Abstract
Genotyping methods are essential to understand the transmission dynamics of Acinetobacter baumannii. We examined the representative genotypes of A. baumannii at different time periods in select locations in Ohio, using two rapid automated typing methods: PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS), a form of multi-locus sequence typing (MLST), and repetitive-sequence-based-PCR (rep-PCR). Our analysis included 122 isolates from 4 referral hospital systems, in 2 urban areas of Ohio. These isolates were associated with outbreaks at 3 different time periods (1996, 2000 and 2005–2007). Type assignments of PCR/ESI-MS and rep-PCR were compared to each other and to worldwide (WW) clone types. The discriminatory power of each method was determined using the Simpson's index of diversity (DI). We observed that PCR/ESI-MS sequence type (ST) 14, corresponding to WW clone 3, predominated in 1996, whereas ST 12 and 14 co-existed in the intermediate period (2000) and ST 10 and 12, belonging to WW clone 2, predominated more recently in 2007. The shift from WW clone 3 to WW clone 2 was accompanied by an increase in carbapenem resistance. The DI was approximately 0.74 for PCR/ESI-MS, 0.88 for rep-PCR and 0.90 for the combination of both typing methods. We conclude that combining rapid automated typing methods such as PCR/ESI-MS and rep-PCR serves to optimally characterize the regional molecular epidemiology of A. baumannii. Our data also sheds light on the changing sequence types in an 11 year period in Northeast Ohio.
Introduction
Acinetobacter baumannii has emerged worldwide as a cause of infection among seriously ill patients [1]. A. baumannii is a frequent cause of outbreaks in hospitals and long term care facilities, where this pathogen is associated with prolonged hospitalizations and possibly increased mortality [2], [3]. Also, military medical facilities treating personnel serving in Iraq and Afghanistan have experienced A. baumannii outbreaks [4], [5], [6]. The remarkable ability of A. baumannii to display resistance to multiple classes of antibiotics, including carbapenems, poses a serious therapeutic challenge and likely contributes to its global success as a healthcare-associated pathogen [1], [7], [8].
Genetic typing is an essential tool in understanding the transmission dynamics and temporal evolution of A. baumannii. For instance, typing of DNA digests using pulsed field gel electrophoresis (PFGE) enhances the epidemiological investigation of outbreaks by demonstrating highly related or indistinguishable isolates, suggesting transmission from a common source or from patient-to-patient [9], [10]. Comparing PFGE typing of A. baumannii from different institutions requires the careful standardization of protocols [11]. In this regard, the combination of PFGE, ribotyping, and amplified fragment length polymorphism (AFLP) permitted the identification of important international clones of A. baumannii from different hospitals in Europe [12], [13], [14]. These strains, European clones 1–3, are also found globally and thus are now termed worldwide (WW) clones [12]. Multi-locus sequence typing (MLST), a method suitable for pathogens with wide genomic, temporal and spatial variations, has permitted further characterization of the population structure, genetic diversity and distinctness of the WW clones of A. baumannii [15]. The cost and time-consuming nature of MLST, although becoming more accessible, limit its current applicability except in specialized circumstances [16].
Rapid and automated typing methods are increasingly applied to A. baumannii. PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) analyzes the base-composition of amplicons from six housekeeping genes, generating a unique signature that corresponds to a sequence type (ST) in a database [17], [18]. These genes, however, differ from the seven housekeeping genes amplified and sequenced to perform MLST. Consequently, the STs generated by PCR/ESI-MS do not correspond to those of MLST schemes. Automated PCR/ESI-MS has been used to investigate A. baumannii from civilian and military treatment facilities revealing sequence types corresponding to the WW clones [3], [6], [17],[19]. Automated repetitive-sequence-based-PCR (rep-PCR) has also been employed to type A. baumannii. Using rep-PCR, a unique profile of bands is generated by the resolution of amplified DNA fragments in a gel matrix. Automated rep-PCR has been applied to the analysis of local outbreaks and to illustrate the global spread of carbapenem-resistant lineages of A. baumannii [3], [12].
Insights into the molecular epidemiology of A. baumannii gained from rapid automated methods aid in surveillance, demonstrate the temporal pattern of strain replacement, and may lead to successful interventions to control this important pathogen. In this study, we investigate temporal changes in the molecular epidemiology of A. baumannii in two urban centers in Ohio, and explore the discriminatory ability of two rapid automated methods, PCR/ESI-MS and rep-PCR.
