Abstract
The transmissible spongiform encephalopathies, more commonly known as the prion diseases, are associated with the production and aggregation of disease-related isoforms of the prion protein (PrPSc). The mechanisms by which PrPSc accumulation causes neurodegeneration in these diseases are poorly understood. In cultured neurons, the addition of PrPSc alters cell membranes, increasing cholesterol, activating cytoplasmic phospholipase A2 (cPLA2), and triggering synapse damage. These effects of PrPSc are dependent upon its glycosylphosphatidylinositol (GPI) anchor, suggesting that it is the increased density of GPIs that occurs following the aggregation of PrPSc molecules that triggers neurodegeneration. This hypothesis was supported by observations that cross-linkage of the normal cellular prion protein (PrPC) also increased membrane cholesterol, activated cPLA2, and triggered synapse damage. These effects were not seen after cross-linkage of Thy-1, another GPI-anchored protein, and were dependent on the GPI anchor attached to PrPC containing two acyl chains and sialic acid. We propose that the aggregation of PrPSc, or the cross-linkage of PrPC, causes the clustering of sialic acid-containing GPI anchors at high densities, resulting in altered membrane composition, the pathological activation of cPLA2, and synapse damage.
Keywords: Glycosylphosphatidylinositol Anchors, Neurobiology, Phospholipase A, Prions, Synapses
Introduction
The cellular prion protein (PrPC)2 is a glycosylphosphatidylinositol (GPI)-anchored glycoprotein (1), which is abundantly expressed on neurons. Although the precise role of PrPC in neurons is unclear, observations that it is concentrated at synapses (2, 3) led to suggestions that it regulates neurotransmission (4). The conversion of PrPC into an alternatively folded, disease-associated conformation (PrPSc) is the key event leading to the prion diseases (5). This change in structure is accompanied by changes in the biochemical properties, including a capacity to self-aggregate, resulting in PrPSc oligomers (6). The accumulation of PrPSc oligomers leads to the loss of synapses and consequently the clinical signs of infection. However, the molecular mechanisms by which PrPSc causes synapse degeneration remain poorly understood.
Although GPIs were originally regarded as simple anchors that tethered proteins to cell membranes, it is now recognized that GPIs are involved in protein sorting and cell signaling (7). An increasing number of GPI-anchored proteins including CD59 (8), Thy-1 (9), CD14 (10), and Fc-ϵ-R1 (11) are associated with cell responses and the activation of multiple cell-signaling pathways. The diversity of responses that follow the engagement of GPI-anchored receptors may reflect diversity in the composition and structure of GPI anchors. Although the core of GPI anchors is conserved (7, 12), they have variable glycan side chains and lipid moieties, and there is increasing interest in the physiological significance of such variations. Cell activation commonly occurs following cross-linkage of GPI-anchored proteins by antibodies (10, 14), suggestive of a density-dependent effect.
Although several studies have examined the role of the GPI in PrPSc formation (15, 16), the first indication that GPI anchors play a role in the pathogenesis of prion disease was when transgenic mice producing anchorless PrPC that were infected with scrapie were reported to produce large amounts of PrPSc without suffering clinical disease (17). However, because these mice produce PrPC lacking the entire GPI anchor, they failed to provide information regarding the biological effects of specific GPI anchor components. The role of GPI anchors in the biological activity of PrPSc has been difficult to determine as aggregated forms of PrPSc protect the GPIs from digestion with phosphatidylinositol-phospholipase C (PI-PLC) (18). However, a shaking technique developed to amplify small amounts of PrPSc for detection (19) allowed successful digestion of the PrPSc-GPI anchor with either PI-PLC or phospholipase A2 (PLA2), resulting in deacylated and monoacylated PrP proteins (20). In the current study, synapse damage induced in cultured neurons by PrPSc was reduced by digestion of PrPSc with phospholipases. Furthermore, the cross-linkage of PrPC mimicked the effects of PrPSc on neurons and triggered synapse damage. These properties of cross-linked PrPC were dependent on the composition of the GPI anchor and were lost after digestion with phospholipases or with neuraminidase, which removes sialic acid from the GPI anchor attached to PrPC. We therefore propose that PrPSc-induced synapse degeneration occurs as a consequence of the clustering of specific GPI anchors at high densities in cell membranes.
EXPERIMENTAL PROCEDURES
Primary Neuronal Cultures
Cortical neurons were prepared from the brains of day 15.5 murine embryos derived from Prnp wild type (+/+) or Prnp knock-out (−/−) mice. After mechanical dissociation and cell sieving, neurons were seeded at 2 × 105 cells/well in 48-well plates (precoated with poly-l-lysine) in Ham's F12 medium containing 5% fetal calf serum (FCS) for 2 h. Cultures were shaken (600 rpm for 5 min), and nonadherent cells were removed by three washes in PBS. Neurons were grown in Neurobasal medium containing B27 components for 10 days. Immunohistochemistry showed that the cells were greater than 95% neurofilament positive. To assess their effect upon synapses or the activation of cytoplasmic PLA2 (cPLA2), neurons were incubated with test preparations, antibodies, or Fab fragments for 24 h. In some assays, neurons were pretreated with PLA2 inhibitors for 1 h before the addition of PrP preparations. For toxicity experiments, neurons were incubated with PrP preparations for 5 days, and cell survival was determined by the addition of 25 μm thiazolyl blue tetrazolium for 3 h.
Cell Membrane Extracts
Treated cells were washed three times in ice-cold PBS and homogenized in an extraction buffer (10 mm Tris-HCl, pH 7.2, 100 mm NaCl, 10 mm EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, and 0.2% SDS) at 106 cells/ml, and cell debris was removed by centrifugation (500 × g for 5 min). Mixed protease inhibitors (4-(2-aminoethyl) benzenesulfonyl fluoride, aprotinin, leupeptin, bestatin, pepstatin A, and E-46) (Sigma) and a phosphatase inhibitor mixture including PP1, PP2A, microcystin LR, cantharidin, and p-bromotetramisole (Sigma) were added to membrane extracts when appropriate.
PrPSc Preparations
To study the effects of PrPSc on synapses supernatants from prion-infected ScGT1 cells containing PrPSc, oligomers were centrifuged (16,000 × g for 30 min) and passed through a 0.2-μm filter to remove large aggregates. The supernatants were concentrated so that they contained 5 ng/ml PrP and dialyzed against Neurobasal medium. Soluble PrPSc was treated with 0.2 units/ml PI-PLC (Bacillus cereus) or 10 units/ml bee venom PLA2 (Sigma) as described previously (20) or with 0.2 units/ml neuraminidase (Clostridium perfringens) (Sigma) plus intermittent shaking for 2 h at 37 °C and heated to denature enzymes. Control preparations were incubated with heat-denatured enzymes.
