Abstract
Osteogenesis imperfecta (OI) is characterized by susceptibility to bone fractures, with a severity ranging from subtle increase in fracture frequency to prenatal fractures. The first scientific description of OI dates from 1788. Since then, important milestones in OI research and treatment have, among others, been the classification of OI into 4 types (the ‘Sillence classification’), the discovery of defects in collagen type I biosynthesis as a cause of most cases of OI and the use of bisphosphonate therapy. Furthermore, in the past 5 years, it has become clear that OI comprises a group of heterogeneous disorders, with an estimated 90% of cases due to a causative variant in the COL1A1 or COL1A2 genes and with the remaining 10% due to causative recessive variants in the 8 genes known so far, or in other currently unknown genes. This review aims to highlight the current knowledge around the history, epidemiology, pathogenesis, clinical/radiological features, management, and future prospects of OI. The text will be illustrated with clinical descriptions, including radiographs and, where possible, photographs of patients with OI.
Key Words: Collagen type I, Fractures, Osteogenesis imperfecta
Introduction
Osteogenesis imperfecta (OI) comprises a heterogeneous group of diseases characterized by susceptibility to bone fractures with variable severity and, in most cases, with presumed or proven defects in collagen type I biosynthesis [Van Dijk et al., 2010c]. Other clinical manifestations include short stature, blue sclerae, dentinogenesis imperfecta, and hearing loss.
Epidemiology
OI has a birth prevalence of approximately 6–7/100,000 [Steiner et al., 1993]. The prevalence and incidence of the OI types are different from each other, with OI type I and OI type IV accounting for considerably more than half of all OI cases [Steiner et al., 1993]. In 1979, Sillence et al. [1979] reported a prevalence of 3–4/100,000 and an incidence of 3.5/100,000 for OI type I in Victoria, Australia. For OI type II the incidence is about 1–2/100,000 [Steiner et al., 1993], prevalence data are not available due to early lethality [Steiner et al., 1993]. OI type III has a prevalence of 1–2/100,000 [Steiner et al., 1993]. An incidence of 1.6/100,000 has been reported in Australia [Sillence et al., 1979]. OI type IV was believed to be a rare entity [Sillence et al., 1979], but this proved not to be the case [Steiner et al., 1993].
History
The earliest known patient with OI probably dates from about 1000 BC and appears to be an Egyptian infant. This conclusion can be drawn after studying the remains of an Egyptian mummy [Lowenstein, 2009].
The first scientific description of OI was given by the Swedish army surgeon Olaus Jakob Ekman (1788) who, in his thesis on ‘congenital osteomalacia’, described a 3-generation family with hereditary bone fragility [Peltier, 1981; Baljet, 2002]. Since then, many names have been used to describe familial bone fragility. Willem Vrolik (1801–1863), a Dutch professor at the Athenaeum Illustre of Amsterdam, introduced the term ‘osteogenesis imperfecta’ [Baljet, 1984] and was one of the first to realize that OI might be due to insufficient intrinsic ‘generative energy’ [Baljet, 2002] as opposed to the result of an acquired disease.
In the 20th century, it became clear that OI was a disease showing remarkable clinical variability with severity ranging from death in the perinatal period to subtle increase in fracture frequency. Looser [1906] made the first classification of OI into congenital and tarda. Attempts were made to further classify OI, and in 1979 the ‘Sillence classification’ [Sillence et al., 1979] was proposed which, though in an adjusted form, is still in use today.
In 1974, bone collagen aggregation abnormalities were described by scanning electron microscopy of bone collagen in 3 patients with OI [Teitelbaum et al., 1974]. Sykes et al. [1977] reported altered relation of 2 collagen types in pepsin digests of skin of OI patients using a method of interrupted polyacrylamide-gel electrophoresis. In 1983, the presence of an internal deletion of about 0.5 kb in 1 allele for the pro-α1(I) chain in a patient with OI [Chu et al., 1983] was discovered. This finding was the beginning of the unraveling of the collagen type I biosynthesis and the pathogenic mechanisms of OI which are still not completely known today.
Classification
In 1979, Sillence et al. [1979] proposed a numerical classification of OI into 4 types based on clinical and genetic findings in OI patients ascertained in Victoria, Australia. This classification distinguished type I (mild OI, blue sclerae, autosomal dominant inheritance), type II (lethal perinatal OI, autosomal recessive inheritance, later subdivided in II-A, -B, and -C based on radiographic features [Sillence et al., 1984], type III (progressively deforming, autosomal recessive inheritance), and type IV (dominantly inherited OI with normal sclerae). It is evident that Sillence et al. [1979] assumed that OI was a heterogeneous condition.
OI was considered for some time to be an autosomal dominant disease with recurrence due to germ line mosaicism since heterozygous collagen type I mutations (COL1A1 or COL1A2 mutations) were discovered in all OI types. The recurrence risk of OI type II was observed to be less than 10% [Byers et al., 1988; Cohn et al., 1990; Cohen-Solal et al., 1991; Pepin et al., 1997]. The Sillence classification was used only for the clinical/radiological classification of OI. Thereafter, studies were reported of families, in some cases consanguineous, with OI not caused by pathogenic variants in the COL1A1 or COL1A2 genes [Wallis et al., 1993]. The original Sillence classification was extended with OI types V–VII based on OI cases with unknown genetic etiology and/or distinctive clinical manifestations [Rauch et al., 2004].
In 2006, the first genetic cause of autosomal recessive lethal OI was discovered, i.e. bi-allelic variants in the CRTAP gene causing complete loss of protein function [Barnes et al., 2006]. Partial loss of function CRTAP variants encoding cartilage-associated protein (CRTAP) were found to cause OI type VII [Morello et al., 2006]. Presently, 6 more causes of recessive OI (causative variants in LEPRE1 [Cabral et al., 2007], PPIB [Van Dijk et al., 2009b; Barnes et al., 2010], SERPINH1 [Christiansen et al., 2010], FKBP10 [Alanay et al., 2010], SP7 [Lapunzina et al., 2010], and SERPINF1 [Becker et al., 2011]) have been described, all but 2 (SP7 and SERPINF1) concerning genes encoding proteins involved in collagen type I biosynthesis.
There is some debate in the literature about how to involve this newly discovered heterogeneity of OI in the classification. One research group proposes to have OI caused by recessive causative variants in PPIB, SERPINH1, FKBP10, SP7, and SERPINF1 added to the current classification as OI type IX [Barnes et al., 2010] and presumably X, XI, XII, and XIII, respectively. Another viewpoint expressed by our research group is that the clinical/radiological characteristics of recessive OI do not substantially differ from OI type II-B, III or IV caused by dominant OI, so we propose to have recessive OI classified as OI type II-B/III/IV-PPIB/SERPINH1/FKBP10/SP7/SERPINF1- related. OI type I and OI type II-A appear to be exclusively caused by causative COL1A1/2 variants [Van Dijk et al., 2009a]. We also proposed to only add a new numerical type when the phenotype of the patients differs from the numerical types described so far [Van Dijk et al., 2010c]. There is also debate as to whether Bruck syndrome type 1 [Breslau-Siderius et al., 1998] and type 2 [van der Slot et al., 2003], characterized clinically by bone fragility and congenital contractures of the large joints, should be classified as subtypes of OI.
The International Nomenclature group for Constitutional disorders of the Skeleton, among others concerning the classification of OI, proposed to retain the Sillence classification into 5 types and free the classification from direct molecular reference. Bruck syndrome 1 and 2 were not listed as subtypes of OI [Warman et al., 2011].
Collagen Type I Biosynthesis
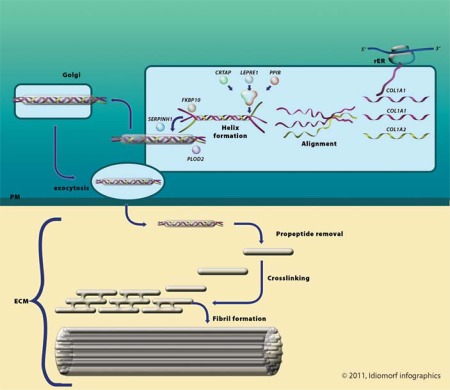
Collagen type I biosynthesis consists of various important steps, which are explained in this section and depicted in figure 1.
Fig. 1.
Collagen type I biosynthesis. rER = Rough endoplasmic reticulum; Golgi = Golgi apparatus; PM = plasma membrane; ECM = extracellular matrix.
Nucleus
Approximately 90% of individuals affected with OI are heterozygous for a causative variant in 1 of the 2 genes, COL1A1 or COL1A2 [Sykes et al., 1990; Körkkö et al., 1998], which encode the pro-α1(I) and pro-α2 (I) chains of type I procollagen, respectively.
Cytoplasm
Procollagen type I is cotranslationally translocated into the lumen of the endoplasmic reticulum [Canty and Kadler, 2005].
Rough Endoplasmic Reticulum
Procollagen type I contains C- and N-terminal propeptides and a large ‘triple helix’ domain comprising predominantly Gly-X-Y triplets. In the rough endoplasmic reticulum, 2 α1(I)-collagen chains encoded by COL1A1 and 1 α2 (I)-collagen chain encoded by COL1A2 align. Interactions between the C propeptides are largely stabilized by interchain disulphide bounds to ensure correct alignment [Prockop et al., 1989]. Protein disulphide isomerase has also been implicated in the formation of inter-chain disulphide bonds [Canty and Kadler, 2005]. The 2 pro-α1 chains and 1 pro-α2 chain then assemble in the C- to N-direction to form a triple helix. During folding, collagen is modified by, among others, specific enzymes that hydroxylate lysine and proline residues and glycosylate hydroxylysyl residues. This post-translational modification stops when triple helix assembly is complete [Engel et al., 1991]. The CRTAP/P3H1/CyPB complex, encoded by the CRTAP, LEPRE1 and PPIB genes, is responsible for the 3-hydroxylation of P986 (p.P1164 counting from the methionine which initiates translation), but the complex most likely also acts as a proline cis-trans isomerase and a molecular chaperone [Ishikawa et al., 2009]. FKBP65 encoded by FKBP10 also acts as a molecular chaperone for type I (pro)collagen [Alanay et al., 2010]. The protein product of PLOD2 hydroxylates telopeptide lysines in the rough endoplasmic reticulum [van der Slot et al., 2003]. HSP47 encoded by SERPINH1 is thought to maintain the stability of the triple helix [Christiansen et al., 2010].
