Abstract
Vibrio anguillarum utilizes quorum sensing to regulate stress responses required for survival in the aquatic environment. Like other Vibrio species, V. anguillarum contains the gene qrr1, which encodes the ancestral quorum regulatory RNA Qrr1, and phosphorelay quorum-sensing systems that modulate the expression of small regulatory RNAs (sRNAs) that destabilize mRNA encoding the transcriptional regulator VanT. In this study, three additional Qrr sRNAs were identified. All four sRNAs were positively regulated by σ54 and the σ54-dependent response regulator VanO, and showed a redundant activity. The Qrr sRNAs, together with the RNA chaperone Hfq, destabilized vanT mRNA and modulated expression of VanT-regulated genes. Unexpectedly, expression of all four qrr genes peaked at high cell density, and exogenously added N-acylhomoserine lactone molecules induced expression of the qrr genes at low cell density. The phosphotransferase VanU, which phosphorylates and activates VanO, repressed expression of the Qrr sRNAs and stabilized vanT mRNA. A model is presented proposing that VanU acts as a branch point, aiding cross-regulation between two independent phosphorelay systems that activate or repress expression of the Qrr sRNAs, giving flexibility and precision in modulating VanT expression and inducing a quorum-sensing response to stresses found in a constantly changing aquatic environment.
Introduction
Quorum sensing is a cell-to-cell communication mechanism that is mediated by small diffusible signal molecules and that is utilized by bacteria to modulate gene expression in response to population density (for review see Ng & Bassler, 2009). Bacteria produce signal molecules, such as N-acylhomoserine lactones (AHLs), which accumulate as the cell number increases. When the concentration of the signal molecules reaches a certain threshold, gene expression is activated or repressed by quorum-sensing systems coordinating bacterial activities as a population, which may provide a selective advantage in natural environments.
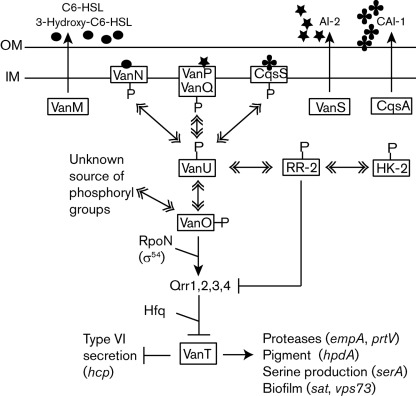
The marine fish pathogen Vibrio anguillarum, like many other vibrios, contains components for multiple quorum-sensing phosphorelay systems (Milton, 2006). The components of these phosphorelay systems in vibrios are believed to regulate gene expression in response to the population density similarly to the quorum-sensing phosphorelay networks of Vibrio harveyi (Ng & Bassler, 2009). A model of the V. anguillarum quorum-sensing phosphorelay signalling systems is given in Fig. 1. In V. anguillarum, components of three sensory phosphorelay quorum-sensing systems have been identified: VanM/N, VanS/PQ (Croxatto et al., 2004) and CqsA/S (predicted by Henke & Bassler, 2004; identified in the incomplete genome sequence, E. Hjerde, D. L. Milton, & N. P. Willassen, unpublished data). These three systems channel signal responses via a phosphorylation cascade to a single regulatory network (VanU/O) leading to activation or repression of a main transcriptional regulator (VanT).
Fig. 1.
V. anguillarum quorum-sensing phosphorelay model. Solid lines with arrows and bars indicate activation and repression of gene expression, respectively. Solid lines with double arrowheads indicate the transfer of phosphoryl groups from one protein to another. Details of the model are given in the Introduction and Discussion. In this study, a model is presented suggesting that the phosphotransferase VanU phosphorylates two response regulators: one response regulator, VanO, activates Qrr sRNA expression, repressing VanT expression, and a second, predicted response regulator (RR-2) is hypothesized to repress Qrr sRNA expression, activating VanT expression. The RR-2 is predicted to be phosphorylated via a histidine kinase (HK-2) of an independent two-component pathway. Abbreviations: OM, outer membrane; IM, inner membrane.
At low cell densities, signal molecules are at a low concentration and the hybrid sensor kinases, VanN, VanQ and CqsS, autophosphorylate and thus initiate a phosphorylation cascade that converges onto the phosphotransferase VanU, which phosphorylates the σ54-dependent response regulator VanO. Upon phosphorylation, VanO is activated and together with the alternative sigma factor RpoN (σ54), induces expression of at least one small regulatory RNA (sRNA) Qrr1 (quorum regulatory RNA). The Qrr sRNA, together with the RNA chaperone Hfq, destabilizes vanT mRNA, repressing expression of the quorum-sensing transcriptional regulator VanT (Croxatto et al., 2004; Weber et al., 2008).
At higher cell densities, when a certain threshold of signal molecules is reached, vanT expression is induced. Three types of signal molecules are produced by the VanM, VanS and CqsA signal synthases. VanM synthesizes both N-hexanoyl-l-homoserine lactone (C6-HSL) and N-(3-hydroxyhexanoyl)-l-homoserine lactone, which are sensed by their cognate sensor kinase VanN (Milton et al., 2001). VanS produces the autoinducer (AI)-2 signal molecule, a furanosyl borate diester, which binds to the periplasmic protein VanP. The VanP-AI-2 complex, in turn, binds the sensor kinase VanQ (Croxatto et al., 2004; Denkin & Nelson, 2004). The CqsA synthase is predicted to synthesize CAI-1, (S)-3-hydroxytridecan-4-one (Higgins et al., 2007), which binds its sensor kinase CqsS. Binding of the signal molecules to the sensor kinases VanN, VanQ and CqsS inhibits kinase activity, allowing phosphatase activity to predominate, leading to dephosphorylation and inactivation of VanO. Thus, qrr1 is not expressed while VanT expression is induced, which leads to gene regulation within the quorum-sensing regulon.
VanT is known to regulate positively the expression of two metalloproteases, EmpA and PrtV, pigment production, exopolysaccharide production, biofilm formation and serine biosynthesis, and to negatively regulate the expression of a main component of the type VI secretion system, the haemolysin co-regulated protein (Hcp) (Croxatto et al., 2002; Weber et al., 2009). In addition, these quorum-sensing systems are an integral part of stress response in V. anguillarum. The stress response sigma factor RpoS indirectly induces VanT expression during late exponential growth by repressing expression of the RNA chaperone Hfq and thus stabilizing vanT mRNA (Weber et al., 2008). Consequently, VanT and RpoS regulate similar cellular functions to coordinate numerous physiological activities for survival in the aquatic environment.
