Abstract
The core assumption driving the use of conditional loss-of-function reagents such as temperature-sensitive mutations is that the resulting phenotype(s) are solely due to depletion of the mutant protein under nonpermissive conditions. However, prior published data, combined with observations presented here, challenge the generality of this assumption at least for telomere biology: for both wild-type yeast and strains bearing null mutations in telomere protein complexes, there is an additional phenotypic consequence when cells are grown above 34°. We propose that this synthetic phenotype is due to a naturally thermolabile activity that confers a telomere-specific defect, which we call the Tmp− phenotype. This prompted a re-examination of commonly used cdc13-ts and stn1-ts mutations, which indicates that these alleles are instead hypomorphic mutations that behave as apparent temperature-sensitive mutations due to the additive effects of the Tmp− phenotype. We therefore generated new cdc13-ts reagents, which are nonpermissive below 34°, to allow examination of cdc13-depleted phenotypes in the absence of this temperature-dependent defect. A return-to-viability experiment following prolonged incubation at 32°, 34°, and 36° with one of these new cdc13-ts alleles argues that the accelerated inviability previously observed at 36° in cdc13-1 rad9-Δ mutant strains is a consequence of the Tmp− phenotype. Although this study focused on telomere biology, viable null mutations that confer inviability at 36° have been identified for multiple cellular pathways. Thus, phenotypic analysis of other aspects of yeast biology may similarly be compromised at high temperatures by pathway-specific versions of the Tmp− phenotype.
Keywords: telomeres, Cdc13, Stn1, temperature sensitivity
TELOMERE research in the budding yeast Saccharomyces cerevisiae has made substantial contributions for ∼30 years, starting with the cloning of yeast telomeres (Szostak and Blackburn 1982; Shampay et al. 1984) and the identification of the first mutant strains with altered telomere length (Carson and Hartwell 1985; Lustig and Petes 1986). Subsequent studies have identified numerous factors that contribute to yeast telomere function. Two key complexes are a telomerase complex (composed of the TLC1 RNA and the Est1, Est2, and Est3 proteins) that is responsible for elongating the G-rich strand of chromosome termini and a heterotrimeric complex that we have called the t-RPA complex (Gao et al. 2007), composed of the essential genes CDC13, STN1, and TEN1, which recruits telomerase to chromosome ends and also confers an essential protective function. In addition, numerous proteins share roles at telomeres and double-strand breaks (Tel1, the Mre11/Rad50/Xrs2 complex, and the Ku heterodimer are three examples), and a cohort of proteins negatively regulate telomere length (Rap1, Rif1, and Rif2, as well as components of DNA replication machinery). Genome-wide efforts have expanded this list with the inclusion of several hundred additional genes (Askree et al. 2004; Gatbonton et al. 2006) that impact telomere function either directly or indirectly. Collectively, this very large panel of defined mutations in known genes has been the basis for numerous in vivo analyses of the consequences of perturbing telomere homeostasis.
In this study, we address an additional factor that impacts chromosome termini even in wild-type yeast: the temperature at which cells are propagated. Compelling evidence for a temperature-induced impact on telomeres was first uncovered with the characterization of yeast strains bearing null mutations in either of the two subunits of the Ku heterodimer. Although viable at lower temperatures, yku70-Δ and yku80-Δ strains exhibit a RAD9-dependent terminal arrest phenotype at 36°, arresting after limited propagation as large-budded cells (Feldmann and Winnacker 1993; Feldmann et al. 1996; Barnes and Rio 1997). This is accompanied by a DNA damage response (Barnes and Rio 1997; Teo and Jackson 2001), arguing that cell death is due to unrepaired DNA damage although the molecular basis for inviability is a subject of some speculation (Fellerhoff et al. 2000; Gravel and Wellinger 2002; Smith et al. 2008). Regardless of the mechanism, the temperature-dependent change in the phenotype of strains bearing null mutations in the Ku heterodimer reveals a thermolabile activity which is Ku-independent. Several other observations are also consistent with a temperature-dependent contribution, as telomeres in wild-type yeast become slightly shorter when cells are propagated at 37° (Grandin and Charbonneau 2001). In addition, est1-Δ null strains have a more exacerbated growth defect when propagated at higher temperatures (Lundblad and Szostak 1989).
These observations suggest that the phenotype of strains with mutations in any gene affecting telomere maintenance might become more severe at higher temperatures. In this study, we systematically analyzed the contribution of temperature to the growth properties of strains bearing null mutations in two key telomere complexes (telomerase and the t-RPA complex), which has revealed a pronounced impact on growth and viability in these null mutant strains at temperatures >34°. This provides further support for a naturally occurring thermolabile activity that, when impaired at higher temperatures, gives rise to a telomere-specific phenotype, which we propose to call the temperature (Tmp−) phenotype. This also raises the possibility that mutations in CDC13, STN1, and TEN1 that exhibit temperature-sensitive (ts) growth may not be ts for activity, but instead are partial loss-of-function mutations at all temperatures. Consistent with this, we present data indicating that the widely used cdc13-1 mutation encodes a protein that is impaired for function even at the permissive temperature of 23°, rather than a thermolabile protein. Similarly, analysis of an extensive panel of stn1− missense mutations, including the previously isolated stn1-13 and stn1-63 mutations, reveals a strikingly similar phenotype: defective telomere maintenance at 23° combined with impaired growth only at temperatures >34°. We propose that this growth phenotype is due to an additive effect, as the result of a hypomorphic stn1− mutation combined with the Tmp− phenotype, rather than a temperature-dependent impairment of STN1 function. A similar explanation potentially applies to a recently reported panel of ten1− mutations (Xu et al. 2009), which we surmise may also be partial loss-of-function mutations rather than ts alleles.
Numerous prior studies with the cdc13-1 mutant strain have been performed at 36°. However, if the cdc13-1 encodes a protein that is severely impaired at all temperatures, and if a naturally thermolabile activity further impairs function above 34°, this raises questions about phenotypic analysis of cdc13-1 strains at 36°. We therefore generated a new panel of cdc13-ts mutant strains that encodes thermolabile proteins and exhibits nonpermissive temperatures from 30° to 33°, thereby allowing a reinvestigation of phenotypes in cells depleted of the Cdc13 protein under conditions where the Tmp− phenotype does not contribute. Previous analysis has indicated that cdc13-1 strains rapidly become inviable following incubation at 36° through a RAD9-dependent mechanism. However, when this experiment was repeated with one of the newly isolated cdc13-ts strains, loss of viability in the absence of RAD9 was minimal at the fully nonpermissive temperature of 32° and became substantial only at 34°–36°. This indicates that inviability is the combined consequence of three defects (in CDC13, RAD9, and the thermosensitive activity) and further suggests that analysis of phenotype(s) of cdc13-ts strains at ≥34° may be monitoring defect(s) that are not solely attributable to loss of CDC13 function.
Materials and Methods
Yeast strains and plasmids
All yeast strains, described in Table 1, were isogenic. Integrated alleles of CDC13 and STN1 were introduced into the genome as URA3 pop-in integrants, Ura− “pop-outs” were selected on 5-FOA, and the status of the CDC13 or STN1 locus was assessed by PCR and sequencing to confirm that the relevant mutation was correctly integrated. A list of the plasmids as well as the starting vectors used for each set of plasmid constructions are in Tables 2 and 3.
Table 1 . Strains used in this study.
| Strain | Genotype |
|---|---|
| JB811 | MATa leu2 trp1 ura3-52 prb− prc− pep4-3 |
| YVL3006 | MATα cdc13-Δ::LYS2 ura3-52 lys2-801 trp1-Δ1 his3-Δ200 leu2-Δ1/pVL438 |
| YVL2394 | MATa stn1-Δ::kanMX6 ura3-52 lys2-801 trp1-Δ1 his3-Δ200 leu2-Δ1 ade2-101/p1046 |
| YVL3584a | MATa/α tlc1-Δ::HIS3/TLC1 |
| YVL3621a | MATa/α tlc1-Δ::HIS3/TLC1 cgi1121-Δ::kanMX6/CGI121 ade2-101/ade2-101 |
| YVL2004a | MATa/MATα cdc13-Δ::LYS2/CDC13 rad24-Δ::HIS3/RAD24 ade2-101/ade2-101 |
| YVL3644a | MATa/MATα cdc13-Δ::LYS2/CDC13 rad24-Δ::HIS3/RAD24 ade2-101/ade2-101 cgi1121-Δ::cNAT/CGI121 |
| YVL2005a | MATa/MATα cdc13-Δ::LYS2/CDC13 rad17-Δ::HIS3/RAD17 ade2-101/ade2-101 |
| YVL2060a | MATa/MATα cdc13-Δ::LYS2/CDC13 mec3-Δ::kanMX6/MEC3 ade2-101/ade2-101 |
| YVL2072a | MATa/MATα cdc13-Δ::LYS2/CDC13 rad50-Δ::kanMX6/RAD50 ade2-101/ade2-101 |
| YVL2090a | MATa/MATα stn1-Δ::kanMX6/STN1 rad24-Δ::HIS3/RAD24 ade2-101/ade2-101 |
| YVL2091a | MATa/MATα ten1-Δ::kanMX6/TEN1 rad24-Δ::HIS3/RAD24 ade2-101/ade2-101 |
| YVL3461a | MATa/MATα stn1-I73A/STN1 |
| YVL3463a | MATa/MATα stn1-I73S/STN1 |
| YVL3474a | MATa/MATα stn1-G137A/STN1 |
| YVL3470a | MATa/MATα stn1-G137S/STN1 |
| YVL3658b | MATa cdc13-S611L |
| YVL3660b | MATa cdc13-F684S |
| YVL3662b | MATa cdc13-S531F |
| YVL3664b | MATa cdc13-N609A |
| YVL3666b | MATa cdc13-F683L |
| YVL3806b | MATa cdc13-S611L rad9-Δ::kanMX6 |
Additional genotype: ura3-52/ura3-52 lys2-801/lys2-801 trp1-Δ1/trp1-Δ1 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1.
