Abstract
Dendritic cells (DCs) are potent antigen-presenting cells capable of promoting or regulating innate and adaptive immune responses against non-self antigens. To better understand the DC biology or to use them for immune intervention, a tremendous effort has been made to improve gene transfer in these cells. Lentiviral vectors (LVs) have conferred a huge advantage in that they can transduce nondividing cells such as human monocyte-derived DCs (MDDCs) but required high amounts of viral particles and/or accessory proteins such as Vpx or Vpr to achieve sufficient transduction rates. As a consequence, these LVs have been shown to cause dramatic functional modifications, such as the activation or maturation of transduced MDDCs. Taking advantage of new pseudotyped LVs, i.e., with envelope glycoproteins from the measles virus (MV), we demonstrate that MDDCs are transduced very efficiently with these new LVs compared to the classically used vesicular stomatitis virus G-pseudotyped LVs and thus allowed to achieve high transduction rates at relatively low multiplicities of infection. Moreover, in this experimental setting, no activation or maturation markers were upregulated, while MV-LV-transduced cells remained able to mature after an appropriate Toll-like receptor stimulation. We then demonstrate that our MV-pseudotyped LVs use DC-SIGN, CD46, and CD150/SLAM as receptors to transduce MDDCs. Altogether, our results show that MV-pseudotyped LVs provide the most accurate and simple viral method for efficiently transferring genes into MDDCs without affecting their activation and/or maturation status.
INTRODUCTION
Dendritic cells (DCs) play a key role in the regulation of the immune system because they can develop into specialized antigen-presenting cells (APCs) (6). This makes them important candidates for gene therapy applications such as anticancer strategies, vaccinations, and the induction of tolerance (7, 11, 21, 39, 89). The immunogenicity of antigens delivered by APCs has been shown in patients with tumors (16) or with from chronic HIV infection (58) due to strong antigen-specific induced T-cell responses (11, 13, 21, 25). In the absence of inflammation, however, DCs do not fully mature and serve to induce self-reactive T-cell tolerance. This inactivation of antigen-specific T cells would be beneficial in treating autoimmune diseases, transplant rejections, and allergies (19, 69, 79). Immature DCs can be derived from monocytes in the periphery, thus representing an accessible and abundant source of cells to produce clinically relevant human monocyte-derived DCs (MDDCs [73]). One obstacle in using these MDDCs is that the genetic modification of these targets suffers from low levels of gene transfer and variable transduction levels when using electroporation, liposome-based transfection, or viral vectors (8, 22, 66, 71, 89). The most attractive vectors capable of introducing a transgene into DCs are adenoviral vectors (50, 77), gamma retroviral vectors (84), and lentiviral vectors (LVs) (8, 9, 22, 60, 66, 89). Among these, the most successful gene transfer vectors for MDDCs are simian immunodeficiency virus (SIV) (60, 66) and human immunodeficiency virus (HIV) lentiviral vectors (49), but a major drawback is that high vector doses (multiplicities of infection [MOIs] up to 500) are needed to achieve close to 100% transduction. Unfortunately, such high vector doses affects DC survival, maturation, and phenotype (10, 82).
In addition, it is well known now that MDDCs show a specific resistance to lentiviral transduction due to a so-called restriction factor that blocks infection in the early phases (41). In the search for a candidate that could relieve this restriction, Vpx, an accessory protein of SIV, was shown to improve SIV and HIV vector transduction levels of MDDCs up to 40-fold (8, 42, 43). It is hypothesized that Vpx counteracts a DC-dependent restriction that dramatically hinders viral infection in MDDCs (8, 42). The cellular protein SAMHD1 has been recently described as such a prominent restriction factor in MDDCs and macrophages (47, 54). Its neutralization by Vpx leads to a promotion of the transduction of myeloid cells by HIV-1-based viruses and a triggering of innate immune responses (54, 59).
We previously engineered lentiviral vectors carrying measles virus (MV) Edmonston (Ed), hemagglutinin (H), and fusion (F) glycoproteins (gps) at their surfaces (H/F-LVs) (26, 27). Most importantly, they represent the first tool to allow efficient transduction of quiescent human T cells and healthy and cancer B cells without inducing entry into the cell cycle and changing their phenotype (26, 27, 57). This is a major breakthrough, since classical VSV-G-LVs are unable to transduce resting lymphocytes due to postentry restrictions at the level of reverse transcription, nuclear import, and proviral genomic integration (12, 52, 85, 86). These H/F-LVs are able to overcome all of these restrictions (27, 29). The natural receptor of MV, used by most clinical isolates is the signaling lymphocyte activating molecule (SLAM [87]). SLAM is constitutively expressed in memory T cells, immature thymocytes, and a proportion of B cells (4, 15). In addition, SLAM is highly expressed on macrophages and on DCs upon maturation (53). The MV Edmonston vaccine strain also gained host cell entry through hCD46 receptor, a member of the complement activation regulators, expressed on all human nucleated cells (64). In accordance, the MV Edmonston gp-pseudotyped LVs conserved their entry through both CD46 and SLAM MV receptors and conserved their tropism for T and B cells (26, 27, 29). Moreover, to allow quiescent lymphocyte transduction, it is necessary that CD46 and SLAM are correctly engaged by these H/F-LVs to trigger an entry mechanism that strongly resembles macropinocytosis (30).
Immature DCs have a low SLAM surface expression (53) but are characterized by the surface expression of DC-specific intercellular adhesion molecule 3 (ICAM-3) grabbing nonintegrin (DC-SIGN, also know as CD209 [38, 70, 76]). Several pathogens, such as dengue virus (65), human T-cell lymphotropic virus type 1 (48), Ebola virus (1), HIV (37), and cytomegalovirus (46), target DC-SIGN, a C-type lectin for their dissemination. Indeed, DC-SIGN easily binds to mannose-containing carbohydrates linked to viral gps (23, 62). DC-SIGN, for example, binds to the HIV-1, HIV-2, and SIV gps and thereby enhances infection of T cells in trans (3, 37, 67). Importantly, DC-SIGN has been identified as a new MV attachment receptor, which does not confer cell entry but enhances DC infection through CD150/SLAM in cis (17) and mediates trans-infection of MV to T lymphocytes (18).
Since MV can bind immature MDDCs through DC-SIGN and the newly engineered H/F-LVs were able to overcome postentry restriction of lentiviral transduction in lymphocytes, we sought to determine whether these vectors were able to overcome specific LV restrictions in MDDCs and genetically modify immature MDDCs. We show here that H/F-LVs attain high-level transduction of immature MDDCs using low vector doses (MOIs from 1 to 10) without any help of exogenous factors to counteract cellular restrictions. In addition, H/F-LV-modified MDDCs conserve their phenotype and can be maturated after transduction. Finally, this new, simple and efficient H/F-LV transduction protocol may provide a sufficient amount of modified APCs to address basic research questions and for therapeutic purposes.
MATERIALS AND METHODS
Cells and reagents.
Elutriated human monocytes from healthy human volunteers were used to generate MDDCs according to the protocol described by Sallusto and Lanzavecchia (73); freshly isolated monocytes were further elutriated by the DTC cell-sorting facility (CHU Nantes/Biogen Ouest, Nantes, France). CD14+ cells (95% purity) were seeded at 106/ml in RPMI 1640 to 10% fetal calf serum (FCS) and 2 mM glutamine supplemented with 100 ng of rhGM-CSF (recombinant human granulocyte-macrophage colony-stimulating factor; Gentaur, Paris, France)/ml and 20 ng of rhIL-4 (recombinant human interleukin-4; Cellgenix, Freiburg, Germany)/ml and then cultured for 4 to 5 days. Day 4 immature MDDCs displayed the following standard phenotype: CD1a+ CD14− HLA-DR+ CD209+ CD80low CD86low CD83− (determined using fluorescein isothiocyanate-conjugated antibodies from BD Biosciences). When required, MDDCs were matured with lipopolysaccharide (LPS; 100 ng/ml; ultrapure LPS from Escherichia coli K-12) or poly(I:C) at 12.5 μg/ml, both purchased from Cayla-Invivogen (Toulouse, France). Human epithelial kidney cells (293T cell line) or HeLa cells were cultured in 8% FCS–Dulbecco modified Eagle medium (DMEM) supplemented with 2 mM glutamine. 293T cells were used for transient DC-SIGN ectopic expression. Briefly, 2 × 105 cells were seeded per six-well in DMEM–10% FCS and transfected with 3 μg of pcDNA expression plasmid diluted in NaCl 150 mM (200 μl, final) using 9.9 μl of ExGen 500 transfection reagent (Euromedex, Souffelweyersheim, France). After an overnight incubation, fresh medium was added, and the transfected cells were cultured for 24 to 48 h until used.
