Photosynthetic microorganisms
can acclimate to a wide range of CO2
concentration, from as low as 0.001% to ≈10%
CO2 (vol/vol in the air in equilibrium with
their environment). Some can even grow in the presence of 40%
CO2 (1). Acclimation to a limiting
CO2 level, well below the
Km(CO2) of their
carboxylating enzyme, Rubisco (ribulose 1,5-bisphosphate
carboxylase/oxygenase), is achieved by substantial physiological and
structural changes at various cell levels (2–4). The most prominent of
these is the induction of a CO2 concentrating
mechanism (CCM), which raises the [CO2] in
close proximity to Rubisco. The latter is for the most part located in
pyrenoids or carboxysomes in eukaryotes and prokaryotes photosynthetic
microorganisms, respectively (5). This CCM involves light
energy-dependent inorganic carbon uptake and accumulation of
HCO within the cell. In cyanobacteria, the
accumulated HCO
within the cell. In cyanobacteria, the
accumulated HCO penetrates the carboxysomes where
carbonic anhydrase (CA) catalyzes the formation of
CO2 in the close vicinity of Rubisco. The
elevated CO2 concentration is thus confined to
the carboxysomes (3). This model is most likely also applicable to
eukaryotic algae where pyrenoids, densely packed with Rubisco and also
containing CA, may have the same function as carboxysomes (2–7). The
elevated [CO2] in these bodies compensates
for the relatively low affinity of Rubisco for
CO2 and consequently also decreases
photorespiration.
penetrates the carboxysomes where
carbonic anhydrase (CA) catalyzes the formation of
CO2 in the close vicinity of Rubisco. The
elevated CO2 concentration is thus confined to
the carboxysomes (3). This model is most likely also applicable to
eukaryotic algae where pyrenoids, densely packed with Rubisco and also
containing CA, may have the same function as carboxysomes (2–7). The
elevated [CO2] in these bodies compensates
for the relatively low affinity of Rubisco for
CO2 and consequently also decreases
photorespiration.
Exciting contributions appearing in this issue of PNAS, which have emerged simultaneously from the laboratories of Hideya Fukuzawa (8) and Donald Weeks (9), have now identified a key component of the transduction pathway between ambient [CO2] and expression of the CO2-responsive genes involved in the CCM. Several CO2-responsive genes already have been identified, both in the green alga Chlamydomonas reinhardtii, which is the subject of Fukuzawa's and Weeks' research, and cyanobacteria. Various approaches have been used, including analyses of changes in the polypeptide patterns (2), detection of low-CO2-induced cDNAs (10), and characterization of mutants impaired in specific steps of the CCM (2, 11–15). Doubtless many more CO2-responsive genes will be reported in the near future as a result of the application of technologies such as DNA microarray and proteomics. More than 40 low CO2-induced genes recently have been detected in the cyanobacterium Synechocystis sp. PCC6803 (T. Ogawa, personal communication) and more than 170 in Chlamydomonas (H. Fukuzawa, personal communication). It should be noted that some of the apparently CO2-sensitive genes may in fact respond to a general stress situation. For example, ndhD3 (directly involved in CO2 uptake in Synechocystis) is up-regulated both by high light (16) and low CO2 (15), suggesting response to the reductive state of photosynthetic electron transport carriers. Further, some CO2-responsive genes may not be involved in the CCM but in other CO2-affected processes such as purine (17) or cyclophilin metabolism (18).
Information is lacking both with regard to the
[CO2] sensor, the DNA elements conferring
CO2 responsiveness on the relevant genes, and the
components of the transduction pathway. On the basis of observed
photosynthetic performance at various ambient CO2
and pH levels, it has been postulated that
[CO2] is sensed at the plasmalemma by specific
receptors (19). Modified acclimation behavior of
Chlamydomonas mutants impaired in phosphoglycolate
phosphatase activity (2) and of a Synechococcus sp. PCC7942
mutant overexpressing this enzyme (20) supported the notion that
metabolites in the photorespiratory cycle may play an important part in
the transduction pathway. The level of photorespiratory metabolites
would be expected to increase with rise in the ratio
[O2]/[CO2] and the
consequent elevated oxygenase activity of Rubisco. These metabolites
might be directly involved or might be sensed as starvation signals.
Provision of organic nutrients down-regulates acclimation, indicating
that ample supply of nutrients is sensed, and can counter acclimation
even under low [CO2]. Conversely, lack of
certain nutrients, including NO , may trigger
acclimation even under sufficient CO2 (21).
Perception of the low CO2 signal may alter during
the course of the cell cycle as demonstrated by use of synchronously
grown Chlamydomonas cells (22–24).
, may trigger
acclimation even under sufficient CO2 (21).
Perception of the low CO2 signal may alter during
the course of the cell cycle as demonstrated by use of synchronously
grown Chlamydomonas cells (22–24).
