Abstract
In this study, the genetic organization of three novel genomic antibiotic resistance islands (AbaRs) in Acinetobacter baumannii isolates belonging to group of European clone II (EC II) comM integrated sequences of 18-, 21-, and 23-kb resistance islands were determined. These resistance islands carry the backbone of AbaR-type transposon structures, which are composed of the transposition module coding for potential transposition proteins and other genes coding for the intact universal stress protein (uspA), sulfate permease (sul), and proteins of unknown function. The antibiotic resistance genes strA, strB, tetB, and tetR and insertion sequence CR2 element were found to be inserted into the AbaR transposons. GenBank homology searches indicated that they are closely related to the AbaR sequences found integrated in comM in strains of EC II (A. baumannii strains 1656-2 and TCDC-AB0715) and AbaR4 integrated in another location of A. baumannii AB0057 (EC I). All of the AbaRs showed structural similarity to the previously described AbaR4 island and share a 12,008-bp backbone. AbaRs contain Tn1213, Tn2006, and the multiple fragments which could be derived from transposons Tn3, Tn10, Tn21, Tn1000, Tn5393, and Tn6020, the insertion sequences IS26, ISAba1, ISAba14, and ISCR2, and the class 1 integron. Moreover, chromosomal DNA was inserted into distinct regions of the AbaR backbone. Sequence analysis suggested that the AbaR-type transposons have evolved through insertions, deletions, and homologous recombination. AbaR islands, sharing the core structure similar to AbaR4, appeared to be distributed in isolates of EC I and EC II via integration into distinct genomic sites, i.e., pho and comM, respectively.
INTRODUCTION
The occurrence of a major nosocomial infectious agent, antibiotic-resistant Acinetobacter baumannii, was attributed to the epidemic spread of international lineages European clones (EC) I and II (6, 7, 10, 11, 14). Multiple antibiotic resistance regions, inserted into the target gene comM and termed resistance islands (AbaR), were reported recently in A. baumannii strains (1, 9, 16, 21, 22). The backbone of AbaR was shown to be comprised of five open reading frames constituting the so-called transposition module (orf1, tniA, tniB, orf2, and orf3), together with two other genes encoding the universal stress protein (uspA) and sulfate permease (sul) (3). Bioinformatic analysis showed that three open reading frames encoded by transposition module showed similarity to transposition-associated proteins of Tn7, which suggested that AbaR had arisen from the ancestral transposon, distantly related to Tn7 (24). Several studies showed the gene uspA in AbaR backbone was intact (AbaR4) or interrupted by a large composite transposon bracketed by two copies of ISPpu12 (renamed as Tn6018 [22]) carrying the resistance genes in AbaR1, AbaR3, and AbaR5 to AbaR19 (3, 16, 21, 22). Recent analysis of these new variants of resistance islands suggested that an AbaR3-like structure was found frequently in strains of EC I and the new variants evolved through deletions or homologous recombination creating truncated derivatives (16).
Little is known regarding the AbaRs in epidemic strains of EC II. The truncated variant of AbaR1, designated AbaR2, which consisted of only the right hand of the island and carried a single copy of ISPpu12, was found in EC II strain ACICU (13). However, a recent study reported that an ISPpu12 composite transposon was absent in the AbaR backbone and that the uspA gene was intact in EC II isolates (3). In the present study, the characterization of three novel AbaRs structures from A. baumannii isolates belonging to EC II is reported.
MATERIALS AND METHODS
Bacterial isolates.
A. baumannii isolates LT-3, LT-11, and LT-V1 were isolated from respiratory tract and joint aspirate samples from patients hospitalized in the intensive care units of two Lithuanian hospitals in 2010 (Lithuanian University of Health Sciences Kauno Klinikos Hospital, Kaunas, Lithuania, and Vilnius University Emergency Hospital, Vilnius, Lithuania). The isolates were identified as A. baumannii by using automated microbiology system Phoenix (BD, USA) and amplified rDNA restriction analysis (ARDRA) (8). Antibiotic susceptibility was tested by using BD Phoenix system and interpreted accordingly the Clinical and Laboratory Standards Institute guidelines.
PFGE and MLST-IP.