Additional genotype: bar1-Δ::cNAT ura3-52 lys2-801 trp-Δ1 his3-Δ200 leu2-Δ1.
Table 2 . Plasmids used in this study.
| Plasmid | Description | Vector backbone |
|---|---|---|
| pVL438 | CEN URA3 CDC13 | |
| pVL440 | CEN LEU2 CDC13 | |
| pVL1086 | CEN LEU2 CDC13-(myc)18 | |
| pVL4222 | CEN LEU2 cdc13-1-(myc)18 | |
| pVL439 | URA3 CDC13 | |
| pVL1492 | CEN LEU2 STN1 | YCplac111 |
| pVL4919 | CEN LEU2 stn1-63 | YCplac111 |
| pVL4330 | CEN LEU2 STN1-(G)9-(myc)7 | pVL1492 |
| pVL4992 | CEN LEU2 stn1-63-(G)9-(myc)7 | pVL4330 |
| pVL4951 | URA3 stn1-I73A | YIplac211 |
| pVL4952 | URA3 stn1-I73S | YIplac211 |
| pVL4958 | URA3 stn1-G137A | YIplac211 |
| pVL4956 | URA3 stn1-G137S | YIplac211 |
Table 3 . New temperature-sensitive mutations in CDC13.
| Parental plasmids | |||
|---|---|---|---|
| Mutation | pVL440 | pVL1086 | pVL439 |
| cdc13-F684S | pVL3945 | pVL5156 | pVL5439 |
| cdc13-S531F | pVL3948 | pVL5157 | pVL5440 |
| cdc13-F683L | pVL3943 | pVL5155 | pVL5453 |
| cdc13-N609A | pVL3956 | pVL5158 | pVL5441 |
| cdc13-S611L | pVL3939 | pVL5154 | pVL5438 |
Genetic methods
Standard genetic methods (telomere length analysis, tetrad dissection, plasmid shuffle, viability assays, and flow cytometry) were performed as previously described (Lendvay et al. 1996; Paschini et al. 2010). For senescence assays, the relevant diploid strains were dissected, and the growth characteristics of tlc1-Δ strains were analyzed by three successive streak-outs on rich media and scored for growth after 3 days. This assay employed very large numbers of isolates to address the high degree of variability displayed by telomerase-defective strains undergoing senescence; senescence was also assessed genotype-blind for the analysis shown in Figure 8 (see Gao et al. 2010 for a more detailed discussion of this protocol).
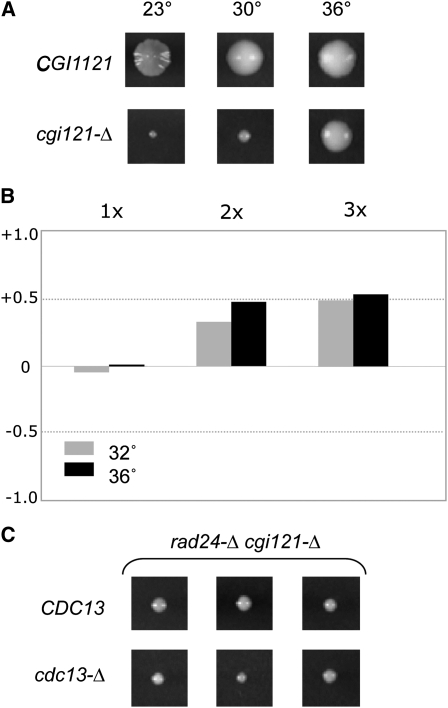
Figure 8 .
Loss of CGI121 function bypasses null mutations in CDC13 and telomerase. (A) Comparison of colony sizes of CGI121 and cgi121-Δ isogenic strains propagated at the indicated temperatures. (B) Comparison of the senescence phenotypes of 26 tlc1-Δ CGI121 isolates with 22 tlc1-Δ cgi121-Δ isolates at 32° and 36°, analyzed as in Figure 2B. (C) Three colonies each of cdc13-Δ cgi121-Δ rad24-Δ and CDC13 cgi121-Δ rad24-Δ generated following dissection of the appropriate heterozygous diploid strain at 23°; see Figure S6 for the relative growth of these two strains at 30° and 36°.
Mutagenesis protocols
Two forward mutagenesis screens of CDC13 were conducted. In the first, pVL440 (containing the intact CDC13 gene) was propagated in an Escherichia coli mutator strain as described previously (Bertuch and Lundblad 2003) to generate a mutant library of 17,000 plasmids, which was transformed into YVL3006 (a cdc13-Δ/p CDC13URA3 shuffle strain). Transformants were subsequently screened for viability at 23° and 36° by replica-plating onto 5-FOA-containing media. In the second screen, a DNA fragment encompassing the DNA-binding domain (DBD) of Cdc13 (amino acids 452–694) was subjected to PCR under error-prone conditions in the presence of 10 mM MnCl2 and one-by-one limiting concentrations of each of the four dNTPs. Pooled PCR products were cotransformed with pVL440 gapped by digestion with BamHI and NruI into the cdc13-Δ/p CDC13URA3 shuffle strain. The resulting 30,000 yeast transformants bearing the gap-repaired plasmids were screened for viability at 23°, 30°, and 36° by replica-plating onto 5-FOA-containing media. For both screens, candidate plasmids were rescued, retransformed to confirm the ts phenotype and subsequently sequenced. Residues for reverse mutagenesis were selected by submitting the Cdc13 DBD or the Stn1 N-terminal OB-fold (oligosaccharide/oligonucleotide binding) domain to the Evolutionary Trace server (http://pdbjets.protein.osaka-u.ac.jp/); residues ranked in the top 10% (for Cdc13) or the top 20 residues (for Stn1) were chosen for mutagenesis.
Results
Telomere-shortening activities are enhanced at 36° in wild-type S. cerevisiae
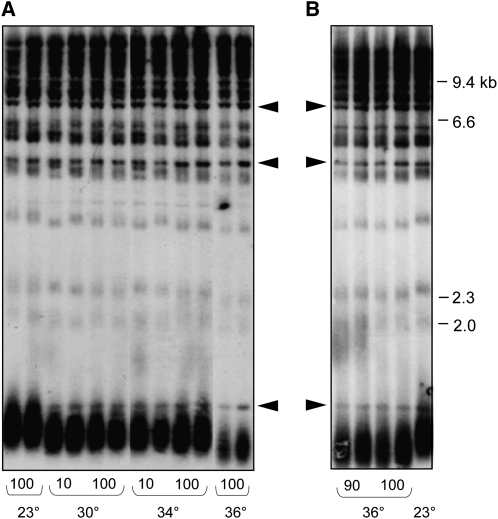
A number of prior studies have indicated that telomere function is impaired by growth at 36°–37°, including even telomere length in wild-type yeast (Grandin and Charbonneau 2001). We re-investigated the effect of temperature on telomere length by propagating two wild-type haploid strains (which were MATα or MATa but otherwise isogenic) in liquid culture at 23°, 30°, 34°, and 36° for ∼100 generations. Comparison of telomere length showed that the samples grown at 30° and 34° underwent a very slight decline in telomere length, relative to the length displayed by the same cultures that had been propagated at 23° (Figure 1A). This change in telomere length homeostasis was reached within 10 generations of growth, with no further length decline upon extended propagation at the higher temperature. At 36°, telomeres underwent a further decline in length, so that the difference in length between cultures grown at 23° vs. 36° was clearly evident (Figure 1B).
Figure 1 .
Telomere length in wild-type S. cerevisiae is temperature-sensitive. (A) Each haploid strain was propagated for ∼100 generations by successive serial dilution of duplicate cultures at the indicated temperatures and genomic DNA prepped for telomere length analysis; two exposures were used to assemble the image to ensure equivalent signal intensity for each lane (as evaluated by comparison of nontelomeric bands, indicated by arrowheads). (B) The same experiment as in A, with samples from 90- and 100-generation cultures grown at 36° displayed immediately adjacent to a sample grown at 23°.