Isolation of PrPC
PrPC was isolated by a mixture of affinity purification and reverse phase chromatography. Briefly, cell membranes from GT1 neuronal cells or from N9 glial cells were isolated following repeated homogenization in distilled water and the removal of cell debris and nuclei by centrifugation (1000 × g for 5 min). Cell membranes were further homogenized in ice-cold 1% Triton X-100 in PBS, 10 mm EDTA, and protease inhibitors for 1 h. Insoluble material was removed by centrifugation (1000 × g for 5 min), and the supernatant was incubated with mAb 4F2 and mixed. After 30 min, 10 μl/ml protein G magnetic microbeads (Miltenyi) were added and mixed for a further 30 min. Antibody-bound complexes were collected using a magnetic bead isolation kit (Miltenyi), and beads were washed twice with cold 1% Triton X-100 and then three times with warm (37 °C) PBS containing 10 mm EDTA and protease inhibitors. Beads were subsequently incubated in PBS containing DNase and sphingomyelinase (Sigma) ± 2 units/ml endoglycosidase F (PNGase) (Sigma), 0.2 units/ml PI-PLC, 10 units/ml PLA2, or 0.2 units/ml neuraminidase for 2 h at 37 °C. PrPC/digested PrPC was dissociated from the mAb/protein G complex using 0.1 m glycine HCl at pH 2.7 and applied to C18 columns. PrPC was eluted under a gradient of acetonitrile in water and 0.1% trifluoroacetic acid; fractions were collected and tested in a PrP ELISA. Fractions containing PrPC were pooled, reloaded onto a new C18 column, and eluted as before. This process was repeated, and the PrPC-containing fractions were lyophilized and stored at −80 °C. Samples were sonicated in culture medium for bioassays. Thy-1 was also isolated from GT1 cell membranes prepared with an anti-Thy-1 mAb (Serotec), protein G magnetic beads, and C18 columns as described above. Fractions eluted from C18 columns were identified by dot blot and pooled and desalted, and the protein concentration was determined by a micro-BCA assay (Pierce).
Western Blot Analysis
Samples were mixed with Laemmli buffer, heated to 95 °C, and subjected to electrophoresis on 15% polyacrylamide gels. Proteins were transferred onto a Hybond-P PVDF membrane (Amersham Biosciences) by semidry blotting. Membranes were blocked with 10% milk powder and incubated with mAb ICSM35 (to detect PrP/cross-linked PrPC). mAb 4H302 (Abcam) was used to detect synaptobrevin, rabbit polyclonal anti-synaptophysin (Abcam), rabbit polyclonal antibody to synapsin-1 (515200, Invitrogen), rabbit polyclonal antibodies to Na,K-ATPase α1 subunit (Abcam), mouse mAb to β-actin, and clone AC-74 (Sigma) followed by a secondary anti-mouse or anti-rabbit IgG or anti-mouse IgG conjugated to peroxidase. Bound antibody was visualized using enhanced chemiluminescence.
Cross-linkage of Proteins
PrPC or Thy-1 preparations were cross-linked using the homobifunctional cross-linking agent dimethyl pimelimidate (Pierce) according to the manufacturer's instructions. Dimethyl pimelimidate is a cross-linking agent that contains an amine-reactive imidoester at each end of a 9.2 Å (seven-atom) spacer arm. Cross-linked proteins and mock-treated controls were desalted using Vivaspin filters (Sartorius) and suspended in culture media. Before use, cross-linked PrPC preparations were centrifuged at 16,000 × g for 10 min and then passed through 0.2-μm and 500-kDa filters and to remove large aggregates.
Isolation of GPI Anchors
GPIs were isolated from PrPC or Thy-1 preparations by digestion with 100 μg/ml proteinase K at 37 °C for 4 h. The GPIs released from PrPC or PLA2-digested PrPC were extracted with water-saturated butan-1-ol and lyophilized. Samples were dissolved in ethanol and separated by high performance thin-layer chromatography (HPTLC) on Silica Gel 60 plates.
Lectin Binding Analysis of GPI Anchors
The presence of specific glycans in GPI anchors was determined using biotinylated lectins. Isolated GPI anchors were bound to nitrocellulose membranes by dot blot and blocked with 5% milk powder. Samples were incubated with biotinylated Sambucus nigra lectin (to detect terminal sialic acid residues bound by α-2,6 or α-2,3 to galactose) or biotinylated concanavalin A to detect mannose (Vector Laboratories). Bound lectins were visualized using ExtrAvidin peroxidase and enhanced chemiluminescence. The blotted GPI anchors were also incubated with mAb 5AB3-11 to show the presence of phosphatidylinositol.
Synaptophysin ELISA
The amount of synaptophysin in neuronal extracts was measured by ELISA as described (21). MaxiSorp immunoplates (Nunc) were coated with a mAb to synaptophysin (MAB368, Chemicon) and blocked with 10% milk powder. Samples were added for 1 h, and synaptophysin was detected using a rabbit polyclonal anti-synaptophysin (Abcam) followed by a biotinylated anti-rabbit IgG (Dako), ExtrAvidin-alkaline phosphatase, and 1 mg/ml 4-nitrophenol phosphate (Sigma). Absorbance was measured on a microplate reader at 405 nm. Samples were expressed as “units of synaptophysin” where 100 units were defined as the amount of synaptophysin in 106 untreated cells.
Activated cPLA2 ELISA
The activation of cPLA2 is accompanied by phosphorylation of the serine 505 residue, which can be measured by phospho-specific antibodies as described (21). Briefly, MaxiSorp immunoplates were coated with 0.5 μg/ml mouse mAb anti-cPLA2, clone CH-7 (Upstate Biotech Millipore) and blocked with 10% milk powder. Samples were incubated for 1 h, and the amount of activated cPLA2 was detected using a rabbit polyclonal anti-phospho-cPLA2 (Cell Signaling Technology). Bound antibodies were detected using biotinylated anti-rabbit IgG (Dako), ExtrAvidin-alkaline phosphatase, and 1 mg/ml 4-nitrophenyl phosphate. Absorbance was measured at 405 nm. Samples were expressed as “units of activated cPLA2,” where 100 units were defined as the amount of activated cPLA2 in 106 untreated neurons.
Cholesterol
The amounts of cholesterol were measured using the Amplex Red cholesterol assay kit (Invitrogen), according to the manufacturer's instructions. Briefly, cholesterol is oxidized by cholesterol oxidase to yield hydrogen peroxide, which reacts with 10-acetyl-3, 7-dihydroxyphenoxazine (Amplex Red reagent) to produce fluorescent resorufin, which is measured by excitation at 550 nm and emission detection at 590 nm. The amounts of cholesterol were calculated by reference to cholesterol standards.
Preparation of Fab Fragments
Fab fragments were prepared from mAbs with the ImmunoPure Fab preparation kit (Pierce) using immobilized papain according to the manufacturer's instructions.
