Abstract
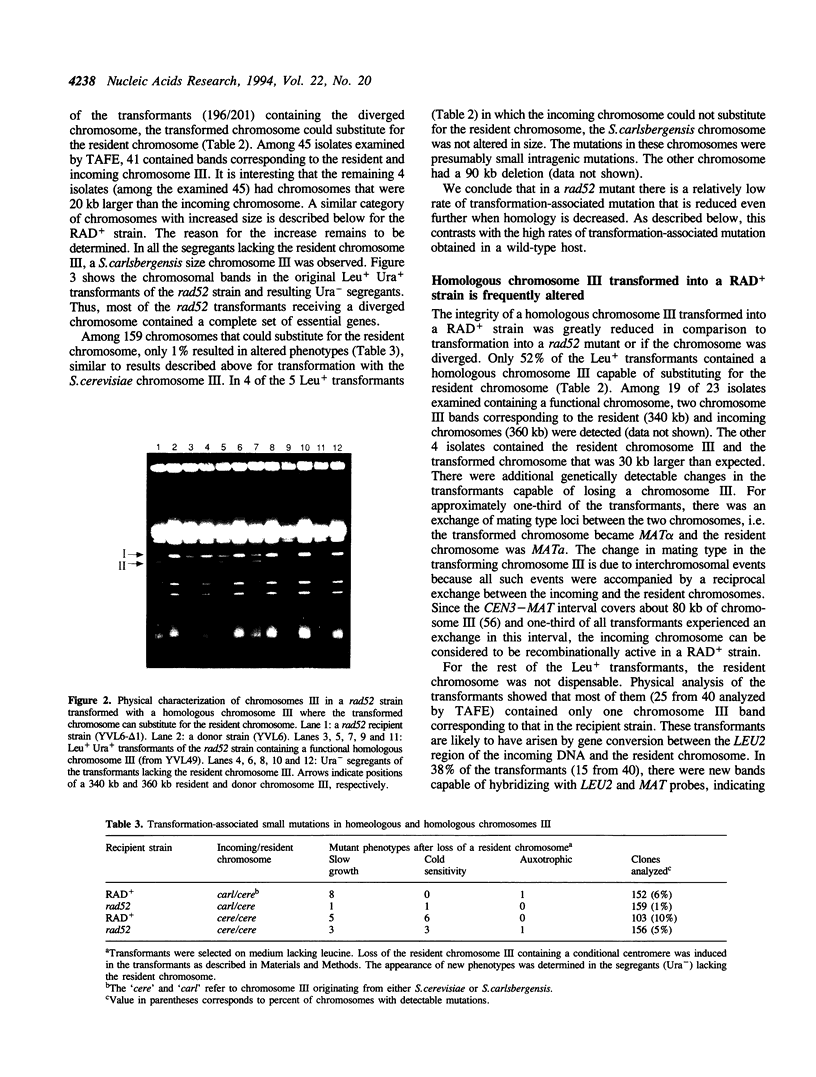
While transformation is a prominent tool for genetic analysis and genome manipulation in many organisms, transforming DNA has often been found to be unstable relative to established molecules. We determined the potential for transformation-associated mutations in a 360 kb yeast chromosome III composed primarily of unique DNA. Wild-type and rad52 Saccharomyces cerevisiae strains were transformed with either a homologous chromosome III or a diverged chromosome III from S. carlsbergensis. The host strain chromosome III had a conditional centromere allowing it to be lost on galactose medium so that recessive mutations in the transformed chromosome could be identified. Following transformation of a RAD+ strain with the homologous chromosome, there were frequent changes in the incoming chromosome, including large deletions and mutations that do not lead to detectable changes in chromosome size. Based on results with the diverged chromosome, interchromosomal recombinational interactions were the source of many of the changes. Even though rad52 exhibits elevated mitotic mutation rates, the percentage of transformed diverged chromosomes incapable of substituting for the resident chromosome was not increased in rad52 compared to the wild-type strain, indicating that the mutator phenotype does not extend to transforming chromosomal DNA. Based on these results and our previous observation that the incidence of large mutations is reduced during the cloning of mammalian DNA into a rad52 as compared to a RAD+ strain, a rad52 host is well-suited for cloning DNA segments in which gene function must be maintained.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abidi F. E., Wada M., Little R. D., Schlessinger D. Yeast artificial chromosomes containing human Xq24-Xq28 DNA: library construction and representation of probe sequences. Genomics. 1990 Jul;7(3):363–376. doi: 10.1016/0888-7543(90)90170-y. [DOI] [PubMed] [Google Scholar]
- Albertsen H. M., Abderrahim H., Cann H. M., Dausset J., Le Paslier D., Cohen D. Construction and characterization of a yeast artificial chromosome library containing seven haploid human genome equivalents. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4256–4260. doi: 10.1073/pnas.87.11.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Riley J. H., Butler R., Smith J. C., Markham A. F. A 3.5 genome equivalent multi access YAC library: construction, characterisation, screening and storage. Nucleic Acids Res. 1990 Apr 25;18(8):1951–1956. doi: 10.1093/nar/18.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailis A. M., Rothstein R. A defect in mismatch repair in Saccharomyces cerevisiae stimulates ectopic recombination between homeologous genes by an excision repair dependent process. Genetics. 1990 Nov;126(3):535–547. doi: 10.1093/genetics/126.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. P., Valdes J., Hummerich H., Baxendale S., Le Paslier D. L., Monaco A. P., Tagle D., MacDonald M. E., Altherr M., Ross M. Characterization of a yeast artificial chromosome contig spanning the Huntington's disease gene candidate region. Nat Genet. 1992 Jun;1(3):180–187. doi: 10.1038/ng0692-180. [DOI] [PubMed] [Google Scholar]
- Boundy-Mills K. L., Livingston D. M. A Saccharomyces cerevisiae RAD52 allele expressing a C-terminal truncation protein: activities and intragenic complementation of missense mutations. Genetics. 1993 Jan;133(1):39–49. doi: 10.1093/genetics/133.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Percival K. J. Transformation of yeast spheroplasts without cell fusion. Anal Biochem. 1987 Jun;163(2):391–397. doi: 10.1016/0003-2697(87)90240-5. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey G. P., Pedersen M. B. DNA sequence polymorphisms in the genus Saccharomyces. V. Cloning and characterization of a LEU2 gene from S. carlsbergensis. Carlsberg Res Commun. 1988;53(3):209–219. doi: 10.1007/BF02904408. [DOI] [PubMed] [Google Scholar]