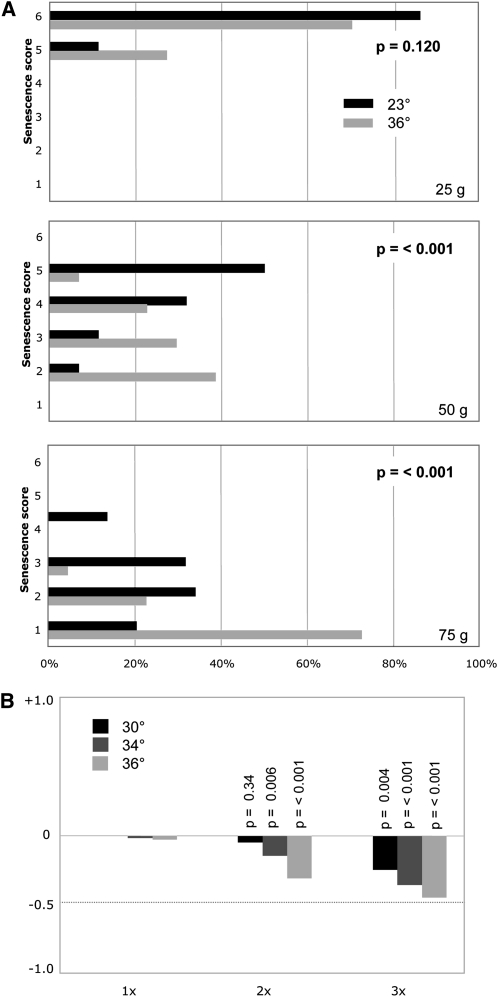
There are two possible explanations for this temperature-dependent effect on telomere length: (i) elongation of telomeres by telomerase is impaired at higher temperatures or (ii) one or more processes that actively shorten telomeres is enhanced at higher temperatures. To distinguish between these two possibilities, the senescence phenotype of a telomerase null strain propagated at 23°, 30°, 32°, 34°, and 36° was examined by successive streak-outs at each temperature, following dissection of a TLC1/tlc1-Δ diploid strain. Senescence was assessed by monitoring the growth characteristics of a large number of isolates to overcome the variability of the senescence phenotype displayed by telomerase-defective strains (Rizki and Lundblad 2001; Gao et al. 2010). Figure 2A compares the growth characteristics of 42 tlc1-Δ isolates grown at 23° vs. 36° for three successive streak-outs, which demonstrates that the senescence progression was clearly exacerbated by growth at 36° even by the second set of streak-outs (which corresponds to ∼50 generations of growth). The histogram in Figure 2B, which summarizes the relative change in the senescence score at 30°, 34°, and 36° relative to 23°, shows that senescence is also accelerated at 30° and 34°, although not to the same degree as at 36°. Thus, at higher temperatures, telomeres are shorter in the presence of telomerase, and senescence is enhanced in the absence of telomerase, which is consistent with the premise that some process by which telomeres are shortened is more active at 34° to 36° (the Tmp− phenotype). We therefore propose that the growth characteristics of the tlc1-Δ strain at elevated temperatures is due to the combined result of the Est− (ever shorter telomeres) and Tmp− phenotypes.
Figure 2 .
(A) Histogram displaying the growth characteristics of 42 tlc1-Δ isolates grown at 23° vs. 36° for three successive streak-outs, corresponding to ∼25, ∼50, and ∼75 generations of growth; isolates were scored for six phenotypic categories, ranging from a scale of 1 (severe senescence) to 6 (comparable to wild-type growth), and a Student’s t-test was used to assess the statistical significance between the two temperatures, as described previously (Gao et al. 2010). (B) Averaged phenotypic scores for the 42 tlc1-Δ isolates propagated at 30°, 34°, or 36° were normalized to growth at 23°, with a negative value indicating enhanced senescence at a given time point, relative to the behavior of the same set of isolates at 23°.
Microcolony growth of cdc13-Δ rad24-Δ and stn1-Δ rad24-Δ strains is reduced at 34°–36°
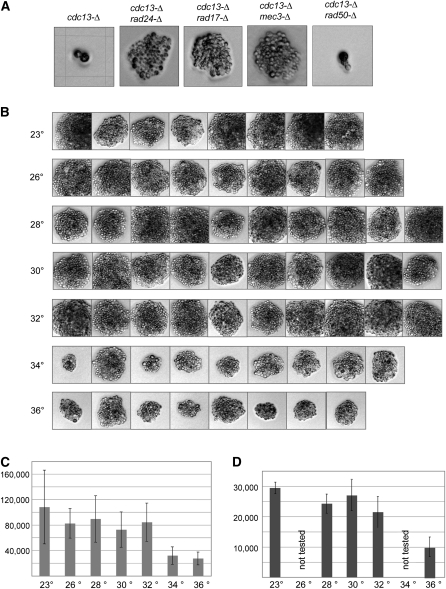
Although cdc13-Δ, stn1-Δ, and ten1-Δ null strains are inviable, several prior observations have suggested that the lethality of strains bearing null mutations in this complex might be partially bypassed by loss of RAD24 function (Weinert et al. 1994; Lydall and Weinert 1995; Small et al. 2008). We examined this by monitoring growth of cdc13-Δ and cdc13-Δ rad24-Δ isolates following dissection of a cdc13-Δ/CDC13rad24-Δ/RAD24 diploid strain. Whereas 22 cdc13-Δ strains were capable of only one to two cell divisions, all 22 cdc13-Δ rad24-Δ newly generated haploid strains underwent sufficient cell divisions to form a microcolony (a representative example is shown in Figure 3A). This behavior extended to the other subunits of the proposed t-RPA complex: 30 of 30 stn1-Δ strains and 22 of 22 ten1-Δ strains arrested after one to two cell divisions, whereas 29 of 30 stn1-Δ rad24-Δ strains and 21 of 22 ten1-Δ rad24-Δ strains produced microcolonies. These cdc13-Δ rad24-Δ, stn1-Δ rad24-Δ, and ten1-Δ rad24-Δ microcolonies were not capable of further propagation, although rare “escaper” clones could be recovered at very low frequencies (data not shown). Figure 3A further demonstrates that the ability to partially bypass cdc13-Δ lethality was a property that extended to other members of the RAD24 epistasis group (Lydall and Weinert 1995; Paulovich et al. 1997), as cdc13-Δ rad17-Δ and cdc13-Δ mec3-Δ strains exhibited a comparable ability to form microcolonies.
Figure 3 .
Microcolony growth of cdc13-Δ rad24-Δ and stn1-Δ rad24-Δ strains is temperature-sensitive. (A) Isogenic diploid strains bearing null mutations of the indicated genotype were dissected, and germinated spores were photographed after growth was complete (48 hr at 30°, 32°, 34°, and 36° or 72 hr at 23°, 26°, and 28°) with a Zeiss Axioskop 50 with a Nikon Digital Sight DS-5M camera; multiple isolates were examined for each genotype, and representative examples are shown. The grid on the cdc13-Δ image corresponds to 62.5 × 62.5 µm; all images were at the same magnification. (B) Photographs of cdc13-Δ rad24-Δ microcolonies from germinated spores generated by dissection of the cdc13-Δ/CDC13 rad24-Δ/RAD24 strain, following incubation for 3 days at the indicated temperature. (C) Each cdc13-Δ rad24-Δ microcolony image shown in B was selected, and the sum of the pixels in the selected area was quantitated using Photoshop. The maximum and minimum values were eliminated from each temperature group; mean and standard variation are shown. (D) Quantitation of stn1-Δ rad24-Δ microcolonies from a stn1-Δ/STN1 rad24-Δ/RAD24 strain, processed as described in B and C. The stn1-Δ rad24-Δ microcolonies were approximately two- to threefold smaller than cdc13-Δ rad24-Δ microcolonies, although the experiments shown in C and D were performed separately, which precluded a more quantitative comparison.
The ability to observe a limited degree of growth in t-RPA null strains in the absence of RAD24 function provided an assay for examining whether this phenotype was also sensitive to temperature. To test this, the cdc13-Δ/CDC13rad24-Δ/RAD24 diploid strain was dissected at 23°, 26°, 28°, 30°, 32°, 34°, and 36°, and cdc13-Δ rad24-Δ spore products were identified. Microcolony size demonstrated a reduction in size (more than twofold) for microcolonies grown at 34° and 36° vs. at lower temperatures (Figure 3, B and C). Similar results were observed when comparing multiple stn1-Δ rad24-Δ isolates following dissection of a stn1-Δ/STN1rad24-Δ/RAD24 diploid strain at 23°–36° (Figure 3D). Thus, similar to the situation with telomerase-defective yeast strains, the consequence of loss of the t-RPA complex is sensitive to elevated temperatures.
Analysis of current temperature-sensitive alleles of CDC13 and STN1
Since even strains with null mutations in CDC13 and STN1 are susceptible to growth temperature, this prompted us to re-examine the characteristics of previously isolated ts mutations in these genes. In particular, we asked whether strains with previously described ts alleles displayed the properties expected for a bona fide temperature-sensitive mutation: fully functional at permissive temperature(s) vs. null (or greatly reduced for function) at nonpermissive temperature(s).
The widely used cdc13-1 strain exhibits reduced viability at 23° and lethality at 25°–26°. Growth is further impaired by the presence of mutations in other telomere-related genes; for example, cdc13-1 tlc1-Δ and cdc13-1 yku80-Δ strains are extremely sick at 23° (Nugent et al. 1996; Polotnianka et al. 1998). The severity of this synthetic phenotype suggested that Cdc13 function was impaired even at permissive temperatures. In fact, examination of steady-state protein levels of the mutant Cdc13-1-(myc)18 protein compared to the wild-type Cdc13-(myc)18 protein (expressed on a CEN plasmid in a wild-type protease-deficient strain) did not reveal behavior consistent with a thermolabile protein. Instead, the Cdc13-1-(myc)18 mutant protein displayed a fourfold reduction in protein levels at 23°, relative to the wild-type protein, and Cdc13-1-(myc)18 levels were not reduced further when the strain was incubated at 36° (Figure 4A). These observations argue that the cdc13-1 mutation results in a hypomorphic protein that is associated with a substantial reduction in protein levels (and presumably function) even at permissive temperatures, thereby explaining the synthetic growth characteristics. The Cdc13-1 protein may be thermosensitive as well, but the lack of change in protein levels at 36° vs. 23° suggests that at least some Cdc13 function is retained at higher temperatures. Consistent with this, in vivo association of the mutant Cdc13-1 protein with telomeres is unchanged at 37° relative to 23° (Vodenicharov and Wellinger 2006), arguing that the Cdc13-1 protein still retains DNA-binding activity and potentially other functions at 36°. We propose that the phenotypes exhibited by a cdc13-1 strain are due to a severe hypomorphic mutation in CDC13 combined with the Tmp− phenotype, rather than due to conditional depletion of the Cdc13 protein.