Statistical Analysis
Comparison of treatment effects was carried out using Student's t test and analysis of variance techniques.
RESULTS
PrPSc-induced Synapse Damage Is GPI-dependent
The loss of synapses, with a corresponding reduction in synaptic proteins, is an early feature of experimental and human prion diseases (22, 23). The addition of ScGT1 cell supernatants containing PrPSc caused a dose-dependent reduction in the amount of synaptophysin in neurons, indicative of synapse damage. In contrast, supernatants from uninfected GT1 cells did not affect the synaptophysin content of neurons (data not shown). Immunodepletion with mAb ICSM35 (which binds to PrPSc) reduced the effects of ScGT1 cell supernatants upon synapses, whereas immunodepletion with mAb ICSM18 (which binds to PrPC but not to PrPSc) had no discernable effect, indicating that the toxic entity within these supernatants was PrPSc (Fig. 1A). The role of GPI anchors in PrPSc-mediated synaptotoxicity was examined by digestion of ScGT1 cell supernatants with PI-PLC or PLA2. The addition of mock-treated PrPSc reduced the synaptophysin content of neurons, whereas PI-PLC-digested PrPSc (deacylated PrPSc) and PLA2-digested PrPSc (monoacylated PrPSc) had little effect (Fig. 1B), indicating that the GPI anchors play a major role in the synaptotoxicity of PrPSc. PrPSc preparations that had been incubated with heated-denatured PI-PLC or PLA2 (100 °C for 5 min) had similar effects as mock-treated PrPSc preparations. To determine whether synapse damage was associated with specific sized oligomers, ScGT1 supernatants were passed through different pore-sized filters. Most of the toxic entities in ScGT1 cell supernatants passed through a 500-kDa filter, indicating that small PrPSc oligomers were responsible for synapse damage rather than larger aggregates (Fig. 1, C and D). More specifically, synapse damage was seen in neurons incubated with supernatants that passed through a 100-kDa filter but not with supernatants that passed through a 50-kDa filter.
FIGURE 1.
PrPSc-induced synapse damage is dependent on GPI anchors. A, the amount of synaptophysin in neurons incubated with ScGT1 cell supernatants containing PrPSc that had been mock-treated (●), immunodepleted by incubation with the PrPSc-reactive mAb ICSM35 (○), or immunodepleted by incubation with the PrPC-reactive mAb ICSM18 (□). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12. B, the amount of synaptophysin in neurons incubated with ScGT1 cell supernatants containing PrPSc that had been incubated with control medium (●), PI-PLC (■), heat-denatured PI-PLC (□), PLA2 (▴), or heat-denatured PLA2 (△). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12. C, the amount of synaptophysin in neurons incubated with ScGT1 cell supernatants containing PrPSc (●), ScGT1 cell supernatants that had been passed through a 50-kDa filter (○), a 100-kDa filter (□), or a 500-kDa filter (■), or ScGT1 supernatants greater than 500 kDa (▴). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed three times, n = 9. D, the amount of synaptophysin in neurons incubated with ScGT1 cell supernatants containing PrPSc that had been separated into different sized oligomers by sequential passage through 50-, 100-, and 500-kDa filters. Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12.
Cross-linked PrPC Damages Synapses
The self-aggregation of PrPSc molecules results in the clustering of GPI anchors at high densities within cell membranes. Because cell activation by some GPI-anchored proteins is associated with the clustering of proteins (24, 25), we hypothesized that the synapse damage induced by PrPSc was mediated by the increased density of GPI anchors that occurs following the aggregation of PrPSc. To test this hypothesis, we sought to mimic the effects of PrPSc by cross-linking PrPC molecules. Cortical neurons were incubated with preparations containing cross-linked PrPC or mock-treated PrPC. Cross-linked PrPC caused a dose-dependent reduction in the synaptophysin content of neurons, indicative of synapse damage that was not seen with mock-treated PrPC (Fig. 2A). The synaptophysin content of neurons was reduced by greater than 80% by 1 ng/ml cross-linked PrPC, which did not affect neuronal survival as measured by thiazolyl blue tetrazolium (98% neuronal survival ± 6 as compared with mock-treated cells 100% ± 6, n = 9, p = 0.53). The synaptophysin content of neurons was not affected by incubation with preparations containing cross-linked Thy-1, a protein that also contains a GPI anchor. Immunoblots showed that incubation with cross-linked PrPC reduced the amounts of the synaptic proteins synaptophysin, synapsin-1, and synaptobrevin in neurons without affecting the amount of β-actin or the plasma membrane marker Na,K-ATPase α1 subunit (Fig. 2B). An immunoblot showed that cross-linked PrPC preparations contained mostly low-n PrPC oligomers and unlinked PrPC (Fig. 2C). Next, PrPC was digested with PI-PLC or PLA2 and isolated on C18 columns. They were subsequently cross-linked and incubated with neurons. Although the addition of cross-linked PrPC reduced the synaptophysin content of neurons, cross-linked deacylated PrPC or cross-linked monoacylated PrPC had no discernible effect (Fig. 2D). Cross-linked PrPC that had been incubated with heat-denatured PI-PLC or PLA2 had similar toxic effects to cross-linked PrPC. Next, cross-linked PrPC preparations were passed through filters with different sized pores to estimate the size of the toxic oligomers. None of the toxic entities within cross-linked PrPC preparations passed through a 50-kDa filter (Fig. 3A). Most of the toxic cross-linked PrPC preparations passed through a 100-kDa filter, indicating that they were mainly low-n oligomers (Fig. 3B).
FIGURE 2.
Cross-linked PrPC-induced synapse damage is dependent on GPI anchors. A, the amount of synaptophysin in neurons incubated with cross-linked PrPC (●), mock-treated PrPC (○), or cross-linked Thy-1 (■). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12. B, immunoblots showing the amount of synaptophysin (i), synapsin-1 (ii), synaptobrevin (iii), β-actin (iv), or Na,K-ATPase α1 subunit (v) in extracts from neurons incubated for 24 h with cross-linked PrPC as shown. C, immunoblot showing preparations of mock-treated PrPC (lane 1) or cross-linked PrPC (lane 2). D, the amount of synaptophysin in neurons incubated with cross-linked PrPC that had been pretreated with control medium (●), PI-PLC (■), heat-denatured PI-PLC (□), PLA2 (▴), or heat-denatured PLA2 (△). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12.
FIGURE 3.
Synapse damage is caused by low-n oligomers of cross-linked PrPC. A, the amount of synaptophysin in neurons incubated with cross-linked PrPC (●) or cross-linked PrPC preparations that had been passed through a 50-kDa filter (○), a 100-kDa filter (■), or a 500-kDa filter (□). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12. B, the amount of synaptophysin in neurons incubated with 1 ng/ml cross-linked PrPC that had been separated into different sizes as shown by sequential passage through 50-, 100-, and 500-kDa filters. Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12.