In this study, the roles of VanU and the Qrr sRNAs in the V. anguillarum quorum-sensing phosphorelay were investigated. Three additional qrr genes were identified and the expression of all four qrr genes was positively regulated by VanO and σ54. Together with Hfq, the Qrr sRNAs destabilized vanT mRNA repressing expression of VanT. Interestingly, expression of all four qrr genes peaked during late exponential growth and exogenously added AHL signal molecules activated expression of the qrr genes at low cell density. Moreover, the phosphotransferase VanU repressed expression of the four qrr genes in a cell-density-independent manner and activated vanT expression post-transcriptionally. A model is presented on how VanU may antagonize the VanO-mediated regulation within the quorum-sensing phosphorelay.
Methods
Strains, plasmids and culture conditions.
Bacterial strains and plasmids are listed in Table 1. V. anguillarum strains were routinely grown in trypticase soy broth containing 1 % sodium chloride (TSB-1 %) at 24 °C with aeration, or on trypticase soy agar (TSA) grown at room temperature. Escherichia coli was routinely grown at 37 °C with aeration in Luria broth (per l: Bacto tryptone, 10 g; Bacto yeast extract, 5 g; sodium chloride, 5 g). Plasmid transfers from E. coli to V. anguillarum were performed as described previously (Milton et al., 1996). The vibrio selective medium TCBS agar (Difco) containing 10 µg chloramphenicol ml−1 was used after conjugation to select against E. coli.
Table 1. Bacterial strains and plasmids used in the study.
| Strain or plasmid | Relevant genotype | Reference or source |
| Strains | ||
| E. coli | ||
| BL21(DE3) | Protein expression E. coli host | Invitrogen |
| V. anguillarum | ||
| NB10 | Wild type V. anguillarum, serotype O1, clinical isolate from the Gulf of Bothnia | Norqvist et al. (1989) |
| NB12 | Cmr, NB10 derivative carrying a mutation in the empA | Milton et al. (1992) |
| AC10 | NB10 derivative carrying an in-frame deletion in vanT | Croxatto et al. (2002) |
| AC11 | NB10 derivative carrying an in-frame deletion in vanO | Croxatto et al. (2004) |
| BW11 | NB10 derivative carrying an in-frame deletion in hfq | Weber et al. (2008) |
| DM71 | NB10 derivative carrying an in-frame deletion in vanU | Croxatto et al. (2004) |
| DM88 | NB10 derivative carrying a vanO gene that encodes an alanine instead of an aspartate at position 56 (D56A) | This study |
| DM89 | NB10 derivative carrying a vanO gene that encodes a glutamate instead of an aspartate at position 56 (D56E) | This study |
| DM105 | NB10 derivative carrying an in-frame deletion of hcp | Weber et al. (2009) |
| OTR83 | NB10 derivative carrying an in-frame deletion in rpoN | O’Toole et al. (1997) |
| SQ1 | NB10 derivative carrying deletions of qrr1 and its promoter | This study |
| SQ2 | NB10 derivative carrying deletions of qrr2 and its promoter | This study |
| SQ3 | NB10 derivative carrying deletions of qrr3 and its promoter | This study |
| SQ4 | NB10 derivative carrying deletions of qrr4 and its promoter | This study |
| SQ5 | NB10 derivative carrying deletions of qrr1, qrr2, qrr3 and qrr4 and their promoters | This study |
| Plasmids | ||
| pDM4 | Cmr, suicide vector with an R6K origin (requires pir) and sacBR of B. subtilis | Milton et al. (1996) |
| pDM4-Qrr1 | Cmr, pDM4 carrying a qrr1 mutant allele that deletes the gene and the promoter (72 bp) | This study |
| pDM4-Qrr2 | Cmr, pDM4 carrying a qrr2 mutant allele that deletes the gene and the promoter (91 bp) | This study |
| pDM4-Qrr3 | Cmr, pDM4 carrying a qrr3 mutant allele that deletes the gene and the promoter (92 bp) | This study |
| pDM4-Qrr4 | Cmr, pDM4 carrying a qrr4 mutant allele that deletes the gene and the promoter (109 bp) | This study |
| pDM4-VanO-D56A | Cmr, pDM4 carrying a vanO mutant allele that encodes an alanine instead of an aspartate at position 56 | This study |
| pDM4-VanO-D56E | Cmr, pDM4 carrying a vanO mutant allele that encodes a glutamate instead of an aspartate at position 56 | This study |
| pDM41 | Cmr, R6K origin, carrying a promoterless RBSII-gfp(ASV)-T0 gene | Weber et al. (2008) |
| pDM41-qrr1 | Cmr, pDM41 carrying a qrr1 : : gfp(ASV) transcriptional gene fusion | Weber et al. (2008) |
| pDM41-qrr2 | Cmr, pDM41 carrying a qrr2 : : gfp(ASV) transcriptional gene fusion | This study |
| pDM41-qrr3 | Cmr, pDM41 carrying a qrr3 : : gfp(ASV) transcriptional gene fusion | This study |
| pDM41-qrr4 | Cmr, pDM41 carrying a qrr4 : : gfp(ASV) transcriptional gene fusion | This study |
| pDM41-vanT·TC | Cmr, pDM41 carrying a vanT : : gfp(ASV) transcriptional gene fusion | Weber et al. (2008) |
| pDM41-vanT3·TL | Cmr, pDM41 carrying a vanT : : gfp(ASV) translational gene fusion | Weber et al. (2008) |
| pETZZ_1a | Kmr, pET-based protein expression vector containing a T7 promoter, coding sequence for a 6×His-tag fused to a double Z-domain, a TEV protease cleavage site, and a multi-cloning site | Bogomolovas et al. (2009) |
| pETZZ_1a-EmpA | Kmr, pETZZ_1a derivative encoding an N-terminal 6×His-tag and a double Z-domain fused to EmpA | This study |
PCR conditions, sequencing and DNA techniques.