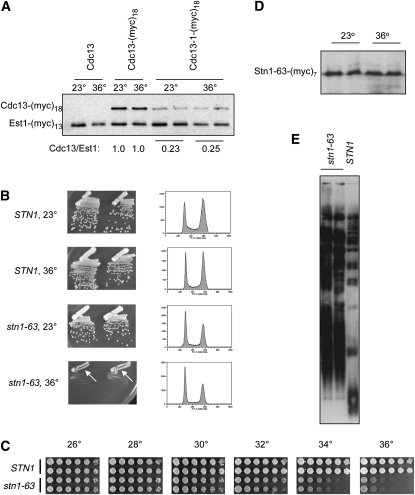
Figure 4 .
The cdc13-1 and stn1-63 mutations behave like hypomorphic alleles. (A) Steady-state protein levels of the wild-type Cdc13-(myc)18 and mutant Cdc13-1-(myc)18 proteins, expressed from a CEN vector; extracts were prepared from strains grown at 23° or at 36° for 3.5 hr and analyzed by anti-myc Western analysis on 8% SDS-PAGE. The strain also expresses an Est1-(myc)13 protein (as an integrated tagged construct), which provided an internal control for protein levels. (B) Comparison of stn1-Δ/pCEN STN1 vs. stn1-Δ/pCEN stn1-63, as assessed by single-colony propagation on rich media (Left panels) or cell-cycle progression of liquid cultures (Right panels) grown at 23° and 36°; arrows indicate that cell division still occurs at 36°. (C) Viability of RAD24 and rad24-Δ versions of the stn1-Δ/pCEN stn1-63 strain at the indicated temperatures; serial dilutions were plated on prewarmed rich media plates and photographed after 2.5 days (for ≥28° incubations) or after 4 days (for 23° and 25° incubations). (D) Steady-state protein levels of the Stn1-63-(myc)7 protein, expressed from a CEN vector, assessed as described in A. (E) Telomere length of the stn1-Δ/pCEN stn1-63 strain compared to the isogenic stn1-Δ/pCEN STN1 strain at 23°.
For STN1, two mutations, stn1-13 and stn1-63, have been used in several previous studies (Grandin et al. 1997; Puglisi et al. 2008). The stn1-13 allele (containing six missense mutations throughout the protein) is only minimally impaired for growth even at 37° (Grandin et al. 1997, 2001; data not shown), and thus it was not included in our subsequent analysis. The stn1-63 strain (Puglisi et al. 2008), which contains a single missense mutation in the essential N-terminal domain (D99E), showed a more substantial impairment for growth at 36° although the strain was not completely inviable at this temperature (Figure 4B). Somewhat surprisingly, FACS analysis revealed that the stn1-63 strain did not exhibit a pronounced defect in cell-cycle progression at 36° (Figure 4B), as would be expected if a subunit of the t-RPA complex had been depleted by a conditional lethal mutation. A more careful examination of the growth of the stn1-63 strain revealed that a reduction in viability was observed only at 34° and 36° (Figure 4C). Steady-state protein levels of the Stn1-63-(myc)7 mutant protein were also unaffected when the culture was shifted to higher temperature (Figure 4D), in contrast to the expectations for a thermolabile protein that should be depleted (or at least diminished) at the nonpermissive temperature. Furthermore, as previously observed (Puglisi et al. 2008), the stn1-63 strain exhibited greatly elongated telomeres even at 23° (Figure 4E), indicating that the Stn1 protein is substantially impaired even at permissive temperature. These data indicate that the stn1-63 mutation, like cdc13-1, encodes a hypomorphic protein that exhibits a temperature-independent defect, rather than a thermolabile mutant protein. We hypothesize that the reduced growth at 34°–36° in the stn1-63 strain background is also an additive effect, due to a partial loss-of-function defect in STN1 in combination with the Tmp− phenotype. This hypothesis is tested further in a later section that examines an expanded panel of missense mutations in STN1.
Since protein levels and/or function appeared to be severely impaired at the presumed permissive temperature for the existing cdc13 and stn1 alleles, we conclude that these alleles are not bona fide conditional lethal reagents. This prompted us to screen for new ts alleles of CDC13 and STN1, as described in the following sections, with the goal of identifying alleles in each gene that are fully functional under permissive conditions (such as 23°) and completely null for function under nonpermissive conditions (such as ≤32°) that would be minimally influenced by the Tmp− phenotype.
Identification of new temperature-sensitive alleles of CDC13 using both forward and reverse mutagenesis
Two forward mutagenesis screens were performed, mutagenizing either the full-length CDC13 gene by passage through an E. coli mutator strain or the essential DBD of CDC13 (Mitton-Fry et al. 2002) by low-fidelity PCR. Both collections of mutagenized plasmids were transformed into a cdc13-Δ shuffle strain kept alive by the presence of a covering CEN URA3CDC13 plasmid, and yeast transformants were screened for ts growth by replica-plating onto media that selected for loss of the covering plasmid (see Materials and Methods for details). Rescued plasmids were subsequently retested following retransformation into the cdc13-Δ shuffle strain and sequenced to identify mutation(s).
Screening the mutagenized full-length CDC13 gene resulted in 26 candidate cdc13-ts alleles, corresponding to 7 unique mutations (Supporting Information, Figure S1). Five alleles contained a single missense mutation: 1 mutation in the N terminus of the protein (cdc13-S56F), 3 mutations in the DBD (cdc13-V530G, cdc13-S531F, and cdc13-D546G) and 1 C-terminal allele (cdc13-T847M). The remaining 21 alleles had a frameshift mutation at either residue 686 (2 isolates) or residue 707 (19 isolates), resulting in truncation of the protein just past the boundary of the DBD (with either 10 or 65 amino acids added to the end of the protein as a result of the frameshift mutation). Recovery of these two frameshift mutations, as well as of cdc13-T847M, was somewhat unexpected because a previously well-characterized allele of CDC13 (cdc13-5), which contained a stop codon introduced at amino acid 694, does not exhibit thermolabile growth (Chandra et al. 2001 and Figure S2).
The second screen, which targeted the DBD of CDC13, yielded 18 alleles with ts phenotypes. Sequence analysis revealed that all but two of these alleles had multiple missense mutations, a common problem with error-prone PCR protocols. However, several clusters of amino acids (aa 525–544, 611–618, and 683–684) appeared to be overrepresented (Figure S2), suggesting that this information might be useful in identifying the causative mutation for at least a subset of these 18 isolates. Therefore, a panel of single missense mutations in residues in these clusters was constructed and tested for ts growth. This analysis identified 6 alleles—cdc13-L529Q, cdc13-V543F, cdc13-S611L, cdc13-G614V, cdc13-F683L, and cdc13-F684S—which conferred a ts phenotype with impaired growth at temperatures ranging from 30° to 36° (Figure S1).
In parallel with these two forward mutagenesis screens, we also employed reverse mutagenesis, using a computational method called Evolutionary Trace (ET) to identify residues in the DBD domain as targets for reverse mutagenesis. ET combines structural information with amino acid diversity to determine the evolutionary pressure at a given residue, which can identify functionally significant residues (Lichtarge et al. 1996; Lichtarge and Sowa 2002). A total of 14 amino acids in Cdc13 with an ET score of ≤10% were selected for mutagenesis (excluded from this set were residues that contact DNA, which are under analysis in a separate study in this laboratory). Each residue was mutated to alanine, and the resulting collection of plasmids were introduced into a cdc13-Δ shuffle strain and assayed as described above. Four of these 14 strains displayed growth defects: one allele, cdc13-D546A, conferred lethality (data not shown), whereas strains expressing alanine mutations in three residues (F547, N609, and F684) exhibited ts growth (Figure S1).