Methods
Ethics Statement
Expedited approval was obtained from the Institutional Review Board at Louis Stokes Cleveland VA Medical Center, IRB # 09084-H04. The bacterial isolates analyzed in this study belong to the microbiological collections of each hospital and were obtained as part of routine clinical care in the past. Furthermore, all patient identifiers had been previously removed and data were analyzed anonymously. Therefore, the Institutional Review Board waived the need to obtain written or verbal consent.
Bacterial Isolates
A total of 122 single-patient isolates of A. baumannii were studied. The isolates were identified and antimicrobial susceptibility testing was performed with VITEK® (bioMérieux, Durham, NC) or Microscan (Siemens Healthcare, Deerfield, IL), at their respective hospitals of origin. These were four tertiary-care hospital systems from two cities in Ohio, which experienced outbreaks of A. baumannii at three different time periods: 1996, 2000 and 2005–2007 (Figure 1). Hospital systems A, B, and D are located in the Cleveland, Ohio regional area and hospital system C is located approximately 140 miles south in Columbus, Ohio. The isolates were obtained from microbiological cultures of various types of specimens (e.g., blood, sputum, urine, wounds) and belonged to different patient populations (i.e., ICU, non-ICU, adult, pediatric, medical, surgical, trauma and burn patients).
Figure 1. Origin, sequence type and antibiotic susceptibility of Acinetobacter baumannii from Ohio.
Distribution of 122 A. baumannii isolates by hospital of origin (hospitals A, B, C and D), year, sequence type (ST) determined by PCR/ESI-MS, worldwide clone type (WW) and susceptibility to carbapenems and ampicillin/sulbactam.
PCR/ESI-MS
Overnight cultures were diluted 1∶50 in Tris-EDTA buffer and were boiled at 99°C for 15 minutes prior to use. PCR/ESI-MS was performed using the Acinetobacter genotyping kit on the T5000™ Biosensor (Ibis Biosciences, Inc., Abbott Molecular, Inc., Carlsbad, CA). The base composition analysis was completed using the provided software. The software compared our amplicons to previously obtained sequences stored in a database in order to assign the sequence type (ST) of each strain, as previously described and validated [17]. PCR/ESI-MS STs were correlated with WW clones 1–3, according to previously published studies [6], [17], [19].
Rep-PCR
Genomic DNA was extracted from bacterial isolates using the UltraClean™ Microbial DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA). PCR amplification was performed using the DiversiLab® (bioMérieux, Athens, GA) Acinetobacter fingerprinting kit, according to the manufacturer's instructions. Rep-PCR products were separated by electrophoresis on microfluidic chips and analyzed with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The resulting band patterns were compared in order to generate a dendrogram using two different statistical methods: Pearson correlation (PC) and the modified Kullback–Leibler (KL). Both methods calculate similarity using relative band intensity, however, PC is more “band intensity” based and KL is more “band presence” driven. As validated in previous studies, isolates having band patterns with ≥95% similarity were considered genetically related strains (genotypic clusters) [3], [12]. In a further analysis, isolates were grouped on the basis of ≥98% similarity, in order to detect dissemination of identical or near-identical strains.
Discriminatory ability of PCR/ESI-MS and rep-PCR
Strain types obtained by PCR/ESI-MS and rep-PCR patterns were compared to establish concordance between the two typing methods. The discriminatory abilities of PCR/ESI-MS and rep-PCR were compared by contrasting the number of unique types determined by each method, and by determining the Simpson's index of diversity (DI). This is an index of discrimination for bacterial typing methods and was calculated using the following formula [20], [21]:
In the above equation, N is the total number of strains in the sample population, S is the total number of types described, and ni is the number of strains belonging to the i th type. DI ranges from 0 to 1; a value closer to 1 represents larger diversity. 95% confidence intervals were estimated for DI, according to previously described equations [22]. DI and 95% confidence intervals were calculated for PCR/ESI-MS, rep-PCR (analyzed with both the KL and PC methods) and for the combination of PCR/ESI-MS and rep-PCR.