PCR was performed as described previously (McGee et al., 1996). When a PCR fragment required minimal errors, the high-fidelity KOD polymerase (Novagen) was used. Unless otherwise stated, conditions for various DNA techniques were according to Sambrook et al. (1989). Reaction conditions for DNA-modifying enzymes and DNA restriction enzymes were according to the manufacturer’s instructions. Editing of DNA sequences was done using the Genetics Computer Group Sequence Analysis software (Devereux et al., 1984) of the Genetics Computer Group (University of Wisconsin). Database searches were done using the blast program from the National Center for Biotechnology Information. Genomic DNA sequencing was done by Eurofins MWG as part of a genome sequencing project (E. Hjerde, D. L. Milton and N. P. Willassen, unpublished data). The sequence data have been submitted to the DDBJ/EMBL/GenBank databases under accession nos JN585990 for qrr2, JN585991 for qrr3 and JN585992 for qrr4.
Mutagenesis methods.
Full gene deletions of qrr1, qrr2, qrr3 and qrr4 were made by allelic exchange using the R6K origin-based suicide vector pDM4 as described previously (Milton et al., 1996). The plasmids pDM4-qrr1, pDM4-qrr2, pDM4-qrr3 and pDM4-qrr4, which were used to make the mutants SQ1, SQ2, SQ3 and SQ4, respectively, carry mutant alleles that delete the entire qrr gene as well as its promoter. The mutant SQ5, which carries deletions of all four qrr genes, was created by performing four successive allelic exchanges of each gene.
Overlap PCR was used to create vanO alleles that encode an amino acid substitution of either an alanine or a glutamate for an aspartate at position 56 in VanO. The alleles were cloned into pDM4 creating pDM4-VanO-D56A and pDM4-VanO-D56E. For easier mutant selection, exchange of the two mutant alleles was done in a ΔvanO mutant (AC11) that carries a deletion of vanO and strains DM88 and DM89 were created, respectively. All mutations were confirmed by sequencing a DNA fragment of the chromosomal locus carrying the mutation that had been amplified by PCR. Primers used to make the alleles are included in Supplementary Table S1 (available with the online version of this paper).
Transcriptional gfp reporter fusions and GFP assays.
Transcriptional gfp reporter gene fusions were created for qrr2, qrr3 and qrr4 using the R6K-origin-based suicide plasmid pDM41 as described previously (Weber et al., 2008). The DNA sequence (300 bp) directly upstream of each gene was fused to a gfp gene that encodes a short-lived green fluorescent protein GFP-ASV with a half-life of 80 min in V. anguillarum. The plasmids pDM41-qrr2, pDM41-qrr3 and pDM41-qrr4 carrying the transcriptional gene fusions were inserted into the promoter region of each gene on the chromosome resulting in a duplication of the promoter region leaving the wild type gene intact. Primers used to create the transcriptional gene fusions are given in Supplementary Table S1.
For GFP assays, strains carrying the gfp gene fusions were grown overnight at 24 °C in TSB-1 % with aeration. Overnight cultures were diluted to OD600 0.001 in TSB-1 % and incubation was continued for 24 h. During the 24 h growth, OD600 was measured at 2, 4, 6, 8, 10, 12, 14, 18 and 24 h to determine growth. For some GFP assays, the cultures were grown until they reached OD600 0.2 or 1.0. To measure fluorescence, a cell number equivalent to OD600 0.2 (1×108 cells ml−1) was removed from each culture. Bacterial cells from each sample were pelleted, washed in 20 µl PBS, pelleted again and resuspended in 20 µl PBS. One microlitre was removed to determine c.f.u. Chloroform (3 µl) was added to the sample, which was then vortexed and incubated for 2 min at room temperature. Cell debris was removed by high speed centrifugation. Fluorescence of a 3 µl sample was measured immediately using a NanoDrop ND-3300. Fluorescence units were divided by the c.f.u. to obtain fluorescence relative to cell number. Wild type without a gene fusion was used to determine the background fluorescence and PBS was used as a blank control. Measurements were done in triplicate and the mean was determined.
AHL induction of qrr gene expression in single cells.
Overnight cultures of the wild type and the ΔvanO and ΔvanU mutants containing one of the qrr : : gfp transcriptional gene fusions were grown overnight in TSB-1 % at 24 °C with aeration. The overnight cultures were diluted in 1 ml TSB-1 % to OD600 0.2 (108 cells ml−1). The cells were pelleted in a microfuge and washed once in 1 ml TSB-1 %. Each cell sample was diluted in TSB-1 % to a concentration of 100 bacteria ml−1 and the final cell number was confirmed by c.f.u. counts. To allow the level of GFP-ASV in the cells to adjust to the low cell density after dilution, the cells were incubated at room temperature for 80 min. Two bacterial samples from each culture were prepared as above. To one tube, C6-HSL signal molecules were added to a final concentration of 10 nM, while no signal molecules were added to the second tube. Since the C6-HSL stock was prepared in acetonitrile, samples were also prepared similarly using solvent alone as a negative control. Both induced and uninduced samples were incubated for 45 min at room temperature to allow expression of GFP-ASV. The cells were pelleted and all but 10 µl of the supernatant was removed. The pellet was resuspended in the remaining 10 µl and applied to a glass coverslip coated with poly-l-lysine. Prolong anti-fade mounting reagent (Invitrogen) was added to the cells and the coverslip was mounted onto a glass slide. Expression of GFP-ASV in the single cells was determined using a Nikon Eclipse 90i microscope. Bacterial cells from the same field of image were visualized using light microscopy to visualize all bacterial cells and fluorescence microscopy to visualize bacteria expressing GFP-ASV. For bright-field images, a Plan Apo VC 60×/1.40 oil objective with differential interference contrast was used. To detect GFP-ASV fluorescence, UV light and an FITC filter was applied with an exposure time of 200 ms. To determine the background fluorescence, the wild type strain without a gfp gene fusion was used. All images were processed with real-time deconvolution using the NIS-Elements software. All images were cropped and processed identically in Adobe Photoshop CS2. This experiment was done using three independent cultures of each strain.
Western analyses.
