrosomonas europaea in soil is explained by the free metal ion activity in pore water. Environ. Sci. Technol. 44, 9201–9206 10.1021/es1026294 (doi:10.1021/es1026294) [DOI] [PubMed] [Google Scholar]
- 66.Anttila J., et al. 2011. Is coproporphyrin III a copper-acquisition compound in Paracoccus denitrificans? Biochim. Biophys. Acta Bioenerg. 1807, 311–318 10.1016/j.bbabio.2010.12.014 (doi:10.1016/j.bbabio.2010.12.014) [DOI] [PubMed] [Google Scholar]
- 67.Enwall K., Throback I. N., Stenberg M., Soderstrom M., Hallin S. 2010. Soil resources influence spatial patterns of denitrifying communities at scales compatible with land management. Appl. Environ. Microbiol. 76, 2243–2250 10.1128/aem.02197-09 (doi:10.1128/aem.02197-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bru D., Ramette A., Saby N. P. A., Dequiedt S., Ranjard L., Jolivet C., Arrouays D., Philippot L. 2011. Determinants of the distribution of nitrogen-cycling microbial communities at the landscape scale. ISME J. 5, 532–542 10.1038/ismej.2010.130 (doi:10.1038/ismej.2010.130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kandeler F., Kampichler C., Horak O. 1996. Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fert. Soils 23, 299–306 10.1007/bf00335958 (doi:10.1007/bf00335958) [DOI] [Google Scholar]
- 70.Ruyters S., Mertens J., T'Seyen I., Springael D., Smolders E. 2010. Dynamics of the nitrous oxide reducing community during adaptation to Zn stress in soil. Soil Biol. Biochem. 42, 1581–1587 10.1016/j.soilbio.2010.05.036 (doi:10.1016/j.soilbio.2010.05.036) [DOI] [Google Scholar]
- 71.Freing A., Wallace D. W. R., Bange H. W. 2012. Global oceanic production of nitrous oxide. Phil. Trans. R. Soc. B 367, 1245–1255 10.1098/rstb.2011.0360 (doi:10.1098/rstb.2011.0360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Portmann R., Daniel J. S., Ravishankara A. R. 2012. Stratospheric ozone depletion due to N2O: influences of other gases. Phil. Trans. R. Soc. B 367, 1256–1264 10.1098/rstb.2011.0377 (doi:10.1098/rstb.2011.0377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Law Y., Ye L., Pan Y., Yuan Z. 2012. Nitrous oxide emissions from wastewater treatment processes. Phil. Trans. R. Soc. B 367, 1265–1277 10.1098/rstb.2011.0317 (doi:10.1098/rstb.2011.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morley N., Baggs E. M., Dörsch P., Bakken L. 2008. Production of NO, N2O and N2 by extracted soil bacteria, regulation by NO2− and O2 concentrations. FEMS Microbiol. Ecol. 65, 102–112 10.1111/j.1574-6941.2008.00495.x (doi:10.1111/j.1574-6941.2008.00495.x) [DOI] [PubMed] [Google Scholar]
- 75.van Groenigen J. W., Velthof G. L., Oenema O., van Groenigen K. J., van Kessel C. 2010. Towards an agronomic assessment of N2O emissions: a case study for arable crops. Eur. J. Soil Sci. 61, 903–913 10.1111/j.1365-2389.2009.01217.x (doi:10.1111/j.1365-2389.2009.01217.x) [DOI] [Google Scholar]
- 76.Crosson E., Balslev-Clausen D., Dore J. (eds). 2011. A new analyzer to measure the abundance of 14N15N16O and 15N14N16O relative to 14N14N16O to help elucidate microbial N2O dynamics in terrestrial ecosystems. In EGU General Assembly, 3–8 April 2011 Vienna, Austria: European Geosciences Union [Google Scholar]
- 77.Standing D., Baggs E. M., Wattenbach M., Smith P., Killham K. 2007. Meeting the challenge of scaling up processes in the plant–soil–microbe system. Biol. Fert. Soils 44, 245–257 10.1007/s00374-007-0249-z (doi:10.1007/s00374-007-0249-z) [DOI] [Google Scholar]
- 78.Beatty P. H., Good A. G. 2011. Future prospects for cereals that fix nitrogen. Science 333, 416–417 10.1126/science.1209467 (doi:10.1126/science.1209467) [DOI] [PubMed] [Google Scholar]
- 79.Wan S., Mottiar Y., Johnson A. M., Goto K., Altosaar I. In press Expression of the Nos operon proteins from Pseudomonas stutzeri in transgenic plants to assemble nitrous oxide reductase. Transgenic Res. (doi:10.1007/s11248-011-9555-1) [DOI] [PubMed] [Google Scholar]
- 80.Pretty J., Toulmin C., Williams S. 2011. Sustainable intensification in African agriculture. Int. J. Agric. Sustainability 9, 5–24 10.3763/ijas.2010.0583 (doi:10.3763/ijas.2010.0583) [DOI] [Google Scholar]
- 81.Pretty J. 2008. Agricultural sustainability: concepts, principles and evidence. Phil. Trans. R. Soc. B 363, 447–465 10.1098/rstb.2007.2163 (doi:10.1098/rstb.2007.2163) [DOI] [PMC free article] [PubMed] [Google Scholar]