Plasmids and constructs.
The gps H and F from the Edmonston MV strain and H-D4 from a clinical MV strain were inserted into pCG plasmids under the control of the cytomegalovirus early promoter. The plasmids pCG-HΔ24 and pCG-FΔ30 code for cytoplasmic tail mutants of H and F, respectively, and were described elsewhere (27). The pCG-H-D4Δ24 construct was generated by the deletion of 24 amino acids from the cytoplasmic tail of the clinical strain H-D4 to allow efficient incorporation on LVs. pCG-H-D4YG-Δ24 was generated by introduction of point mutations N481Y and E492G into H-D4 as described by us previously (30). The pCMV-VSV-G plasmid was described previously (61).
Lentiviral vector production and titration.
HIV-1-derived vectors were generated by transient transfection of 293T cells with a Gag-Pol packaging construct (8.91), a self-inactivating (SIN) HIV vector encoding for green fluorescent protein (GFP) and the envelope gps, as described previously (27). For the codisplay of the HΔ24 gps (HEdΔ24, H-D4Δ24, and H-D4YGΔ24) and FΔ30 gps, 2.75 μg of the H and F envelope gps encoding plasmids were transfected. At 16 h posttransfection, the supernatant was replaced by Opti-MEM medium supplemented with HEPES (Invitrogen, Carlsbad). For VSV-G-LVs, 3 μg of pCMV-G plasmid was transfected. Viral supernatant was harvested at 48 h posttransfection. Low-speed concentration of the vectors was performed by overnight centrifugation of the viral supernatant at 3,000 × g and 4°C. To determine the infectious titers of the HIV vectors, serial dilutions of vector preparations were added to 293T cells or CHO-SLAM cells for the SLAM-tropic vectors (HEdΔ24, H-D4Δ24, and H-D4YGΔ24). The infectious titers are expressed as 293T, Raji, or CHO-SLAM transducing units (TU)/ml in the Table 1. In parallel, the vectors were titrated for physical particles (p24 levels in ng/ml) by measuring p24 antigen by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (RetroTek-Zeptomatrix, Buffalo, NY).
Table 1.
Comparison between physical and infectious titers of MV-derived LVs versus VSV-G-LVs obtained with different cell linesa
| Vector (100× concentrated) | Mean titer ± SD |
|||
|---|---|---|---|---|
| 293T (TU/ml) | CHO-SLAM (TU/ml) | Raji (TU/ml) | p24 (ng/ml) | |
| H/F-LVs | 4.7E6 ± 4,5E6 | 4.1E 7 ± 2.9E7 | 6.7E7 ± 2.1E7 | 155 ± 67 |
| VSV-G-LVs | 4.5E8 ± 1.8E8 | 8.4E7 ± 1.5E7 | 7.4E8 ± 1.1E7 | 182 ± 62 |
Supernatants of 293T producer cells were concentrated 100-fold by low-speed centrifugation. Titers for the 293T, CHO-SLAM, and Raji cells were assessed by adding serial dilutions of each vector preparation to the appropriate target cell. For each vector, the percentage of GFP+ cells was analyzed at day 3 posttransduction. Vectors were titered on 293T, CHO-SLAM, and Raji cells. Titers are expressed as mean transduction units (TU) per ml (n = 6). For the p24 cells, the titers are expressed as the p24 level (in ng/ml) determined by ELISA that represents a measure for the total physical particles present in the vector preparation.
Although we had constant numbers of physical particles (p24 levels, ELISA), due to the need to incorporate H and F gps on the same vector particle, infectious titers of H/F-LVs vary from one preparation to anothor.
Transduction assay.
For lentiviral transduction assays, 2 × 105 MDDCs or other cell lines (i.e., 293T or HeLa) cells were incubated with H/F-, H-D4/F-, or H-C2/F-LVs (D4 and C2 are both CD150/SLAM-tropic MV genotypes) and VSV-G-LVs in RPMI 1640 without FCS in a flat-bottom 96-well plate for 2 h at 37°C at the indicated MOIs. The transduced MDDCs were then washed twice with RPMI 1640 and seeded in RPMI 1640–10% FCS supplemented with 200 ng of rhGM-CSF and 40 ng of rhIL-4/ml and cultured for four additional days to allow detection of GFP expression. When needed, MDDCs were transduced with GFP-encoding LVs in the presence of Vpx-containing virus-like particles (VLPs) generated as described previously by Negre et al. (66). The pSIV3+ plasmid used to generate the Vpx-containing VLP was kindly supplied by Nicolas Manel (Institut Curie, Paris, France [http://dx.doi.org/10.1038/protex.2010.208]) (59). Transduction efficiencies (% GFP+ cells) were evaluated by fluorescence-activated cell sorting (FACS) analysis. Upon transduction by LVs, alpha interferon (IFN-α; PBL Interferonsource, Piscataway, NJ) and IFN-β (Invitrogen, Carlsbad, CA) were quantified by specific ELISA in the culture supernatants of MDDCs at different MOIs.
Receptor blocking assay.
Day 4 to 5 immature MDDCs or DC-SIGN-expressing HEK cells were incubated for 1 h with blocking antibodies at 20 μg/ml: anti-CD46 (monoclonal antibody [MAb] 13/42) was kindly provided by R. Buckland, anti-SLAM (anti-hCD150; clone A12) was purchased from eBioscience (San Diego, CA), and anti-DC-SIGN (clone AZN-D1) was obtained from Beckman Coulter. Alternatively, mannan (at 20 μg/ml in phosphate-buffered saline [PBS]) was used as a positive control of DC-SIGN receptor blocking (Sigma-Aldrich, St. Louis, MO). Subsequently, transductions were performed at an MOI of 10 in the presence of blocking antibodies as described above. Transduction efficiencies (i.e., the % GFP+ cells) were evaluated at 4 days posttransduction by FACS analysis.
Cell cycle analysis.
THP1 cells (ATCC no. TIB-202), fresh monocytes, or MDDCs were harvested, fixed, and permeabilized with a fixation/permeabilization buffer (1% paraformaldehyde and 0.02% saponin in PBS) for 30 min. The cells were then washed twice in washing buffer (PBS, 1% bovine serum albumin [BSA], 0.1% sodium azide, 0.02% saponin) and stained for 30 min with a phycoerythrin (PE)-conjugated anti-Ki67 antibody (clone B56) from BD Biosciences (Le Pont de Claix, France). A final incubation with a 1 μM TO-PRO III solution (Invitrogen) to stain DNA was performed before the cells were analyzed on an LSR II flow cytometer (BD Biosciences).
Flow cytometry.
Phenotype analyses were also performed by flow cytometry on an LSR II flow cytometer with the following antibodies: CD150/SLAM-PE (eBiosciences, San Diego, CA) and CD46-PE, CD14-PE, CD83-PE, CD1a-APC, HLA-DR-PE, CD80-PE, and CD86-PE (BD Biosciences). Anti-human CD209-PE (DC-SIGN) was obtained from R&D Systems (Abingdon, United Kingdom).
Statistical analyses.
Statistics were generated using the GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA). Unpaired sample comparisons were performed using a Mann-Whitney nonparametric rank test.
RESULTS
Genetic modification of immature MDDCs is greatly enhanced using MV-pseudotyped lentiviral vectors.
In an attempt to increase the transduction efficiency of MDDCs, we compared two distinct lentiviral vectors differing only in surface display of envelope glycoproteins (gps), a process called pseudotyping. We indeed generated LVs pseudotyped with the extensively used VSV envelope gp G (VSV-G) or with hemagglutinin (H) and fusion (F) gps from the Edmonston MV strain. Both LVs encoded GFP as a reporter gene for easy detection of gene transfer efficiency in these primary cells. Fresh highly purified human monocytes were induced into DC differentiation by a 4 days of culture in the presence of rIL-4 and GM-CSF.