Contaminating Proteins Reduce Toxicity of Cross-linked PrPC Preparations
Although the preparations used were highly enriched for PrPC, we recognize that they were not pure. To determine whether the presence of contaminating proteins affected their activity, PrPC preparations were mixed with equimolar concentrations of bovine serum albumin (BSA), Thy-1, or deacylated PrPC and cross-linked to produce hetero-oligomers. We report that hetero-oligomers containing 1 ng/ml PrPC and BSA, Thy-1, or deacylated PrPC caused less synapse damage than homo-oligomers of PrPC (Fig. 4).
FIGURE 4.
Contaminating proteins reduce toxicity of cross-linked PrPC. 1 ng/ml PrPC was mixed with control medium (□) or with equimolar concentrations of BSA, Thy-1, or deacylated PrPC (■) as shown. The mixtures were cross-linked and incubated with neurons for 24 h when the amount of synaptophysin remaining in neurons was measured. Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed five times, n = 15.
Removal of Sialic Acids from GPI Anchor Reduces Synaptotoxicity of Cross-linked PrPC
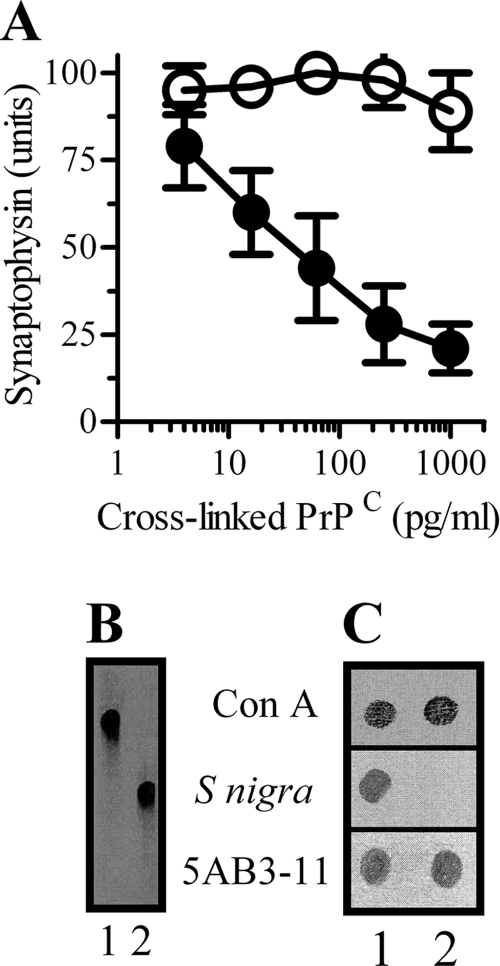
Because the glycan composition of GPIs attached to some proteins is cell line-specific (26), the effects of PrPC derived from a glial cell line were examined. Preparations containing cross-linked PrPC derived from glial cells did not cause synapse damage (Fig. 5A), suggesting that synapse damage occurs in response to the clustering of GPI anchors with a specific glycan composition. HPTLC analysis showed that that the GPIs isolated from PrPC derived from neuron and glial cells were different (Fig. 5B). The GPI attached to PrPC has been reported to contain sialic acid, a rare modification of mammalian GPI anchors (1). To confirm the presence of sialic acid, GPI anchors were probed with biotinylated lectins. S. nigra lectin (which reacts with terminal sialic acid bound by either α-2,6 or α-2,3 to galactose) bound to GPI anchors isolated from neuron-derived PrPC, but not to GPI anchors isolated from glia-derived PrPC. The binding of the mannose-binding lectin concanavalin A or the phosphatidylinositol-specific mAb 5AB3-11 demonstrated that similar amounts of GPI anchors were loaded on each blot (Fig. 5C).
FIGURE 5.
Cross-linked, glia-derived PrPC does not damage synapses. A, the amount of synaptophysin in neurons incubated with cross-linked, neuron-derived PrPC (●) or cross-linked, glia-derived PrPC (○). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed three times, n = 9. B, HPTLC showing GPI anchors isolated from neuron-derived PrPC (lane 1) or glia-derived PrPC (lane 2) and separated in a solution of methanol/chloroform/water on silica 60 plates. C, dot blots showing the binding of biotinylated concanavalin A (Con A), biotinylated S. nigra, and mAb 5AB3-11 to GPI anchors isolated from neuron-derived PrPC (lane 1) or glia-derived PrPC (lane 2).
To examine the effects of the sialic acid on the function of GPI anchors, PrPC was digested with neuraminidase, resulting in desialylated PrPC. HPTLC analysis showed that neuraminidase digestion altered the GPI anchor attached to neuron-derived PrPC (Fig. 6A). Neuraminidase digestion of neuron-derived PrPC resulted in a GPI anchor that bound readily to concanavalin A and the phosphatidylinositol-selective mAb 5AB3-11, but did not bind to S. nigra lectin (Fig. 6B). Because neuraminidase can affect sialic acids expressed on N-linked glycans as well as those on the GPI anchor, the N-linked glycans attached to PrPC were removed by PNGase digestion (27). There was no significant difference in the synaptophysin content of neurons incubated with cross-linked PrPC and those incubated with cross-linked PNGase-digested PrPC, indicating that the N-linked side chains did not play a significant role in synapse damage. PNGase-digested PrPC was then digested with neuraminidase and cross-linked. Preparations containing cross-linked PrPC that had been digested with PNGase and neuraminidase did not cause synapse damage, indicating that the sialic acid expressed upon the GPI anchor was essential for synapse damage (Fig. 6C). Pretreatment with heat-denatured neuraminidase did not affect cross-linked PrPC-induced synapse damage.
FIGURE 6.
Neuraminidase digestion reduced neurotoxicity of cross-linked PrPC. A, HPTLC showing the migration of GPI anchors isolated from PrPC (lane 1) or neuraminidase-digested PrPC (lane 2) separated in a solution of methanol/chloroform/water on silica 60 plates. B, dot blots showing the binding of biotinylated concanavalin A (Con A), biotinylated S. nigra, and mAb 5AB3-11 to GPI anchors isolated from PrPC (lane 1) or neuraminidase-digested PrPC (lane 2). C, the synaptophysin content of neurons incubated with cross-linked PrPC (●) or cross-linked PrPC that had been digested with PNGase and neuraminidase (○), PNGase (□), or PNGase and heat-denatured neuraminidase (■). Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12.
Cross-linked PrPC Increases Membrane Cholesterol in Synapses
The level of cholesterol in cell membranes is an important factor in the progression of prion diseases (28–30). PrPSc increases the amount of cholesterol in cell membranes, and because cholesterol is essential for the maintenance of synapses (31), the effects of PrPSc, PrPC, and cross-linked PrPC upon the amount of cholesterol in synaptosomes was examined. Neurons were treated for 1 h, and synaptosomes were isolated. The amount of cholesterol in treated synaptosomes was significantly increased in comparison with control neurons following incubation with 1 ng of PrPSc or with 1 ng of cross-linked PrPC, but not after incubation with 1 ng of mock-treated PrPC (Table 1). The addition of cross-linked deacylated PrPC or cross-linked monoacylated PrPC did not alter the amount of cholesterol in neuronal membranes, nor did the addition of deacylated PrPSc or monoacylated PrPSc.
