Abstract
Recent findings suggest a close link between long-term meditation practices and the structure of the corpus callosum. Prior analyses, however, have focused on estimating mean fractional anisotropy (FA) within two large pre-defined callosal tracts only. Additional effects might exist in other, non-explored callosal regions and/or with respect to callosal attributes not captured by estimates of FA. To further explore callosal features in the framework of meditation, we analyzed 30 meditators and 30 controls, carefully matched for sex, age, and handedness. We applied a multimodal imaging approach using diffusion tensor imaging (DTI) in combination with structural magnetic resonance imaging (MRI). Callosal measures of tract-specific FA were complemented with other global (segment-specific) estimates as well as extremely local (point-wise) measures of callosal micro- and macro-structure. Callosal measures were larger in long-term meditators compared to controls, particularly in anterior callosal sections. However, differences achieved significance only when increasing the regional sensitivity of the measurement (i.e., using point-wise measures versus segment-specific measures) and were more prominent for microscopic than macroscopic characteristics (i.e., callosal FA versus callosal thickness). Thicker callosal regions and enhanced FA in meditators might indicate greater connectivity, possibly reflecting increased hemispheric integration during cerebral processes involving (pre)frontal regions. Such a brain organization might be linked to achieving characteristic mental states and skills as associated with meditation, though this hypothesis requires behavioral confirmation. Moreover, longitudinal studies are required to address whether the observed callosal effects are induced by meditation or constitute an innate prerequisite for the start or successful continuation of meditation.
Keywords: brain, corpus callosum, DTI, mindfulness, MRI, plasticity
Introduction
Several studies demonstrate structural differences between the brains of meditators and non-meditators (Lazar et al., 2005; Pagnoni and Cekic, 2007; Holzel et al., 2008; Vestergaard-Poulsen et al., 2008; Luders et al., 2009; Grant et al., 2010; Luders et al., 2011a). Moreover, there is accumulating evid ence for actual meditation-induced anatomical alterations (Holzel et al., 2010; Tang et al., 2010). Interestingly, none of the observed differences (or alterations) are expressed equally in both hemispheres but appear confined to a particular hemisphere (or more pronounced in one hemisphere than the other). Although, actual structural asymmetries have not been addressed in the context of meditation, reports exist with respect to functional asymmetries. More specifically, as measured using electroencephalography (EEG), meditation practices seem to increase the leftward bias of frontal brain activity, (Davidson et al., 2003; Moyer et al., 2011). Moreover, EEG and functional imaging studies revealed different activation patterns and/or networks during resting states in meditators compared to controls (Lutz et al., 2004; Faber et al., 2008; Tei et al., 2009; Kilpatrick et al., 2011; Jang et al., 2011; Berkovich-Ohana et al., 2011). Altogether, this seems to suggest a different brain organization in meditators that is likely to be accompanied by a different inter-hemispheric coupling. Therefore, the anatomical substrate for inter-hemispheric communication might be measurably different in long-term meditation practitioners.
The corpus callosum is the largest inter-connecting fiber structure in the human brain, and pioneering evidence for callosal differences between meditators and controls (for meditation-induced callosal alterations, respectively) has been provided in recent diffusion tensor imaging (DTI) studies (Tang et al., 2010; Luders et al., 2011a). For example, we previously reported a significantly larger fractional anisotropy (FA) within the forceps minor (Fminor) in meditators compared to age- and gender-matched controls (Luders et al., 2011a). Group differences, however, were absent for the forceps major (Fmajor). Since fibers of the Fminor cross the midline via the callosal anterior third (while fibers of the Fmajor cross via the callosal splenium), these findings may suggest that anterior regions of the corpus callosum are more involved in the process of meditation than posterior regions. However, the aforementioned findings were based solely on an atlas-based approach that estimated callosal FA only within these two pre-defined regions. Alterations in other callosal sections (perhaps towards the middle of the corpus callosum) may have remained undetected. Moreover, while atlas-based methods that average FA within relatively large search regions may improve statistical power (by increasing signal-to-noise), smaller more localized effects may be missed. Indeed, another DTI study used a voxel-wise approach to estimate FA (albeit not conducted in long-term meditators but rather in meditation-naïve subjects) and revealed “significant FA increases in the body and genu of the corpus callosum” after 11 hours of an Integrative Body-Mind Training spread over one month (Tang et al., 2010). Thus, alterations in callosal mid sections might also exist in long-term practitioners and possibly become evident when increasing the regional specificity of the measurements applied.