Pulsed-field gel electrophoresis (PFGE)-ApaI restriction analysis was performed, and gel images were analyzed using Bionumerics software with the Dice coefficient and a band tolerance set at 0.5% (30). The relatedness of isolates to EC I and II was determined by the multiplex PCR assays, as described previously (29). Genotyping by multilocus sequence typing according to the scheme of the Institut Pasteur (MLST-IP) was undertaken with the primers and conditions described on the Pasteur website (http://www.pasteur.fr/mlst), with the exception of rpoB amplification, which was undertaken with the primers FrpoB (5′-GTTGCTGCTGCAATCAAAGA-3′) and RrpoB (5′-TCTCACCAAAAATTGCACGA-3′), designed on the basis of a published sequence (CP001921). The sequences of A. baumannii AB210 (AEOX00000000), 1656-2 (CP001921), and TCDC-ABO715 (CP002522) were retrieved from GenBank for determination of the sequence type in silico.
DNA extraction.
Genomic DNA was extracted using an Arrow magnetic workstation (NorDiag, Norway) according to the manufacturer's instructions.
Gene detection.
Genes coding for intrinsic β-lactamases (blaOXA-51-like and blaADC) and acquired genes conferring narrow-spectrum resistance to β-lactams (blaTEM-1) or carbapenems (acquired carbapenem hydrolyzing class D β-lactamases blaOXA-23-like, blaOXA-40-like, blaOXA-58-like, and blaOXA-143 and metallo-β-lactamases blaIMP, blaVIM, blaGIM, blaSPM, blaSIM, and blaNDM), aminoglycosides (aadA1, aadA2, aadB, aadA5, strA, strB, aphA1, aphA2, aphA6 aacC1, aacC2, aacC4, aacA4, armA, rmtC, and rmtB), tetracyclines (tetA and tetB), and fenicols (catI, catII, catIII, catB2, catB3, catB8, cmlA, cmlB, and floR), in addition to class 1 integron cassettes and genes coding the adeABC efflux pump, were detected by PCR as described previously (5, 12, 17–19, 20, 23).
AbaR mapping and sequence analysis.
Analysis of characteristic AbaR junction regions and genomic surroundings upstream and downstream 3′ and 5′ parts of comM gene was undertaken as described earlier (15, 25). The comM integrated sequences were amplified by Long-Range PCR (Fermentas, Lithuania), purified and used for DNA sequencing and AbaR mapping. The comM integrated sequence of 18-kb AbaR4a from isolate LT-3 was sequenced by primer walking strategy (primers are listed in Table S1 in the supplemental material). PCR mapping of AbaR backbone was carried out by using the long-range PCR with primers targeting the genes in AbaR4a and DNA restriction analysis (the positions of amplicons, restriction endonucleases, and primers are listed in Fig. S1 and Table S1 in the supplemental material). DNA regions of LT-11 and LT-V1, different from those of integrated sequence in LT-3, as determined by PCR mapping, were sequenced. DNA and protein sequences were analyzed using Blastn, Blastp (www.ncbi.nlm.nih.gov [August 2011]), and CLC Main Workbench software (CLC Bio A/S, Denmark).
Nucleotide sequence accession numbers.
The entire sequences of the AbaR elements have been deposited in the GenBank under the accession numbers JN129845 (AbaR4a), JN129846 (AbaR4b), and JN129847 (AbaR4c).
RESULTS AND DISCUSSION
Characterization of isolates.
A. baumannii isolates LT-3, LT-11, and LT-V1 were resistant to meropenem, imipenem, piperacillin-tazobactam, ceftazidime, piperacillin, ciprofloxacin, and amikacin. LT-11 and LT-V1 were also resistant to gentamicin; LT-11 was resistant to sulbactam/ampicillin. According to PFGE-ApaI macrorestriction analysis, LT-3 and LT-V1 were closely related (cutoff value, 90%), being representative isolates of the clonal outbreaks in these hospitals. The LT-11 isolate was unique (showing a similarity of <70%) and sporadic (data not shown). All isolates belonged to EC II according multiplex PCR analysis and were assigned to ST2 (MLST-IP). PCR screening and DNA sequencing confirmed the presence of the acquired carbapenemase genes blaOXA-72 (LT-3 and LT-V1) and blaOXA-23 (LT-11). The characteristics of isolates are summarized in Table 1.
Table 1.