Golgi Apparatus
After folding, the procollagen molecules are transported through the Golgi apparatus and plasma membrane into the extracellular matrix.
Extracellular Matrix
In the extracellular matrix, cleavage of the N- and C-terminal propeptides occurs, and collagen molecules aggregate to form fibrils. Covalent cross-links occur within and between triple-helical collagen molecules in fibrils. These fibrils converge into collagen type I fibers.
Genotype-Phenotype
Autosomal Dominant OI Types I, II-A, II-B, III, and IV
At present, more than 1,000 distinct variants in the COL1A1 and COL1A2 genes have been identified that give rise to OI (https://oi.gene.le.ac.uk) [Dalgleish, 1997, 1998]. Types of mutation as well as position appear to influence the phenotype.
OI type I is mostly characterized by a 50% reduction of the amount of collagen type I (quantitative or haploinsufficiency effect) usually resulting from variants in 1 COL1A1 allele (frameshift, nonsense, and splice-site alterations) that lead to mRNA instability and haploinsufficiency [Marini et al., 2007b] and sometimes from a deletion of the complete COL1A1 allele [van Dijk et al., 2010a] or from substitutions for glycine by small amino acids (cysteine, alanine and serine) near the amino terminal ends of the triple helical domains in either one COL1A1 or COL1A2 allele.
OI types II–IV are characterized by intertwining of mutated and normal collagen type I chains resulting in production of abnormal collagen type I (dominant-negative effect). This occurs usually due to causative variants in either COL1A1 or COL1A2 that result in substitutions for glycine. Less common causative variants include splice-site alterations, insertion/deletion/duplication events that lead to in-frame sequence alterations and variants in the carboxyl-terminal propeptide coding domains. Most of these variants result in synthesis of an abnormal type I procollagen molecule which has to intertwine with normal pro-α chain(s) [Marini et al., 2007b]. This assembly leads to disturbed helical folding resulting in overprocessing by the enzymes responsible for post-translational modification of (pro)collagen type I. Post-translational overmodification of the triple-helical domain results in alterations visible by SDS-polyacrylamide gel electrophoresis.
Autosomal Recessive OI Types II-B, III, IV
It is estimated that 10% of OI cases will be caused by recessive causative variants in 1 of the currently known genes (CRTAP, LEPRE1, PPIB, SERPINH1, FKBP10, PLOD2, SP7, SERPINF1) or in genes that have yet to be discovered.
CRTAP
CRTAP forms a complex with prolyl-3-hydroxylase-1(P3H1) and Cyclophilin B (CyPB). One known function of the complex is the 3-hydroxylation of a single proline residue at position 986 (P986) in the pro-α1 (I)-collagen chain [Marini et al., 2007a]. It is highly likely that the complex also acts as a cis-trans isomerase and a molecular chaperone [Ishikawa et al., 2009] initiating chain recognition and helical folding [Pyott et al., 2011b]. Most CRTAP variants are reported to cause autosomal recessive lethal/severe OI [Barnes et al., 2006] types II-B and III, but CRTAP variants do not seem to cause OI type II-A [Van Dijk et al., 2009a]. Most causative variants result in null alleles with absence or severe reduction of gene transcripts and proteins [Marini et al., 2010b]. Biochemically, decreased prolyl 3-hydroxylation of P986 is evident as well as post-translational overmodification on (pro)collagen gel electrophoresis [Barnes et al., 2006].
LEPRE1
LEPRE1 encodes P3H1 and pathogenic variants in LEPRE1 cause autosomal recessive lethal/severe OI [Cabral et al., 2007; Baldridge et al., 2008], types II-B/III [Van Dijk et al., 2010b]. In most patients, the causative variants result in null alleles with absence or severe reduction of gene transcripts and proteins [Cabral et al., 2007; Willaert et al., 2009; Marini et al., 2010a]. As in CRTAP-related OI, decreased prolyl 3-hydroxylation of P986 in the pro-α1(I)-collagen chains as well as post-translational overmodification are observed [Cabral et al., 2007].
PPIB
PPIB encodes the protein cyclophilin B, a collagen-specific proline cis-trans isomerase. CyPB is bound in a complex with P3H1 and CRTAP. Recessive variants in PPIB were described to cause OI with decreased P986 3-hydroxylation and post-translational overmodification of type I(pro)collagen in 2 families [Van Dijk et al., 2009b]. These findings were confirmed in other patients with OI types II, III and IV due to PPIB mutations [Pyott et al., 2011a]. Interestingly, in another family, a homozygous PPIB variant in the presumed start codon of PPIB (c.2T>G in the sequence of PPIB described by Price et al. [1991], c.26T>G in the current reference sequence of PPIB (NM_000942.4))appeared to cause only a moderately deforming type of OI without decreased P986 3-hydroxylation and detected post-translational overmodification but with absence of CyPB on Western blot [Barnes et al., 2010]. At this point, it would be correct to state that recessive variants in the PPIB gene can cause OI type II-B, III and IV with, in the majority of cases, decreased P986 3-hydroxylation in the pro-α1 (I)-collagen chains and post-translational overmodification, as is seen in CRTAP and LEPRE1-related OI.
SERPINH1
First detected in Dachshund pedigrees with OI [Drögemüller et al., 2009], bi-allelic causative missense variants in the SERPINH1 gene encoding collagen chaperone protein HSP47 appear to result in severe recessive OI. HSP47 monitors the integrity of the triple helix of type I procollagen at the endoplasmic reticulum/cis-Golgi boundary. When absent, the rate of transit of procollagen type I chains from endoplasmic reticulum to Golgi appears to be increased and the triple helical structure is compromised. Since the action of HSP47 appears not to be required for procollagen type I chain modification, no post-translational overmodification is visible on (pro)-collagen gel electrophoresis [Christiansen et al., 2010].
SERPINF1
Four individuals (2 related and 2 unrelated individuals) with OI type III, and without collagen type I overmodification on electrophoresis, were recently described to harbor bi-allelic causative variants in SERPINF1 leading to a loss of pigment-epithelium-derived factor (PEDF). The causative SERPINF1 variants in the index patient were found by next generation sequencing. PEDF is known mainly for its strong inhibition of angiogenesis. However, expression analyses in bone tissue from wild-type mice and in vitro experiments with murine cell systems support a role for PEDF in bone formation and remodeling. It is speculated that a loss of PEDF causes OI independent of collagen type I biosynthesis [Becker et al., 2011].
SP7
Recently, a homozygous causative variant in SP7 was described in a patient with a moderately severe recessive form of OI with recurrent fractures, mild bone deformities, delayed tooth eruption, normal hearing, and white sclerae. SP7 does not seem to encode a protein involved in the collagen type I biosynthesis pathway. However, it should be noted that in vivo studies to assess the effect on collagen type I production could not be performed [Lapunzina et al., 2010]. SP7 encodes Osterix, an osteoblast-specific transcription factor that has been shown to be essential for bone formation in mice [Sun et al., 2010; Zhou et al., 2010].
Autosomal Recessive OI/Bruck Syndrome
PLOD2 and FKBP10
In 1998, a family was reported with bone fragility, congenital contractures of the large joints and aberrant cross-linking of bone collagen [Breslau-Siderius et al., 1998]. The affected individuals were diagnosed as having Bruck syndrome which is characterized by bone fragility in combination with congenital joint contractures. A locus responsible for Bruck syndrome in this family was mapped to 17p12 [Bank et al., 1999]. In other families with similar clinical and biochemical features, recessive variants in PLOD2, encoding a bone-specific telopeptidase lysyl hydroxylase-2, appeared to be causative [van der Slot et al., 2003; Ha-Vinh et al., 2004]. All 3 causative variants identified in PLOD2 are homozygote missense mutations affecting highly conserved residues in exon 17 of PLOD2. These variants possibly alterate folding and isomerization of the protein, leading to severe reduction of the enzymatic activity [Hyry et al., 2009], which results in aberrant cross-linking of bone collagen due to underhydroxylation of lysine residues in the telopeptides.
However, in the family described by Breslau-Siderius et al. [1998], no recessive variants in PLOD2 were found, leading to a classification of Bruck syndrome in type I (with unknown genetic cause) and II (with recessive variants in PLOD2) [van der Slot et al., 2003]. Recently, however, causative variants in FKBP10 (locus 17q12) encoding FKBP65, were described to cause (a) recessive OI without post-translational overmodication of (pro)collagen type I most closely resembling OI type III [Alanay et al., 2010] or (b) Bruck syndrome [Shaheen et al., 2010; Kelley et al., 2011]. The genetic cause of Bruck syndrome type I in the originally described family has not been reported yet.
Bone Formation and OI
Although causative variants in many genes are now known to result in OI, the pathogenic mechanism leading from causative variant(s) to OI is still largely unknown, which emphasizes the need to study normal bone formation.
Bone consists of cells and mineralized extracellular matrix, it provides support and protection, is an insertion site for muscles and serves as a storage site for calcium and phosphate. Four cell types are active in bone tissue: (1) osteoprogenitor cells (resting cells that can transform into osteoblasts, chondrocytes and adipocytes), (2) osteoblasts, (3) osteocytes (mature osteoblasts), and (4) osteoclasts. The bone matrix (osteoid) is secreted by osteoblasts and consists of type I collagen and ground substance containing proteoglycans and non-collageneous glycoproteins. Resorption of the bone matrix is performed by macrophage-related osteoclasts and is important for adaption to growth, repair and mineral mobilization [Ross et al., 1995].