The current study was designed to further establish the presence and direction of callosal differences between long-term meditators and well-matched controls. For this purpose, we used a refined set of callosal measures complementing global (segment-specific) estimates with extremely local (point-wise) indices. As mentioned above, two prior studies have used DTI to uncover possible effects related to meditation with respect to callosal FA (Tang et al., 2010; Luders et al., 2011a). FA indicates the degree of directional sensitivity of water diffusion within the voxel (Basser and Pierpaoli, 1996), which is generally interpreted as an indicator for fiber connectivity, and is thus considered a suitable measure to explore possible alterations with respect to callosal micro-structure. However, other morphometric measures related to callosal macro-structure, such as area and thickness, may also be functionally relevant – as shown in subjects unselected for meditation (Witelson and Goldsmith, 1991; Schlaug et al., 1995; Luders et al., 2007b; Kurth et al., 2012) – and thus warrant investigation in meditators in addition to FA. We therefore applied a multimodal imaging approach combining DTI-based measures (FA) with MRI-based measures, such as callosal area size and callosal thickness.
Materials and Methods
Subjects
Our sample included 30 meditators (15 men, 15 women) and 30 controls (15 men, 15 women). Meditators and controls were pair-wise matched for age and sex, where the maximum allowed age difference within a sex-matched pair was two years. Age ranged from 24 to 64 years both within meditators (mean ± SD: 47.3 ± 11.7 years) and within controls (mean ± SD: 47.3 ± 11.8 years). Both groups were comparable with respect to their educational background, where 86.7% of all mediators and also 86.7% of all controls had, at least, some college experience. While the scans for the controls were obtained from the ICBM database of normal adults (http://www.loni.ucla.edu/ICBM/Databases/), meditators were newly recruited from various meditation venues. Years of meditation practice ranged from 5 to 46 years (mean ± SD: 20.2 ± 12.2 years), where self-reported meditation styles included Chenrezig, Kriya, Shamatha, Vajrayana, Vipassana, and Zazen. An overview with respect to the subject-specific meditation style as well as amount of practice (i.e., number of years, frequency per week, and duration per session) is provided in Supplementary Table 1. Most subjects (93.3%) were right-handed as based on self-reports of hand preference for selected activities (e.g., writing a letter, holding a racket, throwing a ball). Since two male meditators were left-handed, we selected two left-handed male controls from the ICBM database. All subjects were required to be free of any neurological and psychiatric disorders and gave informed consent according to institutional guidelines (Institutional Review Board of the University of California, Los Angeles). Subjects constituting the current sample (n=60) partly overlap with samples of n=44 (53.33%) and n=54 (78.33%), as analyzed in two studies addressing the effects of meditation on brain structure (Luders et al., 2009; Luders et al., 2011a). Note, variations in sample characteristics across studies are due to the inclusion/exclusion of left-handed subjects, the inclusion/exclusion of subjects older than 64 years, and the exclusion of subjects with artifacts in their T1-weighted/diffusion-weighted data.
Image acquisition
MRI data from all subjects was acquired on a 1.5T Siemens Sonata scanner (Erlangen, Germany) using an 8-channel head coil and a 3D T1-weighted sequence (MPRAGE) with the following parameters: TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, 160 contiguous 1 mm sagittal slices, FOV: 256 mm × 256 mm, matrix: 256 × 256, voxel dimensions: 1.0 × 1.0 × 1.0 mm. DTI data was acquired at the same site (UCLA), on the same 1.5 Tesla scanner (Siemens Sonata), and with the same head coil (8-channel) as the MRI data. The whole-brain sequence included 5 non-diffusion-weighted images (b=0 s/mm2) and 30 directionally sensitized diffusion-weighted images (b=1000 s/mm2) with the following parameters: TR = 6400 ms, TE = 83 ms, 55 brain slices oriented obliquely to the AC–PC line, FOV: 240 × 240 mm, matrix: 96 × 96, voxel dimensions: 2.5 mm × 2.5mm × 2.5 mm.
MRI-based analyses
Automated radio-frequency bias field corrections were applied to correct image volumes for intensity drifts caused by magnetic field inhomogeneities (Shattuck et al., 2001). In addition, all images volumes were placed into the same standard space using automated 6-parameter rigid-body transformations (Woods et al., 1998b). The corpus callosum was then outlined automatically based on the Chan-Vese model for active contours (Chan and Vese, 2001). This resulted in two midsagittal callosal outlines (i.e., the upper and lower callosal boundary) for each subject, as detailed elsewhere (Luders et al., 2006a). Subsequently, each callosal outline was overlaid onto the MR image from which it had been extracted and visually inspected to ensure that automatically generated callosal outlines precisely followed the natural course and boundaries of the corpus callosum. Contours that did not match this criterion were corrected manually by one rater (E.L.).
Segment-specific Area (Analysis I)
To obtain callosal area measures in accordance with traditional parcellation schemes (Witelson, 1989), the automatically obtained upper and lower callosal boundaries were connected at their start/end points, reoriented to maximize callosal length, and divided into five vertical partitions: the splenium (representing the posterior fifth), the isthmus (representing two fifteenths), the posterior and anterior midbody (both representing one sixth), and the callosal anterior third. Subsequently, for each of these five callosal segments the area was calculated (in mm2).