TABLE 1.
Structure of GPI anchor affects membrane cholesterol
The amount of cholesterol in synaptosomes from neurons incubated for 1 h with 1 ng/ml cross-linked PrPC or 1 ng/ml PrPSc that had been digested with enzymes as shown. The amount of cholesterol in synaptosomes derived from untreated neurons was 92 ng/106 cells ± 9. Values shown are the mean amount of cholesterol in synaptosomes (ng/106 cells) ± S.D. from triplicate experiments performed four times, n = 12.
| Treatment | Cholesterol (ng/106 cells) |
|
|---|---|---|
| Cross-linked PrPC | PrPSc | |
| None | 125 ± 9a | 129 ± 17a |
| PI-PLC | 96 ± 13 | 89 ± 9 |
| Denatured PI-PLC | 124 ± 15a | 123 ± 12a |
| PLA2 | 99 ± 10 | 93 ± 8 |
| Denatured PLA2 | 130 ± 11a | 133 ± 14a |
| PNGase | 128 ± 18a | 126 ± 10a |
| Neuraminidase | 123 ± 14a | 128 ± 9a |
a Amount of cholesterol significantly higher than that of synaptosomes from untreated neurons (p < 0.05).
PrPSc and Cross-linked PrPC Activate cPLA2
The activation of cPLA2 is a key event in neurodegenerative diseases including prion and Alzheimer diseases (32–34). More specifically, the activation of cPLA2 at synapses causes synapse damage in cultured neurons (21). In this study, the addition of PrPSc increased the amount of activated cPLA2 in synaptosomes, whereas the addition of deacylated PrPSc or monoacylated PrPSc had little effect (Fig. 7A). Similarly, the addition of cross-linked, neuron-derived PrPC increased the amount of activated cPLA2 in synaptosomes, whereas mock-treated neuron-derived PrPC or cross-linked, glia-derived PrPC had little effect (Fig. 7B). Next, the modifications of GPI anchors that reduced synapse damage in response to cross-linked, neuron-derived PrPC were tested for their effects on the activation of cPLA2 in synaptosomes. We report that cross-linked deacylated PrPC, cross-linked monoacylated PrPC, or cross-linked desialylated PrPC had a lesser effect upon activation of synaptic cPLA2 than cross-linked PrPC (Fig. 7C). To confirm a causal role of activated cPLA2 in the synapse damage induced by cross-linked PrPC, neurons were pretreated with cPLA2 inhibitors. Pretreatment with 1 μm archidonyl trifluoromethyl ketone or 2 μm methyl arachedonyl fluorophosphorate protected neurons against the cross-linked PrPC-induced synapse damage (Fig. 7D).
FIGURE 7.
PrP-induced activation of cPLA2 is dependent on GPI anchors. A, the amount of activated cPLA2 in synaptosomes incubated with PrPSc that had been pretreated with control medium (●), PI-PLC (■), heat-denatured PI-PLC (□), PLA2 (▴), or heat-denatured PLA2 (△). Values shown are the mean units of activated cPLA2 ± S.D. from triplicate experiments performed three times, n = 9. B, the amount of activated cPLA2 in neurons incubated with neuron-derived PrPC (○), cross-linked neuron-derived PrPC (●), or cross-linked glia-derived PrPC (■). Values shown are the mean units of activated ± S.D. from triplicate experiments performed three times, n = 9. C, the amount of activated cPLA2 in neurons incubated with cross-linked PrPC that had been pretreated with control medium (●), PI-PLC (■), heat-denatured PI-PLC (□), PLA2 (△), or neuraminidase (▴). Values shown are the mean units of activated cPLA2 ± S.D. from triplicate experiments performed three times, n = 9. D, the amount of synaptophysin in neurons pretreated with control medium (●), 1 μm archidonyl trifluoromethyl ketone (□), or 2 μm methyl arachedonyl fluorophosphorate (■) and incubated with cross-linked PrPC. Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12.
Cross-linkage of PrPC by mAbs Induces Cell Signaling and Synapse Damage
Some of the effects of PrPSc can be mimicked by the cross-linkage of PrPC with mAbs, which leads to cell signaling in T cells (35) and neurodegeneration (36). Here we show that the addition of mAb 4F2 caused synapse damage to cultured Prnp+/+ neurons, whereas the monovalent Fab fragments prepared from this mAb had little effect (Fig. 8A). Similarly, mAb 4F2 caused a dose-dependent increase in the amount of activated cPLA2 in synaptosomes, whereas Fab fragments had no effect (Fig. 8B).
FIGURE 8.
mAb-mediated cross-linkage of PrPC causes synapse damage. A, the amount of synaptophysin in Prnp+/+ neurons that had been incubated with mAb 4F2 (□) or Fab fragments isolated from 4F2 (■) for 24 h. Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed three times, n = 9. B, the amount of activated cPLA2 in synaptosomes derived from Prnp+/+ neurons that had been incubated with mAb 4F2 (□) or Fab fragments isolated from 4F2 (■) for 24 h. Values shown are the mean units of activated cPLA2 ± S.D. from triplicate experiments performed three times, n = 9.
To examine further the role of the GPI anchor in the cell signaling and neurodegeneration induced by PrPC-reactive mAbs, 106 Prnp−/− neurons were pretreated with different concentrations of PrPC, monoacylated PrPC, or desialylated PrPC for 2 h, and the amount of cell-associated PrPC was determined by ELISA. Greater amounts of monoacylated PrPC were incorporated into neurons than either PrPC or desialylated PrPC (Fig. 9A). Next, 106 Prnp−/− neurons were pretreated with 10 ng of PrPC, monoacylated PrPC, PNGase-digested PrPC, or desialylated PrPC for 2 h and then incubated with serial dilutions of mAb 4F2. The addition of mAb 4F2 did not affect untreated neurons Prnp−/− neurons but caused a dose-dependent reduction in the synaptophysin content of Prnp−/− neurons pretreated with either PrPC or PNGase-digested PrPC. Notably, mAb 4F2 did not cause synapse damage in neurons pretreated with monoacylated PrPC or desialylated PrPC (Fig. 9B). Similarly, mAb 4F2 did not alter the amount of activated cPLA2 in synaptosomes from untreated Prnp−/− neurons but caused a dose-dependent increase in the amount of activated cPLA2 in synaptosomes pretreated with PrPC or with PNGase-digested PrPC. mAb 4F2 did not activate cPLA2 in Prnp−/− synaptosomes pretreated with monoacylated PrPC or desialylated PrPC (Fig. 9C).