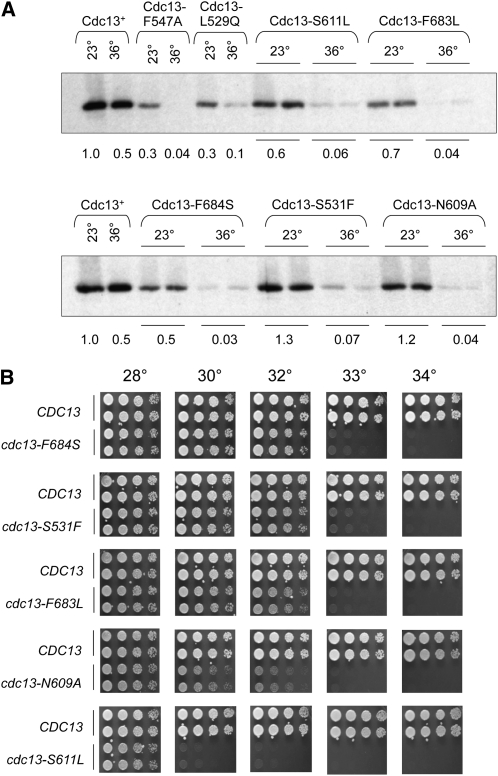
Collectively, these three screens yielded missense mutations in 13 amino acids of Cdc13 that conferred conditional lethal growth. To determine which of these cdc13-ts alleles were fully functional at permissive temperatures but null with regard to both phenotype and protein levels at higher temperatures, we initially examined cell-cycle progression at 23° and 36°. Two mutants (cdc13-V543F and cdc13-T847M) did not exhibit a complete cell-cycle arrest at 36°, and one mutant (cdc13-S56F) had a slight cell-cycle delay even at 23° (data not shown); these three mutants were discarded from further analysis. As a next step, seven mutants that were fully viable at temperatures >28° were examined for Cdc13 protein levels (mutant strains with defects in viability at ≤28° were not analyzed, on the assumption that this would correlate with reduced function and/or protein levels even at permissive temperatures). Each of these seven mutations were introduced into a plasmid construct expressing Cdc13-(myc)18, and extracts prepared from strains expressing these mutant Cdc13-(myc)18 proteins grown at 23° and 36° were examined for protein levels by Western analysis. In contrast to the Cdc13-1 protein (Figure 4A), all seven mutant proteins behaved like thermolabile proteins, with protein levels substantially reduced at 36°, relative to protein levels at 23° (Figure 5A). However, two mutant proteins displayed greater than threefold reduction in protein levels even at 23° (cdc13-F547A and cdc13-L529A) and were excluded from the next stage of analysis.
Figure 5 .
Characterization of new cdc13-ts mutations. (A) Steady-state protein levels of mutant Cdc13-(myc)18 proteins compared to wild-type Cdc13-(myc)18, assessed as in Figure 4A. (B) Viability of the indicated strains, with cdc13-ts mutations integrated into the genome, assessed as in Figure 4C.
Each of the remaining five mutations were integrated into the genome in place of the wild-type CDC13 gene for subsequent analysis to exclude possible artifacts due to variations in plasmid copy number and/or altered gene expression by plasmid-borne alleles. The resulting cdc13-ts strains were screened for effects on viability and cell-cycle progression at a range of temperatures between 23° and 34°. Cell viability assays demonstrated that these new cdc13-ts alleles were fully viable at 28° and inviable at temperatures ranging from 30° to 33° (Figure 5B). It is also worth noting that each of the resulting integrated strains exhibited a ts phenotype that was slightly more severe than the comparable mutation when examined on a CEN plasmid in a cdc13-Δ null strain (compare Figure S1 with Figure 5B). This parallels previous comparisons from our laboratory of the viability of the cdc13-1 allele as an integrated vs. plasmid-borne allele (data not shown), as well as comparisons of integrated vs. plasmid-borne stn1− and ten1− alleles (Paschini et al. 2010).
A return-to-viability experiment demonstrates that phenotypes of cdc13-ts strains at 34°–36° are not solely due to loss of CDC13 function
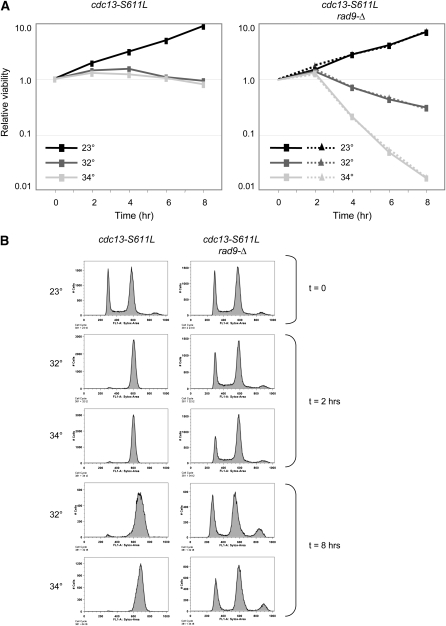
These cdc13-ts mutant strains provide a set of reagents for analysis of CDC13 under conditions where Cdc13 is functional at 23° and fully impaired at temperatures <34°–36°. As a first step in assessing this, we repeated a return-to-viability experiment that has previously been used to analyze Cdc13-related defects in the presence or absence of RAD9 function in a cdc13-1 strain (Weinert and Hartwell 1993; Lydall and Weinert 1995; Addinall et al. 2011). In this protocol, cdc13-1 cells grown at 23° are shifted to nonpermissive temperature for varying time periods and subsequently assessed for viability at permissive temperature (23°). Prior versions of this experiment have used 36° or 38° as the nonpermissive temperature, whereas in the experiment presented here, the cdc13-S611L strain was incubated at 32°, 34°, or 36°. As expected on the basis of the observations in Figure 5B, cdc13-S611L cells failed to undergo cell division when incubated for 8 hr at 32°, which was accompanied by a cell-cycle arrest. The response at 32° was indistinguishable from that at 34° (Figure 6, A and B), confirming that 32° was fully nonpermissive for this cdc13-ts strain.
Figure 6 .
Temperature-dependent effects on viability of a cdc13-S611L rad9-Δ strain. (A) Mid-log cultures of cdc13-S611L (Left) or cdc13-S611L rad9-Δ (Right, duplicate samples) strains grown at 23° were shifted to the indicated temperatures and incubated for up to 8 hr; viability was determined by plating appropriate dilutions on rich media plates that were incubated at 23° for 3 days. Three independent repetitions of this experiment produced essentially identical results. (B) Flow cytometry profile of log-phase cultures of the indicated strains from the experiment shown in A, fixed and stained with SYTOX green.
Consistent with previous analysis of the cdc13-1 strain (Weinert and Hartwell 1993), arrest of the cdc13-S611L strain resulted in no more than a 1.5-fold loss of viability, even after 8 hr at 32°, 34°, or 36° (Figure 6A and data not shown). As was also expected from prior observations, loss of RAD9 function prevented cell-cycle arrest when the cdc13-S611L rad9-Δ strain was incubated at 32° (Figure 6B). Incubation of cdc13-S611L rad9-Δ cells at the nonpermissive temperature (32°) was accompanied by a modest reduction in viability (5-fold by 8 hr). However, when cells were incubated at 34°, there was a striking effect in the absence of RAD9 function, as viability was reduced 65-fold at the 8-hr time point (Figure 6A). Increasing the incubation temperature to 36° reduced viability even further (data not shown). Thus, the RAD9-dependent effect at 34°–36°, which was well above the nonpermissive temperature for the cdc13-S611L strain, indicated that an additional defect that is independent of CDC13 function, but RAD9-dependent, contributed to inviability at 34°–36°.
Reverse mutagenesis of the essential N-terminal domain of STN1
In an attempt to recover conditional lethal alleles of STN1, we employed two reverse mutagenesis strategies that targeted the essential N-terminal domain of the protein. Because the ET protocol was so effective with CDC13 (3 of 14 mutations yielded a thermolabile protein, including 2 of the mutations shown in Figure 5), ET was similarly applied to the predicted OB-fold domain of Stn1. The top 20 residues were mutated to alanine, and a subset were also mutated to serine. These 30 stn1 missense mutations were transformed into a stn1-Δ/p CEN URA3STN1 strain, and yeast transformants were screened for ts growth following loss of the covering plasmid (Figure S3 and data not shown). Roughly half of these mutant strains had no notable growth defect and were eliminated from further analysis. The remaining strains exhibited a range of growth phenotypes, which fell into roughly three categories. One subset (stn1-N67A, stn1-E167A, stn1-W171S, stn1-L181S stn1-L70A, stn1-I73S, and stn1-G77A) grew approximately as well as wild type at lower temperatures but exhibited growth defects at 34°–36° (Figure S3); however, examination of telomere length revealed that substantial telomere elongation had occurred even when these strains were grown at permissive temperature (Figure S4 and data not shown). Strains in the second category (stn1-L41S, stn1-F64A, stn1-F64S, and stn1-L70S) were somewhat impaired for growth at temperatures ranging from 23° to 34°, with the growth phenotype more severe at 36°; all four of these strains exhibited even more extensive telomere elongation at 23° (Figure S3 and Figure S4). The last category (stn1-G77S, stn1-D98A, and stn1-D99A) exhibited the most severe growth defect, as all three strains were barely viable at temperatures up to 32°–34° and inviable at 36° (Figure S3). In every case, the growth defect associated with a given mutation became more pronounced at 36°, a pattern that was very similar to that described above in Figure 4 for stn1-63. Furthermore, cell-cycle progression as assessed by FACS demonstrated that the reduced growth at high temperatures did not exacerbate the relatively modest cell-cycle defect displayed by most of these mutants (Figure S3 and data not shown). Thus, this first attempt at recovering one or more ts alleles of STN1 appeared to be unsuccessful.
However, inspection of the position of this collection of mutations on the predicted structure of the Stn1 protein suggested a possible structural correlation: mutations with the most severe growth defects were located in residues that comprised, or were in close proximity to, the β-barrel of the OB-fold (G77, L70, D98, D99). This suggested that mutagenesis that targeted this particular region of the Stn1 protein might be more successful. Specifically, we directed our attention to a panel of 11 hydrophobic residues (I73, L75, I79, I93, L97, L106, L140, V142, L153, V155, and L158) with side chains located in the interior of the β-barrel of Stn1, on the assumption that (partial) destabilization of the OB-fold might have a higher probability of generating thermosensitive proteins. Each of these 11 residues were mutated to alanine, serine, and tyrosine (based on the results described above for Cdc13, which indicated that restricting mutagenesis to alanine missense mutations might be insufficient). The resulting panel of 33 plasmids bearing stn1 missense mutations in the β-barrel were introduced into a stn1-Δ shuffle strain and examined at a range of temperatures from 23° to 36°. Not unexpectedly, a large number (27%) of the resulting strains were inviable or nearly inviable. Many of the viable strains exhibited a range of growth defects and, once again, the severity of the defect was enhanced in each case when the strains were propagated at 34°–36° (summarized in Figure S5). Furthermore, the majority of the viable strains exhibited elongated telomeres even when the strains were propagated at permissive temperatures (Figure S4). Only two residues, L106 and L140, appeared to be immune to mutagenesis, as the strains expressing mutations in either amino acid exhibited wild-type growth at all temperatures with no telomere length defect, despite the fact that these two bulky hydrophobic residues were predicted to be on the interior of the barrel of the OB-fold (data not shown).