Point-wise Thickness (Analysis II)
To obtain highly localized measures of callosal thickness, anatomical surface-based mesh modeling methods (Thompson et al., 1996a; Thompson et al., 1996b) were employed, as detailed elsewhere (Luders et al., 2006a; Luders et al., 2007a). This resulted in distance measurements at 100 equally spaced points along the callosal axis indicating callosal thickness (in mm) with an extremely high spatial resolution.
DTI-based analyses
For the DTI analyses, we excluded two male subjects (i.e., one 24-year old meditator and one 36-year old control) who had a stripe artifact in their diffusion-weighted images. The remainder of the images were corrected for motion artifacts using a 3D rigid body registration (Woods et al., 1998a) and for eddy current induced distortions using a 2D nonlinear registration algorithm (Woods et al., 1998b), where all diffusion-weighted images were registered to the first non-diffusion-weighted image in the series. Using the CLAPACK library (Anderson et al., 1999) and in-house software written in C, the diffusion tensor was then computed at each voxel using a linear least-squares method to fit the log-transformed data of the signal intensities (Basser et al., 1994). Finally, the resultant eigenvalues were used to compute the FA for thousands of points across the entire brain.
Segment-specific FA (Analysis III)
In correspondence with our MRI-based analyses, we first calculated the mean FA within the five aforementioned callosal segments: splenium, isthmus, posterior midbody, anterior midbody, and anterior third (detailed above: Analysis I).
Point-wise FA (Analysis IV)
To obtain highly localized measures of FA, we conducted Tract-Based Spatial Statistics (TBSS), as described elsewhere (Smith et al., 2006). For the purpose of mapping corpus callosum-specific FA estimates, the resulting overall brain map was masked using a callosal label outlined at the midsagittal section as well as on eight additional sagittal slices to the left and to the right (i.e., altogether comprising 17 slices).
Statistical comparisons
Callosal Measurements were compared between meditators and controls by applying independent sample Student’s t-tests. As a safeguard against type I error, significance values (p) were established both uncorrected and corrected for multiple comparisons. For this purpose, we applied False Discovery Rate (FDR) corrections thresholded at q=0.05 for analyses II and IV (where comparisons were conducted at hundreds of points). In contrast, we applied Bonferroni corrections for analyses I and III (accounting for the five segment-specific comparisons) where p≤0.01 was employed as the appropriate threshold.
Supplemental analysis 1
As mentioned above, in our preceding study (Luders et al., 2011a) we had observed larger FA within Fminor but not within Fmajor (both constituting major fiber tracts of the corpus callosum, as shown in Supplementary Figure 1). In order to determine whether these callosal findings are reproducible in a larger sample, we re-investigated the tract-specific mean FA within Fminor and Fmajor. For this purpose, we utilized the Johns Hopkins University (JHU) white matter tractography atlas (Wakana et al., 2007; Hua et al., 2008), as detailed previously (Luders et al., 2011a). After establishing the two callosal fiber tracts within each subject’s native space, mean FA was computed within Fminor and Fmajor and compared between meditators and controls. As a safeguard against type I error, we applied Bonferroni corrections (accounting for the two tract-specific comparisons) employing p≤0.025 as the appropriate statistical threshold.
Supplemental analysis 2
Last but not least, in order to explore whether there is a link between the amount of individual meditation experience and callosal measures, Pearson’s correlations (after removing the partial effects of age) were used to examine the relationships between the number of meditation years and (a) point-wise callosal thickness as well as (b) point-wise callosal FA. Since correlations were conducted at hundreds of points, FDR corrections thresholded at q=0.05 were applied as a safeguard against type I error.
Results
MRI-based findings
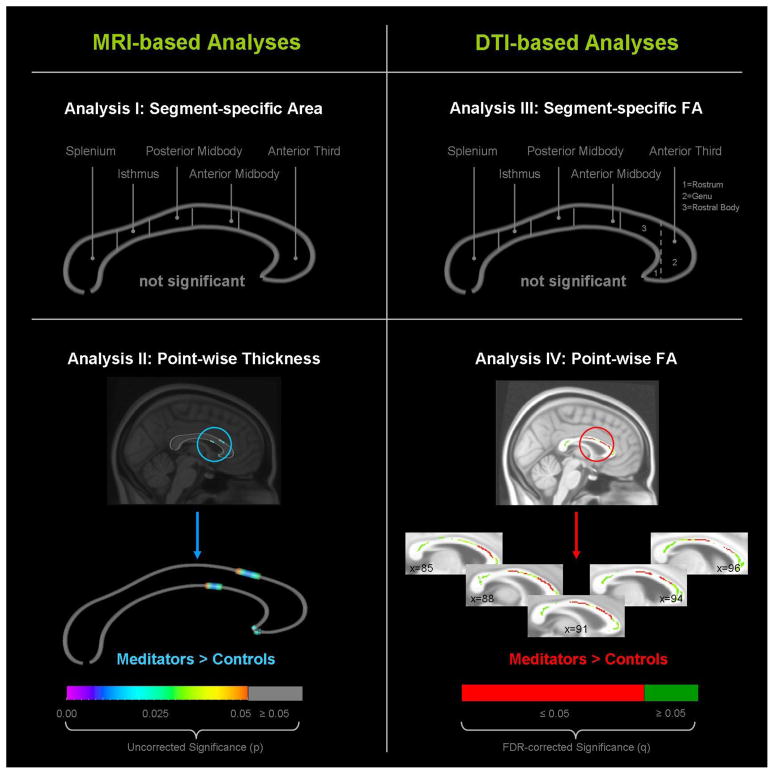
Using the segment-based approach (Analysis I), there were no significant group differences within any of the callosal regions (Figure 1, left top panel). Using the point-wise approach (Analysis II), we observed significantly thicker corpora callosa at the border between anterior midbody and anterior third (Figure 1, left bottom panel). However, findings did not survive FDR correction. There were no regions where controls had a significantly larger callosal thickness than meditators.