Immature MDDCs were transduced with an MOI of 10 and, at 4 days posttransduction, the gene transfer efficacy was evaluated by flow cytometry as the percentage of GFP+ cells (Fig. 1A). In this setting, the H/F-LV was about four times more efficient than VSV-G-LV for the transduction of MDDCs. Moreover, this gain of H/F-LV transduction efficacy was also observed at lower MOIs (MOIs of 1 and 2 compared to an MOI of 10). Even at these low vector doses, H/F-LVs outperformed by far VSV-G-LVs for the transduction of immature MDDCs (Fig. 1B). Of note, the mean fluorescence intensity (MFI) of GFP expression in MDDCs was markedly higher upon H/F-LV-mediated transduction compared to VSV-G-LV-mediated transduction (an approximately 5- to 10-fold increase; Fig. 1C). One possible explanation is that the higher fluorescence intensity may be due to a higher copy number of integrated proviruses per transduced cell for H/F-LVs compared to VSV-G-LVs. However, we did not detect a significant difference in number of proviral integration sites for both LV pseudotypes (MOI of 2; VSV-G-LVs = 3.2 ± 1.1 and H/F-LV = 3.7 ± 1.4 average copy numbers/cell, as assessed by quantitative PCR [data not shown]). It is, however, difficult to compare both vector pseudotypes (VSV-G-LVs and H/F-LVs) using the infectious titers determined on a cell line since the level of VSV-G or MV receptor expression will vary depending on the particular cell line used (Table 1). Therefore, we performed the MDDC transduction by adding equivalent amounts of both vector types, as determined by p24 levels (Table 1). Again, the better transduction efficiency of H/F-LVs compared to VSV-G-LVs was confirmed (Fig. 1D). Taken together, these results showed that H/F-LVs are capable of transferring genes in MDDCs more efficiently than classical VSV-G-LVs, even at low vector doses.
Fig 1.
MV gp-pseudotyped LVs are highly superior to VSV-G-LVs for the transduction of immature MDDCs. (A) Day 5 immature MDDCs were transduced or not by VSV-G-LVs (middle panel) or H/F-LVs (lower panel) encoding GFP (MOI = 10). Transduction efficiencies were analyzed by flow cytometry and plotted as the percentage of GFP+ cells in DC-SIGN expression in immature MDDCs. (B and C) Flow cytometric analyses showing frequencies (B) and GFP MFIs (C) after the transduction of immature MDDCs with increasing doses of VSV-G-LV (empty bars) or H/F-LV (gray bars) particles (MOI = 0, 1, 2, or 10) (only MOI = 10 in panel C) (n = 5). (D) Transduction of day 5 immature MDDCs was performed as described in for panel A but with equivalent total p24 levels of VSV-G-LVs (empty bars) and H/F-LVs (gray bars) (in pg; n = 3). Error bars are indicated above each column.
H/F-LV-mediated gene transfer into immature MDDCs depends on their differentiation status.
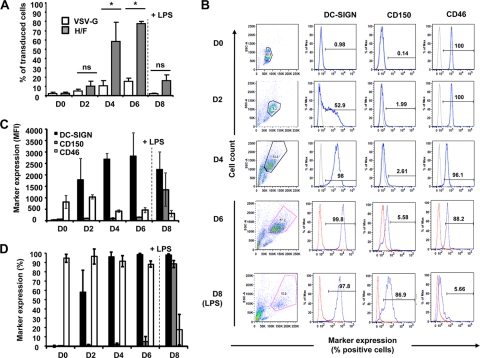
Next, we evaluated whether H/F-LV DC permissiveness changed at different time points of cell differentiation (i.e., 2, 4, and 6 days after differentiation) and whether matured MDDCs could be transduced to a similar extent. In agreement with our previous results, freshly isolated monocytes were very poorly transduced by H/F-LVs (28) (Fig. 2A, day 0 [D0]). Subsequently, we transduced differentiating MDDCs (i.e., days 2, 4, and 6 after differentiation) and LPS-matured MDDCs (100 ng of LPS/ml for 48 h [day 8]) with H/F-LVs or VSV-G-LVs. After 4 days, the transduction rates were measured by flow cytometry. Almost no GFP-expressing cells were detected in H/F-LV-transduced early differentiating MDDCs (day 2; Fig. 2A). In contrast, GFP expression peaked at days 4 and 6 of culture of immature MDDCs but decreased in LPS-matured MDDCs. VSV-G-LV-transduced MDDCs behaved similarly, but the respective transduction efficiencies remained modest (Fig. 2A). Interestingly, D2 MDDCs were transduced by the VSV-G-LVs, although with a very low efficiency. These results suggested that the marked difference in H/F-LV transduction levels were a function of the differentiation status of the MDDCs and could be attributed to either changes in the phenotype or in their proliferation capacity.
Fig 2.
Efficient transduction of immature MDDCs by H/F-LVs coincides with high DC-SIGN and CD46 receptor expression on their surfaces. (A) Flow cytometric analysis of GFP-encoding VSV-G-LV (empty bars)- versus H/F-LV (gray bars)-transduced freshly isolated monocytes (day 0 [D0]), immature MDDCs (days 2, 4, and 6 [D2, D4, and D6]), and LPS-matured MDDCs (100 ng/ml for 48 h). The maturation step is indicated by a dashed line on the graph. Statistical analyses (Mann-Whitney t test) are indicated above each comparison between the VSV-G-LV and H/F-LV transductions. Asterisks indicate P < 0.05, and “ns” stands for “not significant.” Equivalent p24 levels (10 ng) of VSV-G-LVs and H/F-LVs were applied. (B) Analysis of morphological parameters (FSC-A versus SSC-A, left column), as well as DC-SIGN, CD150/SLAM, and CD46 expression (the percentage of positive cells is indicated) by flow cytometry on fresh monocytes (day 0 [D0]), immature MDDCs (days 2, 4, and 6 [D2, D4, and D6]) and LPS-matured MDDCs (D8). The expression levels (C) and frequencies (D) of DC-SIGN, CD150/SLAM, and CD46 markers were evaluated by flow cytometry. The results are displayed as means ± the standard errors of the mean (SEM; n = 6).
Therefore, we first explored the phenotypic changes in the surface expression of MV receptors induced by the differentiation process of monocytes toward MDDCs. Indeed, based on the fact that MV may use CD150/SLAM and/or CD46 as entry receptors in the target cells, we looked at the expression of these two MV receptors in the course of MDDC differentiation. In parallel, we monitored the expression of the C-type lectin DC-SIGN, identified as a hallmark of the rhGM-CSF- or rhIL-4-generated immature MDDCs but also as a potential coreceptor for MV (Fig. 2B and C) (17, 18). The CD46 expression remained almost constant during the course of the DC differentiation in terms of the MFI, but the frequency of CD46+ MDDCs strongly decreased upon LPS-mediated maturation (Fig. 2D). Thus, CD46 may be used as a receptor by H/F-LVs since its downregulation abrogated the transduction of matured MDDCs. However, CD46 may not be sufficient to explain why immature MDDCs are far more susceptible to transduction by these vectors. In agreement with other studies (53), CD150/SLAM was shown to be moderately or lightly expressed on immature MDDCs but dramatically upregulated in LPS-matured MDDCs (Fig. 2B and C). However, these latter cells did not exhibit a high susceptibility to H/F-LV transduction (Fig. 2A). Therefore, CD150/SLAM was at this stage not considered as a major receptor for H/F-LVs on MDDCs even if its involvement cannot be ruled out completely. DC-SIGN, which is known for its ability to promote infection of MDDCs by several viruses, such as MV, clearly was already highly expressed at day 2 of differentiation and peaked between days 4 and 6, depending on the donor (Fig. 2B, C, and D). Taken together, these results demonstrated that H/F-LVs can transduce immature MDDCs with a high efficacy, which correlates with their differentiation status and more precisely with their CD46+ DC-SIGN+ phenotype.
High-level transduced H/F-LVs immature MDDCs reside in the G0 phase of the cell cycle.
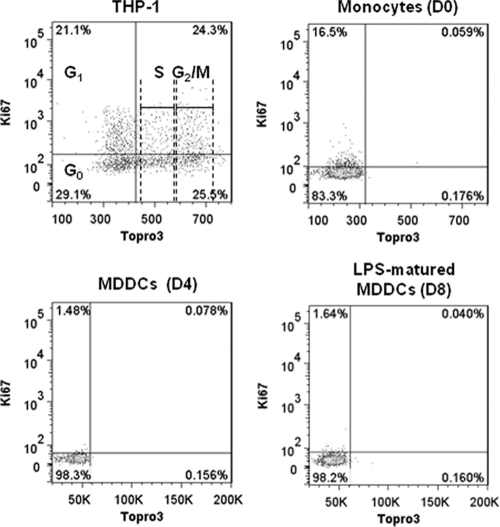
A second important aspect next to MDDC phenotype that needed to be studied was whether the high permissiveness of H/F-LVs correlated with MDDC cycle entry at the time of transduction. It is widely assumed that MDDCs, as well as monocytes, do not proliferate; however, only a few studies have presented convincing arguments supporting that view (2). Thus, we assessed whether H/F-LV-transduced monocytes and immature or mature MDDCs were in the cycle or not by using cellular DNA and Ki-67 antigen staining, followed by FACS detection. Ki-67 antigen is only expressed by cells that have left the G0 phase to enter into the G1, S, G2, or M phase of the cell cycle.