Results
The 122 A. baumannii isolates included in this study were represented by 17 different PCR/ESI-MS STs and by 29 and 27 different rep-PCR types, when analyzed with the KL and PC methods, respectively. Overall, ST 10 (n = 47), ST 14 (n = 38) and ST 12 (n = 14), were the predominant ST types established by PCR/ESI-MS (Table 1 and Figure 1).
Table 1. Predominant PCR/ESI-MS sequence types and rep-PCR types analyzed by Kullback-Leibler (KL) and Pearson correlation (PC) methods.
| PCR/ESI-MS sequence type (ST) | No. | rep-PCR Kullback-Leibler (KL) type | No. | rep-PCR Pearson correlation (PC) type | No. |
| ST 10* | 47 | KL 1 | 16 | PC 1 | 10 |
| ST 12* | 14 | KL 2 | 23 | PC 2 | 20 |
| ST 1* | 3 | KL 3 | 10 | PC 3 | 5 |
| ST 86* | 1 | KL 4 | 7 | PC 4 | 21 |
| KL 5 | 5 | PC 5 | 5 | ||
| Unique | 4 | PC 6 | 2 | ||
| Unique | 2 | ||||
| ST 14 | 38 | KL 5 | 28 | PC 7 | 28 |
| KL 6 | 7 | PC 8 | 7 | ||
| Unique | 3 | Unique | 3 | ||
| ST 54 | 4 | KL 7 | 2 | PC 9 | 3 |
| Unique | 2 | Unique | 1 |
rep-PCR did not differentiate among ST 10, ST 12, ST 1 and ST 86.
Change of predominant sequence types over time
Our results indicate that different sequence types or separate “waves” of A. baumannii spread through different parts of Ohio in past decades. Figures 1 and 2 illustrate the sequence and rep-PCR types of A. baumannii from the four hospital systems in the three different time periods analyzed. Among the 21 isolates from the initial period of 1996, ST 14 was the predominant type (90% of isolates). ST 14 corresponds to WW clone 3. Isolates from 2000 were characterized by the continued predominance of ST 14 (69% of isolates). In the 2000 period however, 31% of isolates belonged to ST 12, corresponding to WW clone 2. Among isolates from the more recent period of 2007, ST 14 was not identified, while ST 12 represented only 8% of isolates. The predominant type in 2005–2007 was ST 10 (63%), which also belongs to WW clone 2 (Figures 1 and 2 and Table 1). Susceptibility to carbapenems and ampicillin/sulbactam was maintained in isolates from 1996 and 2000. More recent isolates, from 2005–2007, displayed increasing resistance to these agents: non-susceptibility to carbapenems was 76.2% among A. baumannii isolates from 2007 (Figure 1).
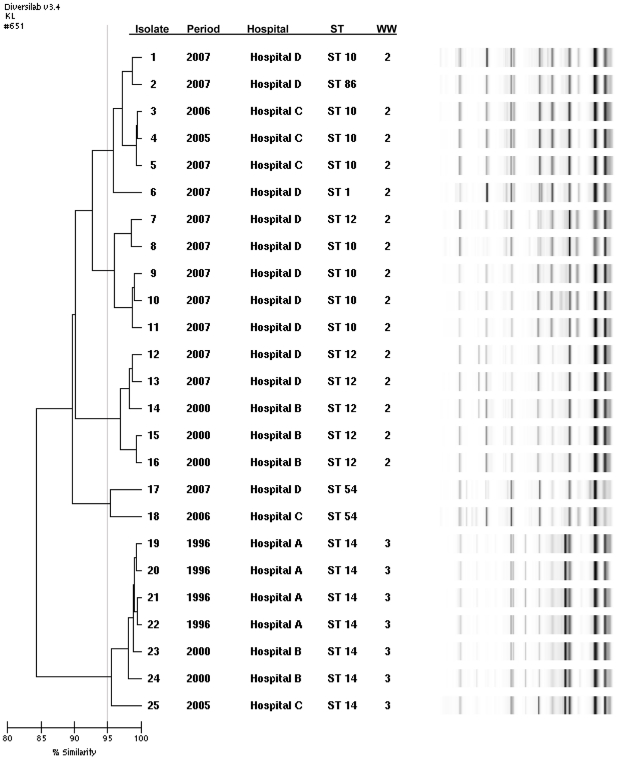
Figure 2. Genetic similarity among A. baumannii isolates.