Bacterial cultures were grown in TSB-1 % at 24 °C with aeration for various times. Extracellular proteins and cell lysates were prepared from a 10 ml culture as described previously (Weber et al., 2009). c.f.u. counts were used to equalize samples to 5×108 cells µl−1 with SDS-PAGE sample loading buffer. Proteins from 5×109 cells were separated using 12.5 or 15 % SDS-PAGE and Western blot analysis was done using Enhanced Chemiluminescence (Amersham Life Sciences) as described previously (Weber et al., 2009). Production of antisera directed against OmpU, VanT and Hcp was as described previously (Wang et al., 2002; Weber et al., 2008, 2009). EmpA was purified and sent to Agrisera AB, Sweden, for polyclonal rabbit antiserum production.
EmpA purification.
Part of the empA gene that encodes the mature EmpA protein was amplified by PCR using the primers EmpA-Acc651 and EmpA-NcoI and cloned into pETZZ_1a, a pET-based 6×His-tag protein expression vector, creating pETZZ_1a-EmpA. The plasmid pETZZ_1a contains a T7 promoter fused to a sequence encoding a 6×His-tag fused to a double Z-domain, an immunoglobulin G-binding domain of protein A from Staphylococcus aureus, and a TEV protease cleavage site (Bogomolovas et al., 2009; Tashiro et al., 1997). The resulting gene fusion encodes a mature EmpA fused to an N-terminal 6×His-ZZ-domain-TEV protein tag. E. coli BL21(DE3) carrying pETZZ_1a-EmpA was grown in 400 ml LB containing 50 µg kanamycin ml−1 at 37 °C to OD600 0.5. At this time, IPTG (0.5 mM final concentration) was added and the culture was shifted to 16 °C and incubated overnight. Cells were pelleted, frozen and then lysed according to Semsey et al. (2002). The soluble fraction was mixed with 2 ml 50 % Ni-NTA bead slurry (Qiagen) and the 6×His-ZZ-domain-EmpA protein was purified according to the manufacturer’s instructions. The eluate was dialysed overnight at 4 °C in buffer containing 50 mM NaH2PO4 pH 8.0, 200 mM NaCl and 10 % glycerol. To cleave the N-terminal 6×His-ZZ-domain tags from EmpA, TEV protease (Invitrogen, 100 µg ml−1) plus 1.0 mM dithiothreitol was incubated with the pure protein for 16 h at 30 °C. TEV protease, which contains a 6×His-tag, and the N-terminal EmpA tags were removed from the protein mix with a Ni-NTA column. Protein concentration was measured using the Bradford reagent (Bio-Rad).
Real-time quantitative RT-PCR (qRT-PCR).
Bacterial cultures, equivalent to 1×108 cells ml−1, were harvested at OD600 1.0 and total RNA was extracted using the RNeasy Mini kit (Qiagen). The iScript one-step real-time qRT-PCR kit with SYBR Green (Bio-Rad) was used to measure transcript levels of vanT in various mutants as described previously (Weber et al., 2008). Calculations for mRNA levels were done according to the standard curve method (Larionov et al., 2005), which normalizes the mRNA amounts to that of the 16S mRNA. Each qRT-PCR was done using three independent cultures, the results were averaged and P-values were determined.
vanT mRNA stability assay.
The vanT mRNA stability assays were performed as described previously (Weber et al., 2008). Briefly, bacterial cultures were grown in TSB-1 % to OD600 0.2 and 1.0. To stop RNA transcription, rifampicin (200 µg ml−1 final concentration) was added to each culture. Before the addition of rifampicin, a 100 µl sample was removed for the zero time point. For mRNA half-life measurements, samples (100 µl) were removed at 2, 5 and 10 min after addition of rifampicin. Total RNA was isolated and mRNA levels were determined using real-time qRT-PCR. The assay was done in triplicate.
Northern analysis.
V. anguillarum cultures were grown at 24 °C in TSB-1 % to OD600 1.0. RNA was isolated using the RNAzol RT reagent (Molecular Research Center). RNAzol RT allows the isolation of mRNA (>200 bases) and microRNA (<200 bases) in separate fractions. For the Northern analyses, an mRNA-containing and a microRNA-containing fraction were collected following the manufacturer’s instructions. After precipitation, these pellets were resuspended in DEPC-treated water and RNA concentrations were measured using the RiboGreen RNA Quant-it reagent (Molecular Probes).
To detect vanT mRNA, 5 µg of the mRNA-containing (>200 bases) RNA fraction was used and to detect the Qrr sRNAs, 3 µg of the microRNA-containing (<200 bases) RNA fraction was used. RNA samples were separated in a 1.2 % (w/v) formaldehyde agarose gel (20 mM MOPS, 5 mM sodium acetate, 1 mM EDTA, 0.24 M formaldehyde) with a formaldehyde running buffer (20 mM MOPS, 5 mM sodium acetate, 1 mM EDTA, pH 7.0, 2.5 M formaldehyde) using 50 V for 3 h. The RNA was transferred to a ZetaProbe GT membrane (Bio-Rad Laboratories) by overnight capillary transfer with 10× SSC (1500 mM sodium chloride, 150 mM sodium citrate). After transfer, RNA was cross-linked to the membrane using UV light. Pre-hybridization (buffer: 7 % SDS, 250 mM sodium phosphate pH 7.2) and probe hybridization (buffer: 7 % SDS, 250 mM sodium phosphate pH 7.2 plus the DIG-labelled probe) were performed at 50 °C for the vanT and qrr probes and at 68 °C for 16S rRNA and 5S rRNA probes. The DIG-labelled DNA probes were generated using the PCR DIG probe synthesis kit (Roche) and primers listed in Supplementary Table S1. After hybridization, membranes were washed twice for 30 min with wash buffer I (5 % SDS, 20 mM sodium phosphate pH 7.2) and wash buffer II (5 % SDS, 20 mM sodium phosphate pH 7.2). Probe detection was done using the DIG luminescence detection kit (Roche).
Protease activity assay.
Cultures were grown to OD600 0.7 and the extracellular proteins in the supernatant were assayed for protease activity using azocasein as substrate, as described previously (Croxatto et al., 2002).