FIGURE 9.
Sialic acids on GPI anchor are required for mAb-induced synapse damage. A, the amount of PrPC in Prnp−/− neurons that had been incubated with different amounts of PrPC (□), monoacylated PrPC (■), or desialylated PrPC (striped bars) for 2 h. Values shown are the mean amount of PrPC (ng/106 neurons) ± S.D. from triplicate experiments performed three times, n = 9. B, the amount of synaptophysin in Prnp−/− neurons that had been pretreated with 10 ng of PrPC (□), 10 ng of PNGase-digested PrPC (●), 10 ng of monoacylated PrPC (■), or 10 ng of desialylated PrPC (○) and incubated with mAb 4F2 for 24 h. Values shown are the mean units of synaptophysin ± S.D. from triplicate experiments performed four times, n = 12. C, the amount of activated cPLA2 in synaptosomes in Prnp−/− neurons that had been pretreated with 10 ng of PrPC (□), 10 ng of PNGase-digested PrPC (●), 10 ng of monoacylated PrPC (■), or 10 ng of desialylated PrPC (○) and incubated with mAb 4F2 for 24 h. Values shown are the mean units of activated cPLA2 ± S.D. from triplicate experiments performed four times, n = 12.
DISCUSSION
The molecular mechanisms by which PrPSc leads to neurodegeneration in prion diseases are poorly understood. Although the differences in the protein structure between PrPC and PrPSc have been extensively studied, the effects of the clustered GPI anchors that result from the aggregation of PrPSc have been largely overlooked. In this study, we show that the clustering of sialic acid-containing GPIs attached to PrP proteins altered the composition of cell membranes, leading to increased activation of cPLA2 and resulting in synapse damage.
The digestion of PrPSc with PI-PLC or PLA2 reduced its effects on synapses, indicating that the synapse damage induced by PrPSc was GPI-dependent. The hypothesis that the neurotoxicity of PrPSc was due to the clustering of their GPI anchors was supported by further observations that the effects of PrPSc on synapses were replicated by cross-linked PrPC. Mock-treated PrPC did not have the same effects, indicating that it is the aggregation of PrPC, and hence the density of GPI anchors, that is the important factor in generating synapse damage. Such observations are consistent with in vivo studies where naturally aggregated PrPC is associated with synaptic abnormalities (37). The concentrations of cross-linked PrPC and PrPSc required for these activities were remarkably similar, results consistent with observations that the conversion from PrPC to PrPSc does not entail changes to the GPI anchor (1). Filtration of ScGT1 supernatants or cross-linked PrPC preparations showed that the toxic entities were predominantly low-n oligomers of less than 100 kDa. The gradual accumulation of PrPSc in prion diseases is thought to cause synapse damage at early stages of infection where the concentrations of PrPSc in the brain are relatively low (23). In this study, the concentrations of PrPSc or cross-linked PrPC required to cause synapse damage in vitro did not cause a significant loss of neuronal viability.
The synaptophysin content of neurons was not affected by incubation with cross-linked Thy-1. The GPI anchors attached to Thy-1 and PrPC have different glycans (1, 38), suggesting that neurodegeneration resulted from the aggregation of GPI anchors with a specific composition. The GPI anchor attached to PrPC is unusual in that it contains sialic acid (1). This sialic acid moiety was a key element in the toxicity of the GPI anchors as the removal of sialic acid resulted in PrPC that, when cross-linked, did not cause synapse damage. The exact role of sialic acid is unclear. It may mediate interactions between PrPC and sialic acid-binding proteins, or it may compete with other sialic acid-containing compounds, such as the sphingolipids for sialic acid-binding proteins. PrPC extracted from hamster brains contained PrPC molecules attached to different GPIs, some of which did not contain sialic acid (1). The composition of the GPI anchor attached to specific proteins may be cell type-specific (26), and we found that the GPI anchor attached to PrPC derived from glial cells (and from some neuronal cell lines)3 does not contain sialic acid. The oligomers formed by cross-linkage of glia-derived PrPC did not cause significant synapse damage, an observation that suggests that PrPSc oligomers made by these cells would also show little toxicity. This observation may help to explain the lack of neuronal damage in the presence of high concentrations of PrPSc in different models of prion infection.
Disturbances in neuronal cholesterol metabolism are a feature of many neurodegenerative diseases (39–41). Both PrPC and PrPSc are found within cholesterol-dense lipid rafts (42, 43), and the addition of either PrPSc or cross-linked PrPC increased the amount of cholesterol in synaptosomes. However, this effect was not specific to cross-linked PrPC; it was also observed following the addition of cross-linked preparations of Thy-1 or desialylated PrPC, suggesting that the increase in cholesterol alone is not sufficient to trigger synapse damage. The effects of PrPSc or cross-linked PrPC on membrane cholesterol were lost after digestion with PI-PLC or PLA2, an observation that is consistent with the idea that most GPI anchors contain saturated fatty acids, which are responsible for the increased solubility of cholesterol (44). Although insufficient in itself to initiate pathology, changes in cholesterol levels may affect synapse function as the increase in cholesterol alters membrane fluidity and cholesterol is required for synapse formation (31).
Because the lipids that surround raft-associated proteins are dependent upon multiple glycan-lipid, protein-lipid, and lipid-lipid interactions (45), the specific molecular structure of the PrPC-GPI anchor may have a direct effect on the size, composition, and function of lipid rafts. This hypothesis is supported by the observations that PrPC and Thy-1 have different GPI anchors (1, 38) and that the domain surrounding PrPC differs from that surrounding Thy-1 (46). Thus, any modification of the GPI anchor attached to PrPC would be expected to alter the composition of the surrounding lipids. We propose that the membranes surrounding PrPC differed from those surrounding either monoacylated PrPC or desialylated PrPC as shown in Fig. 10A.
FIGURE 10.
Sialic acids on GPI anchor are required for activation of cPLA2 by cross-linked PrPC. A, graphic showing a model of PrPC, monoacylated PrPC, desialylated PrPC, and their proposed effects on the surrounding membrane domains including cholesterol, lyso-PLs, saturated PLs, and unsaturated PLs. B, cross-linkage of PrPC with a conventional GPI anchor that contains sialic acid stabilizes and activates cPLA2 within cholesterol-dense lipid rafts. C, cross-linkage of monoacylated PrPC does not sequester cholesterol that is required for the formation of lipid rafts and the activation of cPLA2. D, cross-linkage of desialylated PrPC provides the lipid raft environment, but the lack of sialic acid moieties fails to stabilize cPLA2 within the membrane.