Characteristics of A. baumannii isolates
| Isolate | Hospital/clonalitya | MLST | Resistance profileb | blaOXA-51-like | Acquired CHDL | Gene cassettes of class 1 integron | Other genes conferring antibiotic resistance | AbaR length (kb) |
|---|---|---|---|---|---|---|---|---|
| LT-3 | K/clonal | ST2 | MEM IPM TZP CAZ AN CIP PIP | OXA-66 | OXA-72 | tetB, strA, strB | 18 | |
| LT-11 | K/sporadic | ST2 | MEM IPM TZP SAM CAZ GM AN CIP PIP | OXA-66 | OXA-23 | aacA4-catB8-aadA1 | tetB, strA, strB, aphA1, armA, adeABC | 23 |
| LT-V1 | V/clonal | ST2 | MEM IPM TZP CAZ GM AN CIP PIP | OXA-66 | OXA-72 | aacC1c | tetB, strA, strB, adeABC | 21 |
K, Lithuanian University of Health Sciences Kauno Klinikos Hospital; V, Vilnius University Emergency Hospital. LT3 and LT-V1 were representative isolates of clonal outbreaks in hospitals in 2010, whereas LT-11 was a sporadic isolate. Clonality was determined by PFGE-ApaI restriction analysis.
Antimicrobial susceptibility to MEM (meropenem), IPM (imipenem), TZP (tazobactam/piperacillin), SAM (sulbactam/ampicillin), SCF (sulbactam/cefoperazone), CAZ (ceftazidime), GM (gentamicin), AN (amikacin), CIP (ciprofloxacin), and PIP (piperacillin) was tested.
The 3′-conserved segment (CS) of integron was missing.
Structure of resistance islands.
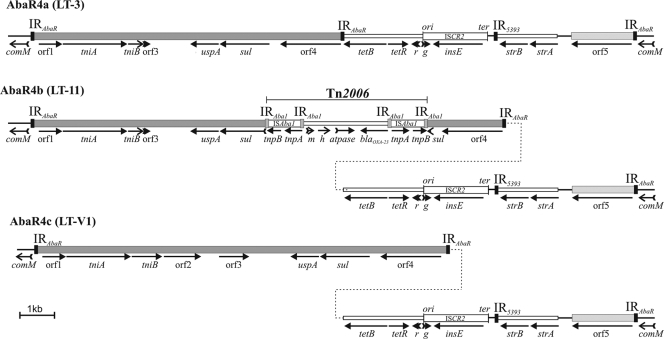
In previous studies, AbaR sequences were found to be integrated into the same position of the comM gene, coding for a hypothetical protein with an ATPase function (25). Long-range PCR amplification of isolates LT-3, LT-V1, and LT-11 with primers targeting the 3′ and 5′ ends of comM gene yielded products of 18, 21, and 23 kb, respectively. Subsequent PCR analysis confirmed the presence of the 3′ and 5′ parts of comM gene and the characteristic junction regions between the AbaR sequences and comM in all isolates. Therefore, A. baumannii isolates LT-3, LT-V1, and LT-11 possessed an integrated sequence located at the same position known for other AbaRs (15, 16, 22, 25). The PCR product of 18 kb from isolate LT-3 was purified and sequenced. The resistance island carries the backbone of AbaR encoding part of the transposition module (orf1 and tniA), an intact universal stress protein (uspA), sulfate permease (sul), and orf4, coding for the protein of unknown function (Fig. 1). However, in LT-3, the transposition module is partially deleted. orf2 and fragments of tniB and orf3 were removed. The backbone of AbaR is fused to a DNA fragment carrying the insertion sequence CR2 element and the tetracycline and streptomycin resistance genes tetB, tetR, strA, and strB. DNA sequencing showed that the resistance island contains the complete transposition module in LT-V1 and carries the insertion of Tn2006, encoding blaOXA-23, in LT-11 (Fig. 1).
Fig 1.
Structure of AbaR4a, AbaR4b, and AbaR4c islands. AbaR-type transposon backbone is shown by filled boxes bounded by inverted terminal repeats (IRAbaR) shown as black bars. The additional inserted regions are indicated by vertical arrows indicating the insertion site. Boxes of different thickness distinguish the various segments: antibiotic resistance genes, ISCR2, and ISAba1. Vertical bars indicate the ori and ter sites of ISCR2 element and the inverted repeats (IR) with a subscript note, indicating the identity of IR. The genes are shown by horizontal arrows with the gene name below. An angled style at the beginning and the head of the arrow indicate 5′ and 3′ truncated genes, respectively. Genes named m, h, g, and r potentially encode a DNA methylase, helicase, phosphoglucosamine mutase (glmM), and the transcription regulator of ArsR family, respectively. Note that orf5 is 85% identical to orf4. The sizes of individual AbaRs are drawn to scale. Sequences of AbaR4a, AbaR4b, and AbaR4c are available from GenBank under accession numbers JN129845, JN129846, and JN129847, respectively.