Results
Identification of four quorum regulatory RNAs (Qrr) that are positively regulated by VanO and σ54
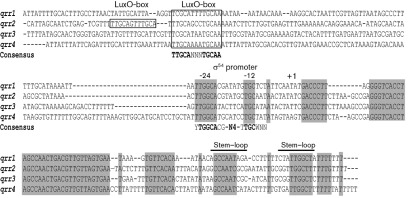
The gene qrr1 in V. anguillarum, which encodes the Qrr1 sRNA, was previously found upstream of the vanOU operon, which encodes two components of the quorum-sensing phosphorelay system (Weber et al., 2008). Although only one Qrr sRNA is found in Vibrio fischeri, multiple qrr genes have been found in Vibrio cholerae and V. harveyi (Lenz et al., 2004; Tu & Bassler, 2007; Miyashiro et al., 2010). Thus, the number of qrr genes present in V. anguillarum was determined. Using the qrr1 sequence, a draft of a V. anguillarum genome sequence was screened and three additional qrr genes, designated qrr2, qrr3 and qrr4, were identified. Fig. 2 shows an alignment of the four genes. All qrr genes are predicted to be single transcriptional units as stem–loop terminators may be predicted at the 3′-end of each gene. Each promoter region contains a consensus σ54 promoter sequence (Barrios et al., 1999) and at least one consensus LuxO box (Lenz et al., 2004), a site to which the LuxO family of σ54 activators bind, suggesting that σ54 (RpoN) and VanO are required for expression of the Qrr sRNAs.
Fig. 2.
DNA alignment of the four V. anguillarum qrr genes and their promoters. The qrr1, qrr2, qrr3 and qrr4 genes and their 5′- and 3′-flanking sequences were aligned using clustal w (Thompson et al., 1994). Regions of the gene sequences with 100 % identity are highlighted in grey. In each promoter region, consensus σ54 promoter sequences were found and designated −24 and −12. The consensus σ54 promoter sequence (YTGGCACG-N4-TTGCWNN, Barrios et al., 1999) is given below the aligned sites. Putative transcriptional start sites are designated +1. Putative LuxO binding sites were found upstream of the promoters and are indicated with a box. The consensus sequence for the LuxO binding site (TTGCA-N3-TGCAA, Lenz et al., 2004) is shown below the aligned sites. Possible stem–loop transcriptional terminators are indicated with solid lines.
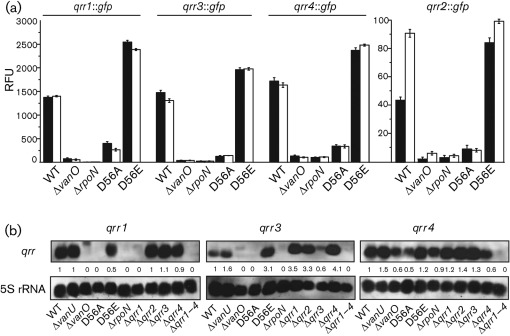
To test the role of RpoN and VanO in regulating expression of the Qrr sRNAs, transcriptional qrr gene fusions were made to a gfp gene encoding an unstable GFP, with a half-life of 80 min in V. anguillarum. Each qrr : : gfp transcriptional gene fusion was measured in the wild type and the ΔvanO and ΔrpoN mutants at OD600 0.2 (low cell density) and OD600 1.0 (high cell density). At both time points, GFP fluorescence for each qrr gene fusion was significantly decreased in both mutants compared with the wild type (Fig. 3a). In addition, the qrr reporter gene fusions were measured in ΔvanO mutants that encode proteins with a single amino acid substitution that is predicted to lock VanO into either an active phosphate-ON state (VanO-D56E) or an inactive phosphate-OFF (VanO-D56A) state (Freeman & Bassler, 1999). Compared with the wild type, GFP expression from all qrr : : gfp gene fusions was decreased in the inactive VanO-D56A mutant but was increased in the active VanO-D56E mutant. Northern analyses (Fig. 3b) confirm that the Qrr1, Qrr3 and Qrr4 sRNAs are expressed at OD600 1.0 and that VanO and RpoN activate their expression. Although some cross-reactivity occurred with the Qrr4 probe, a decrease in transcripts can be seen for the ΔvanO and ΔrpoN mutants. Because the qrr2 transcripts were not detectable using Northern blots, the expression levels of the qrr2 gene were considered to be much lower than those of the other qrr genes. The decreased expression level of qrr2 may be due to two possible VanO-binding sites detected in the qrr2 promoter, whereas only one VanO-binding site is predicted in the promoters of the qrr1, qrr3 and qrr4 genes (Fig. 2).
Fig. 3.
VanO and σ54 positively regulate expression of the four Qrr sRNAs. (a) The transcriptional gene fusions qrr1 : : gfp, qrr2 : : gfp, qrr3 : : gfp and qrr4 : : gfp were localized to the chromosome of each strain. Expression of GFP-ASV was measured as fluorescence at early (OD600 0.2, black bars) and late exponential growth (OD600 1.0, white bars) and is presented as relative fluorescence units (RFU; ±sd), which equals fluorescence units per 108 cells. The expression data for each qrr : : gfp gene fusion are given under a labelled solid line and the qrr2 : : gfp gene fusion data are presented with a different scale from that of the other three gene fusions. (b) Northern analysis of the four Qrr sRNAs. The wild type and various mutant strains were grown to OD600 1.0 and RNA was purified from each strain. For each qrr mRNA, 3 µg microRNA-containing (<200 bases) RNA was separated in a denaturing agarose gel. The mRNA was hybridized to a DIG-labelled PCR fragment amplified from a qrr gene. The transcript detected on each filter is given above each panel. A probe to detect 5S rRNA transcripts was used as a loading and transfer control. Strains used in both images are designated by their mutation at the bottom of the image.