THP-1 cells (transformed monocytoid leukemia cells) were used as a positive control for proliferation to indicate the different cell cycle phases (Fig. 3). Interestingly, a tiny fraction (ca. 15%) of the freshly isolated monocytes was weakly stained by the anti-Ki-67 antibody, suggesting that these cells may not be completely quiescent but rather engaged in the G1 phase of the cell cycle (i.e., KI-67-positive cells but no DNA synthesis [Fig. 3]). These results were similar for several donors and in agreement with recent findings suggesting that a small subset of human blood monocytes may be osteoclast precursors with proliferative properties (14, 24, 55). In addition, we confirmed that immature (day 4) and LPS-matured MDDCs (day 8) resided in the G0 phase of the cell cycle (Fig. 3). In summary, we demonstrated that although immature MDDCs reside in the G0 phase of the cell cycle, they remained highly susceptible to H/F-LV transduction.
Fig 3.
Efficient H/F-LV transduction of MDDCs is not correlated with their cell cycle entry. Cell cycle analyses of a dividing transformed monocytoid cell line (THP-1, upper left) or monocytes (upper right), as well as of immature (lower left) and mature (lower right) MDDCs, were performed using flow cytometry with the Ki-67 proliferation marker and a DNA-specific labeling with the TO-PRO III fluorochrome. The percentages of positive or negative cells for both markers are indicated in each quadrant of the presented dot plots. For the THP-1 cell line, the cell cycle phases are indicated in the dot plot (n = 3).
Vpx complementation increases H/F-LV transduction of MDDCs but induces their maturation.
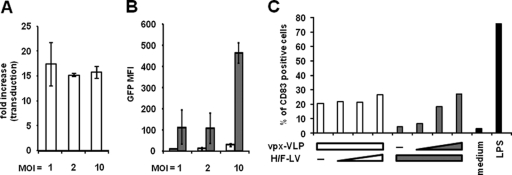
Vpx was previously shown to complex with the SAMHD1 protein in cells of the myeloid lineage, thus leading to the degradation of the complex by the proteasome (47, 54). SAMHD1 has now been characterized as a restriction factor of the transduction of cells from myeloid origin by HIV1. Addition of Vpx-containing VLPs (Vpx-VLPs) to an MDDC culture augments the VSV-G-LV transduction efficiency of these myeloid cells (8, 59). We therefore examined whether Vpx complementation could improve the transduction of MDDCs by H/F-LVs. In the presence of constant levels of Vpx-LVs, we obtained a relatively constant fold increase in MDDC transduction efficiency (i.e, about 15- to 20-fold) compared to transduction in the absence of Vpx-LVs for different H/F-LV vector doses (Fig. 4A). GFP expression levels (MFI) increased, however, with augmented H/F-LV doses (Fig. 4B). We then evaluated DC differentiation using CD83 surface expression, which is a marker of DC maturation/activation, induced by MDDCs transduced with varying or constant amounts of H/F-LV and Vpx-VLPs. The results are presented in Fig. 4C. White histograms show the transduction results with a constant amount of Vpx-VLP (100 μl of supernatant) and no or increasing amounts of H/F-LVs (0 and MOIs of 1, 2.5, and 5). Vpx-VLPs induced maturation in 20 to 30% of MDDCs, as detected by 20 to 30% CD83 surface marking in the absence or presence of different doses of H/F-LVs (Fig. 4C). Conversely, gray histograms show the results for cells transduced with a constant MOI (10) and no or increasing amounts of Vpx-VLPs (10, 25, and 50 μl of supernatant). CD83 expression appeared to be correlated to the increasing amount of Vpx-VLPs added to a constant dose of H/F-LVs (gray bars in Fig. 4C). Altogether, these results demonstrate that the Vpx complementation was responsible for a marked increase of the transduction of MDDCs by H/F-LV vectors but concomitantly for a substantial upregulation of the expression of the maturation marker CD83.
Fig 4.
Vpx complementation potentiates H/F-LV transduction efficacy but increases maturation markers on MDDCs. MDDCs were transduced with increasing MOIs of H/F-LV (MOIs of 1, 2, and 10) in the presence (gray bars) or absence (empty bars) of a constant amount of Vpx-containing VLP supernatants (100 μl). The results are displayed as the transduction fold increase ± the SEM (i.e., the fold increase = [transduction-level H/F-LV + Vpx-VLP]/[transduction-level H/F-LV − Vpx-VLP]) (A) or as GFP MFIs ± the SEM (with or without Vpx-VLP [gray and empty bars, respectively]; n = 3) (B). (C) Flow cytometric analysis of the CD83 maturation marker expression by MDDCs after being either transduced with a constant amount of Vpx-VLPs (100 μl of supernatant) and no or increasing doses of H/F-LV (MOIs of 1, 2.5, and 5; empty bars). Inversely, the gray histograms represent a transduction experiment conducted with a single dose of H/F-LV (MOI = 10) and no or increasing amounts of Vpx-VLPs (10, 25, and 50 μl of supernatant). LPS-matured (100 ng/ml for 24 h) MDDCs were used as a positive control of CD83 expression induction (n = 2).
MDDCs do not get activated or matured upon transduction with H/F-LV but maintain their capacity to mature upon TLR3 agonist exposure.
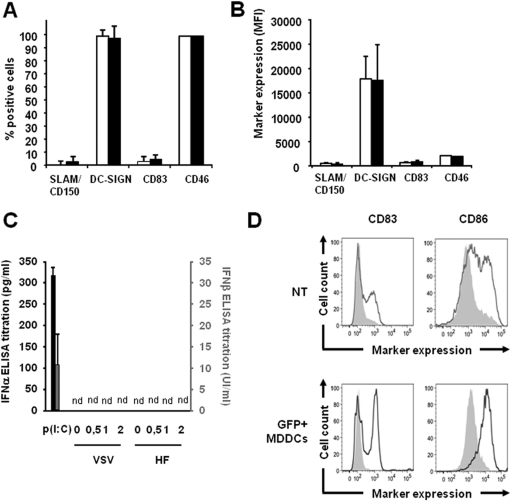
It was of great importance to identify possible phenotypic/functional changes of H/F-LV transduced MDDCs by documenting modulations of some key surface markers on these cells. To do this, the CD46, CD150/SLAM, DC-SIGN, and CD83 expression levels were measured by flow cytometry on MDDCs before (empty bars) and after (black bars) transduction with GFP-encoding H/F-LVs (MOI = 10). We did not observe any differences in terms of percentage of the positive cells (Fig. 5A) and expression levels of the previously listed markers (Fig. 5B). Of note, no upregulation of the maturation marker CD83 could be evidenced (i.e., transductions reaching 80 to 90%) upon H/F-LV-mediated transduction of MDDCs with MOIs ranging from 1 to 20 (data not shown). In this setting, no type I IFN (IFN-α and IFN-β) was secreted by H/F-LV-transduced MDDCs at all (Fig. 5C). In light of their potential utility in immunotherapeutic protocols as adjuvant cells or as antigen-specific stimulators of the immune system in vaccination protocols, we assessed the ability of H/F-LV-transduced cells to mature upon activation by Toll-like receptor (TLR) agonists. At day 4 of culture, the MDDCs were transduced with a GFP-encoding H/F-LV (MOI = 10), and the GFP-positive cells were then sorted by FACS. Sorted cells (GFP+ MDDCs) and untransduced cells (NT, negative control) were then subcultured or not with a suboptimal dose of poly(I:C) (12.5 μg/ml), which is a strong TLR3 agonist. The cells were then analyzed on a flow cytometer, and their CD83 and CD86 expression levels were measured (Fig. 5D). Clearly, untransduced and sorted H/F-LV-transduced MDDCs were capable of undergoing maturation upon TLR3 activation to a similar extent, as shown by their similar increases in CD83 and CD86 expression induction. The minor differences we observed in the experiment displayed in the Fig. 5D in both CD83 and CD86 expression induction after poly(I:C) exposure between NT and GFP+ MDDCs were not seen for all tested MDDC preparations. Altogether, these results demonstrated that H/F-LV-transduced immature MDDCs did not mature after H/F-LV transduction but kept their ability to mature upon an appropriate TLR stimulation.
Fig 5.