Representative A. baumannii isolates typed by rep-PCR, analyzed with the Kullback-Leibler method. Five strain types with >95% similarity are illustrated, and further discriminated by year, hospital of origin, PCR-ESI/MS sequence type (ST), and worldwide (WW) clone types.
There were many instances of almost identical rep-PCR types (defined as ≥98% similar) present in different locations (data not shown): hospital A shared isolates that were 99.2% similar (belonging to ST 14) with hospital B, and a ST 1 isolate from hospital A was 98% similar to ST 10 isolates from hospital D. Strains belonging to ST 10 and ST 68 with ≥98% similarity were found in hospitals C and D, while several ST 10/ST 12 isolates from hospitals B and D were 98.1% similar.
Comparison and discriminatory power of PCR/ESI-MS and rep-PCR
Isolates with the same STs belonged to multiple rep-PCR types (Table 1). For example, the 38 isolates identified as ST 14 were classified into five different rep-PCR types: two predominant types, accounting for 74% and 18% of isolates respectively, and 3 unique types.
The Simpson's index of diversity (DI) was determined to compare the discriminatory ability of PCR/ESI-MS and rep-PCR, analyzed by two different methods (KL and PC). Additionally, DI was calculated for the combined analysis of strain types with both PCR/ESI-MS and rep-PCR. Table 2 shows different DI values for PCR/ESI-MS and rep-PCR, with confidence intervals that do not overlap. The value of DI was larger for the combination of PCR/ESI-MS and rep-PCR, indicating a superior discriminatory power. There is, however, overlap of the confidence interval with that of rep-PCR alone indicating that differences in DI may not be significant. Note that both methods of rep-PCR analysis (KL and PC) have a very similar DI and confidence intervals, indicating essentially identical discriminatory abilities.
Table 2. Simpson's Diversity index (DI) of PCR/ESI-MS, rep-PCR and the combination of PCR/ESI-MS and rep-PCR.
| Typing method | Number of different types | Percentage of isolates with the most frequent type | Simpson's DI | 95% Confidence interval |
| PCR/ESI-MS | 17 | 39 | 0.744 | 0.692–0.796 |
| rep-PCR(Kullback-Leibler) | 29 | 23 | 0.884 | 0.854–0.914 |
| rep-PCR(Pearson correlation) | 27 | 23 | 0.882 | 0.852–0.912 |
| PCR/ESI-MS+rep-PCR (Kullback-Leibler) | 34 | 23 | 0.899 | 0.869–0.929 |
| PCR/ESI-MS+rep-PCR(Pearson correlation) | 33 | 23 | 0.903 | 0.872–0.934 |
Discussion
This decade-long study provided a unique view of the molecular epidemiology of A. baumannii in two urban areas of a Midwestern state in the USA. Of primary importance, we observed that there was a change in the predominant sequence types of A. baumannii associated with outbreaks from the four hospital systems, shifting from carbapenem-susceptible strains corresponding to WW clone 3 in 1996, to carbapenem-resistant strains corresponding to WW clone 2 in 2007. Secondly, we noted discordances in the type assignments and differences in the discriminatory abilities of two rapid automated typing methods. The higher discrimination of rep-PCR makes it useful to chart hospital outbreaks, whereas the PCR/ESI-MS delineates the underlying population structure. Combining both methods may provide a more accurate differentiation of isolates when performing molecular epidemiology studies comprising isolates of different years obtained from several hospitals.
The isolates of A. baumannii analyzed in this study were restricted to outbreak strains rather than those collected prospectively and continuously. Nevertheless, we found, considerable diversity, 17 to 29 strain types, depending on the typing method used. Although our study was limited to isolates from four hospital systems and three different time periods, we believe that our data provide early clues as to the regional transmission dynamics of A. baumannii in Ohio and perhaps even on a global level. The initial description of European clones 1–3 dates from the mid-1990s and was inferred from ribotyping, AFLP and PFGE typing of A. baumannii strains associated with outbreaks throughout that continent [15], [23], [24], [25], [26]. Presently, these clone types of A. baumannii extend beyond Europe. For example, clonal clusters corresponding to what is now termed worldwide (WW) clone 2 are widely disseminated in multiple Chinese cities, and WW clone 2 has been the predominant clonal type in a medical center in Australia for more than a decade [27], [28]. A contemporary international survey of A. baumannii non-susceptible to carbapenems typed with rep-PCR, PFGE and multiplex PCR, which led to change the designation of European clones to WW clones, confirmed the universal presence of clones 1–3, and identified clone 2 as the largest and most widespread type [12]. Our analysis identified an international strain of A. baumannii, WW clone 3, in the Midwestern USA as early as 1996. This surprising result resonates with ongoing questions as to the meaning and origin of these successful global strains [29].