Signal molecules induce the expression of Qrr1–4
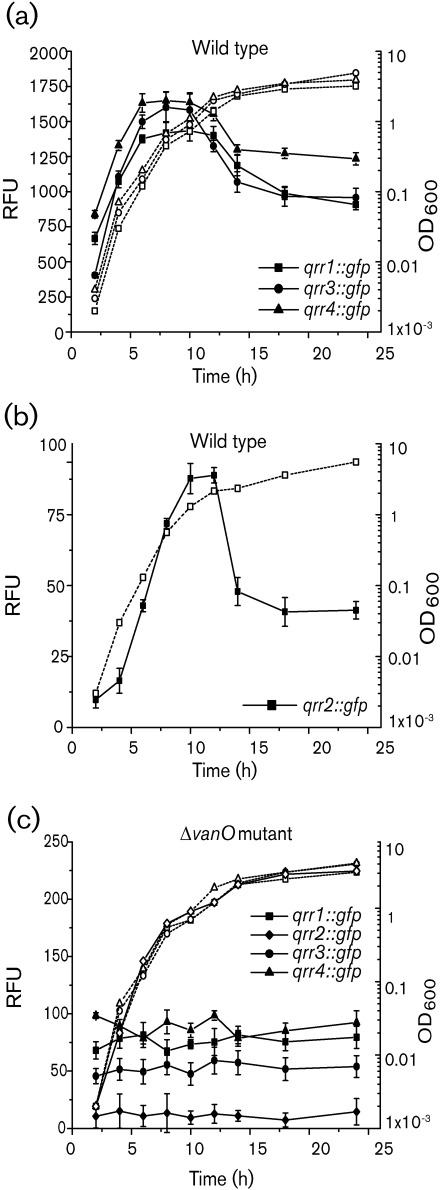
Interestingly, Fig. 3 indicates that the Qrr sRNAs were expressed at high cell density (OD600 1.0). This observation is in contrast with the expression of the qrr genes in V. harveyi, V. cholerae and V. fischeri for which expression peaks during low cell density but decreases significantly at high cell density (Tu & Bassler, 2007; Svenningsen et al., 2008; Miyashiro et al., 2010). This prompted us to determine the expression of the four qrr : : gfp gene fusions in the wild type and the vanO mutant throughout growth. In the wild type, expression profiles of all qrr genes were similar, showing a decreased expression at lower cell densities and a peak of expression during entry into the stationary growth phase (Fig. 4a and b). The expression levels of qrr1, qrr3 and qrr4 were very similar; however, the level of qrr2 expression was at least 20-fold less throughout growth than the expression of qrr1, qrr3 and qrr4. In the vanO mutant, a significant loss of expression of all qrr genes was observed and no peak of expression was observed as the cell density increased (Fig. 4c) confirming a role for VanO in the cell-density-dependent expression of the qrr genes. Moreover, in the wild-type, the expression of all qrr genes at the lowest cell density was significantly higher than expression at any time point in the vanO mutant, suggesting that expression of the qrr genes is induced in the wild type at a lower cell density than was measured for the GFP assays.
Fig. 4.
Expression profiles of the qrr genes during growth in the wild type and the vanO mutant. For the wild type, expression profiles of the transcriptional gene fusions qrr1 : : gfp, qrr3 : : gfp and qrr4 : : gfp are presented in (a), whereas the transcriptional gene fusion qrr2 : : gfp was expressed less than the other qrr genes and is presented separately (b). For the vanO mutant (c), expression profiles of all qrr : : gfp transcriptional gene fusions are shown. For all strains, the gene fusions were expressed from the chromosome. Growth (dotted lines) is indicated as OD600. At each time point, GFP-ASV expression (solid lines) was measured as fluorescence and presented as relative fluorescence units (RFU; ±sd, which equals fluorescence units per 108 cells.
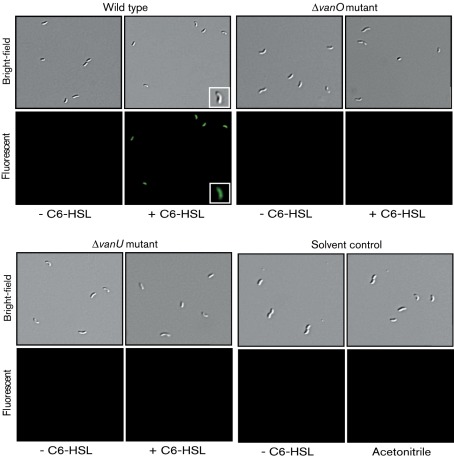
These data suggest that in V. anguillarum, the expression of the Qrr RNAs is induced in the presence of the AHL signal molecules instead of repressed, as is shown for other vibrios. If this is true, then GFP fluorescence produced from the qrr : : gfp gene fusions at a low cell density should increase when AHLs are added to the growth medium exogenously. Since the expression of qrr1, qrr3 and qrr4 was relatively high at the lowest cell density analysed using a fluorometer, fluorescence microscopy was used to visualize expression of the qrr : : gfp gene fusions in the wild type and the ΔvanO mutant in the presence and absence of AHL signal molecules at a cell density of 100 bacteria ml−1. Fig. 5 shows a representative image of single bacterial cells that were analysed for the qrr1 : : gfp gene fusion in the wild type and the ΔvanO mutant. At this cell density in the absence of C6-HSL, GFP fluorescence could not be detected in either the wild type or the ΔvanO mutant. However, if C6-HSL was added exogenously to the growth medium, an intense fluorescence was observed in the wild type but not in the ΔvanO mutant bacterial cells showing that C6-HSL induces expression of the Qrr1 sRNA in the wild type and that this induction is dependent on VanO. Similar results were seen for the qrr2 : : gfp, qrr3 : : gfp and qrr4 : : gfp reporter gene fusions (Supplementary Figs S1, S2 and S3, respectively, all available with the online version of this paper). These data are in sharp contrast with what would be predicted from studies in other Vibrio systems and they raise the question as to whether the Qrr sRNAs have a similar function in V. anguillarum as they do in other vibrios.
Fig. 5.
Analyses of the induction of qrr1 expression by AHL signal molecules in the wild type and the vanO and vanU mutants. Fluorescence microscopy was used to determine the effect of C6-HSL on expression of the qrr1 gene in the various strains at a cell density of 100 cells ml−1. Overnight cultures carrying the transcriptional gene fusion qrr1 : : gfp on the chromosome were washed and diluted in TSB-1 % to a cell density of 100 bacteria ml−1. The cells were allowed to stabilize at low cell density by incubation at room temperature for 80 min, which is the half-life of the unstable GFP-ASV variant in V. anguillarum. Two cell samples were prepared for each culture. To one sample (induced), C6-HSL (10 nM) was added, whereas nothing was added to the second sample (uninduced). The bacteria were incubated at room temperature for 45 min and then mounted onto a glass slide. The same field of vision was imaged using both bright-field differential interference contrast microscopy to show all bacteria and UV light to detect GFP-ASV fluorescence, which detected qrr gene expression in the bacterial cells. Representative images are given for each strain, which are designated above each group of four images. For the induced sample, one bacterial cell was chosen and enlarged in the inset. +/−C6-HSL at the bottom of a column of images indicates the presence or absence of signal molecules. As the C6-HSL was first dissolved in acetonitrile, solvent alone was used as a negative control. Similar images were also obtained using the gene fusions for qrr2, qrr3 and qrr4 in the same strains and are presented as Supplementary Figs S1, S2 and S3.
