Abstract
The adaptation of vascular endothelial cells to shear stress alteration induced by global hemodynamic changes, such as those accompanying exercise or digestion, is an essential component of normal endothelial physiology in vivo. An understanding of the transient regulation of endothelial phenotype during adaptation to changes in mural shear will advance our understanding of endothelial biology and may yield new insights into the mechanism of atherogenesis. In this study, we characterized the adaptive response of arterial endothelial cells to an acute increase in shear stress magnitude in well-defined in vitro settings. Porcine endothelial cells were preconditioned by a basal level shear stress of 15 ± 15 dyn/cm2 at 1 Hz for 24 h, after which an acute increase in shear stress to 30 ± 15 dyn/cm2 was applied. Endothelial permeability nearly doubled after 40-min exposure to the elevated shear stress and then decreased gradually. Transcriptomics studies using microarray techniques identified 86 genes that were sensitive to the elevated shear. The acute increase in shear stress promoted the expression of a group of anti-inflammatory and antioxidative genes. The adaptive response of the global gene expression profile is triphasic, consisting of an induction period, an early adaptive response (ca. 45 min) and a late remodeling response. Our results suggest that endothelial cells exhibit a specific phenotype during the adaptive response to changes in shear stress; this phenotype is different than that of fully adapted endothelial cells.
Keywords: permeability, gene expression, microarray
atherosclerosis arises from endothelial cell dysfunction. Endothelial dysfunction features increased permeability, enhanced expression of adhesion molecules and leukocyte adhesion, enhanced cell turnover, increased oxidant stress, and reduced endothelium-dependent vasodilation (51). Increased endothelial permeability leads to the accumulation of LDL in the intima, which is a key step in atherosclerotic development. The inflammatory response of endothelial cells results in the recruitment of monocytes, which migrate into the intima and differentiate into macrophages, which engulf oxidized LDL and form foam cells. The accumulation of foam cells in the intima leads to the formation of atherosclerotic plaques. Therefore, endothelial permeability and inflammatory status greatly affect arterial atherosusceptibility.
Endothelial cells in the vasculature are constantly exposed to shear stress. Shear-dependent permeability to macromolecules has been examined both in vitro and in vivo. The application of shear stress to static cultured cells increased endothelial permeability to albumin (32) and dextran (50). However, these increases may have been caused by the transient response of endothelial cells to the onset of shear, since other in vitro studies (10, 33) suggest that decreased permeability is associated with higher shear stress after a relatively long-term exposure. Himburg et al. (28) demonstrated that the in vivo endothelial permeability to albumin decreases with increasing time-average shear stress in porcine iliac arteries. Several other shear stress parameters, such as spatial gradients (5, 36), have also been correlated with in vivo permeability.
Endothelial transcriptional activities are also regulated by shear stress. Although the mechanism of endothelial mechanotransduction is not fully understood, a large number of signaling cascades have been found to be induced by shear stress (8, 9, 12, 13, 23, 58). As a result, a significant number of downstream genes are regulated by shear stress, including endothelial nitric oxide synthase (eNOS; Refs. 12, 14, 20), MCP1 (29, 30), Kruppel-like factor 2 (KLF2; Refs. 14, 15), MnSOD (48), NADPH oxidase (30, 56), and endothelin-1 (40, 63). Endothelial transcriptional regulation is sensitive to many parameters of the shear stress profile, including magnitude (26), frequency (27), temporal (2) and spatial (34) gradient, and the presence of flow reversal (27). Generally, prolonged unidirectional shear stress is believed to be atheroprotective since it increases endothelial anti-inflammatory and antioxidative activity.
Endothelial cells in vivo are believed to adapt to the local shear stress, at least in regions with unidirectional flow (23), to assume a quiescent phenotype. However, the local shear stress profiles are not invariant over time and they are altered occasionally by changes in global hemodynamic variables, such as heart rate, flow rate, and flow partition at branches. These changes are caused by a number of normal physiologic events, such as exercise, smoking, sleep, stress, and digestion. The duration of these changes ranges from minutes to hours. During these periods, endothelial cells may undergo transient phenotypic transformation and possibly engage in early remodeling events, as they adapt to the altered shear stress. It is reasonable to expect that the dynamic adaptive endothelial response to changes in shear may be different from the dependence of endothelial permeability and transcription seen in in vitro or ex vivo preparations exposed to long-term unchanging shear levels. It is also possible that the atherosusceptibility of the arterial wall is enhanced during these transients (21).
In an effort to understand the dynamics of the adaptation of endothelial permeability, several animal studies have been previously conducted in our laboratory (22, 25), where shear stress levels in porcine iliac arteries were altered by opening and closing downstream arteriovenous femoral shunts. Endothelial permeability to albumin was found to increase transiently in response to an acute increase in shear stress. To better understand this, we sought to characterize the adaptive response of arterial endothelial cells to an acute increase in shear stress in well-defined in vitro settings in this study. Cultured endothelial cells were preconditioned by a basal level shear of 15 ± 15 dyn/cm2 at 1 Hz (sinusoidal waveform) for 24 h, and shear stress was then increased to 30 ± 15 dyn/cm2. The basal shear stress exhibited the same mean and maximum values as the basal shear stress in the porcine iliac arteries (28). The elevated shear stress also corresponds to the animal studies, where the mean shear stress was raised to twice the basal level by opening the shunt. The basal mean shear stress is also physiologically relevant in humans and is considered to be a healthy and atheroprotective shear stress profile (8, 23). During the 6-h period after shear step-up, endothelial permeability and gene expression were measured at multiple time points. Our results demonstrate that endothelial cells exhibit a specific phenotype during the adaptive response and that the transient phenotype is different from that of fully adapted endothelial cells.