Figure 1. Group differences in callosal attributes.
MRI-based measurements are shown on the left; DTI-based measurements are shown on the right. Global measurements (segment-specific callosal area and FA) are shown in the top panels; local measurements (point-wise callosal thickness and FA) are shown in the bottom panel. The color bars in the bottom panels encode significance. MRI-based findings are provided uncorrected (on the midsagittal section) and DTI-based findings are provided FDR-corrected (on the midsagittal section and two additional slices to the right and to the left).
DTI-based findings
Using the segment-based approach (Analysis III), there were no significant group differences with respect to FA within any of the callosal regions (Figure 1, right top panel). Using the point-wise approach (Analysis IV), we detected significantly increased FA in meditators across large regions of the corpus callosum (Figure 1, right bottom panel). Findings survived FDR corrections and were pronounced within the rostral body of the anterior third, the anterior midbody, and even slightly extending into the posterior midbody, while sparing isthmus and splenium as well as the genu and rostrum of the callosal anterior third (for an illustration of subregions of the anterior third, refer to the right top panel of Figure 1). There were no callosal regions where controls had significantly larger FA than meditators.
Supplemental findings
There were no significant group differences within the Fmajor. In contrast, within the Fminor we detected significantly larger FA in meditators (mean ± SD: 0.262 ± 0.013) than in controls (mean ± SD: 0.245 ± 0.024). This effect remained significant after applying a Bonferroni correction (p=0.002). Significant correlations between the number of meditation years and callosal measures, such as point-wise thickness and point-wise FA, were absent.
Discussion
This study was conducted to follow-up on previous findings (Luders et al., 2011a) suggesting enhanced fiber connectivity in meditators within anterior portions of the corpus callosum, or more specifically within the callosal anterior tip occupied by the Fminor. Since our previous study had only examined mean FA within two pre-defined callosal tracts (Fminor and Fmajor), additional effects in other callosal regions (or with respect to callosal attributes not captured by estimates of FA) might have been overseen. Thus, the current study leveraged a multimodal imaging approach and used an extended set of callosal measures to investigate whether attributes of the corpus callosum are altered in long-term meditators. More specifically, MRI-based measures were complemented by DTI-based measures, and global (segment-specific) estimates were complemented by extremely local (point-wise) measures.
The location of findings and their correspondence with outcomes from other studies
In agreement with our previous study (Luders et al., 2011a), we detected significantly increased FA within Fminor but not Fmajor (Supplemental Analysis 1), supporting the assumption that meditation might more strongly involve anterior rather than posterior callosal regions. Since the Fminor crosses via the callosal anterior third, our tract-specific outcomes are also in line with our point-wise findings indicating a larger callosal thickness (Analysis II) and larger callosal FA (Analysis IV) in meditators within regions of the anterior third (more specifically, the rostral body of the anterior third). Moreover, our current findings seem to agree with outcomes from a prior study by another group (Tang et al., 2010) who revealed pronounced meditation effects within anterior midbody and rostral body (although their cluster was situated a considerable number of slices away from the brain’s midsagittal section).