Qrr sRNAs destabilize vanT mRNA repressing expression of VanT
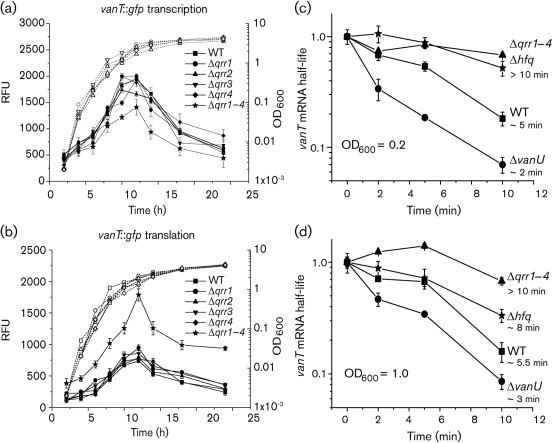
In V. cholerae, V. harveyi and V. fischeri, in the absence of AHL signal molecules, LuxO homologues are phosphorylated by the quorum-sensing phosphorelay and then activate expression of the Qrr sRNAs, which, together with Hfq, target and destabilize the mRNA of the luxR homologues repressing their expression (Lenz et al., 2004; Tu & Bassler, 2007; Svenningsen et al., 2008; Miyashiro et al., 2010). In V. anguillarum, Hfq was previously shown to decrease the mRNA half-life of vanT, a luxR homologue (Weber et al., 2008). Hfq can interact with many different sRNAs to regulate gene expression by either stabilizing or destabilizing mRNA (Gottesman, 2004; Majdalani et al., 2005). To determine if each Qrr sRNA affects the expression of VanT, mutants carrying null deletions of each qrr gene (Δqrr1, Δqrr2, Δqrr3 and qrr4) and one mutant carrying deletions of all four qrr genes (Δqrr1–4) were constructed and transcriptional and translational vanT : : gfp reporter gene fusions were measured in these mutants. Fig. 6(a) and (b) show that deletion of a single qrr gene did not affect the expression of vanT. Northern analyses (Fig. 3) further show that this lack of an effect on vanT expression may be due to an increase in expression of the remaining Qrr sRNAs when one gene is deleted. However, when all four qrr genes were deleted, an increase in the translational vanT : : gfp reporter fusion was observed, which resulted in a decrease in the transcription of vanT due to VanT directly repressing transcription from its own promoter (Croxatto et al., 2004). These data suggest that the Qrr sRNAs act redundantly to repress vanT expression post-transcriptionally.
Fig. 6.
(a, b) Expression of vanT during growth of the wild type, and the Δqrr1, Δqrr2, Δqrr3, Δqrr4 and Δqrr1–4 mutants. The transcriptional (a) and translational (b) vanT : : gfp reporter gene fusions were expressed from the chromosome and were measured during growth of each strain. Growth (dotted lines) is indicated as OD600. At each time point, GFP-ASV expression (solid lines) was measured as fluorescence and presented as relative fluorescence units (RFU), which equals fluorescence units per 108 cells. (c, d) Stability of vanT mRNA in the wild-type and the Δqrr1–4, Δhfq and ΔvanU mutant strains. All strains were grown to OD600 0.2 (c) and 1.0 (d). At these time points, transcription was stopped by the addition of rifampicin (200 µg ml−1). Culture samples were taken at 0, 2, 5 and 10 min. The zero time point was withdrawn before rifampicin addition. Total RNA was isolated from each sample and qRT-PCR was done to determine the amount of vanT mRNA remaining at each time point. The mRNA levels at the zero time point for each sample were set at 1.0. The vanT mRNA was normalized to the 16S rRNA and each time point was normalized to the respective zero time point. The approximate half-life of the vanT mRNA in each strain is given as minutes on the right of each curve.
To test if the Qrr sRNAs destabilize vanT mRNA, the stability of the vanT mRNA at OD600 0.2 and 1.0 was measured in the mutant carrying deletions of all qrr genes (Δqrr1–4) and in a Δhfq mutant (Fig. 6c and d). At both cell densities, the vanT mRNA half-life was approximately 5 min in the wild type, while in the Δqrr1–4 and Δhfq mutants, the half-life of vanT mRNA increased at least 1.5–2.0-fold showing that the Qrr sRNAs play a role in destabilization of the vanT mRNA. Furthermore, these data further confirm that the Qrr sRNAs are expressed and functional at a high cell density.
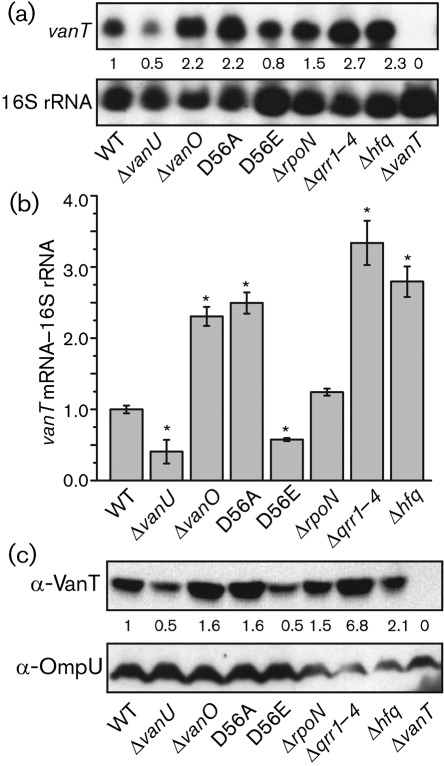
To show that destabilization of vanT mRNA leads to a repressed expression of the VanT transcriptional activator, Northern analyses, qRT-PCR and Western analyses were done using cells grown to a high cell density (OD600 1.0). In addition to the Δqrr1–4 and Δhfq mutants, the ΔvanO and ΔrpoN mutants and the inactive phosphate-OFF (D56A) and the active phosphate-ON (D56E) vanO mutants were analysed (Fig. 7). As expected, the inactive vanO mutant, the Δqrr1–4 mutant and the Δhfq mutant showed a significant increase in vanT mRNA and encoded protein, whereas the constitutively active vanO mutant (D56E) showed a decrease in vanT expression compared with the wild type (Fig. 7). The ΔrpoN mutant gave variable results, from no change to an increase in vanT expression compared with the wild type, suggesting that RpoN, a global regulator, may affect expression of the qrr genes via σ54 activators other than VanO. The data are all consistent with the Qrr sRNAs playing a role in the destabilization of the vanT mRNA and thus repressing expression of this transcriptional regulator.