MATERIALS AND METHODS
Cell culture.
Aortic endothelial cells were harvested from recently euthanized healthy female pigs weighing about 60 kg; procedures preceding and including euthanasia were carried out under a Duke Institutional Animal Care and Use Committe-approved protocol. Cells were cultured with Medium 199 (GIBCO), supplemented with 10% FBS (GIBCO) and antibiotic/antimycotic (Sigma-Aldrich). Cells between passage 2 and 5 were seeded at a density of around 105 cell/cm2 on collagen-coated (Sigma-Aldrich; 10 μg/cm2) glass slides or Transwell polyester membrane with a 0.4-μm pore size (Corning). When cells were tightly confluent, the media were changed to a low serum formulation (2%) in preparation for flow perfusion.
Permeability measurement.
The flow circuit was similar to that in earlier work (27) and is shown in Supplemental Fig. S1 (Supplemental Data; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website). The steady mean flow was provided by a peristaltic roller pump. To superimpose the pulsatile flow, a computer-controlled stepping motor was used to drive a bellows pump. A flow-separation device used an enclosed volume of air to transmit the pressure pulse generated by the bellows pump to the flow circuit, thus minimizing the perfusion volume. The flow system was optimized to generate the desired shear stress profiles with <20 ml media. The volumetric flow rate was monitored using ultrasonic flow probes (Transonic Systems). The flow circuit was placed in a cell culture incubator at 37°C and 5% CO2.
A laminar flow chamber apparatus (Supplemental Fig. S2) was designed to measure the permeability of endothelial cells cultured on a Transwell filter. The filter was glued over a 20-mm diameter opening on an acrylic slide (1/8-inch thick) using medical grade silicone adhesive (Dow Corning). A 1-mm diameter hole was drilled through the side of the chamber to allow a silica optical fiber access to the opening under the filter. The luminal chamber was connected to the flow circuit to apply shear stress to the endothelial cells, while the abluminal one was an enclosed media reservoir with the optical fiber access and ports for media flushing. To eliminate possible convective flux induced by the pressure difference across the cell monolayer, the abluminal chamber was kept closed so that the pressure in the abluminal chamber was the same as that in the luminal chamber.
A fluorescence detection system was designed to measure the concentration of FITC-BSA in the abluminal chamber in real-time (described in detail in Supplemental Data). Briefly, excitation light from a green light-emitting diode was filtered and transmitted through the optical fiber to the abluminal chamber and the emitted light passed through the same fiber to an ultrasensitive photodiode. A LabView (National Instruments) program was used for real-time light-emitting diode control and data acquisition.
Endothelial cells cultured on the filter were preconditioned by basal level shear stress for 24 h. The preconditioning time was determined by a pilot study, in which the endothelial permeability was measured after 18 and 24 h of basal level shear stress. No difference in permeability was found (data not shown). This is consistent with a previous study (47), where endothelial permeability reached a stable level after 12-h exposure to shear stress. Around 20 min before shear step-up, FITC-BSA was added to the perfusion media at a final concentration of 1 mg/ml. The fluorescence detection system was turned on to measure the tracer concentration in the abluminal chamber. The permeability of the preconditioned endothelial cells was obtained from the average rate of change in abluminal concentration, Sp, found from a linear fit to the concentration profile over 5 min (7, 16). After the permeability of the preconditioned endothelial cells was obtained, a step-up change of shear stress to 30 ± 15 dyn/cm2 was applied. Endothelial permeability was then measured at multiple time points until 6 h had elapsed. At each time point, the average rate of change in concentration, S, was calculated as above during a time period of 5 min centered on the measurement time point. The endothelial permeability at each time point was normalized by the pre-step-up value, Sp.
After each flow experiment, endothelial monolayer was fixed using Diff-Quick for 5 min (2 mg/l Fast Green in methanol; VWR) and then crystal violet for 3 min (0.5% crystal violet in 20% methanol; VWR). Monolayer integrity was confirmed under a microscope (Nikon). Data were obtained only from the fully confluent endothelial monolayers.
Microarray study.
In the microarray studies, polytetrafluoroethylene manifolds were added to the flow circuit to distribute the flow evenly to eight identical laminar flow chambers installed in parallel (Fig. Supplemental S3). Pinch valves were used to fine tune the flow rate for each chamber; the flow rates were monitored using Transonic flow probes. This approach minimized experimental variability since all cells were from the same batch, had the same passage number, and were sheared by the same perfusion media. It also facilitated cell sampling, since individual chambers could be detached separately from the flow circuit. The laminar flow chamber was designed as described by Himburg et al. (27).
The preconditioning basal shear stress was applied to cells in the eight chambers simultaneously. After 24 h, one randomly selected flow chamber was quickly detached from the flow circuit, and a tubing segment with matching hydraulic resistance was placed in the circuit to maintain the flow rates in the remaining flow chambers. The integrity of the endothelial monolayer was confirmed under a microscope, and endothelial RNA was then isolated. After the preconditioned control sample was obtained, the mean shear stress was doubled. At each designated time point (5, 15, 45, 90, 180, and 360 min after step-up), one randomly selected chamber was detached from the flow circuit and immediately disassembled for RNA isolation. RNA isolation and amplification were performed as described previously (62). Each sample and a standard reference, derived from static cultured endothelial cells, were hybridized to custom printed Sus Scrofa DNA microarrays (Operon Biotechnologies) by the Duke Microarray Facility. Four experiments were performed.