Information on the DNA elements that may confer responsiveness of gene
expression to [CO2] is scarce. Analysis of the
promoter region of the low CO2-induced
cmpA, encoding a component of an ABC-type
HCO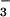 transporter (25) in Synechococcus
PCC7942, led to the identification of enhancing and suppressing
regulatory elements (26). Deletion of the latter potentiates
transcription under high CO2 (20). In
Chlamydomonas, CAH1, which encodes a periplasmic CA, is
among the genes up-regulated by low CO2 in the
light and down-regulated by high CO2 or dark
conditions. Analysis of its promoter region identified silencer and
enhancer cis-elements, which control the promoter under low
CO2 conditions in the light (27), but, before the
publication of the two papers in this issue (8, 9), transcription
factors involved were not recognized.
transporter (25) in Synechococcus
PCC7942, led to the identification of enhancing and suppressing
regulatory elements (26). Deletion of the latter potentiates
transcription under high CO2 (20). In
Chlamydomonas, CAH1, which encodes a periplasmic CA, is
among the genes up-regulated by low CO2 in the
light and down-regulated by high CO2 or dark
conditions. Analysis of its promoter region identified silencer and
enhancer cis-elements, which control the promoter under low
CO2 conditions in the light (27), but, before the
publication of the two papers in this issue (8, 9), transcription
factors involved were not recognized.
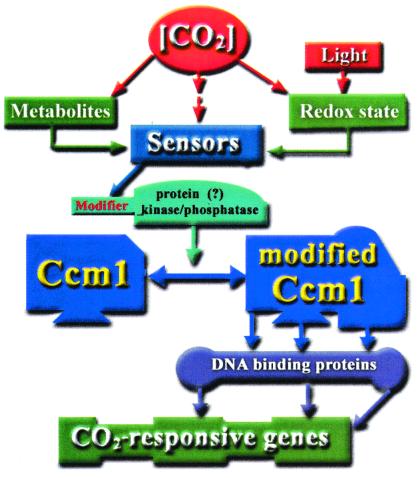
Isolation of high CO2-requiring Chlamydomonas mutants, cia5 and C16, which appear to lack all of the presently recognized responses to low CO2, opened the way to dissection of the signal transduction path between ambient [CO2] and gene expression. Mutant cia5 was produced by random mutagenesis (12) and C16 by gene interruption (28). In the two seminal papers in this issue (8, 9), it is proposed that the newly discovered component of the signal transduction pathway is a transcription factor. A genomic region that complements the phenotype of both cia5 and C16 has been identified (8, 9) and the relevant gene has been designated Cia5 and ccm1, respectively. Ccm1 (CIA5) contains a putative zinc-finger motif in its N-terminal region (where the H54Y mutation in cia5 is located) and a Gln repeat typical of transcription factors. In transformed onion epidermal cells, the Ccm1 apparently was located in the nucleus (9) but this may not be the case in Chlamydomonas (H. Fukuzawa, personal communication). ccm1 apparently is expressed constitutively, and Ccm1 is present under both high and low CO2 conditions. It is thus plausible that Ccm1-dependent transcription of low CO2-induced genes requires posttranslational modification of Ccm1 under low CO2. Strikingly, when the cia5 mutant was complemented with a truncated ccm1 missing the region coding for the last 54 C-terminal amino acids, the transformant expressed low CO2 characteristics even under high CO2 conditions. It remains to be seen whether the putative protein kinase C phosphorylation site in the C terminus of Ccm1 (missing in the truncated construct; ref. 9) participates in the control of CO2-dependent gene expression via phosphorylation/dephosphorylation cycles, or possibly even via phospho-relay cascade (see the proposed scheme in Fig. 1). Use of an anti-CCM1 antibody (H. Fukuzawa, personal communication) indicated that Ccm1 is not the nuclear protein that binds to the promoter region of CAH1 and presumably activates its transcription under limiting CO2. Because CAH1 is among the genes under the control of Ccm1 it follows that if the latter is a transcription factor it must function higher in the hierarchy.
Figure 1.
A possible scheme for the transduction path between ambient [CO2] and gene expression in Chlamydomonas.
In cyanobacteria, transcription of cmpA is under the control of CmpR, belonging to the LysR transcription factor family (29). CcmN, a putative DNA-binding protein, is required for expression of cmpA in low CO2-grown Synechococcus cells. In Synechocystis, transcription of ndhD3 is up-regulated by low CO2 (15) and repressed by NdhR. The latter is itself up-regulated by low CO2 (30).
In the race to break open the “black box” that up to now has enveloped the transduction path between ambient CO2 concentration and expression of the CCM, researchers into Chlamydomonas thus have gained a signal advantage over their colleagues investigating cyanobacteria. Identification of other components of this pathway is likely to follow clarification of the manner in which Ccm1 is modified in response to changing CO2 concentration.
Acknowledgments
Research in this laboratory is supported by grants from the USA-Israel Binational Science Foundation; Program MARS2 (cooperation between the German Bundes Ministerium fur Bildung Wissenschaft, Forschung und Technologie, and the Israeli Ministry of Science); and the Israeli Academy of Science.
Footnotes
See companion papers on pages 5341 and 5347.