No difference in the expression of MV receptors and activation or maturation markers of MDDCs occurs upon transduction with H/F-LVs. (A or C) Results for MDDC phenotypes upon H/F-LV transduction. (D) Phenotypic analysis of poly(I:C)-matured MDDCs with or without transduction. In panels A and B, the percentages (A) and MFI values (B) for positive immature MDDCs for the CD150/SLAM, DC-SIGN, CD83, and CD46 molecules were evaluated by flow cytometry before (white bars) and after (black bars) transduction with a GFP-encoding H/F-LV (MOI of 10 for both). These analyses were performed using the MDDC subpopulation. The results are presented as means ± the SEM (n = 4). (C) IFN-α (black bars) and IFN-β (gray bars) were quantified by ELISA in the supernatants of MDDCs upon transduction with H/F-LVs or VSV-G-LVs at the indicated MOIs. Supernatants were harvested at 24 h posttransduction. Treatment with poly(I:C) at 50 μg/ml was used as a positive control for type I IFN secretion by MDDCs. nd, not detected (neither IFN-α nor IFN-β was recovered in the culture supernatants). The results are presented as means ± the SEM (n = 2). (D) Comparison of CD83 and CD86 expression levels on untransduced (NT) versus H/F-LV-transduced MDDCs. The cells were transduced with a GFP-encoding LV (MOI = 10). GFP-positive MDDCs were positively selected by FACS to achieve nearly 100% transduced cells. Untransduced and GFP+ cells were subcultured with (thick line) or without (gray filled) poly(I:C) (a TLR3 agonist) at 12.5 μg/ml and subsequently stained for the CD83 and CD86 maturation markers (n = 3).
DC-SIGN expression in cell lines enhances their transduction by H/F-LVs.
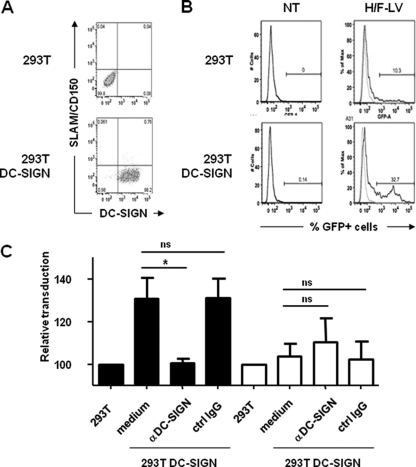
As shown in Fig. 2, H/F-LV-mediated transduction of MDDCs strongly coincided with high levels of DC-SIGN. Thus, we wanted to elucidate whether DC-SIGN could be effectively involved as a coreceptor for the transduction of MDDCs by H/F-LVs. Indeed, DC-SIGN was already described as a crucial receptor capable of promoting the attachment and even entry steps of MV into MDDCs (17, 18). Therefore, 293T cells were transiently transfected with a plasmid encoding DC-SIGN or a control plasmid, and the DC-SIGN expression was evaluated by flow cytometry after 48 h (Fig. 6A). Virtually all transfected cells expressed DC-SIGN, but these 293T cells lacked CD150/SLAM receptor expression. The complement receptor, CD46, was expressed naturally on untransfected 293T at sufficient levels to allow basal transduction activity by H/F-LVs (MOI = 0.1), i.e., ca. 10% of GFP+ parental 293T cells (Fig. 6B, upper panels). Interestingly, DC-SIGN-expressing 293T cells were more susceptible to H/F-LV transduction (exhibiting a 3-fold increase in transduction), suggesting that DC-SIGN might be involved in this process (Fig. 6B, lower panels). To elucidate the role of DC-SIGN in this increased H/F-LV-mediated gene transfer, we conducted experiments using a preincubation step with a neutralizing anti-DC-SIGN MAb. Indeed, the antibody-mediated DC-SIGN neutralization led to a modest but significant decrease in the transduction efficiency (33% inhibition) compared to the same cells treated with an isotypic control or no antibody. In contrast, no significant effect could be observed with VSV-G-LV-transduced DC-SIGN+ 293T cells in the presence of an anti-DCSIGN antibody (Fig. 6C). Taken together, these results demonstrated a possible involvement of the C-type lectin DC-SIGN as a prominent facilitating receptor for H/F-LV transduction.
Fig 6.
Overexpression of DC-SIGN augments H/F-LV transduction efficiency in cell lines. (A) 293T cells were transiently transfected with an empty or a DC-SIGN-encoding expression plasmid for 48 h. The percentages of DC-SIGN+ cells were assessed by flow cytometry. (B) DC-SIGN+ and parental 293T cells were transduced with a GFP-encoding H/F-LV (right two profiles; MOI = 0.1) versus no transduction (NT; left two profiles). Percentages of GFP+ 293T cells are indicated. Isotype controls are displayed as thin dashed lines. (C) DC-SIGN-expressing and parental 293T cells were transduced with GFP-encoding VSV-G-LVs (white bars) or H/F-LVs (black bars) (MOI = 1) with or without (medium) pretreatment with a neutralizing anti-DC-SIGN MAb (clone 1B10) or an isotype control antibody (ctrl IgG). The results are indicated as mean indices ± the SEM (n = 4). Statistical analyses (Mann-Whitney t test) were performed, and significant differences (P < 0.05) between tested experimental conditions are indicated by an asterisk. ns, not significant.
High-level H/F-LV transduction requires DC-SIGN and CD46 expression on MDDCs.
In light of our results above indicating a role of DC-SIGN in H/F-LV cell line gene transfer, we wanted to study the role of DC-SIGN, CD46, and CD150/SLAM receptors in the H/F-LV transduction of MDDCs.
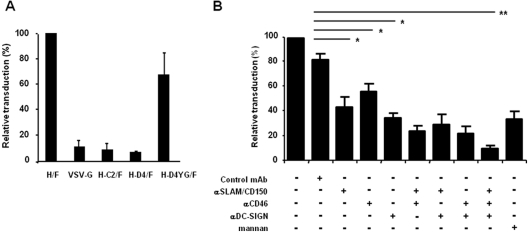
First, we generated H/F-LVs harboring H gps from clinical MV strains, i.e., C2 and D4 genotypes, which are SLAM-tropic (H-C2/F-LVs and H-D4/F-LVs, respectively). In addition, we used an H-D4 gp mutant called H-D4YG/F that also contains, next to the SLAM binding sites, CD46 binding sites in H, as previously described (30). All of these LVs were compared to the Edmonston H and F gp-pseudotyped LVs (H/F-LV) and to the VSV-G-LV (Fig. 7A). The SLAM-tropic H-C2/F-LVs and H-D4/F-LVs very poorly transduced the MDDCs (Fig. 7A). In contrast, the SLAM/CD46-tropic H/F-LVs and H-D4YG/F-LVs were able to transduce immature MDDCs to a similar extent, strongly suggesting that mainly CD46 and less often CD150/SLAM receptors were involved in the transduction of MDDCs by H/F-LVs. This finding was in line with the results obtained with DC-SIGN-expressing 293T cells (Fig. 6B and C). Next, we used an antibody-mediated neutralization assay to evidence the implication of DC-SIGN and CD46 in the highly efficient H/F-LV transduction of MDDCs. Antibodies were used alone or in combination (20 μg/ml each) to evaluate the inhibition of the different receptors (Fig. 7B). Inhibition of CD46, CD150/SLAM, and DC-SIGN separately led to modest but significant decreases in transduction (29, 47, and 57% mean inhibitions, respectively). Combining the neutralizing MAbs by pairs allowed a more pronounced inhibition of transduction (61 to 80% inhibition). When the three blocking antibodies were associated, a maximum inhibition was observed (88%; P < 0.01). Notably, the use of concentrated mannan (50 μg/ml), a DC-SIGN blocking agent, and an anti-DC-SIGN antibody (1B10 clone) led to comparable inhibitions of transduction (56 and 57%, respectively).
Fig 7.
Immature MDDCs require DC-SIGN, CD46, and CD150/SLAM to be efficiently transduced by H/F-LVs. (A) MDDCs were transduced with GFP-encoding LV-pseudotyped H and F gps from the Edmonston MV strain (H/F; CD46 and CD150/SLAM dependent) or F gp and with three distinct H gps from wild-type MV strains (C2 and D4 genotypes). LVs pseudotyped with the wild-type MV strain gps are CD150/SLAM dependent. The H-D4YG gp has been mutated to gain the capacity to utilize CD46 as a coreceptor, in addition to the SLAM receptor (n = 3). (B) MDDCs were transduced with H/F-LVs coding for GFP (MOI = 10) in the presence or absence of neutralizing antibodies against DC-SIGN, CD46, and/or CD150/SLAM, alone or in combination (each at 20 μg/ml). Mannan (50 μg/ml) was used a positive control of C-type lectin functional inhibition. The results are indicated as means ± the SEM (n = 5). Statistical analyses (Mann-Whitney t test) were done, and significant differences are marked by asterisks (*, P < 0.05; **, P < 0.01). ns, not significant.