- Chumakov I. M., Le Gall I., Billault A., Ougen P., Soularue P., Guillou S., Rigault P., Bui H., De Tand M. F., Barillot E. Isolation of chromosome 21-specific yeast artificial chromosomes from a total human genome library. Nat Genet. 1992 Jun;1(3):222–225. doi: 10.1038/ng0692-222. [DOI] [PubMed] [Google Scholar]
- Clancy S., Mann C., Davis R. W., Calos M. P. Deletion of plasmid sequences during Saccharomyces cerevisiae transformation. J Bacteriol. 1984 Sep;159(3):1065–1067. doi: 10.1128/jb.159.3.1065-1067.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Urso M., Zucchi I., Ciccodicola A., Palmieri G., Abidi F. E., Schlessinger D. Human glucose-6-phosphate dehydrogenase gene carried on a yeast artificial chromosome encodes active enzyme in monkey cells. Genomics. 1990 Aug;7(4):531–534. doi: 10.1016/0888-7543(90)90196-2. [DOI] [PubMed] [Google Scholar]
- Dunford R., Vilageliu L., Moore G. Stabilisation of a yeast artificial chromosome containing plant DNA using a recombination-deficient host. Plant Mol Biol. 1993 Mar;21(6):1187–1189. doi: 10.1007/BF00023615. [DOI] [PubMed] [Google Scholar]
- Eliceiri B., Labella T., Hagino Y., Srivastava A., Schlessinger D., Pilia G., Palmieri G., D'Urso M. Stable integration and expression in mouse cells of yeast artificial chromosomes harboring human genes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2179–2183. doi: 10.1073/pnas.88.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S., Vollrath D., Hilton A., Page D. C. The human Y chromosome: overlapping DNA clones spanning the euchromatic region. Science. 1992 Oct 2;258(5079):60–66. doi: 10.1126/science.1359640. [DOI] [PubMed] [Google Scholar]
- Gaensler K. M., Burmeister M., Brownstein B. H., Taillon-Miller P., Myers R. M. Physical mapping of yeast artificial chromosomes containing sequences from the human beta-globin gene region. Genomics. 1991 Aug;10(4):976–984. doi: 10.1016/0888-7543(91)90188-k. [DOI] [PubMed] [Google Scholar]
- Gnirke A., Barnes T. S., Patterson D., Schild D., Featherstone T., Olson M. V. Cloning and in vivo expression of the human GART gene using yeast artificial chromosomes. EMBO J. 1991 Jul;10(7):1629–1634. doi: 10.1002/j.1460-2075.1991.tb07685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnirke A., Huxley C., Peterson K., Olson M. V. Microinjection of intact 200- to 500-kb fragments of YAC DNA into mammalian cells. Genomics. 1993 Mar;15(3):659–667. doi: 10.1006/geno.1993.1121. [DOI] [PubMed] [Google Scholar]
- Goebl M. G., Petes T. D. Most of the yeast genomic sequences are not essential for cell growth and division. Cell. 1986 Sep 26;46(7):983–992. doi: 10.1016/0092-8674(86)90697-5. [DOI] [PubMed] [Google Scholar]
- Green E. D., Riethman H. C., Dutchik J. E., Olson M. V. Detection and characterization of chimeric yeast artificial-chromosome clones. Genomics. 1991 Nov;11(3):658–669. doi: 10.1016/0888-7543(91)90073-n. [DOI] [PubMed] [Google Scholar]
- Harris S., Rudnicki K. S., Haber J. E. Gene conversions and crossing over during homologous and homeologous ectopic recombination in Saccharomyces cerevisiae. Genetics. 1993 Sep;135(1):5–16. doi: 10.1093/genetics/135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A., Bloom K. Genetic manipulation of centromere function. Mol Cell Biol. 1987 Jul;7(7):2397–2405. doi: 10.1128/mcb.7.7.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C., Hagino Y., Schlessinger D., Olson M. V. The human HPRT gene on a yeast artificial chromosome is functional when transferred to mouse cells by cell fusion. Genomics. 1991 Apr;9(4):742–750. doi: 10.1016/0888-7543(91)90369-p. [DOI] [PubMed] [Google Scholar]