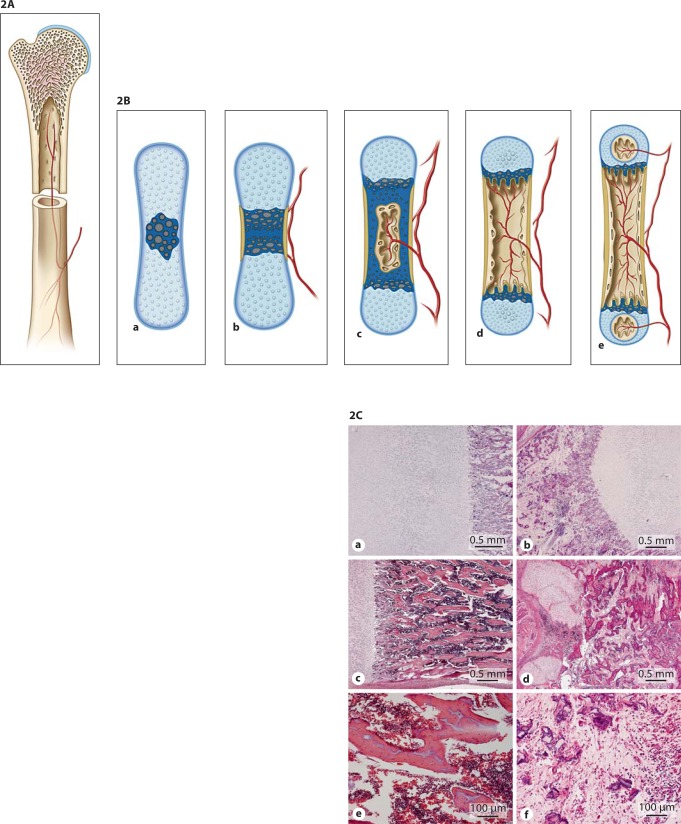
Bone consists of an outer layer of mature compact bone (cortex) largely composed of cylindrical units called osteons or Haversian systems. The inner layer of adult spongy bone is arranged as trabeculae or spicules (fig. 2A). Bone is formed by endochondral (long bones, ribs and vertebrae) (fig. 2B) or intramembranous (flat bones of skull and face, mandible, clavicle, ileum) ossification. Intramembranous ossification takes place by direct differentiation of mesenchymal cells into osteoblasts that secrete osteoid. Osteoblasts retreat or become entrapped as osteocytes in the osteoid. The osteoid calcifies to form spicules of spongy bone, the spicules unite and form trabeculae. Endochondral bone formation is characterized by the presence of a cartilaginous model in which chondrocytes differentiate [Ross et al., 1995] (fig. 2B).
Fig. 2.
A Compact and spongy bone. B Endochondral ossification. Endochondral ossification occurs when mesenchymal cells differentiate into chondroblasts that produce a cartilage matrix. This cartilage acquires the shape of the bone that will be formed. A periosteal collar of bone forms around the diaphysis. Osteoblasts in this region are engaged in periosteal bone formation, which is responsible for the growth in thickness of long bones. In the centre of the diaphysis chondroblasts hypertrophy, the cartilage matrix becomes calcified, blood vessels and connective tissue cells evade the calcified cartilage, creating a marrow cavity (primary ossification center). Trabeculae of calcified cartilage (primary and secondary spongiosa) remain at the 2 ends of the cavity on which endochondral bone forms (secondary ossification centers). CHistologic abnormalities in OI compared to a control. Panels a, c and e show histology of a normal control femur at 18 weeks of gestational age (GA). Panels b, d and f are from an 18-week GA type II OI case. The transition zone of cartilage to primary spongiosa is sharp in both cases (a, b). Minor disruption can occur due to fractures and scar formation in the primary spongiosa (not shown). Panel d shows extensive metaplastic cartilage formation at the sight of a fracture. The bony trabeculae are hypercellular and the marrow is fibrotic. Panel e shows normocellular trabeculae and hematopoietic marrow between these trabeculae. In the severe OI cases, the trabeculae are thin, irregular and hypercellular in comparison with normal trabeculae (f). The marrow in between is fibrotic with hardly any hematopoiesis (d, f).
Paracrine factors and transcription factors appear to be active in the transition of cartilage to bone. For example, SOX9 is important for differentiation of mesenchymal cells into chondroblast (mutations in SOX9 cause campomelic dysplasia [Unger et al., 1993]), whereas RUNX2 (mutations in RUNX2 cause cleidocranial dysplasia [Mundlos et al., 1997]) is critical for differentiation of prehypertrophic chondrocytes into preosteoblasts and activation of Osterix (mutations in SP7, encoding Osterix, cause OI [Lapunzina et al., 2010]), which activates bone-specific proteins [Gilbert, 2010].
Mineralized cartilage is replaced by bone through remodeling. During life, bone is remodeled through the action of osteoblasts and osteoclasts, replacing old bone with new bone. Normally, remodeling is coupled such that the level of resorption is equal to the level of formation and bone density remains constant. The continued growth of the long bones is dependent on the presence of epiphyseal cartilage (epiphyseal plate) between the primary and secondary ossification centers. This continues to form new cartilage which is replaced by bone, resulting in increased length of the bone. Growth continues until the cartilage in the plate is replaced by bone.
A study of iliac bone specimens of 70 children with OI type I, III and IV compared to 27 age-matched controls reported that in OI bone thickness is reduced because of delayed periosteal bone formation. Trabeculae are reduced in number and abnormally thin. The overall bone formation is increased but counteracted by increased activity of bone resorption [Rauch and Glorieux, 2004]. Figure 2C compares bone tissue of an individual affected with OI to a control.
Prenatal and Postnatal Diagnosis of OI with Clinical Examples
The severity of clinical features of OI at birth ranges from no clinical features to prenatally lethal skeletal abnormalities. The clinical variability of OI led to a classification of OI originally in 4 types [Sillence et al., 1979]. In 2004, OI type V, VI and VII were added to this classification. Important for the prenatal as well as postnatal diagnosis of OI is that a continuum of severity is observed in OI with clinical overlap between OI types I and IV, II and III, III and IV. Tables 1 and 2 represent an overview of the clinical and radiological characteristics of OI type I–VI. In our opinion, the addition of OI type VII and the newly proposed OI types VII–XII are unnecessary [Van Dijk et al., 2010c]. Figures 3, 4, 5, 6, 7 are clinical pictures and radiographs of patients with OI type I, II, III, IV with case descriptions in the legend.
Table 1.
Clinical characteristics of OI
| Type | Ia | II-Ab | II-Bb | IIIa | IV | Vc | VId |
|---|---|---|---|---|---|---|---|
| Inheritance | AD | AD | AD/ARe | AD/ARf | AD/ARg | AD | AR |
| Severity | mild | perinatal lethal | severe | moderate-mild | moderate | moderate | moderate |
| Congenital fractures | no | yes | yes | usually | rarely | no | no |
| Bone deformity | rarely | very severe | severe | moderate-severe | mild-moderate | moderate | moderate |
| Sclerae | predominantly blueh | dark bluei | dark bluei | blue/grey/whitej | normal-greyk | normal | normal |
| Stature | normall | severely shortm | severely shortn | very shorto | variable shortp | variable | mild short stature |
| Joint hypermobility | yesq | yes | yes | yesq | variable | variable | variabler |
| Hearing loss | present in ∼60% of casess | NA | NA | commont | present in ∼42% of casesu | no | no |
| Dentinogenesis imperfecta (DI) | variablev | NA | NA | yesw | variable | no | no |
| Respiratory complications | no | yesx | yesx | yesy | no | no | no |
| Neurological complications | no | NA | NA | yesz | no | no | no |
OI type I, II, III, and IV constitute the original Sillence classification [Sillence et al., 1979].
OI type II has been divided in OI type II-A, II-B and II-C on the base of radiological characteristics [Sillence et al., 1984]. II-C is an uncertain entity that is extremely rare; it is left out of consideration in this table.
OI type V was added to the Sillence classification based on distinct clinical/radiological (limitations in the range of pronation/supination in one or both forearms associated with a radiologically apparent calcification of the interosseous membrane) and histological features (irregular arrangement or meshlike appearance of lamellae) in patients originally diagnosed with OI type IV in the absence of COL1A1I2 mutations [Glorieux et al., 2000].
OI type VI was added to the Sillence classification because of distinct histological features (increase in both osteoid surface and thickness pointing to a mineralization defect) in the absence of COL1A1/2 mutations in patients originally diagnosed with OI type IV but without abnormalities of collagen type I on electrophoresis. Autosomal recessive inheritance is presumed because of 2 consanguineous families with recurrence of OI in 1 of these families [Glorieux et al., 2002].
Recessive variants in CRTAP, LEPRE1 and PPIB have been described to result in a clinical/radiological phenotype indistinguishable from OI type II-B [Van Dijk et al., 2009a, b, 2010b].
Recessive variants in CRTAP, LEPRE1, PPIB, SERPINH1, SERPINF1, FKBP10 can result in a clinical/radiological phenotype of OI type III [Bal-dridge et al., 2008; Van Dijk et al., 2009a, b; Alanay et al., 2010; Christiansen et al., 2010; Becker et al., 2011; Pyott et al., 2011a].
Recessive variants in CRTAP, PPIB, SP7 can result in a clinical/radiological phenotype of OI type IV [Morello et al., 2006; Barnes et al., 2010; Lapunzina et al., 2010; Pyott et al., 2011a].
In infants <1 year, blue sclerae can be observed as a normal phenomenon due to a thin, scleral envelope and the underlying darkly pigmented choroid layer [Aase, 1990]. This normal blue sclera usually disappears by 1 year of age. In OI, the presence of blue sclera is not associated with any significant ocular pathology [Zack et al., 2007]. A correlation between blue sclerae and reduced corneal thickness has been described [Evereklioglu et al., 2002] but has also been challenged by opposite findings [Sarathchandra et al., 1999]. In spite of also other explanations [Eichholtz and Miiller, 1972; Lanting et al., 1985], the etiology of the blue sclerae in OI type I is unclear [Zack etal., 2007].
The blue sclera in OI type II, as well as the blue sclera that can be observed in OI type III (and sometimes in OI type IV), are probably the result of the light reflected from the pigmented layers of the eye because of abnormal slender collagen fibrils and reduced tissue thickness [Chan et al., 1982; Pedersen and Bramsen, 1984; Zack et al., 2007].
Short stature has been described in OI type I, but most patients do not meet the criteria of growth deficiency (>-2 SD). However, in most instances they will be shorter than family members [Marini et al., 1995].
The cause of short stature in OI is unclear. Children with OI type III will typically have a final adult stature in the range of a prepubertal child. The final stature of a child with OI type IV approximates that of an early teenager [Marini, 2010a].
Generalized hypermobility is described in OI type I [Engelbert et al., 1997]. Joint hypermobility (ligamentous laxity) is also a feature of OI types II-A, B and III [Spranger et al., 2003].
Of the first 16 patients with OI type VI, 50% had ligamentous laxity [Glorieux et al., 2002].
According to a Finnish study in adult patients with OI, conductive or mixed hearing loss in late adolescence is observed in 60.4% of patients with OI type 1,42.3% of patients with OI type IV and is common in OI type III. The hearing loss in OI resembles that of otosclerosis. In most cases, the hearing loss is initially conductive and later mixed or sensorineural. It is common in adults and usually progressive [Kuurila et al., 2002].