Analysis of integrated stn1− alleles
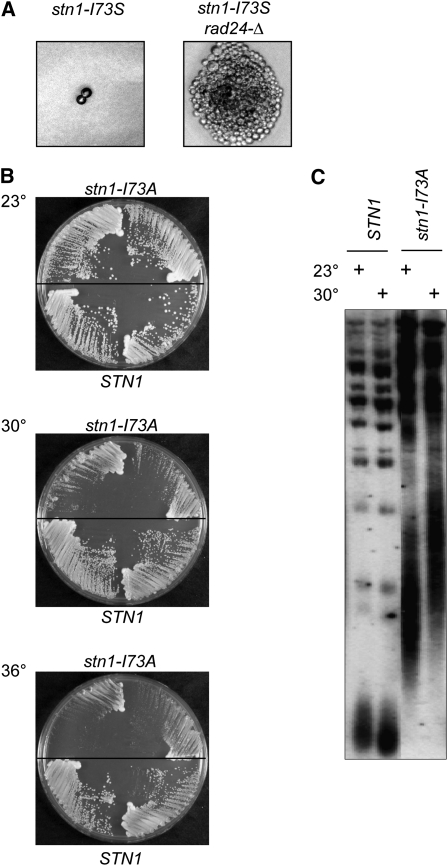
Very few, if any, of the panel of stn1− mutations described above behaved as expected for a thermolabile protein. However, in a previous study, we observed differences in viability when comparing stn1− alleles present on a plasmid in a stn1-Δ strain vs. integrated into the genome (Paschini et al. 2010). Since this current set of stn1− mutations was also expressed on a CEN plasmid in a stn1-Δ strain, we considered the possibility that fluctuations in plasmid copy number might mask a ts phenotype. To test this, diploid strains that were heterozygous for STN1 were constructed by integrating candidate stn1-ts alleles into the genome (see Materials and Methods for details), and haploid stn1 strains were generated by dissection. Five alleles (stn1-I73A, stn1-I73S, stn1-I79S, stn1-G137A, and stn1-G137S) were chosen for this analysis on the basis of the magnitude of the difference comparing growth at 23° vs. 36° when assessing the plasmid-based phenotype in a stn1-Δ strain (Figure S3 and Figure S5).
Dissection of the stn1-I73S/STN1 diploid revealed that the haploid stn1-I73S strain was inviable, as germinated stn1-I73S spores were capable of undergoing only one to two divisions even at 23° (Figure 7A). This indicates that stn1-I73S is a null mutation, since stn1-I73S and stn1-Δ strains resulted in the same phenotype following dissection. Furthermore, germinated stn1-I73S rad24-Δ spores were capable of forming microcolonies (Figure 7A), similar to our observations for stn1-Δ rad24-Δ. Therefore, the apparent ts plasmid-based phenotype (viable at 23°–32° and inviable at ≥34°) was not due to a thermolabile protein; consistent with this, Western analysis did not reveal any change in steady-state levels of the Stn1-I73S protein when examined by Western analysis from extracts grown at 23° vs. 36° (data not shown). We postulate that viability of the stn1-Δ strain expressing the plasmid-borne stn1-I73S mutation was due to increased plasmid copy number in response to selective pressure for viability.
Figure 7 .
Characterization of stn1-I73S and stn1-I73A strains. (A) Photomicrographs of germinated stn1-I73S and stn1-I73S rad24-Δ spores. (B) Single-colony streak-outs of isogenic stn1-I73A and STN1 haploid strains (generated by dissection of a stn1-I73A/STN1 diploid) incubated at 23°, 30°, and 36°. (C) Telomere-length analysis of stn1-I73A and STN1 isolates from B after ∼40 generations of growth at 23° or 30°.
In contrast, dissection of the other heterozygous strains gave rise to viable stn1− haploid strains. Among this set of four strains, only the stn1-I73A strain exhibited a possible thermosensitive phenotype, as the mutant strain exhibited a notable growth defect at 30° compared to 23°, although growth was once again more severely affected at 34°–36° (Figure 7B). This slow gradient of impairment suggested that the stn1-I73A mutation might encode a partially defective protein even at permissive temperatures. Consistent with this, telomere length was substantially affected in the stn1-I73A strain even at 23°, although telomeres were further elongated when the strain was propagated at higher temperatures (Figure 7C). Thus, we conclude that stn1-I73A encodes a protein that is most likely both hypomorphoric and thermolabile, with a partial loss of function at permissive temperature that is further exacerbated by growth at higher temperatures.
Re-examination of a cdc13-1 suppressor reveals a complex genetic interaction between null mutations, cold sensitivity, and the Tmp− phenotype
Several genome-wide screens for genes that enhance or suppress the ts growth of a cdc13-1 strain have yielded several hundred genes, implicating a wide number of molecular pathways in telomere capping (Downey et al. 2006; Addinall et al. 2008). The analysis above indicates that temperature alone can impact telomere-related phenotypes, which adds an additional layer of complexity when interpreting the results from cdc13-1-based screens. To investigate this more closely, we re-examined one candidate (CGI121) recovered from a cdc13-1 suppressor screen. Since loss of Cgi121 function is capable of rescuing the temperature-dependent growth defects displayed by the hypomorphic cdc13-1 strain and a yku80-Δ null strain (Downey et al. 2006), we asked whether a cgi121-Δ mutation would have a similar impact on the senescence phenotype of a tlc1-Δ strain or on the microcolony growth of a cdc13-Δ rad24-Δ strain.
Dissection of cgi121-Δ/CGI121 diploids revealed an unexpected surprise, however: the cgi121-Δ strain was itself cold-sensitive for growth. A comparison of colony sizes for CGI121 vs. cgi121-Δ strains following sporulation at 23°, 30°, and 36° demonstrated that the cgi121-Δ strain exhibited a substantial growth defect at 23°, which was largely alleviated by growth at 36° (Figure 8A). A re-examination of previously published data by Durocher and colleagues indicates that a cold-sensitive growth defect had been previously observed for cgi121-Δ as well as for a strain with a null mutation in BUD32 (another member of the same complex) (Downey et al. 2006), supporting the results reported here. Thus, the growth phenotype of a cgi121-Δ strain is the consequence of two activities: loss of Cgi121 function combined with an activity that is naturally impaired for function at 23° but not at 36°.
To assess for effects on senescence, tlc1-Δ and tlc1-Δ cgi121-Δ strains were propagated for ∼75 generations following dissection at either 32° or 36° (at lower temperatures, the significant growth defect due to cgi121-Δ overwhelmed the senescence phenotype). As shown in Figure 8B, loss of Cgi121 function significantly attenuated senescence at both temperatures. Thus, a null mutation in CGI121 acts as a partial bypass suppressor of a null mutation in telomerase, at least at high temperatures. In contrast, the genetic interaction between null mutations in CGI121 and CDC13 was more complex. At 23°, the cgi121-Δ mutation was capable of bypassing the near-lethality of a cdc13-Δ rad24-Δ strain: CDC13 was no longer an essential gene in a cgi121-Δ rad24-Δ background, such that CDC13cgi121-Δ rad24-Δ and cdc13-Δ cgi121-Δ rad24-Δ strains gave rise to equally sized (although small) colonies (Figure 8C). However, at higher temperatures, the difference between these two sets of strains became more obvious (Figure S6). This reveals a complex genetic interaction between the cold-sensitive growth properties of the suppressor strain (cgi1121-Δ) and the temperature-enhanced phenotypes of the cdc13-Δ rad24-Δ strain, as four genetic factors contributed to the growth characteristics of the cdc13-Δ cgi121-Δ rad24-Δ strain at different temperatures (loss of Cdc13 function, loss of Cgi121 function, the Tmp− phenotype, and the cold-sensitive activity). Thus, whether the enhanced growth properties of the cdc13-Δ cgi121-Δ rad24-Δ strain are due to a genetic relationship between CDC13 and CGI121 cannot be determined.
Discussion
The results presented here, as well as in several prior studies, establish that there is an additional phenotypic consequence at telomeres when cells are grown at higher temperatures, particularly >34°. This phenotype can be observed even in wild-type yeast and also contributes to the severity of phenotypes displayed by yeast strains bearing null mutations in the Ku heterodimer (Feldmann and Winnacker 1993; Boulton and Jackson 1996; Feldmann et al. 1996), telomerase (Figure 2), and the t-RPA complex (Figure 3). We propose that the enhanced phenotype at higher temperatures is a synthetic genetic effect, as illustrated schematically in Figure 9, due to a mutation in a telomere-related complex combined with an additional defect that becomes particularly apparent at 34°–36°.