- Imai T., Olson M. V. Second-generation approach to the construction of yeast artificial-chromosome libraries. Genomics. 1990 Oct;8(2):297–303. doi: 10.1016/0888-7543(90)90285-3. [DOI] [PubMed] [Google Scholar]
- Kunz B. A., Peters M. G., Kohalmi S. E., Armstrong J. D., Glattke M., Badiani K. Disruption of the RAD52 gene alters the spectrum of spontaneous SUP4-o mutations in Saccharomyces cerevisiae. Genetics. 1989 Jul;122(3):535–542. doi: 10.1093/genetics/122.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larin Z., Monaco A. P., Lehrach H. Yeast artificial chromosome libraries containing large inserts from mouse and human DNA. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4123–4127. doi: 10.1073/pnas.88.10.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larionov V., Kouprina N., Eldarov M., Perkins E., Porter G., Resnick M. A. Transformation-associated recombination between diverged and homologous DNA repeats is induced by strand breaks. Yeast. 1994 Jan;10(1):93–104. doi: 10.1002/yea.320100109. [DOI] [PubMed] [Google Scholar]
- Ling L. L., Ma N. S., Smith D. R., Miller D. D., Moir D. T. Reduced occurrence of chimeric YACs in recombination-deficient hosts. Nucleic Acids Res. 1993 Dec 25;21(25):6045–6046. doi: 10.1093/nar/21.25.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. D., Pilia G., Johnson S., D'Urso M., Schlessinger D. Yeast artificial chromosomes spanning 8 megabases and 10-15 centimorgans of human cytogenetic band Xq26. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):177–181. doi: 10.1073/pnas.89.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M. K., Campbell E., Deaven L., Moyzis R. Low-frequency chimeric yeast artificial chromosome libraries from flow-sorted human chromosomes 16 and 21. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1063–1067. doi: 10.1073/pnas.90.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M. K., Shero J. H., Cheung M. C., Kan Y. W., Hieter P. A., Antonarakis S. E. Construction of human chromosome 21-specific yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9991–9995. doi: 10.1073/pnas.86.24.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Walker A. P., Millwood I., Larin Z., Lehrach H. A yeast artificial chromosome contig containing the complete Duchenne muscular dystrophy gene. Genomics. 1992 Mar;12(3):465–473. doi: 10.1016/0888-7543(92)90436-v. [DOI] [PubMed] [Google Scholar]
- Mézard C., Pompon D., Nicolas A. Recombination between similar but not identical DNA sequences during yeast transformation occurs within short stretches of identity. Cell. 1992 Aug 21;70(4):659–670. doi: 10.1016/0092-8674(92)90434-e. [DOI] [PubMed] [Google Scholar]
- Neil D. L., Villasante A., Fisher R. B., Vetrie D., Cox B., Tyler-Smith C. Structural instability of human tandemly repeated DNA sequences cloned in yeast artificial chromosome vectors. Nucleic Acids Res. 1990 Mar 25;18(6):1421–1428. doi: 10.1093/nar/18.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., van der Aart Q. J., Agostoni-Carbone M. L., Aigle M., Alberghina L., Alexandraki D., Antoine G., Anwar R., Ballesta J. P., Benit P. The complete DNA sequence of yeast chromosome III. Nature. 1992 May 7;357(6373):38–46. doi: 10.1038/357038a0. [DOI] [PubMed] [Google Scholar]