Comparative analysis of the AbaR regions in LT-3, LT-11, LT-V1, and AbaR4-type islands.
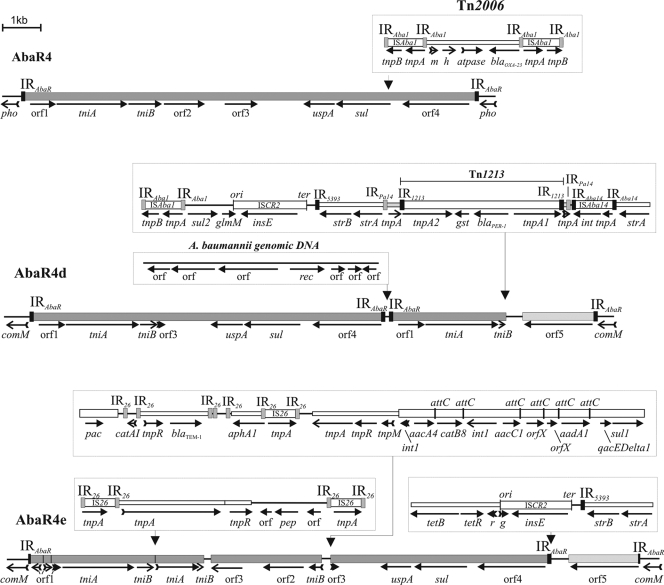
GenBank homology searches indicated that the AbaR sequences of LT-3, LT-V1, and LT-11 are closely related to AbaR4, integrated in AB57_0566 locus in A. baumannii strain AB0057 (EC I), and to the AbaR sequences of A. baumannii strains AB210, 1656-2, and TCDC-ABO715, which belonged to EC II and which were assigned to ST2 (MLST-IP) corresponding to international CC2 (4, 28, 29). For clarity, the structures of these related sequences, found in the GenBank DNA database, are illustrated in Fig. 2. Since the islands identified in the present study and found in the database clearly showed structural similarity to the earlier-described AbaR4, these islands were named for simplicity as follows: AbaR4a in LT-3 (JN129845, bases 1 to 17996), AbaR4b in LT-11 (JN129846, bases 1 to 22810), AbaR4c in LT-V1 (JN129847, bases 1 to 20846), AbaR4d in A. baumannii 1656-2 (CP001921, bases 257120 to 291743), and AbaR4e in A. baumannii TCDC-AB0715 (CP002522, bases 245943 to 291133).
Fig 2.
Structure of AbaR4, AbaR4d, and AbaR4e islands. Features are as described in Fig. 1. The gene pac potentially encodes a puromycin N-acetyltransferase (usually designated orf5 in class 1 integrons). The sizes of individual AbaRs are drawn to scale. Sequences of AbaR4, AbaR4d, and AbaR4e are available from GenBank under accession numbers NC_011586, CP001921, and CP002522, respectively.
Comparative structural analysis showed that AbaR4a to AbaR4e share a 12,008-bp backbone, which is composed of the transposition module, the genes uspA, sul, and orf4. 5′-comM-associated orf5 showed significant identity in DNA (85%) to orf4. The partial copies of the transposition module were found in the backbone of AbaR4d and AbaR4e, which appear to have undergone multiple events of deletions, insertions, and inversions and might be generated through homologous recombination between DNA sequences carrying separate copies of AbaR backbone (Fig. 2). The direct repeats and imperfect inverted terminal repeats (ITR), flanking the AbaR4-type integrated sequences, are the same as found for the other AbaRs (2, 9, 21). ITRs of AbaR4e were found to be truncated by five and seven nucleotides, probably due to DNA recombination and deletion.
Antibiotic resistance regions of AbaR4-type islands.