Interestingly, the callosal genu of the anterior third seems to be largely absent of meditation effects, which might contradict findings by Tang et al. who reported meditation-induced increases of FA in the “genu”. It is likely, however, that the aforementioned study used a different definition of genu (i.e., different from our parcellation scheme as shown in Figure 1) as their genu is depicted as almost the entire anterior half of the corpus callosum. Alternatively, the increased FA within the callosal “genu” as observed by Tang et al. (but not in our study) might imply that different callosal regions are involved and/or affected during skill acquisition than during the continued practice of an acquired skill: Tang’s longitudinal study included meditation-naïve subjects scanned before and after only one month of practice, while the current cross-sectional study included long-term meditation practitioners with an average practice experience of more than twenty years. As suggested previously, the learning of a new skill might have more impact on the structure of the brain than the continued practice of an already acquired skill (Driemeyer et al., 2008). Likewise, learning a new skill might also involve different brain regions than those relevant for maintaining an ongoing practice. Moreover, it is possible that the observed effects in Tang’s longitudinal study constitute actual meditation-induced effects while outcomes of our cross-sectional study solely reflect innate differences in the brains of meditation practitioners which, perhaps, attracted a person towards meditation and/or helped foster a continued, regular and intense practice over many years. Detailed discussions on the aspect of nature versus nurture in the context of meditation are provided elsewhere (Luders et al., 2009; Luders et al., 2011a). Last but not least, possible discrepant findings could also imply that different meditation styles (e.g., IBMT practice versus non-IBMT practices) involve or affect different callosal regions, similar as has been shown for brain activation during different meditation tasks (Wang et al., 2011).
Notwithstanding, regardless whether the “genu”-specific findings constitute congruent or discrepant effects, both Tang’s study and our current study seem to suggest that links between meditation and callosal attributes are not confined exclusively to the callosal anterior third but also extend to callosal fibers towards the middle of the corpus callosum. That is, Tang et al. (2010) revealed meditation-induced effects within the callosal “genu” as well as within the callosal “body”, and our current study revealed significant differences between meditators and controls not only within the rostral body but also further posteriorly, involving large parts of the anterior midbody and even the posterior midbody.
The dependence of findings on the measurement applied
We revealed evidence for thicker corpora callosa in meditators using the MRI-based point-wise callosal thickness approach (Analysis II). However, these findings were confined to a region at the border between rostral body and anterior midbody and did not survive FDR correction. In contrast, we observed statistically robust effects when using the DTI-based point-wise FA approach (Analysis IV) indicating larger FA within the entire rostral body and anterior midbody that even extended towards the posterior midbody. If we assume that exploring the thickness of the corpus callosum captures more macroscopic effects, while investigating FA reveals effects at the microscopic level, our observation is in agreement with the idea that macroscopic changes occur only as a consequence of microscopic changes. That is, if FA alterations – possibly reflecting alterations with respect to fiber number, density, or myelination – are sufficiently pronounced, then they might also become evident on a different level (e.g., via measuring callosal thickness).
Similarly it stands to reason that effects isolated at a (sub-)voxel resolution need to be spread over a considerably large region before they will become evident using coarser measurements (e.g., segment-specific estimates). As discussed previously (Luders et al., 2006b), group differences with respect to callosal features might be overseen when measurements lack the necessary spatial sensitivity (i.e., when conducting analyses within pre-defined regions using traditional parcellation boundaries). For example, if groups differ in callosal morphology only for a small portion of a segment (e.g., the rostral body, rather than the entire anterior third) or if callosal alterations occur at the border between two segments (e.g., between anterior midbody and anterior third), effects with respect to the entire callosal segment (e.g., anterior third or anterior midbody) might not reach statistical significance. Since group differences with respect to segment-specific area (Analysis I) and segment-specific FA (Analysis III) were absent, our study might indeed suggest that links between callosal anatomy and meditation are spatially confined – at least, at the midsagittal section of the brain. Interestingly though, when following the course of callosal fibers as shown in Supplementary Figure 1 and comparing FA averaged within the entire tract of the Fminor (rather than extracting callosal features at or close to midline), striking group differences became evident with significantly larger FA in meditators (supplemental findings). The robustness of this effect (observed via a coarse/more global measure) suggests that callosal features in mediators are not only altered at midline (i.e., where callosal measures are usually obtained) but also – and perhaps even more pronounced – away from midline. Similar effects related to meditation practices have been observed in an aforementioned study (Tang et al., 2010) which revealed the strongest “callosal” changes within the left hemisphere (around x=−13 and x=−17) and not at midline.
Altogether these findings underscore the importance (a) of integrating different imaging modalities (e.g., MRI and DTI), (b) of extracting both macroscopic and microscopic features (e.g., area/thickness and FA), and (c) of complementing global estimates with local measures (e.g., segment-specific FA and point-wise FA) when establishing the presence and direction of group differences with respect to callosal attributes.