OI type I and IV have been subdivided in the past based on absence of DI (IA, IVA) and presence of DI (IB, IVB) [Levin et al., 1978].
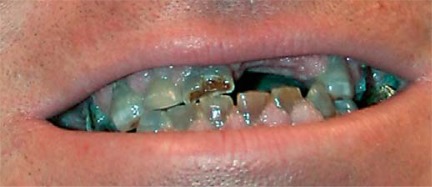
In DI observed in patients affected with OI (fig. 9), the dentine structure of both primary and secondary dentitions are abnormal with teeth appearing typically amber and translucent and showing significant attrition [Barren et al., 2008].
Infants with OI type II die mostly perinatally of respiratory insufficiency or pneumonias. Children with OI type III develop vertebral collapse and kyphoscoliosis later in life, which contribute to restrictive lung disease. They are at risk for developing multiple pneumonias. Lung disease can progress into cor pulmonale [Marini, 2010a].
There is a risk of basilar invagination in patients with OI type III [Marini, 2010a].
Table 2.
Radiological characteristics of OI types I-VI
| Type: I | II-A | II-B | III | IV | V | VI | |
|---|---|---|---|---|---|---|---|
| Radiographic features in perinatal/infantile period | |||||||
| Skull | wormian bones | severely diminished mineralization, wormian bones | diminished mineralization, wormian bones | diminished mineralization, wormian bones | diminished mineralization, sometimes with wormian bones | possibly diminished mineralization, sometimes with wormian bones | 8/11 patients reported had no wormian bones |
| Ribs | no fractures | short, broadened ribs with continuous beading/fractures | thin ribs with discontinuous beading/fractures | thin ribs with discontinuous beading/fractures | no congenital fractures | no congenital fractures | no congenital fractures |
| Vertebrae | normal at birth | platyspondyly at birth | platyspondyly at birth | platyspondyly at birth | normal at birth | normal at birth | normal at birth |
| Extremities | normal modeling at birth | thick, short, crumpled shafts of the long bones | short, deformed long tubular bones | short, deformed long tubular bones | bowing of long bones | calcification of osseous membrane in forearms; bowing of long bones | bowing of long bones |
| Other | usually no congenital fractures or osteopenia | generalized osteopenia; multiple fractures with callus formation | generalized steopenia; multiple fractures with callus formation | generalized osteopenia multiple fractures with callus formation | generalized oteopenia | generalized osteopenia | generalized osteopenia |
| Radiographic features later in life | |||||||
| slender shafts of tubular bones with thin cortex and poorly trabeculated spongiosa; vertebral compression fractures | not applicable | not applicable | kyphoscoliosis with compressed vertebral bodies; thin, severely deformed long tubular bones often with popcorn calcifications; deformed skull with temporal bulge and platybasia; coxa vara | progressive bowing of long bones in some patients; vertebral compression fractures; coxa vara | progressive bowing of long bones in some patients; vertebral compression fractures; coxa vara | progressive bowing of long bones in some patients; vertebral compression fractures; coxa vara | |
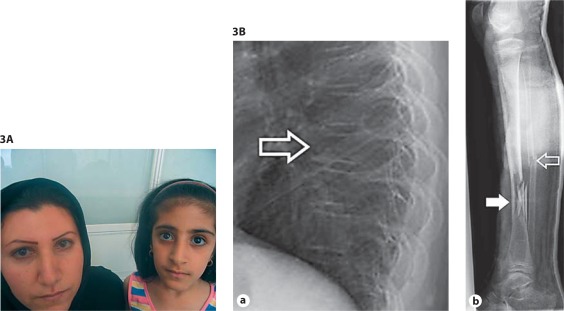
Fig. 3.
A Clinical pictures of patients with OI type I. Blue sclerae in a mother and daughter with OI type I. Clinical synopsis: An Iranian mother and daughter were referred because of suspected OI. The mother, 28 years old, has had 3 fractures in the wrist, ankle and femur occurring between 2–3 years of age. She developed hearing loss at the age of 13–14. She has strikingly blue sclerae, her height is 160 cm (−1.5 SD) and she has no evidence of dentinogenesis imperfecta (DI). The daughter, 8 years old, has had no fractures, a normal height of 125 cm (−1 SD) and no DI or hearing loss. Only deep blue sclerae can be observed. Coincidentally, she appears to have decreased vision due to deterioration of cone cells. Radiographs and bone densitometry of both mother and daughter were normal. There were no family members affected with OI. Molecular analyses of the COL1A1/2 genes revealed a heterozygous variant in the COL1A1 gene creating a premature stop codon (c.1081C>T; p.Arg361X). B Radiographs of a patient with OI type I. a Lateral radiograph showing osteopenia and mid thoracic vertebral compression fracture (open arrow). Note a general decrease in height, compared to adjacent vertebral bodies, and some wedging. b Oblique mid-diaphyseal fibula fracture (open arrow) and a communitive diaphyseal tibia fracture (arrow). Clinical synopsis: The patient is a 10-year-old girl with OI type I due to a deletion of the whole COL1A1 allele, who had 8 fractures so far. A fracture of the femur was noted shortly after birth. Her length is 128.5 cm (−2.5 SD), head circumference is 53.5 cm (0 SD) and bone density of the hips and lumbar vertebral column is between −4 and −7 SD, as measured by dual X-ray absorptiometry. She has wormian bones, a scoliosis and grey-blue sclerae typical for OI. She has no DI [van Dijk et al., 2010a].
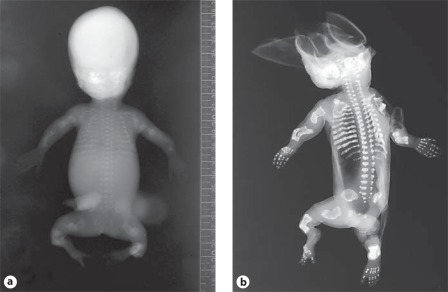
Fig. 4.
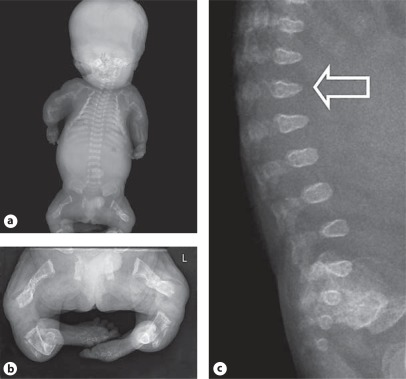
A OI type II-A in a preterm infant. Skeletal overviews without (a) and with (b) silver nitrate impregnation show generalized osteopenia with diminished ossification of the calvarian bones and gross skeletal deformation. No vertebral anomalies are seen. The ribs are broad with continuous fractures (continuous beading). The long bones show multiple fractures and are shortened, deformed and broadened. Clinical synopsis: It concerned the second pregnancy of a nonconsanguineous Caucasian couple. At a gestational age of 21 weeks, ultrasonographic abnormalities were seen. All long bones were severely shortened (<p5). Bowing of the humeri and the femora was observed. The skull appeared somewhat doligocephalic with a remarkable clear imaging of the cerebrum and could be deformed by pressure of the ultrasound transducer. The parents decided to terminate the pregnancy, and a child was born at a gestational age of 22+3 weeks with a birth weight of 160 g. A skeletal overview revealed an almost total absence of skull mineralization and multiple fractures of the ribs and the long bones consistent with a diagnosis of OI type II-A. Bone histology showed hypercellular irregular trabeculae with multiple fractures, fibrotic marrow and metaplastic cartilage formation. On collagen electrophoresis, post-translational overmodification was observed. A causative variant in the COL1A1 gene was found (c.2300G>A; p.Gly767Asp). B Perinatal OI type II-A. a Skeletal overview of a 35+4-week-old fetus with OI type II-A shows multiple fractures of both the long bones as well as the ribs. Note the under mineralization of the skull and its deformity on the left side. b Detail of the lower extremities shows multiple consolidated fractures resulting in deformed growth of the femurs, tibiae and fibulae. c Lateral radiograph of the lumbar spine shows mild platyspondyly (arrow). Clinical synopsis: It concerned the first pregnancy of a nonconsanguineous Caucasian couple. At a pregnancy duration of 35+4 weeks, a boy was delivered by caesarean section. Apgar scores were 4/4/4 after 1, 5 and 10 min, respectively. Birth weight was 1,100 g. Blue sclerae with proptosis were visible. The thorax was small and bell-shaped. Fractures of the ribs were felt. Gasping breath with insufficient thorax excursions was apparent. Auscultation of the heart revealed bradycardia with no other anomalies. The skin was thin and fragile with a large skin defect temporo-occipital. The extremities were short and deformed. The child died 30 min after birth due to severe respiratory and circulatory insufficiency. A skeletal overview was consistent with a diagnosis of OI. Histology showed decreased osteoid formation at the level of the primary spongiosis in combination with decreased numbers of osteoblasts and osteoclasts. A causative variant in the COL1A1 gene c.1804G>A; p.Gly602Arg was found.
Fig. 5.
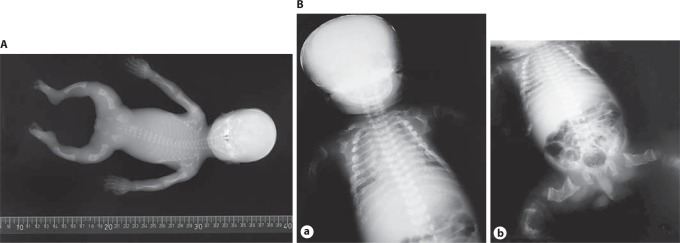
A OI type II-B in a preterm infant. Fetal anteroposterior radiographs of proband 1 from family 1 at 21+2 weeks of gestation show: normal skull mineralization for gestational age, slender ribs without fractures, incomplete ossification of T5 and T12, somewhat irregular proximal metaphyses of the humeri, radii and ulnae, and bowing of the ulnae. Bowed femora with fractures and some loss of modeling, bowed tibiae and fibula are apparent, possibly with fractures. Clinical synopsis: The affected individual was delivered after termination of pregnancy at 22+1 weeks of gestation. She was the second child of nonconsanguineous North European parents. During pregnancy, the diagnosis OI was suspected based on advanced ultrasounds. Bone histology was indicative of OI. Overmodification of collagen type I in fibroblasts was evident on electrophoresis. A homozygous causative variant c.556_559delAAGA in exon 5, resulting in p.Lys186GlnfsX8, was detected in the PPIB gene [van Dijk et al., 2009b]. B Perinatal OI type II-B. a Radiograph shows diminished but visible mineralization of the calvarium. The ribs show multiple fractures in a discontinuous pattern with normal rib parts in between callus formation (discontinuous beading). There are bilateral clavicular fractures. b The long bones of the lower extremities are broadened, deformed and shortened as a result of multiple fractures. There is no complete loss of modeling of the femora. Clinical synopsis: It concerned the first pregnancy of a nonconsanguineous Caucasian couple. A boy was born at term. A severe skeletal dysplasia was suspected. A skeletal overview showed decreased skull mineralization and multiple fractures of the ribs and the long bones. A causative variant in the COL1A2 gene was found (c.1720G>A; p.Gly574Ser).