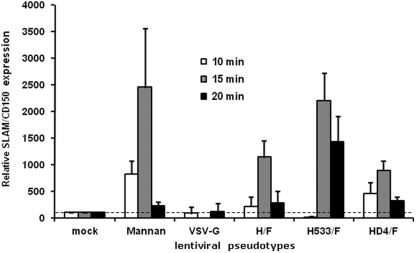
Interestingly, we have been able to inhibit H/F-LV MDDC transduction to some extent with an anti-CD150/SLAM antibody (Fig. 7B), although we showed above that CD150/SLAM was expressed at low levels on immature MDDCs (Fig. 2B, C, and D). Nevertheless, one cannot rule out that very small amounts of such MV receptors are enough to participate in the H/F-LV-mediated transduction of MDDCs. Indeed, a recent report offered strong evidence that DC-SIGN signaling through interaction with a synthetic ligand such as mannan or MV itself may lead to a transient but dramatic expression of CD150/SLAM at the cell surfaces of MDDCs (5). We then performed experiments using either mannan or LVs pseudotyped with VSV-G, H/F, H-D4/F gps and another H mutant, Ha533/F gps. Ha533 was derived from the Edmonston H gp by a single point mutation introduced in amino acid 533 which abolished binding to SLAM while conserving binding to CD46 receptor. The CD150/SLAM expression was determined by flow cytometry at 10, 15, and 20 min of stimulation. The results are displayed as relative expressions comparatively to a reference index (The MFI of CD150/SLAM expression on MDDCs upon incubation at 37°C in the absence of vector was set to 100). Importantly, MDDCs incubated with H/F-LVs showed a high-level upregulation of SLAM expression at 15 min compared to the control cells (Fig. 8). In contrast, a very short incubation with the VSV-G-LV did not induce CD150/SLAM overexpression. The SLAM-tropic H-D4/F-LVs had an effect similar to that of the H/F-LVs (Fig. 8). Interestingly, incubation with the CD46-tropic Ha533/F-LVs seemed to increase the duration of CD150/SLAM overexpression (Fig. 8). This was not surprising since this mutant did not bind CD150/SLAM and was thus unable to induce its internalization. These results strongly suggested that H gp, when displayed on LVs, interacts with DC-SIGN and induced CD150/SLAM expression at the MDDC surface at sufficient levels to participate in the capture and entry phases of the H/F-LV transduction. These data can be reconciled with our previous observation showing anti-SLAM-mediated neutralization of transduction (Fig. 7B). In conclusion, these results strongly suggest that not only CD46 and DC-SIGN but also CD150/SLAM might be implied in highly efficient H/F-LV-mediated transduction of MDDCs even if their respective implications appear to be different.
Fig 8.
Fast kinetics of SLAM-induced expression on MDDCs. CD150/SLAM surface expression was monitored at different time points (empty bars for 10 min, gray bars for 15 min, and black bars for 20 min of induction) by flow cytometry upon interaction with LVs displaying different gps (VSV-G, H/F, H-D4/F, and Ha533/F; H-D4 is a clinical SLAM-tropic H gp, and H533 is mutant H but not able to bind CD150/SLAM, which only uses CD46 as a receptor) or saturating amounts of mannan. The results are representative of three independent experiments. The dashed line indicates the basal CD150/SLAM expression level on MDDCs without any stimulation. The results are indicated as means ± the SEM (n = 3).
DISCUSSION
DCs are major protagonists of the innate and adaptive immune responses in mammalians. They are endowed with unique danger signal-sensing, antigen-processing, and migratory properties (6). Genetic modification of such cells for basic research, as well as for clinical applications, is a major and strategic issue. Tremendous efforts have been made during the last 10 years to improve gene transfer into in vitro-generated DCs from several human origins, i.e., CD34+ hematopoietic stem cells (HSC; cord blood or G-CSF mobilized cells), adult blood monocytes, and more recently embryonic stem (ES) and induced pluripotent stem (iPS) cells (75, 80). In the present study, we were able to improve gene transfer into immature MDDCs using MV gp-pseudotyped LVs, resulting in a far better efficacy than the widely used VSV-G-LVs, and this was accomplished without inducing any change in the maturation or activation phenotype of these cells. Moreover, these high MDDC transduction levels were achieved in the absence of additives, and H/F-LV transduction did not interfere with the maturation capacity of these immature DCs. These H/F-LVs are unique since we and others previously demonstrated that they allowed genetic modification of quiescent T and B cells, which are very poorly permissive for classical VSV-G-LVs (26–28, 34, 35). We can conclude from this that they are excellent tools for efficient gene modification of immature MDDCs. Indeed, the H/F-LV transduction efficacy often yielded 70 to 85% with MOIs ranging from 10 to 20. Even very low MOIs (1 to 2) allowed very high transduction efficiencies (40 to 60%). At the low vector doses used in the present study, no other previously published work has demonstrated an average MDDC transduction efficacy of 80% using HIV-1 derived vectors in the absence of accessory molecules such as Vpr or Vpx, previously described as transduction helpers in DCs (42, 51). Remarkably, in addition to the high percentage of transduction detected, the expression levels per cell (i.e., the MFI values) were also mostly higher in H/F-LV-transduced cells than in VSV-G-LV-transduced cells. Considering the fact that the same internal promoter driving GFP expression was used in both types of LVs, we supposed that H/F-LVs penetrated into the cells, integrated into the host genome, and allowed gene expression more rapidly than VSV-G-pseudotyped vectors in MDDCs. This is supported by the evidence that H/F-LVs may enter cells by the engagement of a very fast receptor-mediated endocytosis pathway and not by direct fusion at the plasma membrane in lymphocytes (26, 30). Even if VSV-G and F envelope gps share similar fusion properties, the VSV-G fusion activity requires a low-pH step, suggesting that it may also need to be internalized in low-pH endosomes to promote viral entry (68, 72, 90). Thus, the observed differences in our study in terms of the transduction rate and transgene expression level are likely to be due to the type of receptor(s) targeted by the H/F-LVs which is specifically displayed by MDDCs.
Based on antibody-mediated inhibition and on the use of mutant H/F-LVs, we demonstrated that CD46 was a prominent receptor used by H/F-LVs on MDDCs. This was in agreement with previous works confirming the Edmonston MV tropism for CD46 (20, 64). The poor transduction observed with LVs pseudotyped with two SLAM-tropic clinical MV strain H gps are in line with the reduction of H/F-LV transduction after blocking the CD46 receptor. Moreover, introduction of mutations able to restore the ability to interact with CD46 in the clinical strain H-D4 gp allowed this mutant H-D4YG/F-LV to transduce MDDCs at efficiencies comparable to those of the initial H/F-LVs. We have shown previously for T and B cells (30) that SLAM attachment is not sufficient for transduction of these quiescent cells and that H-D4/F-LVs cannot transduce these cells. By introducing binding sites for CD46 into the H-D4 gp (H-D4YG), the corresponding LV H-D4YG/F pseudotypes permitted the transduction of T and B cells (30), and now we show here that this is also true for MDDC transduction (Fig. 7A).
It has been reported that wild-type MVs have the ability to infect MDDCs by targeting the C-type lectin DC-SIGN (17, 18). In accordance with these data, we demonstrated that DC-SIGN plays a role in the high-level transduction of MDDCs by H/F-LVs. Interestingly, DC-SIGN appears to be targeted by multiple native viruses (36), and it was used as a major DC specific target receptor for redirecting LV vector tropism both in vitro and in vivo, i.e., mainly dermal DCs (31, 32, 63, 89). In addition, DC-SIGN has been shown to mediate suppression of innate immune responses via the TLR-dependent expression of the regulatory cytokine IL-10 by DCs (44, 45). Thus, one can speculate that such an interaction between the lectin and H/F-LVs may be responsible for reversibly keeping transduced MDDCs in an immature state. Indeed, neither CD83/CD86 expression upregulation nor type I IFN (IFN-α and IFN-β) secretion could be documented in H/F-LV-transduced MDDCs (Fig. 5D).
CD150/SLAM has been extensively described as a central MV receptor for clinical and vaccinal strains, e.g., Edmonston (83, 88). Here, we confirmed results reported by Kruse et al. showing that CD150/SLAM was only expressed on mature MDDCs (53). Interestingly, upon short-term incubations of MDDCs with H/F-LVs or with mannan, a ligand of DC-SIGN, we documented a transient but significant expression of the CD150/SLAM molecule on the MDDCs that may be sufficient to allow an efficient transduction process. These data are in agreement with a recent publication demonstrating the same phenomenon upon incubation of MDDCs with wild-type MV (5). Although the SLAM-tropic H-D4/F-LVs induced SLAM expression to a similar level, they were not able to transduce efficiently MDDCs. MDDC transduction occurred only when CD46 binding sites were inserted by selective mutation into the H-D4-YG/F-LVs in addition to the SLAM binding sites already present on the same H gp molecule. Moreover, the H-533 mutant gp, which prevents SLAM binding, whereas CD46 binding is maintained, showed persistence of SLAM expression, since SLAM was not engaged by the H-533 gp.