A control set of experiments was performed to determine whether the statically cultured endothelial cells adapted sufficiently to the basal level shear stress during the preconditioning time of 24 h. Endothelial cells were exposed to the basal level shear stress for 24 and 30 h. Control samples were kept under static conditions for 24 h. For the two preconditioning times (24 and 30 h), the expression value of each gene under shear stress was normalized by the corresponding static control.
Microarray data analysis.
The arrays were scanned using GenePix (Molecular Devices), and the digitized data were imported into Genespring (Agilent). Quality filtering and normalization were performed using Genespring. For each gene, the expression levels at all time points after step-up were log 2-normalized to the expression value of the preconditioned control sample. Significance analysis of microarrays (57; Stanford) was used to identify the differentially expressed genes at each time point relative to the preconditioned controls. GeneGo (GeneGo bioinformatics software) was used to perform gene ontology and pathway analysis. Microarray data are available at NCBI Gene Expression Omnibus (GSE26505).
Real-time quantitative-PCR.
Real-time quantitative-PCR was performed as described by Zhang et al. (62). GAPDH was used as the housekeeping gene. Primers used had previously been designed and tested for efficiency (27, 34, 35, 62). Supplemental Table S1 lists all the primers used in this study. The 2−ΔΔCT method was used to quantify expression levels of the target genes relative to the standard control sample.
RESULTS
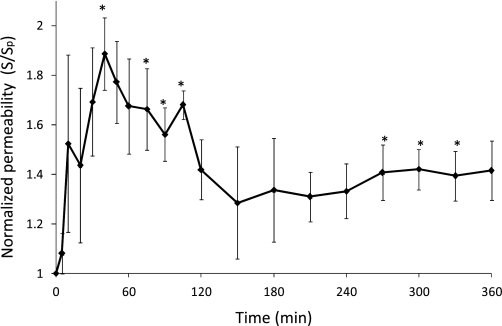
Alterations in endothelial permeability in response to elevated shear stress. Figure 1 plots the normalized endothelial permeability at each time point during the adaptive response to increased shear stress. The permeability values between 40–105 and 270–330 min after the step-up were significantly increased relative to the pre-step-up value (P < 0.05, two-tailed one sample t-test), demonstrating a dynamic response of endothelial permeability to changes in shear stress. Endothelial permeability increased within the first few minutes in response to the acute increase in shear stress magnitude. It peaked at 40 min, exhibiting a 1.9-fold increase compared with the preconditioned value. Cell layer permeability then decreased slowly from 40 to 150 min. After 150 min, it stabilized at an elevated value, ∼1.3 times the preconditioned value.
Fig. 1.
Normalized endothelial permeability during the adaptive response to a step increase in shear stress magnitude. Time 0: start of the shear step-up (after 24-h preconditioning under basal shear stress). Endothelial permeability values are normalized to the preconditioned (time 0) value (3.65 ± 0.82 × 10−6 cm/s). S, average rate of change in concentration; Sp, average rate of change in abluminal concentration. Error bars are SE; n = 4. *P < 0.05.
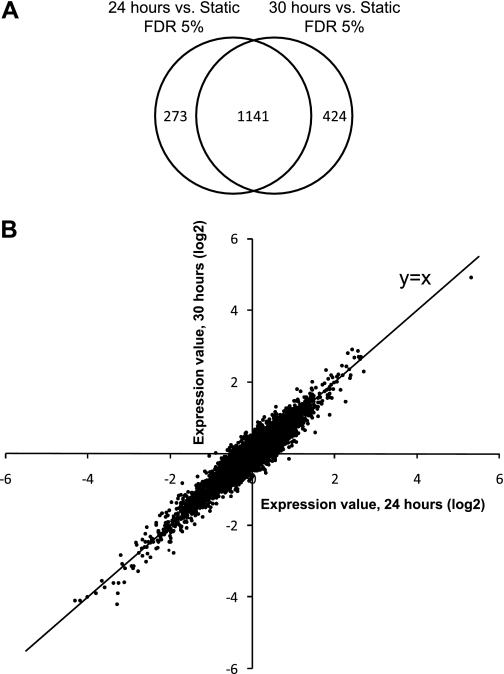
Endothelial transcriptional profile after long-term exposure to basal level shear stress. In the control set of experiments, endothelial cells were exposed to basal level shear stress for 24 and 30 h. When the false discovery rates (FDR) were controlled at 5% in significance analysis of microarrays, 17.1 and 18.7% of the genes on the array were significantly differentially expressed after shear exposure for 24 and 30 h compared with static controls, respectively. The lists of genes identified at 24 and 30 h overlapped considerably (Fig. 2A). This result demonstrated that most of the genes regulated by shear stress were consistently regulated after 24- and 30-h exposure. All 1,838 genes in Fig. 2A are regarded as shear sensitive genes in the following discussion.
Fig. 2.
Gene expression of endothelial cells exposed to 24 and 30 h of basal shear stress. A: Venn diagram displaying the overlap of differentially expressed genes identified at 24 and 30 h (relative to static controls). B: scatter plot of gene expression profiles. Expression value of each gene was normalized by the corresponding static control value. x: Expression value after 24 h of shear stress; y: expression value after 30 h of shear stress.