Fig. 6.
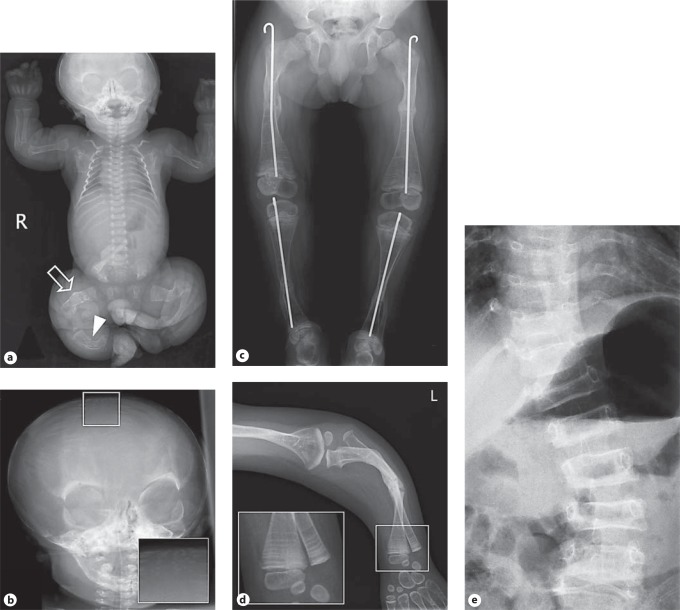
A Clinical pictures of a patient with OI type III. a–c White sclerae, severe kyphoscoliosis with thoracal deformation, severe shortening and bowing of arms and legs. Clinical synopsis: a 10-year-old Iranian girl was born with fractures of the humerus and the tibia. According to the parents, their daughter has had about 100 fractures up to now. Her length is 88 cm (<<−3 SD; 0 SD for a 2-year-old child), weight is 13 kg (0.5 SD (weight for length)) and her head circumference is 49 cm (+2 SD (HC for age)). She has white sclerae. DI was apparent. The skin was soft. Cognition was normal. Molecular analysis of the COL1A1/2 genes is currently being performed. B Radiographs of a patient with OI type III. a The skull shows normal mineralization. The spinal column shows normal development and no fractures. The ribs are slender without fractures. No fractures of humeri, radii and ulnae are visible. Multiple fractures of femora with loss of modeling (arrow) can be observed in combination with fracture and bowing of right tibia (arrowhead). b Wormian bones (see inset), broad skull. c At the age of 5 years, radiographs of the lower extremities show osteopenia and multiple fractures for which surgical intervention, using intramedullary rods, has been performed. No popcorn epiphyses are observed. Multiple growth acceleration lines are visible due to intravenous biphosphonate treatment and calcium suppletion (arrow). d Radiograph of the left arm shows normal epiphyses, with a broad metaphysis of the distal humerus. Note mid-diaphyseal fractures of the radius and ulna. Dislocation of the radial head is observed. e AP Spine shows platypondyly and scoliosis. Clinical synopsis: In the first pregnancy, abnormalities of the extremities were observed in the fetus at 23 weeks of gestation. At the 24th week of gestation, short upper and lower extremities were observed indicative of a severe skeletal dysplasia. At a gestation of 40+4 weeks, a Caucasian boy was born. He had a round face with shallow orbits, greyish sclerae, small thorax, rhizomelic shortening of the upper and lower extremities, abducted position of the legs, and normocephaly. A skeletal overview showed multiple fractures suggestive of OI type II-B/III. The child is alive at the age of 4 years with a current diagnosis of OI type III due to a homozygous CRTAP mutation (intron 1, c.471+2C>A) [van Dijk et al., 2009a].
Fig. 7.
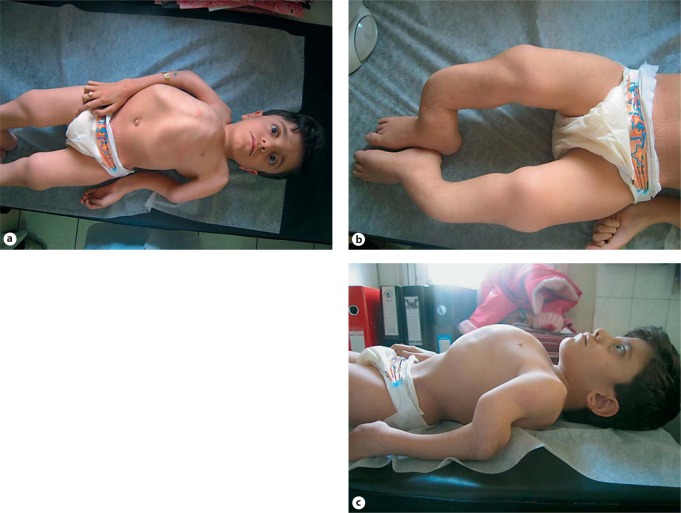
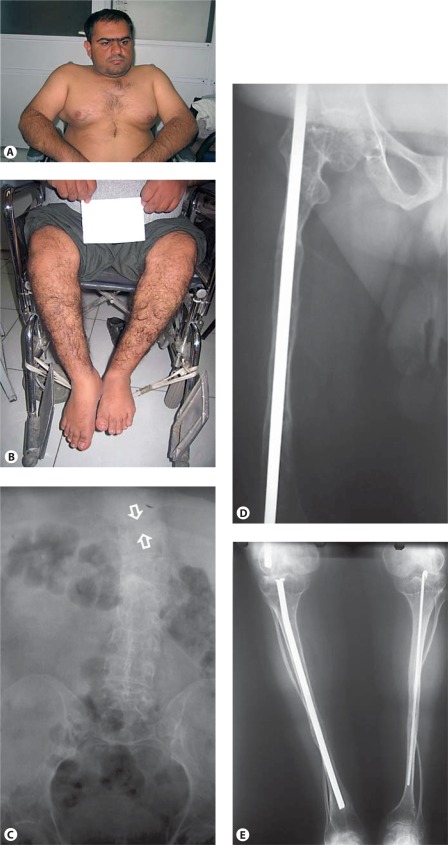
Clinical pictures and radiographs of a patient with OI type IV. A, B White sclerae, muscular upper extremities, wheelchair bound. C AP radiograph of the abdomen, of poor quality, shows a compression fracture of T10 (between arrows). D, E Radiographs of the lower extremities show reduced bone density and thin tibia shafts with both fibula being very thin and tortuous. Intramedullary rods are in position. Clinical synopsis: A 33-year-old Iranian man consulted a clinical geneticist when his wife was pregnant as he wanted to be informed about the chance of recurrence of OI in his unborn child. His height and head circumference are, respectively, 145 cm (−5.5 SD) and 57 cm (−0.5 SD). He claims he has had multiple fractures first occurring at 2 years of age. Unfortunately, no documented medical history is available. His sclerae are greyish. No hearing loss or DI is present. His father and sister were also known to be affected with OI. MLPA analysis of the COL1A1 gene showed a partial deletion of COL1A1 (exon 6–51).
Prenatal Diagnosis of OI
OI type II and III (fig. 4, 5, 6) can prenatally be diagnosed with ultrasonography since they are likely to have prenatal fractures that can be observed. Increased nuchal translucency can be the earliest (nonspecific) sonographic sign of OI type II [Viora et al., 2002]. Sonographic signs of OI type II can be detected as early as 14 weeks [Marini et al., 2007b] due to reduced echogenicity of the fetal bones, followed by multiple fractures at various stages of healing and deformity of the long bones, ribs and skull [Morgan and Marcus, 2010]. Sonographic details of OI type III are usually visible from 18 weeks [Marini et al., 2007b], whereas OI type IV may occasionally be detected after 20 weeks [Marini et al., 2007b] and may consist of bowing of the long bones with or without shortening and without evidence of fractures or osteopenia. Prenatal ultrasonographic diagnosis of OI type I is unreliable. For differential diagnosis, campomelic dysplasia and perinatal lethal hypophosphatasia can be considered [Morgan and Marcus, 2010]. Pathognomonic radiological/ultrasonographic features of campomelic dysplasia are cervical spine abnormalities, scapular hypoplasia, narrow iliac wings, bowing of the femora and the tibiae, and club feet [Unger et al., 1993]. Distinguishing features of perinatal lethal hypophosphatasia are (i) deficient ossification of the spine notably in the thoracic region with patchy ossification of ribs and vertebrae, and (ii) deep cupping of the metaphyses of the long bones [Zankl et al., 2008].
The ultrasonographic prenatal diagnosis of OI can be confirmed by laboratory investigations either by (a) performing a chorion villus biopsy with cultured chorion villi cells showing abnormal production of collagen type I, visible as post-translational overmodification on procollagen electrophoresis, or (b) by performing a chorion villus biopsy/amniocentesis to obtain fetal DNA for molecular analysis of genes involved in OI. Recently, guidelines have been established for the laboratory diagnosis of OI. Molecular analysis of the COL1A1/2 genes will be the first step, followed by DNA analysis of the other causative genes upon re-evaluation and confirmation of the diagnosis OI [van Dijk et al., 2011].
Prenatal or preimplantation genetic diagnosis with the intention of terminating a pregnancy or not selecting embryos carrying the causative variant(s) is possible in the case of identification of known disease-causing variants [van Dijk et al., 2011].