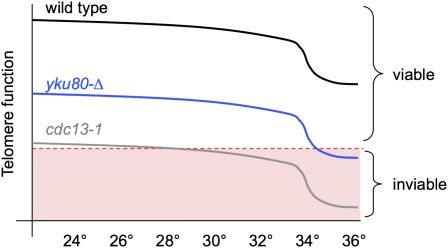
Figure 9 .
A schematic depiction of the additive contribution of the Tmp− phenotype to the viability of strains bearing mutations in telomere-related proteins based on a decline in “telomere function” that occurs with increasing temperature. Since the molecular basis for the Tmp− phenotype is unknown, telomere function is defined as “the collection of activities that maintain chromosome ends as fully replicated and capped telomeres.”
This temperature-dependent defect also complicates the analysis of missense mutations in subunits of each of these complexes, particularly for the essential genes CDC13, STN1, and TEN1, which suggests that a number of mutations in these three genes have been incorrectly categorized as temperature-sensitive alleles and are instead hypomorphic alleles. In particular, several observations argue against the long-standing assumption that cdc13-1 encodes a conditional mutation. Even at 23°, cdc13-1 cells have reduced protein levels (Figure 4A) and thus presumably reduced CDC13 activity, which is consistent with the synthetic lethality even at 23° that occurs when cdc13-1 is combined with other telomere-specific mutations (Nugent et al. 1996; Polotnianka et al. 1998). Furthermore, Cdc13-1 protein levels (Figure 4A), as well as the ability of the mutant protein to associate with telomeres (Vodenicharov and Wellinger 2006), are unchanged following a shift to 36°. Given that the defect encoded by cdc13-1 is so substantial that it is barely compatible with viability at 23°, we suggest that the combination of the cdc13-1 mutation with the defect that gives rise to the Tmp− phenotype is responsible for inviability at 25°–26°. Although the Tmp− phenotype is most obvious at 34°–36° (where it affects the viability of telomerase- and Ku-defective strains), telomere length in wild-type cells is slightly reduced even at 30° relative to 23°. Furthermore, in yku80-Δ cells, the robust DNA damage response that occurs at 37° (as measured by autophosphorylation of Rad53) can also be detected at 30° although to a lesser degree (Teo and Jackson 2001). These observations are consistent with the idea that the Tmp− phenotype can confer a synthetic defect even at lower temperatures (as illustrated in Figure 9) in the presence of a severe mutation such as cdc13-1.
The Tmp− phenotype is potentially the basis for incorrectly assigning ts properties to hypomorphic mutations in STN1 and TEN1 as well. In the extensive panel of stn1− missense mutations reported here, every stn1− strain that exhibited a telomere length defect at 23° (corresponding to mutations in 24 amino acids) was accompanied by reduced viability at 34°–36°, a characteristic also displayed by the previously reported stn1-13 and stn1-63 mutant strains. This behavior is also similar to a recently reported panel of ten1− mutations, which confer extremely elongated telomeres at permissive temperature, with growth impaired at 36° but not at lower temperatures (Xu et al. 2009). We suggest that these ten1 alleles are also hypomorphs, rather than mutations that confer thermolabile Ten1 function. This suggestion also extends to a stn1-td degron construct that retains a significant degree of viability at 37° after switching on the degron (Vodenicharov and Wellinger 2006). Collectively, we propose that there are currently no bona fide stn1-ts or ten1-ts reagents that are wild type at permissive temperature and depleted for the essential function encoded by STN1 or TEN1 following a temperature shift.
Implications for analysis of telomere function at 36°
Although we were unsuccessful in our attempts to identify conditional lethal mutations in STN1, we did recover five new cdc13-ts alleles with nonpermissive temperatures between 30° and 33°. Unlike cdc13-1, these five mutations encode Cdc13 proteins that retain wild-type, or near wild-type, protein levels at 23° but are reduced by >10-fold at 36°. All five mutations map to the DNA-binding domain (Mitton-Fry et al. 2002), with three residues (S531, N609, and S611) in close physical proximity (data not shown). This suggests a region of the DBD that might be particularly prone to thermosensitive perturbations (alternatively, the recovery of mutations in these three residues may be simply a reflection of some bias in how the mutagenesis screens were conducted).
The identification of these new cdc13-ts strains allowed us to re-investigate a prior observation about the viability of Cdc13-depleted strains at nonpermissive temperatures. Previous experiments, which have monitored the viability of cdc13-1 vs. cdc13-1 rad9-Δ strains following prolonged incubation at 36° (Weinert and Hartwell 1993; Lydall and Weinert 1995), have concluded that loss of Cdc13 function creates structure(s) that, if left unrepaired, are lethal in the absence of Rad9 function (presumably as a consequence of unchecked cell-cycle progression). We re-examined this observation using a newly generated cdc13-ts allele (cdc13-S611L) that was nonpermissive for growth at 30° (Figure 5), which allowed us to compare the loss of viability in the absence of RAD9 at 32°, 34°, and 36°. Even though 32° was fully nonpermissive for CDC13 function, loss of viability was minimal. However, elevating the temperature to 34° or 36° resulted in a substantial further reduction in viability. Collectively, these observations suggest that, even in the absence of RAD9, loss of CDC13 function does not lead to inviability per se. Instead, we propose that lethality is the consequence of an additional defect occurring at 34°–36°, when combined with rad9-Δ and cdc13 depletion. If this proposal is correct, this suggests that hypotheses about the role of Cdc13 at telomeres as well as models derived from cdc13-1-induced DNA damage based on observations made at 34°–36° may need to be revisited, as the resulting phenotypes may be due to a more intricate set of genetic interactions than simply depletion of Cdc13. This point is further illustrated by the complex epistatic interaction that we observed between cgi121-Δ and cdc13-Δ mutations at different temperatures (Figure 8).
These results also have implications for genome-wide suppression and enhancer screens that have monitored viability of cdc13-1 and yku70-Δ strains at temperatures ranging from 36° to 37.5° (Downey et al. 2006; Addinall et al. 2008, 2011). At least some subset of genes recovered from these screens is presumably due to a genetic interaction with the Tmp− phenotype, rather than with either CDC13 or YKU70. Although this does not negate the importance of these studies, the potential for such genes to further our understanding of telomere biology will require a fuller understanding of what telomeric process(es) are impaired by elevated temperature.
In addition, the impairment due to the reduction in Cdc13-1 protein levels suggests that a subset of genes recovered from such screens are involved in protein stability and/or turnover, rather than in telomere biology. This would explain how STM1, which modulates translation by regulating formation of the 80S subunit of ribosomes (Balagopal and Parker 2011), functions as a high copy suppressor of cdc13-1 (Hayashi and Murakami 2002). Similarly, the recovery of san1-Δ as a robust suppressor of cdc13-1 (Downey et al. 2006; Addinall et al. 2008) is consistent with a role for San1 in mediating degradation of misfolded nuclear proteins (Fredrickson et al. 2011). More generally, cdc13-1 suppressors that are involved ribosome function, protein degradation, RNA processing, protein transport, and/or biosynthesis may have little direct relationship to telomere function.
What is the molecular basis for the Tmp− phenotype?
This phenotype appears to be the consequence of a telomere-specific activity that is naturally temperature-labile even in wild-type cells, as evidenced by telomere shortening at higher temperatures. This defect is presumably also responsible for the inviability observed in yku70-Δ and yku80-Δ strains, the enhanced senescence of telomerase-null strains, and the reduced microcolony growth of RAD24-deficient cdc13-Δ, stn1-Δ, and ten1-Δ null strains at 36°. Consistent with the premise that a common defect is responsible, the appearance of inviability in Ku-depleted cells exhibits phenotype lag (Barnes and Rio 1997). Similarly, the additive effect of temperature on telomerase-defective strains becomes more pronounced in later generations (Figure 2).
The temperature-induced inviability that occurs in yku70-Δ and yku80-Δ strains propagated at 36° has been characterized in detail by multiple laboratories, although with somewhat differing conclusions as to the molecular basis for the causative lesion (Fellerhoff et al. 2000; Teo and Jackson 2001; Gravel and Wellinger 2002; Maringele and Lydall 2002; Smith et al. 2008). Several studies have lent support to the idea that an altered terminal DNA structure, which is generated when telomeres fall below a minimal length, is responsible. The shortening rate at 36° in Ku-depleted cells is inconsistent with a telomerase defect (Gravel and Wellinger 2002), which is also supported by our results indicating that the temperature-dependent reduction in telomere length is not due to an impaired ability of telomerase at 36°. These observations therefore argue for an active shortening mechanism that occurs at high temperature. In the absence of Ku function, telomere-proximal regions replicate early (Cosgrove et al. 2002; Lian et al. 2011). If high temperature further exacerbates this altered replication timing profile (e.g., as a consequence of the shorter cell-cycle time at 36°), we suggest that this could lead to incomplete replication of the duplex telomeric DNA tract by the conventional DNA replication machinery and hence to telomere shortening.
Regardless of the molecular defect that underlies the Tmp− phenotype, this telomere-specific response is just one aspect of a pleiotrophic set of responses by cells to higher temperatures. Cells have a well-orchestrated mechanism for response to temperature fluctuations. Thus, other cellular pathways may also experience an analogous version of the Tmp− phenotype. The results described here also raise a cautionary note about using phenotypic analysis as the sole basis for categorizing ts mutations. This suggests that efforts to create genome-wide epistasis maps using a comprehensive array of temperature-sensitive reagents may need to take this into account (Ben-Aroya et al. 2008; Li et al. 2011).