Only two genes, FLJ25476 and an unknown gene, were identified as differentially expressed between 24 and 30 h, even when the FDR was relaxed to 20%. Figure 2B shows the overall comparison between the expression profiles at 24 and 30 h. All genes are tightly distributed around the 45° line, indicating that these two expression profiles were highly similar. Therefore, we conclude that the gene expression of cultured endothelial cells is stable after exposure to the basal shear stress for 24 h and hence that 24 h is an appropriate preconditioning time for the subsequent microarray study.
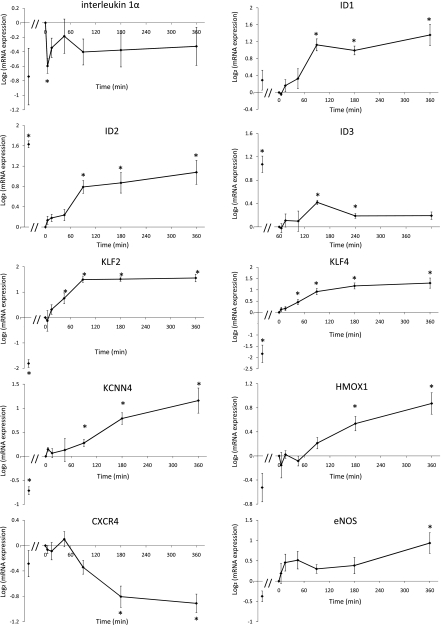
Differentially expressed genes in response to an acute elevation in shear stress. The number of genes that were differentially expressed at each time point after the step-up, relative to their expression in preconditioned cells, is given in Table 1. A limited number of genes were called at earlier time points, and more genes were identified after 90 min. At FDR<10%, 86 genes were sensitive to the step-up at some time point. The size of this gene list is small compared with the control study, where 1,838 genes were found to differ between static cultured and preconditioned endothelial cells. Only 40 of the 86 genes were also called in the control set of experiments. The list of genes identified at each time point is presented in Supplemental Table S2. Several known atherosclerosis-relevant genes are on the list. Among these genes, KLF2, KLF4, heme oxygenase-1 (HMOX1), and GADD45β were upregulated, and BMP4, IL-1α, and IL-8 were downregulated. The vasomotion related genes eNOS and Ca2+-dependent K+ channel 4 (KCNN4) were also upregulated. Several cytoskeletal, junctional, and cell adhesion genes, such as actin, MHY9, PCDH7, and COL4A1, were upregulated by the acute increase in shear stress, suggesting a cell remodeling process. The temporal expression profiles of these genes are shown in Fig. 3.
Table 1.
Numbers of identified genes at different time points after shear step-up
| Estimated FDR <5% |
Estimated FDR <10% |
|||
|---|---|---|---|---|
| Time | Upregulated | Downregulated | Upregulated | Downregulated |
| 5 min | 0 | 2 | 0 | 2 |
| 15 min | 3 | 0 | 3 | 0 |
| 45 min | 2 | 0 | 9 | 0 |
| 90 min | 13 | 0 | 19 | 0 |
| 180 min | 16 | 6 | 20 | 7 |
| 360 min | 16 | 5 | 48 | 6 |
FDR, false discovery rates.
Fig. 3.
Temporal expression profiles of selected genes following shear step-up. All expression values were normalized to the preconditioned value (time 0). In each panel, the first data point, immediately adjacent to the y-axis, represents the expression in static cultured endothelial cells obtained from the control set of experiments, also normalized to the preconditioned value. *P < 0.05, one-sample t-test against zero. ID1, ID2, ID3, inhibitors of DNA binding 1, 2, and 3; CXCR4, chemokine (C-X-C motif) receptor 4; KLF2 and KLF4, Kruppel-like factor 2 and 4; Ca2+-dependent K+ channel 4; HMOX1, heme oxygenase-1; eNOS, endothelial nitric oxide synthase.
In addition to the significantly differentially expressed genes, we also examined an a priori selected set of genes that are known to participate in atherogenesis and respond to shear stress. The expression values of these genes are presented in Supplemental Table S3. Overall, the elevated shear stress had a predominantly anti-inflammatory and antioxidative atheroprotective effect on the endothelial cells.
Our microarray study revealed a much more limited response to an increase in shear in preconditioned cells than in static cells (86 vs. 1,838 genes). More importantly, 46 of the 86 genes in preconditioned cells that were responsive to the elevation in shear were not identified in the control set of experiments, suggesting that different shear sensing mechanisms may be operative in preconditioned cells, which are more representative of the in vivo state. Furthermore, for almost half of the genes that were identified in both control and step-up experiments, the responses to the preconditioning and elevated shear are opposite in direction [e.g., inhibitors of DNA binding 2 and 3 (ID2 and ID3) in Fig. 3]. Thus our results suggest that static and preconditioned cells respond differently to an imposed increase in shear stress.
The expression values of IL-1α, eNOS, and KLF2 at each time point were validated using real-time quantitative PCR as shown in Supplemental Fig. S4.
Temporal response of global gene expression to an acute elevation in shear stress.