These results suggest that it is the self-aggregation of PrPSc, or cross-linkage of PrPC, that results in high densities of the PrP-attached GPI anchors that lead to synapse damage and neuronal death. The composition and function of platforms formed following the coalescence of lipid rafts are dependent upon an induced-fit model (47). Lipid rafts can coalesce to form membrane platforms in which signaling complexes assemble (48). Such platforms are frequently termed signalosomes when their constituent parts combine to activate cells. For example, in T and B cell receptor signaling, the coalescence of membrane proteins in the outer leaflet of lipid rafts affects the cytoplasmic leaflet and its association with signaling molecules (11, 49, 50) and leads to cell activation (51). PrPC is associated with several signaling molecules including tyrosine kinases (52), protein kinase A (53), and cPLA2 (21). Here we show that the PrPSc-induced or cross-linked PrPC-induced activation of cPLA2, which is recognized as a key event in some neurodegenerative diseases (13), was dependent upon sialic acid and two acyl chains attached to the GPI anchor. Thus, we speculate that the platform formed by cross-linkage of PrPC contains cholesterol and a high density of sialic acids that are required to activate cPLA2 (Fig. 10B). In contrast, the cross-linkage of monoacylated PrPC does not sequester cholesterol (Fig. 10C), and cross-linkage of desialylated PrPC does not provide the density of sialic acids that are required to activate cPLA2 (Fig. 10D). The key role of cPLA2 in neurodegeneration was supported by our observation that pharmacological inhibition of cPLA2 reduced the ability of cross-linked PrPC to trigger synapse degeneration.
In summary, we show that the PrPSc-induced synapse degeneration was dependent upon the composition of its GPI anchors and could be mimicked by cross-linked PrPC. The clustering of GPI anchors containing sialic acid activates cPLA2 and triggers synapse damage. Such observations provide insight into the complex signaling processes that result in prion-induced synapse damage. Moreover, they demonstrate that novel therapies designed to modify GPI anchors may be useful in the treatment of prion diseases.
This work was supported by a grant from the European Commission FP6 “Neuroprion” Network of Excellence.
C. Bate and A. Williams, unpublished data.
- PrP
- prion protein
- PrPC
- cellular prion protein
- PrPSc
- disease-related isoform of PrP
- GPI
- glycosylphosphatidylinositol
- PLA2
- phospholipase A2
- cPLA2
- cytoplasmic PLA2
- PI-PLC
- phosphatidylinositol-phospholipase C
- PL
- phospholipase
- HPTLC
- high performance thin-layer chromatography
- PNGase
- endoglycosidase F.
REFERENCES
- 1. Stahl N., Baldwin M. A., Hecker R., Pan K. M., Burlingame A. L., Prusiner S. B. (1992) Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry 31, 5043–5053 [DOI] [PubMed] [Google Scholar]
- 2. Herms J., Tings T., Gall S., Madlung A., Giese A., Siebert H., Schürmann P., Windl O., Brose N., Kretzschmar H. (1999) Evidence of presynaptic location and function of the prion protein. J. Neurosci. 19, 8866–8875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salès N., Rodolfo K., Hässig R., Faucheux B., Di Giamberardino L., Moya K. L. (1998) Cellular prion protein localization in rodent and primate brain. Eur. J. Neurosci. 10, 2464–2471 [DOI] [PubMed] [Google Scholar]
- 4. Brown D. R. (2001) Prion and prejudice: normal protein and the synapse. Trends Neurosci. 24, 85–90 [DOI] [PubMed] [Google Scholar]
- 5. Prusiner S. B. (1998) Prions. Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. (1983) Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35, 349–358 [DOI] [PubMed] [Google Scholar]
- 7. Mayor S., Riezman H. (2004) Sorting GPI-anchored proteins. Nat. Rev. Mol. Cell Biol. 5, 110–120 [DOI] [PubMed] [Google Scholar]
- 8. Kimberley F. C., Sivasankar B., Paul Morgan B. (2007) Alternative roles for CD59. Mol. Immunol. 44, 73–81 [DOI] [PubMed] [Google Scholar]
- 9. Rege T. A., Hagood J. S. (2006) Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 20, 1045–1054 [DOI] [PubMed] [Google Scholar]
- 10. Lund-Johansen F., Olweus J., Symington F. W., Arli A., Thompson J. S., Vilella R., Skubitz K., Horejsi V. (1993) Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur. J. Immunol. 23, 2782–2791 [DOI] [PubMed] [Google Scholar]
- 11. Field K. A., Holowka D., Baird B. (1995) Fcϵ RI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc. Natl. Acad. Sci. U.S.A. 92, 9201–9205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikezawa H. (2002) Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol. Pharm. Bull. 25, 409–417 [DOI] [PubMed] [Google Scholar]
- 13. Sun G. Y., Xu J., Jensen M. D., Simonyi A. (2004) Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J. Lipid Res. 45, 205–213 [DOI] [PubMed] [Google Scholar]
- 14. Horejsí V., Drbal K., Cebecauer M., Cerný J., Brdicka T., Angelisová P., Stockinger H. (1999) GPI-microdomains: a role in signaling via immunoreceptors. Immunol. Today 20, 356–361 [DOI] [PubMed] [Google Scholar]
- 15. Taraboulos A., Scott M., Semenov A., Avrahami D., Laszlo L., Prusiner S. B., Avraham D. (1995) Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 129, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaneko K., Vey M., Scott M., Pilkuhn S., Cohen F. E., Prusiner S. B. (1997) COOH-terminal sequence of the cellular prion protein directs subcellular trafficking and controls conversion into the scrapie isoform. Proc. Natl. Acad. Sci. U.S.A. 94, 2333–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chesebro B., Trifilo M., Race R., Meade-White K., Teng C., LaCasse R., Raymond L., Favara C., Baron G., Priola S., Caughey B., Masliah E., Oldstone M. (2005) Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308, 1435–1439 [DOI] [PubMed] [Google Scholar]
- 18. Caughey B., Neary K., Buller R., Ernst D., Perry L. L., Chesebro B., Race R. E. (1990) Normal and scrapie-associated forms of prion protein differ in their sensitivities to phospholipase and proteases in intact neuroblastoma cells. J. Virol. 64, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atarashi R., Wilham J. M., Christensen L., Hughson A. G., Moore R. A., Johnson L. M., Onwubiko H. A., Priola S. A., Caughey B. (2008) Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat. Methods 5, 211–212 [DOI] [PubMed] [Google Scholar]
- 20. Bate C., Tayebi M., Williams A. (2010) The glycosylphosphatidylinositol anchor is a major determinant of prion binding and replication. Biochem. J. 428, 95–101 [DOI] [PubMed] [Google Scholar]