References
- 1.Sasaki T, Pronina N A, Maeshima M, Iwasaki I, Kurano N, Miyachi S. Plant Biol. 1999;1:68–75. [Google Scholar]
- 2.Spalding M H. In: CO2 Acquisition, Acclimation to Changing Carbon Availability. Rochaix J D, Goldschmidt-Clermont M, Merchant S, editors. Dordrecht, The Netherlands: Kluwer; 1998. pp. 529–547. [Google Scholar]
- 3.Kaplan A, Reinhold L. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:539–570. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 4.Badger M R, Andrews T J, Whitney S M, Ludwig M, Yellowlees D C, Leggat W, Price G D. Can J Bot. 1998;76:1052–1071. [Google Scholar]
- 5.Moroney J V, Somanchi A. Plant Physiol. 1999;119:9–16. doi: 10.1104/pp.119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan A, Schwarz R, Lieman-Hurwitz J, Reinhold L. Plant Physiol. 1991;97:851–855. doi: 10.1104/pp.97.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raven J A. Adv Bot Res. 1997;27:85–209. [Google Scholar]
- 8.Fukuzawa H, Miura K, Kucho K I, Saito T, Kohinata T, Ohyama K. Proc Natl Acad Sci USA. 2001;98:5347–5352. doi: 10.1073/pnas.081593498. . (First Published April 3, 2001; 10.1073/pnas.081593498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang Y, Zhang J, Weeks D P. Proc Natl Acad Sci USA. 2001;98:5341–5346. doi: 10.1073/pnas.101534498. . (First Published April 17, 2001; 10.1073/pnas.101534498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somanchi A, Handley E R, Moroney J V. Can J Bot. 1998;76:1003–1009. [Google Scholar]
- 11.Suzuki K, Marek L F, Spalding M H. Plant Physiol. 1990;93:231–237. doi: 10.1104/pp.93.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moroney J V, Husic H D, Tolbert N E, Kitayama M, Manuel L J, Togasaki R K. Plant Physiol. 1989;89:897–903. doi: 10.1104/pp.89.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultemeyer D, Amoroso G, Fock H. Planta. 1995;196:217–224. [Google Scholar]
- 14.Karlsson J, Clarke A K, Chen Z Y, Hugghins S Y, Park Y I, Husic H D, Moroney J V, Samuelsson G. EMBO J. 1998;17:1208–1216. doi: 10.1093/emboj/17.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohkawa H, Sonoda M, Katoh H, Ogawa T. Can J Bot. 1998;76:1035–1042. [Google Scholar]
- 16.Hihara, Y., Kamei, A., Kanehisa, M., Kaplan, A. & Ikeuchi, M. (2001) Plant Cell, in press. [DOI] [PMC free article] [PubMed]
- 17.Schwarz R, Lieman-Hurwitz J, Hassidim M, Kaplan A. Plant Physiol. 1992;100:1987–1993. doi: 10.1104/pp.100.4.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somanchi A, Moroney J V. Plant Mol Biol. 1999;40:1055–1062. doi: 10.1023/a:1006262123918. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda Y, Bozzo G G, Colman B. Can J Bot. 1998;76:1072–1083. [Google Scholar]
- 20.Kaplan A, Ronen Tarazi M, Zer H, Schwarz R, Tchernov D, Bonfil D J, Schatz D, Vardi A, Hassidim M, Reinhold L. Can J Bot. 1998;76:917–924. [Google Scholar]
- 21.Beardall J, Johnston A, Raven J. Can J Bot. 1998;76:1010–1017. [Google Scholar]
- 22.Marcus Y, Schuster G, Michaels A, Kaplan A. Plant Physiol. 1986;80:604–607. doi: 10.1104/pp.80.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawat M, Moroney J V. Plant Physiol. 1995;109:937–944. doi: 10.1104/pp.109.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson M, Villand P, Gardestrom P, Samuelsson G. Plant Physiol. 1998;116:637–641. doi: 10.1104/pp.116.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omata O, Price D G, Badger M R, Okamura M, Gohta S, Ogawa T. Proc Natl Acad Sci USA. 1999;96:13571–13576. doi: 10.1073/pnas.96.23.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronen-Tarazi M, Schwarz R, Bouevitch A, Lieman-Hurwitz J, Erez J, Kaplan A. In: Molecular Ecology of Aquatic Microbes. Joint I, editor. Berlin: Springer; 1995. , NATO ASI Series, Vol. G38, pp. 323–334. [Google Scholar]
- 27.Kucho K, Ohyama K, Fukuzawa H. Plant Physiol. 1999;121:1329–1337. doi: 10.1104/pp.121.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuzawa H, Ishizaki K, Miura K, Matsueda S, Ino-ue T, Kucho K, Ohyama K. Can J Bot. 1998;76:1092–1097. [Google Scholar]
- 29.Omata T, Gohta S, Takahashi Y, Harano Y, Maeda S-I. J Bacteriol. 2001;183:1891–1898. doi: 10.1128/JB.183.6.1891-1898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figge R M, Cassier-Chauvat C, Chauvat F, Cerff R. Mol Microbiol. 2001;39:455–468. doi: 10.1046/j.1365-2958.2001.02239.x. [DOI] [PubMed] [Google Scholar]