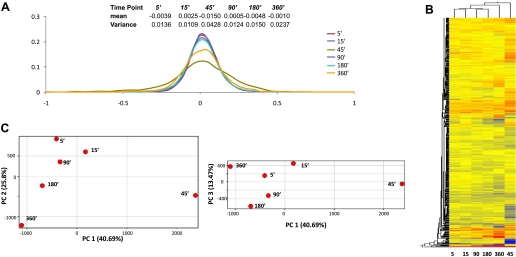
To understand how the endothelial transcriptome was dynamically controlled in response to the elevation of shear stress magnitude, the expression of all genes on the array was examined. A statistical summary and histogram of the transcriptional profile at each time point are presented in Fig. 4A, where the means and variance of the expression values (relative to the preconditioned cells) of all genes were calculated for each time point. The variance at 45 min is much larger than that at other time points, suggesting that the endothelial transcriptome was undergoing considerable change in response to the elevated shear stress. This inference was further confirmed by the scatter plots in Supplemental Fig. S5, which shows that the transcriptional profile at 45 min is substantially different from that at any other time point.
Fig. 4.
A: statistical summary and histograms of the transcriptional profiles at each time point. B: hierarchical clustering of the global expression profiles at all time points. C: principal component analysis. Principal component 1 (PC1) contains 40.69% of the total variance. PC2 and PC3 contain 25.80 and 13.47% of the total variance, respectively. Each time point is plotted in the projected planes of PC1 vs. PC2, and PC1 vs. PC3.
To further understand this result, all genes were clustered in Fig. 4B. The hierarchical tree suggests that the 5- and 15-min time points have similar transcriptional profiles, and so do the time points at 90 and 180 min. The 45-min time point is at the root of the hierarchical tree, indicating a distinct global transcriptional profile. Principal component analysis was applied to the six time points (Fig. 4C) and confirmed this finding. The expression profiles at the six time points are projected onto the plane of principal components (PC) 1 and 2 and the plane of PC1 and PC3. The first three principal components account for 80% of the total variability. As shown in Fig. 4C, the 45-min time point is far from all other time points in PC space. Furthermore, the path of the projected points over time shows that from 5 to 45 min, the PC1 values increase; however, after 45 min, the values of PC1 decrease over time. This suggests that the 45-min time point is not only different with respect to gene expression but may also be a critical turning point.
We next sought to understand how these expression histories affect endothelial cellular function by performing gene ontology and pathway analysis using GeneGo. The GeneGo results are presented in Supplemental Fig. S6. Pathway analysis demonstrated that the overall activity of the top 10 identified pathways was greatest at 45 min and that the activity of cytoskeletal remodeling pathways was greatest at 180 min. Although further research is warranted, our results suggest that the adaptive response of global gene expression to an acute increase in shear stress magnitude is triphasic, consisting of an induction period, an early adaptive response (ca. 45 min), and a late remodeling response.
DISCUSSION
In this study, we demonstrated that a step increase in shear stress immediately increases endothelial permeability to BSA; the permeability nearly doubled after 40-min exposure to the elevated shear. This observation supports our hypothesis that an acute increase in endothelial permeability can occur when endothelial cells adapt to a new hemodynamic environment, owing in part to cytoskeletal reorganization and junctional remodeling. Endothelial permeability declined after 40 min; however, it remained above the baseline (preconditioned) level until the experiment concluded 6 h after the shear stress step-up. Although it is not clear from this study whether endothelial permeability would have eventually decreased to the preconditioned level, it is reasonable to believe so since several in vitro studies have associated lower permeability with higher shear stress magnitude following a relatively long-term shear exposure (10, 33, 61). Thus the increased permeability observed in our study is more likely a transient alteration of endothelial phenotype resulting from the shear increase per se. A recently published study supports this notion. Warboys et al. (61) demonstrated that an acute exposure (1 h) to shear stress significantly increased porcine endothelial permeability to albumin in vitro (relative to static cultured cells), while a chronic exposure (7–9 days) decreased the permeability.
Friedman et al. (22) and Hazel et al. (25) investigated the adaptive response of endothelial permeability to shear stress alteration in vivo. An ad hoc mathematical model of adaptation was developed to fit the experimental data (22, 25). In applying the model, the effect of long-term shear levels in the physiological range on in vivo permeability was assumed to be minor. The mathematical model predicted a rapid increase in endothelial permeability following a step increase in shear stress and a permeability peak at 7 min. The permeability then approached the initial value asymptotically with a time constant of 45 min. Compared with our in vitro result, the calculated in vivo endothelial permeability peaks much earlier (7 min in vivo vs. 40 min in vitro) and decreases much faster. The differences are not too surprising since in vitro measurements of endothelial permeability often differ substantially from in vivo measurements (46). Perhaps equally important, the instantaneous endothelial permeability was measured directly in our study, while the in vivo values were obtained from a mathematical model where numerous assumptions were made. Nevertheless, our in vitro study and the previous in vivo study consistently confirm the transient increase in endothelial permeability in response to an acute increase in shear stress magnitude. It is important to point out that the detailed mechanisms of the induced increase and subsequent decrease in permeability remain unknown. Further studies are necessary to find out whether the increase is due to endothelial cytoskeletal reorganization and junctional remodeling, as hypothesized in our study or is regulated by other mechanisms, such as shear stimulated generation of reactive oxygen species.
Our microarray study provides the first detailed temporal map of endothelial transcriptional activity during the adaptive response to altered shear stress. We have identified 86 genes that are sensitive to an elevation in shear stress.