Possible functional implications and underlying mechanisms
A larger callosal thickness is likely to reflect more fibers crossing the corpus callosum, as has been demonstrated with respect to the link between callosal area size and small diameter fibers (Aboitiz et al., 1992). Likewise, higher callosal FA measures might suggest that fibers are more numerous, more myelinated, more densely packed, or more coherent in orientation (or perhaps a conglomerate of these microscopic characteristics). Thicker callosal regions as well as larger callosal FA in meditators might therefore be an indicator of increased interhemispheric connectivity, possibly reflecting a better integration of information between hemispheres. Although this hypothesis cannot be tested without behavioral confirmation, the anatomically implied enhanced integration of both hemispheres might aid in achieving certain mental states associated with meditation, such as a heightened awareness or present-moment consciousness. A stronger involvement of both hemispheres might also link with specific mental skills, such as the ability to successfully control the fluctuations of the mind (Baerentsen et al., 2009). As reviewed elsewhere (Thomason and Thompson, 2010), the gross geometry of axons influences the ability to rapidly relay electrical signals. Altered callosal attributes in meditators may therefore reflect a specific (innate) cerebral setup potentially facilitating the successful practice of meditation by increasing interhemispheric communication. However, it is also possible that regular meditating (and engaging the brain in a way that both hemispheres are strongly involved) has led to the observed callosal differences between meditators and controls, the more so as electrical activity has been demonstrated to be a contributor in the process of myelin formation (Demerens et al., 1996). It is similarly likely that both mechanisms are responsible for the observed callosal alterations in meditators: Experiencing the benefits of successful meditation practices due to advantageous white matter organization may foster ongoing and long-lasting meditation efforts which, in turn, strengthen the underlying anatomical substrates. However, only longitudinal studies can establish such differential effects of nature and nurture.
Callosal alterations in meditators might also be associated with cortical alterations. Given that the current study focused on analyzing the corpus callosum (rather than the cerebral cortex), the subsequent discussion with respect to potential relationships between callosal and cortical findings (and their possible functional significance) is considered speculative. However, highlighting the spatial correspondence of meditation effects across different studies may help identifying key brain regions, networks, and system involved in the process of meditation. For example, increased numbers of cortical neurons might require increased numbers of callosal connections to allow an efficient crosstalk between both hemispheres. Specifically, callosal fibers traveling through anterior third and anterior midbody (where effects were found to be most pronounced) are suggested to primarily connect (pre)frontal as well as (supplemental) motor regions (Witelson, 1989; Zarei et al., 2006; Hofer and Frahm, 2006). Thus, the observed callosal alterations might be closely related to previously reported thicker cortices in meditators (larger gray matter volumes or larger gray matter densities, respectively) located within the right middle and superior frontal gyrus (Lazar et al., 2005), the left inferior and superior frontal gyrus (Vestergaard-Poulsen et al., 2008), and the right orbito-frontal cortex (Luders et al., 2009). Moreover, positive correlations between accumulated meditation hours and gray matter concentration have been observed bilaterally within the orbito-frontal cortex (Holzel et al., 2008). Furthermore, functional studies revealed increased asymmetries of frontal brain activity due to meditation practices (Davidson et al., 2003; Moyer et al., 2011) as well as activations in (pre)frontal regions when examining functional correlates of mindfulness/meditation (Newberg et al., 2001; Creswell et al., 2007; Newberg et al., 2010; Engstrom and Soderfeldt, 2010). Last but not least, meditation practices have also been related to greater functional connectivity and/or resting state activity in (pre)frontal regions (Lutz et al., 2004; Faber et al., 2008; Kilpatrick et al., 2011; Jang et al., 2011; Berkovich-Ohana et al., 2011). The exact functional implications of alterations within callosal sections connecting the (pre)frontal cortices remain to be established, though might relate to the general regulation mechanism of stilling the mind. However, other aspects and core characteristics associated with meditation such as (self)awareness, emotional regulation, and response control have been shown to engage (pre)frontal regions and may thus constitute a behavioral consequence of the aforementioned cerebral characteristics.
Findings within callosal sections presumably involved in transferring motor information are more difficult to interpret and remain puzzling in the context of meditation. However, it is relevant to note that previous examinations of callosal fiber compositions (Aboitiz et al., 1992) have revealed links primarily between callosal size and fiber number for small-diameter fibers only. Fibers connecting the (supplemental) motor cortices are larger in diameter (Aboitiz and Montiel, 2003). Therefore, it is possible that an increased callosal thickness occurring in callosal regions assumed to contain motor fibers, does not necessarily reflect behavioral correlates of motor function. Interestingly, however, our callosal findings in (supplemental) motor regions seem to correspond well with previous observations of enhanced gray matter in meditators bilaterally within central and paracentral areas (Lazar et al., 2005; Luders et al., 2009) and of enhanced fiber connectivity in long-term meditators bilaterally within the cortico-spinal tract (Luders et al., 2011a), with most of its fibers originating from primary motor and premotor areas (Westerhausen et al., 2007). Moreover, fMRI studies reported bilateral cortical activations in supplementary motor areas during the onset of meditation (Baerentsen et al., 2009) as well as sustained changes of cerebral blood flow within the right precentral gyrus after the end of meditation tasks (Wang et al., 2011). Nevertheless, further research will be necessary to elucidate the significance of meditation effects observed in those callosal regions.