Previously, identification of WW clones 1–3 in the USA occurred among A. baumannii obtained from patients at the Walter Reed Army Medical Center in 2003–2005, and was subsequently confirmed in isolates from 2006–2007 [6], [19]. In these studies, genetic typing with PCR/ESI-MS determined that WW clones 1–3 were represented. Similarly, contemporary A. baumannii from the National Naval Medical Center also included AFLP types belonging to WW clones 1–3 [30]. This is in contrast with isolates obtained in 2005–2007 and in 2008–2009 from civilian hospitals across the United States (California, Arizona, Kentucky, Illlinois, Pennsylvania, New York, Florida, Missouri and Nevada), where WW clone 2 predominated and WW clones 1 and 3 were rarely found [19], [31]. Surveys of isolates recovered in 2001–2004 from 17 European countries revealed the co-dominance of WW clones 1 and 2, to the exclusion of WW clone 3 [32]. The emergence in Italy and Greece of WW clone 2 among isolates obtained between 2005 and 2009 also supports this shift. [33], [34]. Our data confirms the replacement of WW clone 3 and the predominance of strains related to WW clone 2 among A. baumannii causing outbreaks in civilian hospitals in Ohio.
The determination of WW clones of A. baumannii has proven to be consistent, even when diverse typing methods, such as rep-PCR and PCR/ESI-MS, are used [12], [17]. For instance, isolates classified by PCR/ESI-MS as ST 1, ST 10, and ST 86 (all belonging to WW clone 2) shared the same rep-PCR type. Of note, the base composition of ST 1, ST 12, and ST 86 are single locus variants of ST 10 in the PCR/ESI-MS scheme: they only differ in one of the 6 housekeeping genes examined. Nevertheless, disagreement in type assignment, for instance between PFGE and PCR/ESI-MS or rep-PCR, can also occur [17]. In this study, there was a trend towards a higher discriminatory ability using both PCR/ESI-MS and rep-PCR, suggesting a significant benefit in combining these two typing methods for A. baumannii. The value of complementary typing methods has been previously recognized for Aspergillus and Staphylococcus [14], [35], [36], [37]. Our data also demonstrate that the two different methods of analysis for rep-PCR (KL and PC) result in very similar discriminatory abilities (Table 2), but separate isolates into slightly different groupings (Table 1). The presence of almost identical rep-PCR types in different hospitals, even after increasing the threshold of similarity from ≥95% to ≥98%, suggests potential clonal dissemination of A. baumannii. This data must be interpreted carefully, however, since we cannot provide other epidemiological evidence of patient-to-patient spread occurring between hospitals.
The factors behind the widespread distribution of WW clones of A. baumannii are not fully elucidated. Resistance to carbapenems may in part explain the success of WW clone 2 in Ohio (and globally), but we must keep in mind that the understanding of antibiotic resistance and clonality in A. baumannii is evolving [38]. Commonly, up regulation of the intrinsic β-lactamase gene bla OXA-51-like is considered the most prevalent mechanism of carbapenem resistance among A. baumannii, whereas the bla OXA-23-like gene is the predominant acquired mechanism of carbapenem resistance, followed by the presence of bla OXA-58-like and bla OXA-24/40-like genes [12]. Nevertheless, isolates belonging to WW clone 2 are frequently susceptible to carbapenems [25], [27], [28]. This is underscored by our analysis, where WW clone 2 isolates from 2000 were susceptible to carbapenems. Furthermore, in the years 2005–2007, there was coexistence of WW clone 2 isolates resistant and susceptible to carbapenems (Figure 1). Two previously published, larger analyses of the carbapenem-resistant isolates included in this study revealed bla OXA-23 and bla OXA-24/40 in approximately 45% and 10% of strains from hospital D, respectively [3]. In hospital C only 13% of isolates harbored bla OXA-23, but 80% of isolates had ISAbaI linked to bla OXA-66, a bla OXA-51-like gene [39].