Postnatal Diagnosis of OI
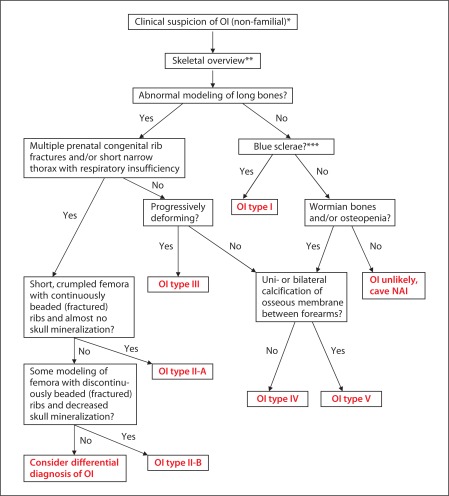
OI types I–V are clinically diagnosed peri- (types II, III and sometimes IV) and postnatally (all types). A flow diagram for the postnatal diagnosis of OI is presented in figure 8. Clinical case examples are presented in figures 3, 6 and 7. For differential diagnosis, rare genetic conditions such as Bruck syndrome (MIM 259450, 609220), Osteoporosis pseudoglioma syndrome (MIM 259770), Cole-Carpenter syndrome (MIM 112240), Hajdu-Cheney (MIM 102500), and gerodermia osteodysplastica (MIM 231070) can be considered. Furthermore, idiopathic juvenile osteoporosis (MIM 259750) and isolated dentinogenesis imperfecta (MIM 125490) can be important differential diagnostic considerations. The most common to be considered is nonaccidental injury (NAI), frequently in cases of suspected OI type I or IV [Bilo et al., 2010]. Fractures resulting from NAI occur in 24/10,000 children under 3 years of age, whereas the OI prevalence is 1/10,000–20,000 [Marlowe et al., 2002]. In the study by Marlowe et al. [2002], the reported incidence of OI among children evaluated for NAI was 2–5%. Differentiation between OI and NAI is aided by an experienced clinician familiar with OI [Ablin et al., 1990]. A positive family history, the presence of blue sclerae, osteoporosis, multiple wormian bones in the skull, and platyspondyly point to OI [Chapman and Hall, 1997]. The laboratory confirmation of OI is made preferably by DNA analysis of the genes involved in OI or by decreased or abnormal production of (pro)collagen type I by fibroblasts measured on procollagen electrophoresis.
Fig. 8.
Flow schedule for postnatal diagnosis of OI. * Recurrent fractures, shortening of limbs, deformation of bones, short stature, early osteoporosis, blue sclerae, hearing loss, dental problems, and joint laxity. ** Particularly in case of short stature and/or disproportiate stature and/or clinical deformity of long bones. *** In infants <1 year, blue sclerae can be a normal phenomenon.
Genetic Counseling
In a large majority of patients, OI type I is caused by dominant (de novo or recurrent) causative variants in the COL1A1/2 genes.
OI types II–IV can be autosomal dominantly and autosomal recessively inherited. In case of autosomal dominant inheritance, the causative variant is either de novo or, with clinically unaffected parents, recurrent due to germ line mosaicism in a parent. The empirical recurrence risk is a mixture of recurrence risk due to gonadal mosaicism and autosomal recessive inheritance.
Pepin et al. [1997] reported a 2% empirical recurrence risk of lethal OI for families with one previous affected child. In a recent study conducted by Pyott et al. [2011b], a recurrence rate of 1.3% was observed for lethal OI (based on 1 recurrence in 76 families with 1 previous affected child). Interestingly, it was reported that approximately 16% of parents with 1 affected child due to a causative variant in COL1A1/2 was mosaic in somatic cells [Pyott et al., 2011b], resulting in a higher risk of recurrence of OI in these families.
Identifying the causative variant(s) and DNA analysis of the parents in case of a causative variant in COL1A1/2 is necessary for an accurate estimate of the recurrence risk, which is important for genetic counseling in case of early prenatal diagnosis and preimplantation genetic diagnosis [Pyott et al., 2011b].
Management
When the diagnosis OI has been established, the affected individual should preferentially be evaluated by a multidisciplinary team [Steiner et al., 1993]. Important members of the team would be orthopedic surgeons, rehabilitation physicians, endocrinologists, physical therapists, and pediatricians. Referral to other disciplines can take place upon individual needs and for routine surveillance such as dental controls. Management consists of pharmacological treatment, orthopedic treatment, physical medicine, dental treatment, treatment for hearing loss, and prevention of primary (e.g. basilar impression) and secondary (e.g. problems due to general medical disciplines) complications [Steiner et al., 1993].
Pharmacological Treatment
Oral and intravenous bisphosphonates are commonly prescribed for all OI types, adults and children since the first publication of the effect of bisphosphonate treatment in children with severe OI [Glorieux et al., 1998]. The main rationale for bisphosphonate therapy is based on several (not placebo-controlled) clinical trials that showed improvements of bone mineral density in individuals with OI. Nitrogenous bisphosphonates disrupt osteoclast formation, survival and cytoskeletal dynamics, and non-nitrogenous bisphosphonates initiate osteoclast apoptosis. A recently published systematic review of bisphosphonate treatment in OI [Philippi et al., 2009] concluded that in a relatively small group of patients, there is significant improvement in bone mineral density in individuals affected with OI and treated with oral or intravenous bisphosphonates. However, the most important question arising is whether increase in bone mineral density leads to fracture reduction and functional improvement; this has not been answered yet and warrants further investigations [Philippi et al., 2009]. The use of growth hormone to affect short stature in types III and IV OI [Marini et al., 2003] is still under active investigation [Marini et al., 2010a].
Orthopedic Treatment
In case of decreased bone mineralization, high fracture frequency and/or bone deformities, intramedullary rods will be placed in the majority of patients with OI types III and IV and sometimes in OI type I [Monti et al., 2010]. These rods are inserted in the bone marrow canal in the center of the long bones and are used to align and stabilize fractures (fig. 6Ba and 7C, D). Severe scoliosis occurs most often in patients with OI type III (fig. 6Be) and sometimes IV and appears not to be related to the number of vertebral compression fractures. Since severe scoliosis can lead to pulmonary insufficiency, corrective surgery is often performed when the curvature is less than 60° [Marini et al., 2010a]. In case of anesthesia, precautions should be undertaken during intubation because of possible cervical fragility, and the patient should be carefully monitored during surgery because of the (possibly weak) association with hyperthermia during anesthesia [Monti et al., 2010; Oakley and Reece, 2010]. Nonsurgical management consists of bracing and splinting interventions [Monti et al., 2010].
Physical Medicine Treatment (Rehabilitation)
An intensive rehabilitation program is necessary especially in OI types III and IV [Monti et al., 2010] with early intervention such as correct positioning of the child and proper head support, muscle strengthening (isotonic) and aerobic conditioning [Marini et al., 2010a].
Dental Treatment
In patients with dentinogenesis imperfecta, fractures and excessive wear of fragile teeth often occurs (fig. 9). This can be treated by capping teeth with hard polymers in order to prevent infections and facial deformities due to the loss of (parts of) teeth and/or malocclusion [Monti et al., 2010].
Fig. 9.
DI in a patient with OI type III.
Treatment for Hearing Loss
Hearing loss often occurs in adults with OI. Initially it concerns conductive hearing loss, but as the hearing loss progresses, a significant sensorineural component emerges. Surveillance for hearing loss is advised after adolescence every 3–5 years [Steiner et al., 1993]. Initially, hearing aids will be sufficient. As the hearing loss progresses, stapedectomy can be considered for which successful outcomes have been reported; however, long-term hearing restoration may be unsatisfactory due to fragility of the ossicular middle ear structures. Cochlear implantation has been reported because of the sensorineural hearing loss, but data are too limited to draw conclusions on effectiveness [Marini et al., 2010a].
Basilar Invagination
Basilar invagination is a rare complication occurring in adults with OI type III when the top of the C2 vertebra migrates upward which may lead to (partial) closure of the foramen magnum with hydrocephalus, pressure on the brain stem, syrinx formation, and hindbrain herniation, requiring ventricular shunt placement or surgery. Only prolonged orthotic immobilization has been proven to stabilize symptoms and arrest progression [Monti et al., 2010].
Pregnancy and Mode of Delivery
Pregnant women with OI who have significant skeletal deformity and short stature should be monitored in high-risk prenatal care centers, not only for maternal reasons but also because of the risk that the fetus is affected with OI as well [Steiner et al., 1993]. In a large retrospective study of 167 pregnancies in which a child was affected with OI, there was an unusually high rate of breech presentation at term (37%). Cesarean delivery did not decrease fracture rates at birth in infants with OI types I, III and IV nor did it prolong survival in OI type II. Prenatal diagnosis did not influence the mode of delivery in most instances [Cubert et al., 2001].
Future Prospects for OI Therapy
Gene Therapy: Silencing or Replacement of the Allele Containing the Causative Variant
Ninety percent of OI patients have a causative variant in the COL1A1/2 genes encoding either the (pro)-α1 chain or the (pro)-α2 chain of collagen type I. OI types II–IV result from intertwining of mutated and normal collagen type I chains resulting in production of abnormal collagen type I (dominant-negative effect), whereas OI type I is mostly due to decreased or nonexpression of 1 COL1A1 allele (haploinsufficiency effect). It was therefore thought that the future treatment of OI should consist of so-called antisense suppression therapy, directed towards decreasing or silencing of the allele containing the causative variant. This would transform a severe type of OI into a mild OI type. Various murine models (oim [Saban et al., 1996], brtl IV [Kozloff et al., 2004], crtap–/– [Morello et al., 2006], Mov-13 [Bonadio et al., 1990], OASIS–/– [Sekiya et al., 2010]) have been developed, which can be used for the purpose of investigating gene therapy in OI. However, the various antisense techniques (short oligonucleotides [Wang et al., 1996], ribozymes [Grassi et al., 1997], silencing RNA [Millington-Ward et al., 2004]) all lack true specificity against the mutant transcript. Other problems are stability of the antisense molecules apart from specific problems which each technique. As such, antisense suppression therapy is currently limited to in vitro studies [Monti et al., 2010].
Another approach is the replacement of cells harboring the causative variant with normal cells by bone marrow transplantation leading to engraftment of functional mesenchymal stem cells that differentiate into bone cells [Undale et al., 2009]. This approach may hold promises for OI treatment as is illustrated by various in vitro and in vivo studies (children with severe OI) [Horwitz et al., 1999, 2001, 2002; Panaroni et al., 2009]. Further studies are warranted to evaluate whether bone marrow transplantation could be a possible treatment for patients with OI.