Limitations and Future Studies
In later years of adulthood, brain white matter undergoes significant changes due to accelerated rates of fiber loss and demyelination (Minati et al., 2007; Kochunov et al., 2009; Michielse et al., 2010; Malykhin et al., 2011) that likewise affect the corpus callosum. We tried minimizing the impact of possible aging effects by excluding subjects older than 64 years and by carefully matching meditators and controls with respect to age. Nevertheless, with subjects ranging from 24 to 64 years, the age span in this study is large. Similarly, the current study included meditators practicing various meditation styles. While this allows investigation of the neural correlates of common and overlapping elements of meditation (rather than specific elements inherent to certain meditation styles), it might account for increased variance and hence the lack of significant group differences for more gross callosal measurements or less robust regional differences. It is possible that, by narrowing the age range and/or by focusing on a single meditation style, future studies will reveal even more pronounced group differences. In addition, separating the meditation sample into subgroups with different levels of experience may provide further insights into how callosal features are linked to meditation practices and how such associations change with increasing experience. For example, it was demonstrated that meditators with an average lifetime practice of 19,000 hours show more brain activation during meditation than novices, while meditators with an average practice of 44,000 hours show less activation (Brefczynski-Lewis et al., 2007). Future studies with larger numbers of subjects and sufficient statistical power may thus address if callosal effects are changing similarly, depending on whether non-meditators are compared to meditators with only little, moderate, or extreme amounts of experience.
Meditators and controls of the current study were matched for handedness. However, handedness was established based on self-reported hand preference for only a few selected activities. Future studies might consider extending the list of selected key items using well-established handedness inventories or perhaps even combining self-reports of hand preference with actual measures of hand performance. On a related note, although meditators and controls were comparable with respect to educational background, we did not obtain data on individual intelligence. Since callosal morphology seems to be associated with intelligence and/or specific cognitive performance (Luders et al., 2007b; Hutchinson et al., 2009; Voineskos et al., 2010; Luders et al., 2011b), future studies might ensure (either by including these variables as covariates in statistical analyses or by applying careful matching processes) that these potential moderators do not significantly affect study outcomes.
Our study leveraged a multimodal imaging approach using both MRI- and DTI-based measures. Moreover, we examined different callosal features, while additionally exploring how outcomes vary with respect to the coarseness of the measurement. Nevertheless, the set of applied callosal measurements, although extensive, is not exhaustive, and future studies might include alternative (or additional) measures sensitive to different aspects of neurobiology. Given that the corpus callosum constitutes a white matter structure, DTI-based measures appear especially appropriate — the more so as meditation effects were more apparent in the DTI as opposed to the MRI data. The current study focused on DTI-based FA, a commonly used measure to explore white matter microstructure. However, other DTI-based parameters, such as axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD), or a regional fiber coherence index (r-FCI) (Zhou and Leeds, 2005), may provide additional or more specific information by indexing possible alterations in axonal integrity (myelin integrity or fiber coherence, respectively).
Supplementary Material
Acknowledgments
We warmly thank all participants for their dedication and partaking in our study. We are also grateful to Trent Thixton who assisted with the acquisition of the image data. For generous support the authors thank the Brain Mapping Medical Research Organization, the Robson Family and Northstar Fund, and the following Foundations: Brain Mapping Support, Pierson-Lovelace, Ahmanson, Tamkin, William M. & Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community, Jennifer Jones-Simon, and Capital Group Companies. This study was additionally supported by the NIH, including NIMH (MH092301), NICHHD (K99HD065832) and NCRR (RR013642, RR12169, and RR00865), as well as NIBIB, NIDA, NINDS and NCI. Further support was provided by grants from the Human Brain Project (P20-MHDA52176 and 5P01-EB001955).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz J Med Biol Res. 2003;36:409–420. doi: 10.1590/s0100-879x2003000400002. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Anderson EE, Bai Z, Bischof C, Blackford S, Demmel J, Dongarra J, Du Croz J, Greenbaum A, Hammarling S, McKenney A, Sorensen D. LAPACK Users’ Guide. 3. Philadelphia, PA: Society for Industrial and Applied Mathematics; 1999. [Google Scholar]
- Baerentsen KB, Stodkilde-Jorgensen H, Sommerlund B, Hartmann T, msgaard-Madsen J, Fosnaes M, Green AC. Cogn Process. 2009. An investigation of brain processes supporting meditation. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced changes in gamma band activity - Implications for the default mode network, self-reference and attention. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process. 2001;10:266–277. doi: 10.1109/83.902291. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning--revisited. PLoS ONE. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom M, Soderfeldt B. Brain activation during compassion meditation: a case study. J Altern Complement Med. 2010;16:597–599. doi: 10.1089/acm.2009.0309. [DOI] [PubMed] [Google Scholar]