- 21. Bate C., Tayebi M., Williams A. (2010) Phospholipase A2 inhibitors protect against prion- and Aβ-mediated synapse degeneration. Mol. Neurodegener. 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cunningham C., Deacon R., Wells H., Boche D., Waters S., Diniz C. P., Scott H., Rawlins J. N., Perry V. H. (2003) Synaptic changes characterize early behavioral signs in the ME7 model of murine prion disease. Eur. J. Neurosci. 17, 2147–2155 [DOI] [PubMed] [Google Scholar]
- 23. Jeffrey M., Halliday W. G., Bell J., Johnston A. R., MacLeod N. K., Ingham C., Sayers A. R., Brown D. A., Fraser J. R. (2000) Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol. Appl. Neurobiol. 26, 41–54 [DOI] [PubMed] [Google Scholar]
- 24. Sharma P., Varma R., Sarasij R. C., Ira, Gousset K., Krishnamoorthy G., Rao M., Mayor S. (2004) Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116, 577–589 [DOI] [PubMed] [Google Scholar]
- 25. Suzuki K. G., Fujiwara T. K., Sanematsu F., Iino R., Edidin M., Kusumi A. (2007) GPI-anchored receptor clusters transiently recruit Lyn and Gα for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177, 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McConville M. J., Ferguson M. A. (1993) The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J. 294, 305–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan K. M., Stahl N., Prusiner S. B. (1992) Purification and properties of the cellular prion protein from Syrian hamster brain. Protein Sci. 1, 1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kempster S., Bate C., Williams A. (2007) Simvastatin treatment prolongs the survival of scrapie-infected mice. Neuroreport 18, 479–482 [DOI] [PubMed] [Google Scholar]
- 29. Mok S. W., Thelen K. M., Riemer C., Bamme T., Gültner S., Lütjohann D., Baier M. (2006) Simvastatin prolongs survival times in prion infections of the central nervous system. Biochem. Biophys. Res. Comm. 348, 697–702 [DOI] [PubMed] [Google Scholar]
- 30. Haviv Y., Avrahami D., Ovadia H., Ben-Hur T., Gabizon R., Sharon R. (2008) Induced neuroprotection independently from PrPSc accumulation in a mouse model for prion disease treated with simvastatin. Arch. Neurol. 65, 762–775 [DOI] [PubMed] [Google Scholar]
- 31. Mauch D. H., Nägler K., Schumacher S., Göritz C., Müller E. C., Otto A., Pfrieger F. W. (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 [DOI] [PubMed] [Google Scholar]
- 32. Farooqui A. A., Horrocks L. A., Farooqui T. (2007) Modulation of inflammation in brain: a matter of fat. J. Neurochem. 101, 577–599 [DOI] [PubMed] [Google Scholar]
- 33. Malaplate-Armand C., Florent-Béchard S., Youssef I., Koziel V., Sponne I., Kriem B., Leininger-Muller B., Olivier J. L., Oster T., Pillot T. (2006) Soluble oligomers of amyloid-β peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol. Dis. 23, 178–189 [DOI] [PubMed] [Google Scholar]
- 34. Sanchez-Mejia R. O., Newman J. W., Toh S., Yu G. Q., Zhou Y., Halabisky B., Cissé M., Scearce-Levie K., Cheng I. H., Gan L., Palop J. J., Bonventre J. V., Mucke L. (2008) Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer disease. Nat. Neurosci. 11, 1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stuermer C. A., Langhorst M. F., Wiechers M. F., Legler D. F., Von Hanwehr S. H., Guse A. H., Plattner H. (2004) PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 18, 1731–1733 [DOI] [PubMed] [Google Scholar]
- 36. Solforosi L., Criado J. R., McGavern D. B., Wirz S., Sánchez-Alavez M., Sugama S., DeGiorgio L. A., Volpe B. T., Wiseman E., Abalos G., Masliah E., Gilden D., Oldstone M. B., Conti B., Williamson R. A. (2004) Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303, 1514–1516 [DOI] [PubMed] [Google Scholar]
- 37. Chiesa R., Piccardo P., Biasini E., Ghetti B., Harris D. A. (2008) Aggregated, wild-type prion protein causes neurological dysfunction and synaptic abnormalities. J. Neurosci. 28, 13258–13267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W., Anand R., Williams A. F. (1988) Complete structure of the glycosyl phosphatidylinositol membrane anchor of rat brain Thy-1 glycoprotein. Nature 333, 269–272 [DOI] [PubMed] [Google Scholar]
- 39. Cenedella R. J. (2009) Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids 44, 477–487 [DOI] [PubMed] [Google Scholar]
- 40. Puglielli L., Tanzi R. E., Kovacs D. M. (2003) Alzheimer disease: the cholesterol connection. Nat. Neurosci. 6, 345–351 [DOI] [PubMed] [Google Scholar]
- 41. Stefani M., Liguri G. (2009) Cholesterol in Alzheimer disease: unresolved questions. Curr. Alzheimer Res. 6, 15–29 [DOI] [PubMed] [Google Scholar]
- 42. Vey M., Pilkuhn S., Wille H., Nixon R., DeArmond S. J., Smart E. J., Anderson R. G., Taraboulos A., Prusiner S. B. (1996) Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl. Acad. Sci. U. S. A. 93, 14945–14949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naslavsky N., Stein R., Yanai A., Friedlander G., Taraboulos A. (1997) Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J. Biol. Chem. 272, 6324–6331 [DOI] [PubMed] [Google Scholar]
- 44. Schroeder R., London E., Brown D. (1994) Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. U. S. A. 91, 12130–12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson R. G., Jacobson K. (2002) A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825 [DOI] [PubMed] [Google Scholar]
- 46. Brügger B., Graham C., Leibrecht I., Mombelli E., Jen A., Wieland F., Morris R. (2004) The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. J. Biol. Chem. 279, 7530–7536 [DOI] [PubMed] [Google Scholar]
- 47. Pike L. J. (2004) Lipid rafts: heterogeneity on the high seas. Biochem. J. 378, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simons K., Ikonen E. (1997) Functional rafts in cell membranes. Nature 387, 569–572 [DOI] [PubMed] [Google Scholar]
- 49. Eisenberg S., Shvartsman D. E., Ehrlich M., Henis Y. I. (2006) Clustering of raft-associated proteins in the external membrane leaflet modulates internal leaflet H-ras diffusion and signaling. Mol. Cell. Biol. 26, 7190–7200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gri G., Molon B., Manes S., Pozzan T., Viola A. (2004) The inner side of T cell lipid rafts. Immunol. Lett. 94, 247–252 [DOI] [PubMed] [Google Scholar]
- 51. Montixi C., Langlet C., Bernard A. M., Thimonier J., Dubois C., Wurbel M. A., Chauvin J. P., Pierres M., He H. T. (1998) Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17, 5334–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mouillet-Richard S., Ermonval M., Chebassier C., Laplanche J. L., Lehmann S., Launay J. M., Kellermann O. (2000) Signal transduction through prion protein. Science 289, 1925–1928 [DOI] [PubMed] [Google Scholar]
- 53. Chiarini L. B., Freitas A. R., Zanata S. M., Brentani R. R., Martins V. R., Linden R. (2002) Cellular prion protein transduces neuroprotective signals. EMBO J. 21, 3317–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]