We demonstrated here that the CD46, DC-SIGN, and CD150/SLAM molecules might cooperate in the induction of these high H/F-LV-mediated MDDC transduction levels. The MDDC target cells apparently have the same requirements as primary lymphocytes: both binding to CD46 and SLAM are needed to permit their efficient transduction (30). However, DC-SIGN has an another function consisting of MV-LV vector capture and binding to the DCs, probably approaching the particles to the MV receptors, CD46 and SLAM. Thus, DC-SIGN would be involved in increasing vector binding to the cell but not in entry of the LV particle, which was also suggested for Edmonston MV (17, 18). In summary, we suggest that DC-SIGN plays a role in increasing H/F-LV attachment to MDDCs, while SLAM and CD46 binding may allow entry in a way that assures efficient vector integration into the genome.
Recently, two different groups described a new MV receptor designated EpR expressed on epithelial cells (56, 81). This receptor was shown to interact with a particular region of the H gp from MV, raising the possibility that such a receptor whose expression, if upregulated in the course of the differentiation from monocytes, could take part in an enhanced attachment and/or entry of H/F-LVs in MDDCs compared to VSV-G-LVs.
Until recently most of the studies involving lentiviruses as tools to transfer genes into human DCs reported the use of Vpr/Vpx-encoding SIV-derived or HIV-1-based vectors transcomplemented with Vpr/Vpx-encoding VLPs (8, 40, 42, 43, 51, 59, 74). Vpx is an accessory protein from SIV and HIV-2 viruses known to enable the inhibition of a host defense mechanism against infection, depending in part on the Cul4-based E3 ubiquitin ligase complex (40, 43). Vpx has been recently described as an interactant of the cellular host protein SAMHD1 identified as a major restriction factor of the transduction of myeloid cells by HIV-1-based viruses (47, 54). The innovative study by Berger et al. showed remarkable improvement in MDDC gene transfer after cotransduction of DCs by a VSV-G-pseudotyped LV with Vpx-containing VLPs (8). The issue of the DC maturation markers was not assessed in that study. Here, we obtained similar results with H/F-LVs based on minimal HIV-1 genomes, thus limiting the risk of inducing activation and maturation of transduced MDDCs. Unfortunately, we also documented an increased expression of the MDDC maturation marker CD83 in a dose-dependent manner when transductions were performed in the presence of Vpx. In this setting, other activation markers, such as CD80 and CD86, were also overexpressed upon transduction (data not shown). In accordance with previous results (33, 51, 78), we confirmed that Vpx allowed for macrophage and monocyte transduction by H/F-LVs (data not shown). In conclusion, we believe that using LVs based on minimal HIV-1 genomes may limit the induction of maturation and modifications of functional properties of transduced MDDCs. We have reported here that the recently designed H/F-LVs are superior to VSV-G pseudotypes for transducing human MDDCs. MV-derived LVs do not require accessory proteins, such as Vpx or Vpr, to infect these nondividing cells at low MOIs. High percentages of transduced MDDCs could be regularly achieved in this setting with an acceptable experimental variability across donors and without inducing any maturation signs. Indeed, this improved protocol of lentivirus-mediated gene transfer into human DCs might be useful in the near future to address basic questions in DC biology or in DC-based immunotherapeutic interventions.
ACKNOWLEDGMENTS
This study was supported by grants from the Region Pays de la Loire (J.-M. Humbert's salary; MOMHI project), the Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales (ANRS), the Agence Nationale de la Recherche (ANR), the European Research Council (ERC-2008-AdG-233130-HEPCENT), and the European Community (FP7-HEALTH-2007-B/222878 [PERSIST] and FP7-GENTHALTHER Erare).
The authors have no conflicting financial interests, and the various funding agencies had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1. Alvarez CP, et al. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841–6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ardeshna KM, et al. 2000. Monocyte-derived dendritic cells do not proliferate and are not susceptible to retroviral transduction. Br. J. Haematol. 108:817–824 [DOI] [PubMed] [Google Scholar]
- 3. Arrighi JF, et al. 2004. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 200:1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aversa G, et al. 1997. SLAM and its role in T cell activation and Th cell responses. Immunol. Cell Biol. 75:202–205 [DOI] [PubMed] [Google Scholar]
- 5. Avota E, Gulbins E, Schneider-Schaulies S. 2011. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS Pathog. 7:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banchereau J, et al. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 [DOI] [PubMed] [Google Scholar]
- 7. Banchereau J, Palucka AK. 2005. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 5:296–306 [DOI] [PubMed] [Google Scholar]
- 8. Berger G, et al. 2011. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat. Protoc. 6:806–816 [DOI] [PubMed] [Google Scholar]
- 9. Breckpot K, Aerts JL, Thielemans K. 2007. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene Ther. 14:847–862 [DOI] [PubMed] [Google Scholar]
- 10. Breckpot K, et al. 2007. Activation of immature monocyte-derived dendritic cells after transduction with high doses of lentiviral vectors. Hum. Gene Ther. 18:536–546 [DOI] [PubMed] [Google Scholar]
- 11. Breckpot K, Heirman C, Neyns B, Thielemans K. 2004. Exploiting dendritic cells for cancer immunotherapy: genetic modification of dendritic cells. J. Gene Med. 6:1175–1188 [DOI] [PubMed] [Google Scholar]
- 12. Cavalieri S, et al. 2003. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood 102:497–505 [DOI] [PubMed] [Google Scholar]
- 13. Cheng WF, et al. 2006. Sindbis virus replicon particles encoding calreticulin linked to a tumor antigen generate long-term tumor-specific immunity. Cancer Gene Ther. 13:873–885 [DOI] [PubMed] [Google Scholar]
- 14. Clanchy FI, Holloway AC, Lari R, Cameron PU, Hamilton JA. 2006. Detection and properties of the human proliferative monocyte subpopulation. J. Leukoc. Biol. 79:757–766 [DOI] [PubMed] [Google Scholar]
- 15. Cocks BG, et al. 1995. A novel receptor involved in T-cell activation. Nature 376:260–263 [DOI] [PubMed] [Google Scholar]
- 16. Davis ID, Jefford M, Parente P, Cebon J. 2003. Rational approaches to human cancer immunotherapy. J. Leukoc. Biol. 73:3–29 [DOI] [PubMed] [Google Scholar]
- 17. de Witte L, Abt M, Schneider-Schaulies S, van Kooyk Y, Geijtenbeek TB. 2006. Measles virus targets DC-SIGN to enhance dendritic cell infection. J. Virol. 80:3477–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Witte L, et al. 2008. DC-SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T-lymphocytes. PLoS Pathog. 4:e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dorig RE, Marcil A, Chopra A, Richardson CD. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295–305 [DOI] [PubMed] [Google Scholar]
- 21. Esslinger C, et al. 2003. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8+ T cell responses. J. Clin. Invest. 111:1673–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Esslinger C, Romero P, MacDonald HR. 2002. Efficient transduction of dendritic cells and induction of a T-cell response by third-generation lentivectors. Hum. Gene Ther. 13:1091–1100 [DOI] [PubMed] [Google Scholar]
- 23. Feinberg H, Mitchell DA, Drickamer K, Weis WI. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163–2166 [DOI] [PubMed] [Google Scholar]
- 24. Finnin M, Hamilton JA, Moss ST. 1999. Characterization of a CSF-induced proliferating subpopulation of human peripheral blood monocytes by surface marker expression and cytokine production. J. Leukoc. Biol. 66:953–960 [DOI] [PubMed] [Google Scholar]
- 25. Firat H, et al. 2002. Use of a lentiviral flap vector for induction of CTL immunity against melanoma: perspectives for immunotherapy. J. Gene Med. 4:38–45 [DOI] [PubMed] [Google Scholar]
- 26. Frecha C, et al. 2009. Efficient and stable transduction of resting B lymphocytes and primary chronic lymphocyte leukemia cells using measles virus gp displaying lentiviral vectors. Blood 114:3173–3180 [DOI] [PubMed] [Google Scholar]
- 27. Frecha C, et al. 2008. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood 112:4843–4852 [DOI] [PubMed] [Google Scholar]
- 28. Frecha C, Fusil F, Cosset FL, Verhoeyen E. 2011. In vivo gene delivery into hCD34+ cells in a humanized mouse model. Methods Mol. Biol. 737:367–390 [DOI] [PubMed] [Google Scholar]