Conclusion
Osteogenesis imperfecta is a complex hereditary disease with (i) a remarkable clinical variability warranting a logical classification system; (ii) causative recessive or dominant variants in 8 different genes with 6 of 8 genes encoding proteins involved in collagen type I biosynthesis; (iii) a need for multidisciplinary management and further investigations of therapeutic approaches such as bisphosphonates, growth hormone therapy and gene therapy.
References
- Aase JM. Diagnostic Dysmorphology. New York: Plenum Medical Book Company; 1990. [Google Scholar]
- Ablin DS, Greenspan A, Reinhart M, Grix A. Differentiation of child abuse from osteogenesis imperfecta. AJR Am J Roentgenol. 1990;154:1035–1046. doi: 10.2214/ajr.154.5.2108539. [DOI] [PubMed] [Google Scholar]
- Alanay Y, Avaygan H, Camacho N, Utine GE, Boduroglu K, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86:551–559. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge D, Schwarze U, Morello R, Lennington J, Bertin TK, et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat. 2008;29:1435–1442. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baljet B. Willem Vrolik as a teratologist [in Dutch] Ned Tijdschr Geneeskd. 1984;128:1530–1534. [PubMed] [Google Scholar]
- Baljet B. Aspects of the history of osteogenesis imperfecta (Vrolik's syndrome) Ann Anat. 2002;184:1–7. doi: 10.1016/S0940-9602(02)80023-1. [DOI] [PubMed] [Google Scholar]
- Bank RA, Robins SP, Wijmenga C, Breslau-Siderius LJ, Bardoel AF, et al. Defective collagen cross-linking in bone, but not in ligament or cartilage, in Bruck syndrome: indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proc Natl Acad Sci USA. 1999;96:1054–1058. doi: 10.1073/pnas.96.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AM, Chang W, Morello R, Cabral WA, Weis M, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355:2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AM, Carter EM, Cabral WA, Weis M, Chang W, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MJ, McDonnell ST, Mackie I, Dixon MJ. Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet J Rare Dis. 2008;3:31. doi: 10.1186/1750-1172-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88:362–371. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilo RAC, Robben SGF, van Rijn RR. Forensic Aspects of Paediatric Fractures: Differentiating Accidental Trauma from Child Abuse. ed 1. Heidelberg: Springer; 2010. [Google Scholar]
- Bonadio J, Saunders TL, Tsai E, Goldstein SA, Morris-Wiman J, et al. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1990;87:7145–7149. doi: 10.1073/pnas.87.18.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau-Siderius EJ, Engelbert RH, Pals G, van der Sluijs JA. Bruck syndrome: a rare combination of bone fragility and multiple congenital joint contractures. J Pediatr Orthop B. 1998;7:35–38. [PubMed] [Google Scholar]
- Byers PH, Bonadio JF, Cohn DH, Starman BJ, Wenstrup RJ, Willing MC. Osteogenesis imperfecta: the molecular basis of clinical heterogeneity. Ann NY Acad Sci. 1988;543:117–128. doi: 10.1111/j.1749-6632.1988.tb55324.x. [DOI] [PubMed] [Google Scholar]
- Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Chan CC, Green WR, de la Cruz ZC, Hillis A. Ocular findings in osteogenesis imperfecta congenita. Arch Ophthalmol. 1982;100:1458–1463. doi: 10.1001/archopht.1982.01030040437014. [DOI] [PubMed] [Google Scholar]
- Chapman S, Hall CM. Non-accidental injury or brittle bones. Pediatr Radiol. 1997;27:106–110. doi: 10.1007/s002470050078. [DOI] [PubMed] [Google Scholar]
- Christiansen HE, Schwarze U, Pyott SM, AlSwaid A, Al Balwi M, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86:389–398. doi: 10.1016/j.ajhg.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ML, Williams CJ, Pepe G, Hirsch JL, Prockop DJ, Ramirez F. Internal deletion in a collagen gene in a perinatal lethal form of osteogenesis imperfecta. Nature. 1983;304:78–80. doi: 10.1038/304078a0. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal L, Bonaventure J, Maroteaux P. Dominant mutations in familial lethal and severe osteogenesis imperfecta. Hum Genet. 1991;87:297–301. doi: 10.1007/BF00200907. [DOI] [PubMed] [Google Scholar]
- Cohn DH, Starman BJ, Blumberg B, Byers PH. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1) Am J Hum Genet. 1990;46:591–601. [PMC free article] [PubMed] [Google Scholar]
- Cubert R, Cheng EY, Mack S, Pepin MG, Byers PH. Osteogenesis imperfecta: mode of delivery and neonatal outcome. Obstet Gynecol. 2001;97:66–69. doi: 10.1016/s0029-7844(00)01100-5. [DOI] [PubMed] [Google Scholar]
- Dalgleish R. The human type I collagen mutation database. Nucleic Acids Res. 1997;25:181–187. doi: 10.1093/nar/25.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish R. The Human Collagen Mutation Database 1998. Nucleic Acids Res. 1998;26:253–255. doi: 10.1093/nar/26.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drögemüller C, Becker D, Brunner A, Haase B, Kircher P, et al. A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet. 2009;5:e1000579. doi: 10.1371/journal.pgen.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholtz W, Müller D. Electron microscopy findings on the cornea and sclera in osteogenesis imperfecta [in German] Klin Monbl Augenheilkd. 1972;161:646–653. [PubMed] [Google Scholar]
- Engel J, Prockop DJ. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu Rev Biophys Biophys Chem. 1991;20:137–152. doi: 10.1146/annurev.bb.20.060191.001033. [DOI] [PubMed] [Google Scholar]
- Engelbert RH, van der Graaf Y, van ER, Beemer FA, Helders PJ. Osteogenesis imperfecta in childhood: impairment and disability. Pediatrics. 1997;99:E3. doi: 10.1542/peds.99.2.e3. [DOI] [PubMed] [Google Scholar]
- Evereklioglu C, Madenci E, Bayazit YA, Yilmaz K, Balat A, Bekir NA. Central corneal thickness is lower in osteogenesis imperfecta and negatively correlates with the presence of blue sclera. Ophthalmic Physiol Opt. 2002;22:511–515. doi: 10.1046/j.1475-1313.2002.00062.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. ed 9. Sunderland: Sinauer Associates; 2010. [Google Scholar]
- Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000;15:1650–1658. doi: 10.1359/jbmr.2000.15.9.1650. [DOI] [PubMed] [Google Scholar]
- Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002;17:30–38. doi: 10.1359/jbmr.2002.17.1.30. [DOI] [PubMed] [Google Scholar]
- Grassi G, Forlino A, Marini JC. Cleavage of collagen RNA transcripts by hammerhead ribozymes in vitro is mutation-specific and shows competitive binding effects. Nucleic Acids Res. 1997;25:3451–3458. doi: 10.1093/nar/25.17.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha-Vinh R, Alanay Y, Bank RA, Campos-Xavier AB, Zankl A, et al. Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am J Med Genet A. 2004;131:115–120. doi: 10.1002/ajmg.a.30231. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyry M, Lantto J, Myllyharju J. Missense mutations that cause Bruck syndrome affect enzymatic activity, folding, and oligomerization of lysyl hydroxylase 2. J Biol Chem. 2009;284:30917–30924. doi: 10.1074/jbc.M109.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Wirz J, Vranka JA, Nagata K, Bächinger HP. Biochemical characterization of the prolyl 3-hydroxylase 1.cartilage-associated protein.cyclophilin B complex. J Biol Chem. 2009;284:17641–17647. doi: 10.1074/jbc.M109.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BP, Malfait F, Bonafe L, Baldridge D, Homan E, et al. Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J Bone Miner Res. 2011;26:666–672. doi: 10.1002/jbmr.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körkkö J, Ala-Kokko L, De Paepe A, Nuytinck L, Earley J, Prockop DJ. Analysis of the COL1A1 and COL1A2 genes by PCR amplification and scanning by conformation-sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with osteogenesis imperfecta type I: identification of common sequences of null-allele mutations. Am J Hum Genet. 1998;62:98–110. doi: 10.1086/301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff KM, Carden A, Bergwitz C, Forlino A, Uveges TE, et al. Brittle IV mouse model for osteogenesis imperfecta IV demonstrates postpubertal adaptations to improve whole bone strength. J Bone Miner Res. 2004;19:614–622. doi: 10.1359/JBMR.040111. [DOI] [PubMed] [Google Scholar]
- Kuurila K, Kaitila I, Johansson R, Grénman R. Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. Ann Otol Rhinol Laryngol. 2002;111:939–946. doi: 10.1177/000348940211101014. [DOI] [PubMed] [Google Scholar]
- Lanting PJ, Borsboom PC, te Meerman GJ, ten Kate LP. Decreased scattering coefficient of blue sclerae. Clin Genet. 1985;27:187–190. doi: 10.1111/j.1399-0004.1985.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Lapunzina P, Aglan M, Temtamy S, Caparrós-Martin JA, Valencia M, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet. 2010;87:110–114. doi: 10.1016/j.ajhg.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LS, Salinas CF, Jorgenson RJ. Classification of osteogenesis imperfecta by dental characteristics. Lancet. 1978;1:332–333. doi: 10.1016/s0140-6736(78)90108-3. [DOI] [PubMed] [Google Scholar]
- Looser E. Zur Kenntnis der Osteogenesis Imperfecta Congenita et Tarda (sogenannte idiopathische Osteopsatyrosis) [in German] Mittlg Grenzgebiete Med Chir. 1906;15:161–207. [Google Scholar]
- Lowenstein EJ. Osteogenesis imperfecta in a 3,000-year-old mummy. Childs Nerv Syst. 2009;25:515–516. doi: 10.1007/s00381-009-0817-7. [DOI] [PubMed] [Google Scholar]
- Marini JC: Osteogenesis Imperfecta (2010a). https://www.endotext.org/parathyroid/parathyroid17/parathyroidframe17.htm
- Marini JC, Bordenick S, Chrousos GP. Endocrine aspects of growth deficiency in OI. Connect Tissue Res. 1995;31:S55–S57. doi: 10.3109/03008209509116835. [DOI] [PubMed] [Google Scholar]
- Marini JC, Hopkins E, Glorieux FH, Chrousos GP, Reynolds JC, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res. 2003;18:237–243. doi: 10.1359/jbmr.2003.18.2.237. [DOI] [PubMed] [Google Scholar]