In conclusion, an approach combining two different typing methods increases our understanding of the molecular epidemiology of A. baumannii in Ohio, revealing the regional predominance, temporal evolution, and progression of certain strain types. Furthermore, our observations also help illustrate the potential utility of combining rapid automated typing methods, such as PCR/ESI-MS and rep-PCR, in the study of A. baumannii. More importantly, we also show that WW clone 3 was present in Ohio at almost the same time as it was described in Europe. Our data also demonstrates the replacement of WW clone 3 with WW clone 2 as the predominant clone type, a pattern that is similar to national and international trends. Despite limitations that challenge our understanding of the transmission dynamics of A. baumannii, rapid and automated molecular typing methods such as PCR/ESI-MS and rep-PCR have demonstrated significant utility in evaluating and documenting the dissemination of A. baumannii in various geographic locations and time periods.
Footnotes
Competing Interests: One of the authors, Federico Perez, received grant funding from Steris Corporation. Scott T. Zoll, Christian Massire, Mark W. Eshoo, and David. J. Ecker are or were employees of Ibis Biosciences Inc, Abbott Molecular Inc., during the completion of this study. This company develops and sells the Ibis T5000 Biosensor, which was used for this study. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: Funding for this study was provided by the Steris Corporation to FP. RAB is supported by the Veterans Affairs Merit Review Program, the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grants RO1-AI072219-05) and the Geriatric Research, Education and Clinical Care Center VISN 10. PNR is supported by a Research Career Scientist Award from the Department of Veterans Affairs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 2.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 3.Perez F, Endimiani A, Ray AJ, Decker BK, Wallace CJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother. 2010;65:1807–1818. doi: 10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb Mortal Wkly Rep. 2004;53:1063–1066. [PubMed] [Google Scholar]
- 5.Scott P, Deye G, Srinivasan A, Murray C, Moran K, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 6.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol. 2003;24:284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 10.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundmann HJ, Towner KJ, Dijkshoorn L, Gerner-Smidt P, Maher M, et al. Multicenter study using standardized protocols and reagents for evaluation of reproducibility of PCR-based fingerprinting of Acinetobacter spp. J Clin Microbiol. 1997;35:3071–3077. doi: 10.1128/jcm.35.12.3071-3077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 13.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 14.Dijkshoorn L, Aucken H, Gerner-Smidt P, Janssen P, Kaufmann ME, et al. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol. 1996;34:1519–1525. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 17.Ecker JA, Massire C, Hall TA, Ranken R, Pennella TT, et al. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol. 2006;44:2921–2932. doi: 10.1128/JCM.00619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hujer KM, Hujer AM, Endimiani A, Thomson JM, Adams MD, et al. Rapid determination of quinolone resistance in Acinetobacter spp. J Clin Microbiol. 2009;47:1436–1442. doi: 10.1128/JCM.02380-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wortmann G, Weintrob A, Barber M, Scott P, Zoll ST, et al. Genotypic evolution of Acinetobacter baumannii strains in an outbreak associated with war trauma. Infect Control Hosp Epidemiol. 2008;29:553–555. doi: 10.1086/588221. [DOI] [PubMed] [Google Scholar]
- 20.Hunter PR. Reproducibility and indices of discriminatory power of microbial typing methods. J Clin Microbiol. 1990;28:1903–1905. doi: 10.1128/jcm.28.9.1903-1905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanc DS, Hauser PM, Francioli P, Bille J. Molecular typing methods and their discriminatory power. Clin Microbiol Infect. 1998;4:61–63. doi: 10.1111/j.1469-0691.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 22.Grundmann H, Hori S, Tanner G. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J Clin Microbiol. 2001;39:4190–4192. doi: 10.1128/JCM.39.11.4190-4192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huys G, Cnockaert M, Nemec A, Dijkshoorn L, Brisse S, et al. Repetitive-DNA-element PCR fingerprinting and antibiotic resistance of pan-European multi-resistant Acinetobacter baumannii clone III strains. J Med Microbiol. 2005;54:851–856. doi: 10.1099/jmm.0.45986-0. [DOI] [PubMed] [Google Scholar]