The quantity of IL-1α mRNA was sharply reduced within the first 5 min after exposure to increased shear stress; after which transcription recovered but never reached the preconditioned levels. IL-1α can induce the expression of endothelial-leukocyte adhesion molecules, such as E-selection and VCAM-1 (44). Evidently, the inhibition of endothelial-leukocyte adhesion through shear-dependent IL-1α suppression has both a transient and long-term component.
After 90 min, ID1, ID2, and ID3 were significantly upregulated. ID family genes regulate cell cycle and cell fate, and they play essential roles in angiogenesis (3, 24, 39). Specifically, it has been demonstrated that ID1 can increase the proliferation activity of endothelial cells (45, 52) and also preserve endothelial cell commitment in stem cell derived endothelial cells (31). Furthermore, Ling et. al. (38) suggested that ID1 is one of the upstream regulators of the master mediator of inflammatory response NF-κB. ID1 can activate the NF-κB signaling pathway to promote cell survival against apoptosis induced by TNFα. ID2 was also found to be able to promote endothelial cell growth and migration (3, 11). Most recently, ID3 has been shown to be an important atheroprotective factor, and loss of ID3 was linked to increased intima-media thickness (17). Thus ID1, ID2, and ID3 may be important mediators of any pathobiological effects that might accompany endothelial adaptation to altered shear stress.
KLF2 and KLF4 were up-regulated at 90 min and later time points. KLF2 and KLF4 are well-known shear stress sensitive genes (14, 15) and important regulators of endothelial activation in response to proinflammatory stimuli (53). Overexpression of KLF2 in endothelial cells induces eNOS expression and increases eNOS activity. KLF2 can inhibit the induction of VCAM-1 and E-selectin in response to proinflammatory cytokines (53). KLF2 also helps the cell to maintain its antithrombotic function by regulating key factors including thrombomodulin, tissue factor, and plasminogen activator inhibitor-1 (37).
Gene KCNN4, encoding the intermediate-conductance calcium-activated potassium channel, was upregulated at 180 and 360 min. KCNN4 is a critical mediator of endothelial hyperpolarization and endothelium-derived hyperpolarizing factor (EDHF)-mediated dilation (6, 19). Specifically, upregulation of KCNN4 can increase endothelial and smooth muscle cell hyperpolarization and EDHF signaling in response to dilation cues such as increased shear stress (1, 4, 59). One study (42) demonstrated that KCNN4-mediated EDHF signaling is a dominant contributor to flow-induced coronary arteriolar vasodilatation in patients with cardiovascular disease. Thus two important vascular dilation mediators, KCNN4 and eNOS, were both upregulated by the prolonged exposure to elevated shear stress. In response to increased blood flow in vivo, endothelial cells employ NO- and EDHF-mediated pathways to dilate the blood vessels, thus reducing the shear stress. However, in our in vitro setting, endothelial cells were unable to regulate the ambient shear stress, and they attempted to adapt to the altered hemodynamic environment by continuing to produce flow-induced dilation mediators.
HMOX1, an enzyme induced in response to oxidative stress, was also upregulated at 180 and 360 min. HMOX1 has been recently recognized as an important antioxidative, cytoprotective, and anti-inflammatory gene (43, 60). Free heme catalyzes the production of reactive oxygen species, leading to endothelial dysfunction. Thus HMOX1, which converts heme to carbon monoxide and biliverdin/bilirubin, is protective against the development of atherosclerosis and has been proposed as a novel therapeutic target (18, 49). Overexpression of HMOX1 in endothelial cells also inhibits the expression of the inflammatory adhesion molecules E-selectin and VCAM-1 by inhibiting the activation of NF-κB (54). Thus the upregulation of HMOX1 during endothelial adaptation can be a critical atheroprotective process that reduces the oxidative and inflammatory levels in these endothelial cells.
Chemokine (C-X-C motif) receptor 4, CXCR4, was downregulated at both 180 and 360 min. CXCR4, a receptor for stromal-cell derived factor-1, mediates angiogenic sprouting in response to hypoxia (55) in endothelial cells. A low expression level of CXCR4 inhibits endothelial apoptosis, and overexpression of CXCR4 induces the transcription of cytokines MCP-1 and IL-8. The levels of CXCR4 expression in endothelial cells from atherosclerotic lesions were greater than that in cells from healthy arteries (41).
Overall, our data suggest that the transient expression accompanying an acute elevation of shear stress is predominantly anti-inflammatory and atheroprotective; at the same time, the cell layer becomes more leaky to blood-borne macromolecules. With respect to atherogenesis, the extent to which the former transient effect offsets the latter is unknown. Therefore, it is not clear from this study how the elevated shear stress during daily exercise will affect overall arterial atherosusceptibility.