- Faber PL, Steiner ME, Lehmann D, Pascual-Marqui RD, Jancke L, Esslen M, Giora R. Deactivation of the medial prefrontal cortex in experienced Zen meditators. Abstract Brain Topogr. 2008;20:165–180. [Google Scholar]
- Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10:43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research: Neuroimaging. 2010 doi: 10.1016/j.pscychresns.2010.08.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. SCAN. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AD, Mathias JL, Jacobson BL, Ruzic L, Bond AN, Banich MT. Relationship between intelligence and the size and composition of the corpus callosum. Exp Brain Res. 2009;192:455–464. doi: 10.1007/s00221-008-1604-5. [DOI] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang DH, Byun MS, Kwon SJ, Choi CH, Kwon JS. Increased default mode network connectivity associated with meditation. Neurosci Lett. 2011;487:358–362. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, Tillisch K, Mayer EA, Naliboff BD. Impact of Mindfulness-Based Stress Reduction training on intrinsic brain connectivity. Neuroimage. 2011;56:290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Ramage AE, Lancaster JL, Robin DA, Narayana S, Coyle T, Royall DR, Fox P. Loss of cerebral white matter structural integrity tracks the gray matter metabolic decline in normal aging. Neuroimage. 2009;45:17–28. doi: 10.1016/j.neuroimage.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Mayer EA, Thompson PM, Toga AW, Luders E. The right inhibition? Callosal correlates of hand performance in healthy children and adolescents. Human Brain Mapping. 2012 doi: 10.1002/hbm.22060. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage. 2011a;57:1308–1316. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Di Paola M, Tomaiuolo F, Thompson PM, Toga AW, Vicari S, Petrides M, Caltagirone C. Callosal morphology in Williams syndrome: a new evaluation of shape and thickness. Neuroreport. 2007a;18:203–207. doi: 10.1097/WNR.0b013e3280115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007b;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006a;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006b;17:1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Narr KL, Zamanyan A, Chou YY, Gutman B, Dinov ID, Toga AW. The link between callosal thickness and intelligence in healthy children and adolescents. Neuroimage. 2011b;54:1823–1830. doi: 10.1016/j.neuroimage.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45:672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc Natl Acad Sci USA. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin N, Vahidy S, Michielse S, Coupland N, Camicioli R, Seres P, Carter R. Structural organization of the prefrontal white matter pathways in the adult and aging brain measured by diffusion tensor imaging. Brain Struct Funct. 2011;216:417–431. doi: 10.1007/s00429-011-0321-1. [DOI] [PubMed] [Google Scholar]
- Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. Neuroimage. 2010;52:1190–1201. doi: 10.1016/j.neuroimage.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging in the aging brain: a conceptual review. J Geriatr Psychiatry Neurol. 2007;20:3–21. doi: 10.1177/0891988706297089. [DOI] [PubMed] [Google Scholar]
- Moyer CA, Donnelly MP, Anderson JC, Valek KC, Huckaby SJ, Wiederholt DA, Doty RL, Rehlinger AS, Rice BL. Frontal electroencephalographic asymmetry associated with positive emotion is produced by very brief meditation training. Psychol Sci. 2011;22:1277–1279. doi: 10.1177/0956797611418985. [DOI] [PubMed] [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, d’Aquili E. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res. 2001;106:113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wintering N, Waldman MR, Amen D, Khalsa DS, Alavi A. Cerebral blood flow differences between long-term meditators and non-meditators. Conscious Cogn. 2010 doi: 10.1016/j.concog.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol Aging. 2007;28:1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA. 2010;107:15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei S, Faber PL, Lehmann D, Tsujiuchi T, Kumano H, Pascual-Marqui RD, Gianotti LR, Kochi K. Meditators and non-meditators: EEG source imaging during resting. Brain Topogr. 2009;22:158–165. doi: 10.1007/s10548-009-0107-4. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion Imaging, White Matter, and Psychopathology. Annu Rev Clin Psychol. 2010 doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J Neurosci. 1996a;16:4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996b;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P, van BM, Skewes J, Bjarkam CR, Stubberup M, Bertelsen J, Roepstorff A. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2008 doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van ZP, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Rao H, Korczykowski M, Wintering N, Pluta J, Khalsa DS, Newberg AB. Cerebral blood flow changes associated with different meditation practices and perceived depth of meditation. Psychiatry Res. 2011;191:60–67. doi: 10.1016/j.pscychresns.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Huster RJ, Kreuder F, Wittling W, Schweiger E. Corticospinal tract asymmetries at the level of the internal capsule: is there an association with handedness? Neuroimage. 2007;37:379–386. doi: 10.1016/j.neuroimage.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 (Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Goldsmith CH. The relationship of hand preference to anatomy of the corpus callosum in men. Brain Res. 1991;545:175–182. doi: 10.1016/0006-8993(91)91284-8. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XJ, Leeds NE. Assessing glioma cell infiltration using a fiber coherence index: a DTI study. Proc Int Soc Magn Reson Med.2005. p. 365. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.