- 24.Nemec A, Dijkshoorn L, van der Reijden TJ. Long-term predominance of two pan-European clones among multi-resistant Acinetobacter baumannii strains in the Czech Republic. J Med Microbiol. 2004;53:147–153. doi: 10.1099/jmm.0.05445-0. [DOI] [PubMed] [Google Scholar]
- 25.Nemec A, Krizova L, Maixnerova M, Diancourt L, van der Reijden TJ, et al. Emergence of carbapenem resistance in Acinetobacter baumannii in the Czech Republic is associated with the spread of multidrug-resistant strains of European clone II. J Antimicrob Chemother. 2008;62:484–489. doi: 10.1093/jac/dkn205. [DOI] [PubMed] [Google Scholar]
- 26.Di Popolo A, Giannouli M, Triassi M, Brisse S, Zarrilli R. Clin Microbiol Infect; 2010. Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Zhou J, Zhou H, Yang Q, Wei Z, et al. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother. 2010;65:644–650. doi: 10.1093/jac/dkq027. [DOI] [PubMed] [Google Scholar]
- 28.Runnegar N, Sidjabat H, Goh HM, Nimmo GR, Schembri MA, et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii in a single institution over a 10-year period. J Clin Microbiol. 2010;48:4051–4056. doi: 10.1128/JCM.01208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS microbiology reviews. 2011;35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 30.Petersen K, Cannegieter SC, van der Reijden TJ, van Strijen B, You DM, et al. Diversity and clinical impact of Acinetobacter baumannii colonization and infection at a military medical center. J Clin Microbiol. 2010;49:159–66. doi: 10.1128/JCM.00766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams-Haduch JM, Onuoha EO, Bogdanovich T, Tian GB, Marschall J, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011;49:3849–3854. doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towner KJ, Levi K, Vlassiadi M. Genetic diversity of carbapenem-resistant isolates of Acinetobacter baumannii in Europe. Clin Microbiol. 2008;Infect14:161–167. doi: 10.1111/j.1469-0691.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- 33.Gogou V, Pournaras S, Giannouli M, Voulgari E, Piperaki ET, et al. Evolution of multidrug-resistant Acinetobacter baumannii clonal lineages: a 10 year study in Greece (2000–09). J Antimicrob Chemother. 2011;66:2767–2772. doi: 10.1093/jac/dkr390. [DOI] [PubMed] [Google Scholar]
- 34.Minandri F, D'Arezzo S, Antunes LC, Pourcel C, Principe L, et al. J Clin Microbiol Jan 11 [Epub ahead of print]; 2012. Evidence of diversity among epidemiologically related carbapenemase-producing Acinetobacter baumannii belonging to the international clonal lineage II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin D, Lehmann PF, Hamory BH, Padhye AA, Durry E, et al. Comparison of three typing methods for clinical and environmental isolates of Aspergillus fumigatus. J Clin Microbiol. 1995;33:1596–1601. doi: 10.1128/jcm.33.6.1596-1601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemec A, Dijkshoorn L, Jezek P. Recognition of two novel phenons of the genus Acinetobacter among non-glucose-acidifying isolates from human specimens. J Clin Microbiol. 2000;38:3937–3941. doi: 10.1128/jcm.38.11.3937-3941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faria NA, Carrico JA, Oliveira DC, Ramirez M, de Lencastre H. Analysis of typing methods for epidemiological surveillance of both methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Clin Microbiol. 2008;46:136–144. doi: 10.1128/JCM.01684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams MD, Chan ER, Molyneaux ND, Bonomo RA. Genomewide analysis of divergence of antibiotic resistance determinants in closely related isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:3569–3577. doi: 10.1128/AAC.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivasan VB, Rajamohan G, Pancholi P, Stevenson K, Tadesse D, et al. Genetic relatedness and molecular characterization of multidrug resistant Acinetobacter baumannii isolated in central Ohio, USA. Ann Clin Microbiol Antimicrob. 2009;8:21. doi: 10.1186/1476-0711-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]