- Marini JC, Cabral WA, Barnes AM, Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle. 2007a;6:1675–1681. doi: 10.4161/cc.6.14.4474. [DOI] [PubMed] [Google Scholar]
- Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007b;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini JC, Cabral WA, Barnes AM. Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res. 2010b;339:59–70. doi: 10.1007/s00441-009-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe A, Pepin MG, Byers PH. Testing for osteogenesis imperfecta in cases of suspected non-accidental injury. J Med Genet. 2002;39:382–386. doi: 10.1136/jmg.39.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington-Ward S, McMahon HP, Allen D, Tuohy G, Kiang AS, et al. RNAi of COL1A1 in mesenchymal progenitor cells. Eur J Hum Genet. 2004;12:864–866. doi: 10.1038/sj.ejhg.5201230. [DOI] [PubMed] [Google Scholar]
- Monti E, Mottes M, Fraschini P, Brunelli P, Forlino A, et al. Current and emerging treatments for the management of osteogenesis imperfecta. Ther Clin Risk Manag. 2010;6:367–381. doi: 10.2147/tcrm.s5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, et al. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006;127:291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Marcus PS. Prenatal diagnosis and management of intrauterine fracture. Obstet Gynecol Surv. 2010;65:249–259. doi: 10.1097/OGX.0b013e3181dbc50b. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- Oakley I, Reece LP. Anesthetic implications for the patient with osteogenesis imperfecta. AANA J. 2010;78:47–53. [PubMed] [Google Scholar]
- Panaroni C, Gioia R, Lupi A, Besio R, Goldstein SA, et al. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood. 2009;114:459–468. doi: 10.1182/blood-2008-12-195859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen U, Bramsen T. Central corneal thickness in osteogenesis imperfecta and otosclerosis. ORL J Otorhinolaryngol Relat Spec. 1984;46:38–41. doi: 10.1159/000275682. [DOI] [PubMed] [Google Scholar]
- Peltier LF. The classic: congenital osteomalacia. Olaus Jacob Ekman. Clin Orthop Relat Res. 1981:3–5. [PubMed] [Google Scholar]
- Pepin M, Atkinson M, Starman BJ, Byers PH. Strategies and outcomes of prenatal diagnosis for osteogenesis imperfecta: a review of biochemical and molecular studies completed in 129 pregnancies. Prenat Diagn. 1997;17:559–570. [PubMed] [Google Scholar]
- Philippi CA, Remmington T, Steiner RD. The Cochrane Collaboration. England: John Wiley & Sons Ltd.; 2009. Bisphosphonate therapy for osteogenesis imperfecta. [Google Scholar]
- Price ER, Zydowsky LD, Jin MJ, Baker CH, McKeon FD, Walsh CT. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci USA. 1991;88:1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Constantinos D, Dombrowski KE, Hojima Y, Kadler KE, et al. Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable disorders of connective tissue. Am J Med Gen. 1989;34:60–67. doi: 10.1002/ajmg.1320340112. [DOI] [PubMed] [Google Scholar]
- Pyott SM, Schwarze U, Christiansen HE, Pepin MG, Leistritz DF, et al. Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes. Hum Mol Genet. 2011a;20:1595–1609. doi: 10.1093/hmg/ddr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott SM, Pepin MG, Schwarze U, Yang K, Gretchen S, Byers PH. Recurrence of perinatal lethal osteogenesis imperfect in sibships: parsing the risk between parental mosaicism for dominant mutations and autosomal recessive inheritance. Genet Med. 2011b;13:125–130. doi: 10.1097/GIM.0b013e318202e0f6. [DOI] [PubMed] [Google Scholar]
- Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- Ross MH, Romrell RJ, Kaye GI. Histology, A Text and Atlas. ed 3. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- Saban J, Zussman MA, Havey R, Patwardhan AG, Schneider GB, King D. Heterozygous oim mice exhibit a mild form of osteogenesis imperfecta. Bone. 1996;19:575–579. doi: 10.1016/s8756-3282(96)00305-5. [DOI] [PubMed] [Google Scholar]
- Sarathchandra P, Pope FM, Ali SY. Morphometric analysis of type I collagen fibrils in the osteoid of osteogenesis imperfecta. Calcif Tissue Int. 1999;65:390–395. doi: 10.1007/s002239900719. [DOI] [PubMed] [Google Scholar]
- Sekiya H, Murakami T, Saito A, Hino S, Tsumagari K, et al. Effects of the bisphosphonate risedronate on osteopenia in OASIS-deficient mice. J Bone Miner Metab. 2010;28:384–394. doi: 10.1007/s00774-009-0142-y. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Al-Owain M, Sakati N, Alzayed ZS, Alkuraya FS. FKBP10 and Bruck syndrome: phenotypic heterogeneity or call for reclassification? Am J Hum Genet. 2010;87:306–307. doi: 10.1016/j.ajhg.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence DO, Barlow KK, Garber AP, Hall JG, Rimoin DL. Osteogenesis imperfecta type II delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet. 1984;17:407–423. doi: 10.1002/ajmg.1320170204. [DOI] [PubMed] [Google Scholar]
- Spranger J, Brill P, Poznanski A. An atlas of genetic disorders of skeletal development. Oxford: Oxford University Press; 2003. Bone Dysplasias. [Google Scholar]
- Steiner RD, Pepin MG, Byers PH. Osteogenesis Imperfecta, in Pagon RA, Bird TD, Dolan CR, Stephens K (eds): GeneReviews (University of Washington, Seattle 1993). http://www.ncbi.nlm.nih.gov/books/NBK1116/
- Sun HB, Chen JC, Liu Q, Guo MF, Zhang HP. Substance P stimulates differentiation of mice osteoblast through up-regulating Osterix expression. Chin J Traumatol. 2010;13:46–50. [PubMed] [Google Scholar]
- Sykes B, Francis MJ, Smith R. Altered relation of two collagen types in osteogenesis imperfecta. N Engl J Med. 1977;296:1200–1203. doi: 10.1056/NEJM197705262962104. [DOI] [PubMed] [Google Scholar]
- Sykes B, Ogilvie D, Wordsworth P, Wallis G, Mathew C, et al. Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Hum Genet. 1990;46:293–307. [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Kraft WJ, Lang R, Avioli LV. Bone collagen aggregation abnormalities in osteogenesis imperfecta. Calcif Tissue Res. 1974;17:75–79. doi: 10.1007/BF02547215. [DOI] [PubMed] [Google Scholar]
- Undale AH, Westendorf JJ, Yaszemski MJ, Khosla S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc. 2009;84:893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger S, Scherer G, Superti-Furga A: Campomelic dysplasia. GeneReviews (University of Washington, Seattle 1993). http://www.ncbi.nlm.nih.gov/books/NBK1116/
- van der Slot AJ, Zuurmond AM, Bardoel AF, Wijmenga C, Pruijs HE, et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- Van Dijk FS, Nesbitt IM, Nikkels PG, Dalton A, Bongers EM, et al. CRTAP mutations in lethal and severe osteogenesis imperfecta: the importance of combining biochemical and molecular genetic analysis. Eur J Hum Genet. 2009a;17:1560–1569. doi: 10.1038/ejhg.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk FS, Nesbitt IM, Zwikstra EH, Nikkels PG, Piersma SR, et al. PPIB mutations cause severe osteogenesis imperfecta. Am J Hum Genet. 2009b;85:521–527. doi: 10.1016/j.ajhg.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk FS, Huizer M, Kariminejad A, Marcelis CL, Plomp AS, et al. Complete COL1A1 allele deletions in Osteogenesis Imperfecta. Genet Med. 2010a;12:736–741. doi: 10.1097/GIM.0b013e3181f01617. [DOI] [PubMed] [Google Scholar]
- van Dijk FS, Nikkels PG, den Hollander NS, Nesbitt IM, van Rijn RR, et al: Lethal/severe osteogenesis imperfecta in a large family: a novel homozygous LEPRE1 mutation and bone histological findings. Pediatr Dev Pathol 2010b, E-pub ahead of print. [DOI] [PubMed]
- Van Dijk FS, Pals G, van Rijn RR, Nikkels PG, Cobben JM. Classification of Osteogenesis Imperfecta revisited. Eur J Med Genet. 2010c;53:1–5. doi: 10.1016/j.ejmg.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Van Dijk FS, Byers PH, Dalgleish R, Malfait F, Maugeri A, Rohrbach M: EMQN best practice guidelines for the laboratory diagnosis of osteogenesis imperfecta. Eur J Hum Genet 2011, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- Viora E, Sciarrone A, Bastonero S, Errante G, Campogrande M, et al. Increased nuchal translucency in the first trimester as a sign of osteogenesis imperfecta. Am J Med Genet. 2002;109:336–337. doi: 10.1002/ajmg.10332. [DOI] [PubMed] [Google Scholar]
- Wallis GA, Sykes B, Byers PH, Mathew CG, Viljoen D, Beighton P. Osteogenesis imperfecta type III: mutations in the type I collagen structural genes, COL1A1 and COL1A2, are not necessarily responsible. J Med Genet. 1993;30:492–496. doi: 10.1136/jmg.30.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Marini JC. Antisense oligodeoxynucleotides selectively suppress expression of the mutant alpha 2(I) collagen allele in type IV osteogenesis imperfecta fibroblasts. A molecular approach to therapeutics of dominant negative disorders. J Clin Invest. 1996;97:448–454. doi: 10.1172/JCI118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Gen. 2011;155A:943–968. doi: 10.1002/ajmg.a.33909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willaert A, Malfait F, Symoens S, Gevaert K, Kayserili H, et al. Recessive osteogenesis imperfecta caused by LEPRE1 mutations: clinical documentation and identification of the splice form responsible for prolyl 3-hydroxylation. J Med Genet. 2009;46:233–241. doi: 10.1136/jmg.2008.062729. [DOI] [PubMed] [Google Scholar]
- Zack P, Zack LR, Surtees R, Neville BG. A standardized tool to measure and describe scleral colour in osteogenesis imperfecta. Ophthalmic Physiol Opt. 2007;27:174–178. doi: 10.1111/j.1475-1313.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Zankl A, Mornet E, Wong S. Specific ultrasonographic features of perinatal lethal hypophosphatasia. Am J Med Genet A. 2008;146A:1200–1204. doi: 10.1002/ajmg.a.32202. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang Z, Feng JQ, Dusevich VM, Sinha K, et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci USA. 2010;107:12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]