In contrast to EC I-related AbaRs, which carry multiple antibiotic resistance regions, inserted in the uspA gene and flanked by two directly repeated copies of ISPpu12, a less complex structure in EC II-related AbaRs was observed. Tn1213, Tn2006, and the multiple fragments, perhaps derived from transposons Tn3, Tn10, Tn21, Tn1000, Tn5393, and Tn6020, insertion sequences ISCR2, IS26, ISAba1, and ISAba14, class 1 integron, and chromosomal DNA, were found inserted into distinct regions (coding for sul, tniB, and orf3) or fused to the backbone elements of AbaR (Fig. 2). Some genes, such as strA, strB, tetB, and tetR, have been found in most AbaR4-type islands. sul2, aphA1, aacA4, catB8, aacC1, aadA1, sul1, blaTEM-1, blaPER-1, and blaOXA-23 have been identified in AbaR4d or AbaR4e. A recent study showed the novel variant of AbaR4-type resistance island carrying two transposition modules, and the DNA fragments similar to those observed in AbaR4a to AbaR4c (tetB, tetR, ISCR2, strA, and strB) and AbaR4d (A. baumannii genomic DNA insertion) inserted into the backbone (31).
AbaR4-type islands contain ISCR2.
Importantly, ISCR2, an atypical class of insertion sequences, was found integrated in AbaR4a-e (Fig. 1 and 2). Such sequences, which are known to move by a mechanism of rolling-circle transposition, are believed to be responsible for the mobilization of virulence and antibiotic resistance genes and thought to represent a powerful system, which can mobilize any section of DNA (27). These elements can cotranspose DNA adjacent to their terminal terIS sequence, mediated by a single copy of the ISCR (26). ISCR1 and ISCR3 elements were identified in AbaR1, suggesting that they are implicated in the acquisition and transposition of the resistance genes (27).
AbaR4-type islands insert into the pho target in isolates of EC I.
A. baumannii AB0057, belonging to EC I group, is known to possess resistance island AbaR3 integrated in comM and resistance island AbaR4 integrated in a locus AB57_0566 (2). A recent study reported that the isolates of EC I, carrying the second genomic AbaR4-type island found at a distinct insertion site, are widespread in United Kingdom (28). Bioinformatic analysis showed that this target gene encodes the putative phosphatase (similar to ABAYE3309) and, therefore, in the present study was designated as the target pho. Sequence analysis showed that pho-integrated AbaR4 contains terminal imperfect inverted repeats, similar to comM specific islands and distinct direct repeats (ACTGA instead of ACCGC).
Concluding remarks.
We showed here that AbaR islands found in isolates of EC II share a core structure similar to that of AbaR4 and common backbone elements for transposition. The results presented here and previously published data demonstrate the similar AbaR-type transposon structures in EC I and EC II isolates and indicate that both AbaRs originated independently from a common larger progenitor. AbaR4-type islands have evolved through the multiple events of insertions, deletions, and homologous recombination and could be distributed in isolates of EC I and EC II through the integration into distinct genomic sites: pho and comM, respectively. Recent studies report the worldwide domination of A. baumannii strains related to EC I and II, show the selective advantage for strains of this lineage, and require monitoring of further genetic evolution.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Research Council of Lithuania (grant MIP-90/2010).
We thank Mervyn Richardson, Basic, Crawley, England, for assistance in reading and commenting on this article.