- 29. Frecha C, Levy C, Cosset FL, Verhoeyen E. 2010. Advances in the field of lentivector-based transduction of T and B lymphocytes for gene therapy. Mol. Ther. 18:1748–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frecha C, et al. 2011. Measles virus glycoprotein-pseudotyped lentiviral vector-mediated gene transfer into quiescent lymphocytes requires binding to both SLAM and CD46 entry receptors. J. Virol. 85:5975–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Froelich S, Tai A, Kennedy K, Zubair A, Wang P. 2011. Pseudotyping lentiviral vectors with aura virus envelope glycoproteins for DC-SIGN-mediated transduction of dendritic cells. Hum. Gene Ther. 10:1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Froelich S, Tai A, Kennedy K, Zubair A, Wang P. 2011. Virus-receptor mediated transduction of dendritic cells by lentiviruses enveloped with glycoproteins derived from Semliki forest virus. PLoS One 6:e21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fujita M, et al. 2008. Vpx is critical for reverse transcription of the human immunodeficiency virus type 2 genome in macrophages. J. Virol. 82:7752–7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Funke S, et al. 2008. Targeted cell entry of lentiviral vectors. Mol. Ther. 16:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Funke S, et al. 2009. Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity. Gene Ther. 16:700–705 [DOI] [PubMed] [Google Scholar]
- 36. Geijtenbeek TB, den Dunnen J, Gringhuis SI. 2009. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 4:879–890 [DOI] [PubMed] [Google Scholar]
- 37. Geijtenbeek TB, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597 [DOI] [PubMed] [Google Scholar]
- 38. Geijtenbeek TB, et al. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575–585 [DOI] [PubMed] [Google Scholar]
- 39. Gilboa E, Nair SK, Lyerly HK. 1998. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol. Immunother. 46:82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goujon C, et al. 2008. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J. Virol. 82:12335–12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goujon C, et al. 2003. Heterologous human immunodeficiency virus type 1 lentiviral vectors packaging a simian immunodeficiency virus-derived genome display a specific postentry transduction defect in dendritic cells. J. Virol. 77:9295–9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goujon C, et al. 2006. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther. 13:991–994 [DOI] [PubMed] [Google Scholar]
- 43. Goujon C, et al. 2007. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. 2009. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1, and Helicobacter pylori. Nat. Immunol. 10:1081–1088 [DOI] [PubMed] [Google Scholar]
- 45. Gringhuis SI, et al. 2007. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 26:605–616 [DOI] [PubMed] [Google Scholar]
- 46. Halary F, et al. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653–664 [DOI] [PubMed] [Google Scholar]
- 47. Hrecka K, et al. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jain P, et al. 2009. DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. J. Virol. 83:10908–10921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jirmo AC, et al. 2010. Monocytes transduced with lentiviral vectors expressing hepatitis C virus nonstructural proteins and differentiated into dendritic cells stimulate multi-antigenic CD8+ T cell responses. Vaccine 28:922–933 [DOI] [PubMed] [Google Scholar]
- 50. Kaplan JM, et al. 1999. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J. Immunol. 163:699–707 [PubMed] [Google Scholar]
- 51. Kaushik R, Zhu X, Stranska R, Wu Y, Stevenson M. 2009. A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell Host Microbe 6:68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Korin YD, Zack JA. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 72:3161–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kruse M, et al. 2001. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1β. J. Immunol. 167:1989–1995 [DOI] [PubMed] [Google Scholar]
- 54. Laguette N, et al. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lari R, Kitchener PD, Hamilton JA. 2009. The proliferative human monocyte subpopulation contains osteoclast precursors. Arthritis Res. Ther. 11:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leonard VH, et al. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 118:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levy C, et al. 2010. Lentiviral vectors and transduction of human cancer B cells. Blood 116:498–500 [DOI] [PubMed] [Google Scholar]
- 58. Lu W, Arraes LC, Ferreira WT, Andrieu JM. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 10:1359–1365 [DOI] [PubMed] [Google Scholar]
- 59. Manel N, et al. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mangeot PE, et al. 2000. Development of minimal lentivirus vectors derived from simian immunodeficiency virus (SIVmac251) and their use for gene transfer into human dendritic cells. J. Virol. 74:8307–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maurice M, et al. 2002. Efficient gene transfer into human primary blood lymphocytes by surface-engineered lentiviral vectors that display a T cell-activating polypeptide. Blood 99:2342–2350 [DOI] [PubMed] [Google Scholar]
- 62. Mitchell DA, Fadden AJ, Drickamer K. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR: subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939–28945 [DOI] [PubMed] [Google Scholar]
- 63. Morizono K, et al. 2010. Redirecting lentiviral vectors pseudotyped with Sindbis virus-derived envelope proteins to DC-SIGN by modification of N-linked glycans of envelope proteins. J. Virol. 84:6923–6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Naniche D, et al. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Navarro-Sanchez E, et al. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:723–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Negre D, et al. 2000. Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther. 7:1613–1623 [DOI] [PubMed] [Google Scholar]
- 67. Pohlmann S, et al. 2001. DC-SIGN interactions with human immunodeficiency virus: virus binding and transfer are dissociable functions. J. Virol. 75:10523–10526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Puri A, et al. 1988. Activation of vesicular stomatitis virus fusion with cells by pretreatment at low pH. J. Biol. Chem. 263:4749–4753 [PubMed] [Google Scholar]
- 69. Rea D, et al. 2001. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 166:5236–5244 [DOI] [PubMed] [Google Scholar]
- 70. Relloso M, et al. 2002. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-β, and anti-inflammatory agents. J. Immunol. 168:2634–2643 [DOI] [PubMed] [Google Scholar]
- 71. Ribas A, Butterfield LH, Glaspy JA, Economou JS. 2002. Cancer immunotherapy using gene-modified dendritic cells. Curr. Gene Ther. 2:57–78 [DOI] [PubMed] [Google Scholar]
- 72. Roche S, Albertini AA, Lepault J, Bressanelli S, Gaudin Y. 2008. Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell Mol. Life Sci. 65:1716–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sallusto F, Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schule S, et al. 2009. Restriction of HIV-1 replication in monocytes is abolished by Vpx of SIVsmmPBj. PLoS One 4:e7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Senju S, et al. 2011. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. [DOI] [PubMed] [Google Scholar]
- 76. Soilleux EJ, Morris LS, Trowsdale J, Coleman N, Boyle JJ. 2002. Human atherosclerotic plaques express DC-SIGN, a novel protein found on dendritic cells and macrophages. J. Pathol. 198:511–516 [DOI] [PubMed] [Google Scholar]
- 77. Song W, Tong Y, Carpenter H, Kong HL, Crystal RG. 2000. Persistent, antigen-specific, therapeutic antitumor immunity by dendritic cells genetically modified with an adenoviral vector to express a model tumor antigen. Gene Ther. 7:2080–2086 [DOI] [PubMed] [Google Scholar]
- 78. Srivastava S, et al. 2008. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 4:e1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Steinman RM, Hawiger D, Nussenzweig MC. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711 [DOI] [PubMed] [Google Scholar]
- 80. Su Z, Frye C, Bae KM, Kelley V, Vieweg J. 2008. Differentiation of human embryonic stem cells into immunostimulatory dendritic cells under feeder-free culture conditions. Clin. Cancer Res. 14:6207–6217 [DOI] [PubMed] [Google Scholar]
- 81. Tahara M, et al. 2008. Measles virus infects both polarized epithelial and immune cells by using distinctive receptor-binding sites on its hemagglutinin. J. Virol. 82:4630–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tan PH, et al. 2005. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood 105:3824–3832 [DOI] [PubMed] [Google Scholar]
- 83. Tatsuo H, Ono N, Tanaka K, Yanagi Y. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–897 [DOI] [PubMed] [Google Scholar]
- 84. Temme A, et al. 2002. Efficient transduction and long-term retroviral expression of the melanoma-associated tumor antigen tyrosinase in CD34+ cord blood-derived dendritic cells. Gene Ther. 9:1551–1560 [DOI] [PubMed] [Google Scholar]
- 85. Unutmaz D, KewalRamani VN, Marmon S, Littman DR. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Verhoeyen E, et al. 2003. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood 101:2167–2174 [DOI] [PubMed] [Google Scholar]
- 87. Yanagi Y, Ono N, Tatsuo H, Hashimoto K, Minagawa H. 2002. Measles virus receptor SLAM (CD150). Virology 299:155–161 [DOI] [PubMed] [Google Scholar]
- 88. Yanagi Y, Takeda M, Ohno S. 2006. Measles virus: cellular receptors, tropism and pathogenesis. J. Gen. Virol. 87:2767–2779 [DOI] [PubMed] [Google Scholar]
- 89. Yang L, et al. 2008. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 26:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yao Y, Ghosh K, Epand RF, Epand RM, Ghosh HP. 2003. Membrane fusion activity of vesicular stomatitis virus glycoprotein G is induced by low pH but not by heat or denaturant. Virology 310:319–332 [DOI] [PubMed] [Google Scholar]