Supplementary Material
Acknowledgments
We thank Madeleine Jennewein and Monika Walterscheid who each contributed to the initial stages of one of the forward mutagenesis cdc13-ts screens; Edward K. Mandell for conducting the Evolutionary Trace analysis on Cdc13; and other members of the Lundblad laboratory for many helpful discussions during the course of this work. This research was supported by grant GM55867 and Cancer Center Core grant P30-CA014195 from the National Institutes of Health and by funding from the F. M. Kirby Foundation, the G. Harold and Leila Y. Mathers Charitable Foundation, and the Chapman Foundation.
Footnotes
Communicating editor: J. Sekelsky
Literature Cited
- Addinall S. G., Downey M., Yu M., Zubko M. K., Dewar J., et al. , 2008. A genomewide suppressor and enhancer analysis of cdc13–1 reveals varied cellular processes influencing telomere capping in Saccharomyces cerevisiae. Genetics 180: 2251–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addinall S. G., Holstein E. M., Lawless C., Yu M., Chapman K., et al. , 2011. Quantitative fitness analysis shows that NMD proteins and many other protein complexes suppress or enhance distinct telomere cap defects. PLoS Genet. 7: e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askree S. H., Yehuda T., Smolikov S., Gurevich R., Hawk J., et al. , 2004. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc. Natl. Acad. Sci. USA 101: 8658–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal V., Parker R., 2011. Stm1 modulates translation after 80S formation in Saccharomyces cerevisiae. RNA 17: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G., Rio D., 1997. DNA double-strand-break sensitivity, DNA replication, and cell cycle arrest phenotypes of Ku-deficient Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Aroya S., Coombes C., Kwok T., O’Donnell K. A., Boeke J. D., et al. , 2008. Toward a comprehensive temperature-sensitive mutant repository of the essential genes of Saccharomyces cerevisiae. Mol. Cell 30: 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuch A. A., Lundblad V., 2003. The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol. Cell. Biol. 23: 8202–8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton S. J., Jackson S. P., 1996. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res. 24: 4639–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M., Hartwell L., 1985. CDC17: an essential gene that prevents telomere elongation in yeast. Cell 42: 249–257. [DOI] [PubMed] [Google Scholar]
- Chandra A., Hughes T. R., Nugent C. I., Lundblad V., 2001. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 15: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove A. J., Nieduszynski C. A., Donaldson A. D., 2002. Ku complex controls the replication time of DNA in telomere regions. Genes Dev. 16: 2485–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey M., Houlsworth R., Maringele L., Rollie A., Brehme M., et al. , 2006. A genome-wide screen identifies the evolutionarily conserved KEOPS complex as a telomere regulator. Cell 124: 1155–1168. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Winnacker E. L., 1993. A putative homolgue of the human autoantigen Ku from Saccharomyces cerevisiae. J. Biol. Chem. 268: 12895–12900. [PubMed] [Google Scholar]
- Feldmann H., Driller L., Meier B., Mages G., Kellermann J., et al. , 1996. HDF2, the second subunit of the Ku homologue from Saccharomyces cerevisiae. J. Biol. Chem. 271: 27765–27769. [DOI] [PubMed] [Google Scholar]
- Fellerhoff B., Eckardt-Schupp F., Friedl A. A., 2000. Subtelomeric repeat amplification is associated with growth at elevated temperature in yku70 mutants of Saccharomyces cerevisiae. Genetics 154: 1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson E. K., Rosenbaum J. C., Locke M. N., Milac T. I., Gardner R. G., 2011. Exposed hydrophobicity is a key determinant of nuclear quality control degradation. Mol. Biol. Cell 22: 2384–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Cervantes R. B., Mandell E. K., Otero J. H., Lundblad V., 2007. RPA-like proteins mediate yeast telomere function. Nat. Struct. Mol. Biol. 14: 208–214. [DOI] [PubMed] [Google Scholar]
- Gao H., Toro T. B., Paschini M., Braunstein-Ballew B., Cervantes R. B., et al. , 2010. Telomerase recruitment in Saccharomyces cerevisiae is not dependent on Tel1-mediated phosphorylation of Cdc13. Genetics 186: 1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatbonton T., Imbesi M., Nelson M., Akey J. M., Ruderfer D. M., et al. , 2006. Telomere length as a quantitative trait: genome-wide survey and genetic mapping of telomere length-control genes in yeast. PLoS Genet. 2: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandin N., Charbonneau M., 2001. Hsp90 levels affect telomere length in yeast. Mol. Genet. Genomics 265: 126–134. [DOI] [PubMed] [Google Scholar]
- Grandin N., Reed S. I., Charbonneau M., 1997. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 11: 512–527. [DOI] [PubMed] [Google Scholar]
- Grandin N., Damon C., Charbonneau M., 2001. Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J. 20: 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel S., Wellinger R. J., 2002. Maintenance of double-stranded telomeric repeats as the critical determinant for cell viability in yeast cells lacking Ku. Mol. Cell. Biol. 22: 2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N., Murakami S., 2002. STM1, a gene which encodes a guanine quadruplex binding protein, interacts with CDC13 in Saccharomyces cerevisiae. Mol. Genet. Genomics 267: 806–813. [DOI] [PubMed] [Google Scholar]
- Lendvay T. S., Morris D. K., Sah J., Balasubramanian B., Lundblad V., 1996. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics 144: 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Vizeacoumar F. J., Bahr S., Li J., Warringer J., et al. , 2011. Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat. Biotechnol. 29: 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H. Y., Robertson E. D., Hiraga S., Alvino G. M., Collingwood D., et al. , 2011. The effect of Ku on telomere replication time is mediated by telomere length but is independent of histone tail acetylation. Mol. Biol. Cell 22: 1753–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtarge O., Sowa M. E., 2002. Evolutionary predictions of binding surfaces and interactions. Curr. Opin. Struct. Biol. 12: 21–27. [DOI] [PubMed] [Google Scholar]
- Lichtarge O., Bourne H. R., Cohen F. E., 1996. An evolutionary trace method defines binding surfaces common to protein families. J. Mol. Biol. 257: 342–358. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Szostak J. W., 1989. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57: 633–643. [DOI] [PubMed] [Google Scholar]
- Lustig A. J., Petes T. D., 1986. Identification of yeast mutants with altered telomere structure. Proc. Natl. Acad. Sci. USA 83: 1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall D., Weinert T., 1995. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science 270: 1488–1491. [DOI] [PubMed] [Google Scholar]
- Maringele L., Lydall D., 2002. ExoI-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Δ mutants. Genes Dev. 16: 1919–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton-Fry R. M., Anderson E. M., Hughes T. R., Lundblad V., Wuttke D. S., 2002. Conserved structure for single-stranded telomeric DNA recognition. Science 296: 145–147. [DOI] [PubMed] [Google Scholar]
- Nugent C. I., Hughes T. R., Lue N. F., Lundblad V., 1996. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274: 249–252. [DOI] [PubMed] [Google Scholar]
- Paschini M., Mandell E. K., Lundblad V., 2010. Structure prediction-driven genetics in Saccharomyces cerevisiae identifies an interface between the t-RPA proteins Stn1 and Ten1. Genetics 185: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich A. G., Margulies R. U., Garvik B. M., Hartwell L. H., 1997. RAD9, RAD17, and RAD24 are required for S phase regulation in Saccharomyces cerevisiae in response to DNA damage. Genetics 145: 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotnianka R. M., Li J., Lustig A. J., 1998. The yeast Ku heterodimer is essential for protection of the telomere against nucleolytic and recombinational activities. Curr. Biol. 8: 831–834. [DOI] [PubMed] [Google Scholar]
- Puglisi A., Bianchi A., Lemmens L., Damay P., Shore D., 2008. Distinct roles for yeast Stn1 in telomere capping and telomerase inhibition. EMBO J. 27: 2328–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki A., Lundblad V., 2001. Defects in mismatch repair promote telomerase-independent proliferation. Nature 411: 713–716. [DOI] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H., 1984. DNA sequences of telomeres maintained in yeast. Nature 310: 154–157. [DOI] [PubMed] [Google Scholar]
- Small V. Y., Chuang C., Nugent C. I., 2008. Rad24 truncation, coupled with altered telomere structure, promotes cdc13–1 suppression in S. cerevisiae. Cell Cycle 7: 3428–3439. [DOI] [PubMed] [Google Scholar]
- Smith S., Banerjee S., Rilo R., Myung K., 2008. Dynamic regulation of single-stranded telomeres in Saccharomyces cerevisiae. Genetics 178: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H., 1982. Cloning yeast telomeres on linear plasmid vectors. Cell 29: 245–255. [DOI] [PubMed] [Google Scholar]
- Teo S. H., Jackson S. P., 2001. Telomerase subunit overexpression suppresses telomere-specific checkpoint activation in the yeast yku80 mutant. EMBO Rep. 2: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodenicharov M. D., Wellinger R. J., 2006. DNA degradation at unprotected telomeres in yeast is regulated by the CDK1 (CDC28/Clb) cell cycle kinase. Mol. Cell 24: 127–137. [DOI] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H., 1993. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics 134: 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A., Kiser G. L., Hartwell L. H., 1994. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 8: 652–665. [DOI] [PubMed] [Google Scholar]
- Xu L., Petreaca R. C., Gasparyan H. J., Vu S., Nugent C. I., 2009. TEN1 is essential for CDC13-mediated telomere capping. Genetics 183: 793–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.