- Pachnis V., Pevny L., Rothstein R., Costantini F. Transfer of a yeast artificial chromosome carrying human DNA from Saccharomyces cerevisiae into mammalian cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5109–5113. doi: 10.1073/pnas.87.13.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri G., Romano G., Casamassimi A., D'Urso M., Little R. D., Abidi F. E., Schlessinger D., Lagerström M., Malmgren H., Steen-Bondeson M. L. 1.5-Mb YAC contig in Xq28 formatted with sequence-tagged sites and including a region unstable in the clones. Genomics. 1993 Jun;16(3):586–592. doi: 10.1006/geno.1993.1234. [DOI] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Modification and transfer into an embryonal carcinoma cell line of a 360-kilobase human-derived yeast artificial chromosome. Mol Cell Biol. 1990 Aug;10(8):4163–4169. doi: 10.1128/mcb.10.8.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Skaanild M., Nilsson-Tillgren T. Lack of DNA homology in a pair of divergent chromosomes greatly sensitizes them to loss by DNA damage. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2276–2280. doi: 10.1073/pnas.86.7.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. M., Burke D. T., Leung J. C., Koos D. S., Chen H., Tilghman S. M. Genomic analysis using a yeast artificial chromosome library with mouse DNA inserts. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2456–2460. doi: 10.1073/pnas.89.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Little R. D., Freije D., Abidi F., Zucchi I., Porta G., Pilia G., Nagaraja R., Johnson S. K., Yoon J. Y. Yeast artificial chromosome-based genome mapping: some lessons from Xq24-q28. Genomics. 1991 Dec;11(4):783–793. doi: 10.1016/0888-7543(91)90001-u. [DOI] [PubMed] [Google Scholar]
- Schlessinger D. Yeast artificial chromosomes: tools for mapping and analysis of complex genomes. Trends Genet. 1990 Aug;6(8):248, 255-8. doi: 10.1016/0168-9525(90)90207-m. [DOI] [PubMed] [Google Scholar]
- Selleri L., Eubanks J. H., Giovannini M., Hermanson G. G., Romo A., Djabali M., Maurer S., McElligott D. L., Smith M. W., Evans G. A. Detection and characterization of "chimeric" yeast artificial chromosome clones by fluorescent in situ suppression hybridization. Genomics. 1992 Oct;14(2):536–541. doi: 10.1016/s0888-7543(05)80263-0. [DOI] [PubMed] [Google Scholar]
- Silverman G. A., Jockel J. I., Domer P. H., Mohr R. M., Taillon-Miller P., Korsmeyer S. J. Yeast artificial chromosome cloning of a two-megabase-size contig within chromosomal band 18q21 establishes physical linkage between BCL2 and plasminogen activator inhibitor type-2. Genomics. 1991 Feb;9(2):219–228. doi: 10.1016/0888-7543(91)90245-a. [DOI] [PubMed] [Google Scholar]
- Sleister H. M., Mills K. A., Blackwell S. E., Killary A. M., Murray J. C., Malone R. E. Construction of a human chromosome 4 YAC pool and analysis of artificial chromosome stability. Nucleic Acids Res. 1992 Jul 11;20(13):3419–3425. doi: 10.1093/nar/20.13.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. High frequency excision of Ty elements during transformation of yeast. Nucleic Acids Res. 1986 Apr 11;14(7):2989–3001. doi: 10.1093/nar/14.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Borstel R. C., Cain K. T., Steinberg C. M. Inheritance of spontaneous mutability in yeast. Genetics. 1971 Sep;69(1):17–27. doi: 10.1093/genetics/69.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicksteed B. L., Collins I., Dershowitz A., Stateva L. I., Green R. P., Oliver S. G., Brown A. J., Newlon C. S. A physical comparison of chromosome III in six strains of Saccharomyces cerevisiae. Yeast. 1994 Jan;10(1):39–57. doi: 10.1002/yea.320100105. [DOI] [PubMed] [Google Scholar]