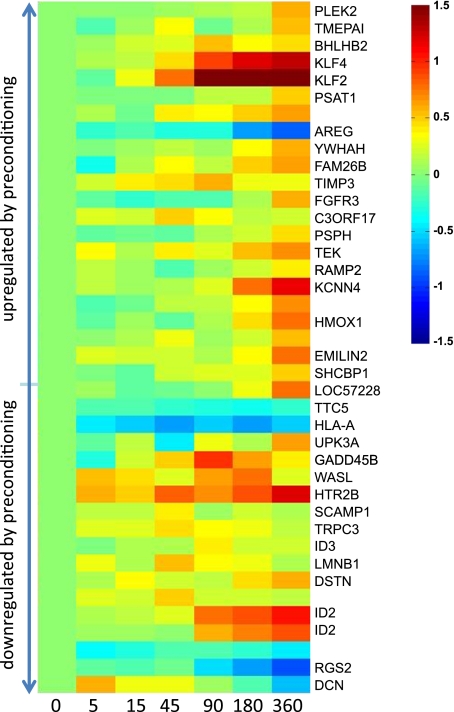
One of the most interesting findings in this study is that static cultured cells and preconditioned cells respond differently to the same level of increase in shear stress (0 to 15 dyn/cm2 for static cells and 15 to 30 dyn/cm2 for preconditioned cells). Approximately 2,000 genes were identified as differentially expressed by initially static cells in response to the preconditioning shear stress. However, only 86 genes were sensitive to the further increase in shear stress applied in the step-up experiment. Of the 86 identified genes, only 40 were sensitive to the application of the preconditioning shear stress to static cultured cells. The expression values of these 40 genes during the adaptive response are shown in Fig. 5. The genes are ranked by their expression values after 24 h exposure to the preconditioning shear stress, relative to their expression in static cultured cells. The top-ranked genes were upregulated greatly when the static cultured cells were exposed to the preconditioning shear stress, and the genes at the bottom were downregulated significantly. As shown in Fig. 5, the expression levels of some genes, such as KLF2 and KCNN4, were consistently upregulated (or downregulated) by the preconditioning shear stress and by the elevation in shear stress. However, for other genes, such as ID2 and AREG, the preconditioning shear stress and the shear stress increase had the opposite effects on expression. Thus one cannot predict from the transcriptional response of static cultured cells how a particular gene will be regulated during the adaptive response to a changed shear stress. The expression of the a priori selected atherogenesis-relevant genes (Supplemental Table S3) further confirmed this. Although most of these well-studied genes responded to the application of the preconditioning shear stress to static cells, only a few were responsive to the further increases in shear stress applied in our study. For some of these genes, the responses to the preconditioning and elevated shear stresses are opposite in direction. Since the preconditioned endothelial cells are more representative of in vivo cells than static cultured cells, the genes and their regulation patterns identified in our step-up studies certainly merit more study.
Fig. 5.
Heat map of the expression values of the genes that were sensitive to both the preconditioning and elevated shear stress. Each row is the time course of the expression of a single gene during the adaptive response. Expression values are normalized to the preconditioned value. Genes are ranked based on their responses to the preconditioning shear stress, i.e., the top-ranked genes were upregulated (relative to the static cultured cells) by the preconditioning shear, and the genes at the bottom were downregulated.
Certain genes were seen to respond to a step increase in shear stress only transiently. Such genes would not be detected in conventional shear stress experiments, in which gene expression is measured only after enough time has presumably elapsed (ca. 24 h) for the cells to exhibit a steady-state response. The transiently regulated genes identified in our study may be important in vivo regulators of vascular response to the local hemodynamic environment, since changes in this environment occur frequently, eliciting extended periods of adaptation during the lifetime of the cell.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-050442 and an American Heart Association Fellowship (09PRE2080238; to J. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z. and M.H.F. conception and design of research; J.Z. performed experiments; J.Z. analyzed data; J.Z. and M.H.F. interpreted results of experiments; J.Z. prepared figures; J.Z. drafted manuscript; M.H.F. edited and revised manuscript; M.H.F. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Charles S. Wallace for help and the Duke Microarray Facility for services.
Present address of J. Zhang: W. L. Gore and Associates, 301 Airport Rd., Elkton, MD (e-mail: zhangji@wlgore.com).
REFERENCES
- 1. Abraham SM, Clark AR. Dual-specificity phosphatase 1: a critical regulator of innate immune responses. Biochem Soc Trans 34: 1018–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NF kappa B, and egr-1. Arterioscler Thromb Vasc Biol 19: 996–1003, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Benezra R. Role of Id proteins in embryonic and tumor angiogenesis. Trends Cardiovasc Med 11: 237–241, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Brakemeier S, Kersten A, Eichler I, Grgic I, Zakrzewicz A, Hopp H, Kohler R, Hoyer J. Shear stress-induced up-regulation of the intermediate-conductance Ca(2+)-activated K(+) channel in human endothelium. Cardiovasc Res 60: 488–496, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Buchanan JR, Jr, Kleinstreuer C, Truskey GA, Lei M. Relation between non-uniform hemodynamics and sites of altered permeability and lesion growth at the rabbit aorto-celiac junction. Atherosclerosis 143: 27–40, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Cancel LM, Fitting A, Tarbell JM. In vitro study of LDL transport under pressurized (convective) conditions. Am J Physiol Heart Circ Physiol 293: H126–H132, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng 36: 554–562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chien S. Molecular basis of rheological modulation of endothelial functions: importance of stress direction. Biorheology 43: 95–116, 2006 [PubMed] [Google Scholar]
- 10. Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol 292: H3190–H3197, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Coma S, Amin DN, Shimizu A, Lasorella A, Iavarone A, Klagsbrun M. Id2 promotes tumor cell migration and invasion through transcriptional repression of semaphorin 3F. Cancer Res 70: 3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85: 9–23, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167: 609–618, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeMaio L, Tarbell JM, Scaduto RC, Jr, Gardner TW, Antonetti DA. A transmural pressure gradient induces mechanical and biological adaptive responses in endothelial cells. Am J Physiol Heart Circ Physiol 286: H731–H741, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Doran AC, Lehtinen AB, Meller N, Lipinski MJ, Slayton RP, Oldham SN, Skaflen MD, Yeboah J, Rich SS, Bowden DW, McNamara CA. Id3 is a novel atheroprotective factor containing a functionally significant single-nucleotide polymorphism associated with intima-media thickness in humans. Circ Res 106: 1303–1311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dulak J, Loboda A, Jozkowicz A. Effect of heme oxygenase-1 on vascular function and disease. Curr Opin Lipidol 19: 505–512, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, Hoyer J, Kohler R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol 138: 594–601, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 291: H985–H1002, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Friedman MH, Fry DL. Arterial permeability dynamics and vascular disease. Atherosclerosis 104: 189–194, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Friedman MH, Henderson JM, Aukerman JA, Clingan PA. Effect of periodic alterations in shear on vascular macromolecular uptake. Biorheology 37: 265–277, 2000 [PubMed] [Google Scholar]