Footnotes
Published ahead of print 30 January 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Adams MD, Chan ER, Molyneaux ND, Bonomo RA. 2010. Genomewide analysis of divergence of antibiotic resistance determinants in closely related isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams MD, et al. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnin RA, Poirel L, Nordmann P. 29 September 2011. AbaR-type transposon structures in Acinetobacter baumannii.J. Antimicrob. Chemother. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 4. Chen CC, et al. 2011. Genome sequence of a dominant, multidrug-resistant Acinetobacter baumannii strain, TCDC-AB0715. J. Bacteriol. 193:2361–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho YJ, et al. 2009. Genetic basis of resistance to aminoglycosides in Acinetobacter spp. and spread of armA in Acinetobacter baumannii sequence group 1 in Korean hospitals. Diagn. Microbiol. Infect. Dis. 64:185–190 [DOI] [PubMed] [Google Scholar]
- 6. D'Arezzo S, et al. 2011. Changing carbapenemase gene pattern in an epidemic multidrug-resistant Acinetobacter baumannii lineage causing multiple outbreaks in central Italy. J. Antimicrob. Chemother. 66:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diancourt L, et al. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dijkshoorn L, Van Harsselaar B, Tjernberg I, Bouvet PJ, Vaneechoutte M. 1998. Evaluation of amplified ribosomal DNA restriction analysis for identification of Acinetobacter genomic species. Syst. Appl. Microbiol. 21:33–39 [DOI] [PubMed] [Google Scholar]
- 9. Fournier PE, et al. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grosso F, et al. 2011. OXA-23-producing Acinetobacter baumannii: a new hot spot of diversity in Rio de Janeiro? J. Antimicrob. Chemother. 66:62–65 [DOI] [PubMed] [Google Scholar]
- 11. Higgins PG, Dammhayn C, Hackel M, Seifert H. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:233–238 [DOI] [PubMed] [Google Scholar]
- 12. Higgins PG, Lehmann M, Seifert H. 2010. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 35:305. [DOI] [PubMed] [Google Scholar]
- 13. Iacono M, et al. 2008. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 52:2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karah N, et al. 2011. Emergence of OXA-carbapenemase- and 16S rRNA methylase-producing international clones of Acinetobacter baumannii in Norway. J. Med. Microbiol. 60:515–521 [DOI] [PubMed] [Google Scholar]
- 15. Krizova L, Nemec A. 2010. A 63-kb genomic resistance island found in a multidrug-resistant Acinetobacter baumannii isolate of European clone I from 1977. J. Antimicrob. Chemother. 65:1915–1918 [DOI] [PubMed] [Google Scholar]
- 16. Krizova L, Dijkshoorn LA, Nemec A. 2011. Diversity and evolution of AbaR genomic resistance islands in Acinetobacter baumannii strains of European clone I. Antimicrob. Agents Chemother. 55:3201–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Madsen L, Aarestrup FM, Olsen JE. 2000. Characterization of streptomycin resistance determinants in Danish isolates of Salmonella typhimurium. Vet. Microbiol. 75:73–82 [DOI] [PubMed] [Google Scholar]
- 18. Magnet S, Courvalin P, Lambert T. 2001. Resistance–nodulation–cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng LK, Martin I, Alfa M, Mulvey M. 2001. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell Probes 15:209–215 [DOI] [PubMed] [Google Scholar]
- 20. Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70:119–123 [DOI] [PubMed] [Google Scholar]
- 21. Post V, Hall RM. 2009. AbaR5, a large multiple-antibiotic resistance region found in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:2667–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Post V, White PA, Hall RM. 2010. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1162–1170 [DOI] [PubMed] [Google Scholar]
- 23. Povilonis J, et al. 2010. Transferable class 1 and 2 integrons in Escherichia coli and Salmonella enterica isolates of human and animal origin in Lithuania. Foodborne Pathog. Dis. 7:1185–1192 [DOI] [PubMed] [Google Scholar]
- 24. Rose A. 2010. TnAbaR1: a novel Tn7-related transposon in Acinetobacter baumannii that contributes to the accumulation and dissemination of large repertoires of resistance genes. Biosci. Horizons 3:40–48 [Google Scholar]
- 25. Shaikh F, et al. 2009. ATPase genes of diverse multidrug-resistant Acinetobacter baumannii isolates frequently harbor integrated DNA. J. Antimicrob. Chemother. 63:260–264 [DOI] [PubMed] [Google Scholar]
- 26. Tavakoli N, et al. 2000. IS1294, a DNA element that transposes by RC transposition. Plasmid 4:66–84 [DOI] [PubMed] [Google Scholar]
- 27. Toleman MA, Walsh TR. 2011. Combinatorial events of insertion sequences and ICE in Gram-negative bacteria. FEMS Microbiol. Rev. 35:912–935 [DOI] [PubMed] [Google Scholar]
- 28. Turton JF, Baddal B, Perry C. 2011. Use of the accessory genome for characterization and typing of Acinetobacter baumannii. J. Clin. Microbiol. 49:1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turton JF, Gabriel SN, Valderrey C, Kaufmann ME, Pitt TL. 2007. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin. Microbiol. Infect. 13:807–815 [DOI] [PubMed] [Google Scholar]
- 30. Turton JF, et al. 2004. A prevalent, multiresistant clone of Acinetobacter baumannii in southeast England. J. Hosp. Infect. 58:170–179 [DOI] [PubMed] [Google Scholar]
- 31. Zhou H, et al. 25 July 2011. Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob. Agents Chemother. Jul 25. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.