- 23. Hahn C, Schwartz MA. The role of cellular adaptation to mechanical forces in atherosclerosis. Arterioscler Thromb Vasc Biol 28: 2101–2107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamik A, Wang B, Jain MK. Transcriptional regulators of angiogenesis. Arterioscler Thromb Vasc Biol 26: 1936–1947, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Hazel AL, Grzybowski DM, Friedman MH. Modeling the adaptive permeability response of porcine iliac arteries to acute changes in mural shear. Ann Biomed Eng 31: 412–419, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol 18: 527–533, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol 293: H645–H653, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol 286: H1916–H1922, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho CM. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J 17: 1648–1657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93: 1225–1232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, Studer L, Lee G, Lyden D, Benezra R, Zaninovic N, Rosenwaks Z, Rabbany SY, Rafii S. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol 28: 161–166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jo H, Dull RO, Hollis TM, Tarbell JM. Endothelial albumin permeability is shear dependent, time dependent, and reversible. Am J Physiol Heart Circ Physiol 260: H1992–H1996, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Kudo S, Tsuzaka M, Ikeda M, Tanishita K. Albumin permeability across endothelial monolayers under long-term shear stress. JSME Int J Series C 48: 419–424, 2005 [Google Scholar]
- 34. LaMack JA, Friedman MH. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am J Physiol Heart Circ Physiol 293: H2853–H2859, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Lamack JA, Himburg HA, Friedman MH. Distinct profiles of endothelial gene expression in hyperpermeable regions of the porcine aortic arch and thoracic aorta. Atherosclerosis 195: e35–e41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LaMack JA, Himburg HA, Li XM, Friedman MH. Interaction of wall shear stress magnitude and gradient in the prediction of arterial macromolecular permeability. Ann Biomed Eng 33: 457–464, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr, Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 96: e48–57, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Ling MT, Wang X, Ouyang XS, Xu K, Tsao SW, Wong YC. Id-1 expression promotes cell survival through activation of NF-kappaB signalling pathway in prostate cancer cells. Oncogene 22: 4498–4508, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401: 670–677, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol Cell Physiol 263: C389–C396, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Melchionna R, Porcelli D, Mangoni A, Carlini D, Liuzzo G, Spinetti G, Antonini A, Capogrossi MC, Napolitano M. Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J 19: 629–631, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Miura H, Wachtel RE, Liu Y, Loberiza FR, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circulation 103: 1992–1998, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Morse D, Choi AMK. Heme oxygenase-1: from bench to bedside. Am J Respir Crit Care Med 172: 660–670, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Murase T, Kume N, Hase T, Shibuya Y, Nishizawa Y, Tokimitsu I, Kita T. Gallates inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of leukocyte adhesion molecules in vascular endothelial cells. Arteriosclerosis Thromb Vasc Biol 19: 1412–1420, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Nishiyama K, Takaji K, Uchijima Y, Kurihara Y, Asano T, Yoshimura M, Ogawa H, Kurihara H. Protein kinase A-regulated nucleocytoplasmic shuttling of Id1 during angiogenesis. J Biol Chem 282: 17200–17209, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng 30: 430–446, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol 176: 719–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, Evans PC. Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J 21: 3553–3561, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Peterson SJ, Frishman WH, Abraham NG. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev 17: 99–111, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Phelps JE, DePaola N. Spatial variations in endothelial barrier function in disturbed flows in vitro. Am J Physiol Heart Circ Physiol 278: H469–H476, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Sakurai D, Tsuchiya N, Yamaguchi A, Okaji Y, Tsuno NH, Kobata T, Takahashi K, Tokunaga K. Crucial role of inhibitor of DNA binding/differentiation in the vascular endothelial growth factor-induced activation and angiogenic processes of human endothelial cells. J Immunol 173: 5801–5809, 2004 [DOI] [PubMed] [Google Scholar]
- 53. SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 199: 1305–1315, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol 172: 3553–3563, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115: 5102–5110, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA 93: 10417–10422, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 98: 176–185, 2006 [DOI] [PubMed] [Google Scholar]
- 59. van Bavel E. Shear stress and intermediate-conductance calcium-activated potassium channels. Cardiovasc Res 60: 457–459, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Wagener FADTG, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev 55: 551–571, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Warboys CM, Eric Berson R, Mann GE, Pearson JD, Weinberg PD. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am J Physiol Heart Circ Physiol 298: H1850–H1856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang J, Burridge KA, Friedman MH. In vivo differences between endothelial transcriptional profiles of coronary and iliac arteries revealed by microarray analysis. Am J Physiol Heart Circ Physiol 295: H1556–H1561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686–692, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.