Fig. 7.
Expression of vanT mRNA and VanT protein in the wild type and various mutant strains. (a) Northern analysis of vanT mRNA. Total RNA was isolated from cultures grown to OD600 1.0. To detect vanT mRNA, 5 µg mRNA-containing (>200 bases) RNA fraction was separated in a denaturing agarose gel and hybridized to a DIG-labelled fragment amplified by PCR from the vanT gene. A probe to detect 16S rRNA transcripts was used as a loading/transfer control. The transcript detected on each filter is given to the left of each panel. (b) Real-time qRT-PCR of vanT mRNA. Total RNA was isolated from cultures grown to OD600 1.0. Real-time qRT-PCR was done as described in Methods. The vanT mRNA was normalized to 16S rRNA transcripts. P-values less than 0.001 are considered significant and are indicated by an asterisk. Error bars indicate sd. (c) Western blot analysis of VanT. Culture samples from the wild type and mutant strains were taken at OD600 1.0. Proteins from equal cell numbers were separated by using 12.5 % SDS-PAGE and Western blot analysis was done using a VanT antiserum. An OmpU antiserum was used as a loading/transfer control. After detection of VanT, the blot was stripped and OmpU antiserum was applied. For (a–c), the strains used are indicated by their mutation. For (a) and (c), the vanT mutant was included as a negative control.
Qrr sRNAs modulate expression of genes regulated by VanT
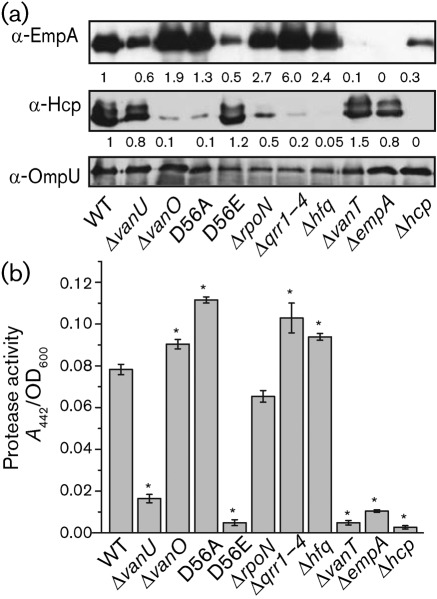
VanT positively regulates expression of the extracellular metalloprotease EmpA (Croxatto et al., 2002) and negatively regulates the expression of Hcp, a protein secreted by the type VI secretion system (T6SS) (Weber et al., 2009). Thus, the Qrr sRNAs may be predicted to modulate expression of EmpA and Hcp indirectly by repressing VanT expression. Western analyses (Fig. 8a) showed that in comparison with the wild type, mutants with a derepressed VanT expression (the inactive VanO-D56A mutant and the ΔvanO, ΔrpoN, Δqrr1-4 and Δhfq deletion mutants) showed an increase in EmpA and a decrease in Hcp protein levels, whereas the active VanO-D56E mutant, which was decreased in VanT expression, and a VanT mutant showed a reverse correlation for both proteins. In addition, total protease activity (Fig. 8b) was measured using spent culture supernatants of the mutants, as VanT activates the expression of a second extracellular metalloprotease PrtV (Weber et al., 2009). When VanT expression was increased or decreased in the mutant strains, the total protease activity also increased or decreased, respectively. Thus, an increase in VanT levels in these mutants strongly correlates with an increase in EmpA, which is positively regulated by VanT, and a decrease in Hcp, which is negatively regulated by VanT. However, the Δhcp mutant did not express EmpA and was decreased in total protease activity. These results were not unexpected since Hcp, which makes up part of the T6SS, was previously shown to modulate the expression of VanT and thus the expression of EmpA (Weber et al., 2009).
Fig. 8.
Qrr sRNAs modulate expression of genes regulated by VanT. (a) Western blot analyses of the extracellular proteins EmpA and Hcp. Bacterial cultures were grown for 16 h and supernatant proteins were precipitated with 5 % TCA. Supernatant proteins from equal cell numbers were separated by using 12.5 % SDS-PAGE. Western blot analysis was done using antisera raised against EmpA and Hcp. An OmpU antiserum was used as a loading/transfer control. V. anguillarum produces outer membrane vesicles and OmpU is localized within these vesicles and precipitates with the extracellular proteins (data not shown). (b) Total protease activity of extracellular proteins was determined by measuring azocasein degradation. For both (a) and (b), the strains used are indicated by their mutation. For negative controls, the empA and the hcp mutant were also included. Error bars in (b) indicate sd.
VanU activates vanT expression post-transcriptionally by repressing expression of the qrr1–4 genes
The data presented support the hypothesis that the Qrr sRNAs function to destabilize vanT mRNA, as is the case for VanT homologues in other vibrios. However, these data still do not explain how an increase in signal molecules results in an increase in Qrr sRNAs. From studies in V. harveyi (Timmen et al., 2006; Neiditch et al., 2005, 2006), the signal molecules are believed to inhibit kinase activity of the hybrid sensor kinases, allowing phosphatase activity to predominate. Thus, VanO is inactivated by dephosphorylation resulting in loss of expression of the qrr genes and induction of the quorum sensing regulon. One possible explanation for this observation is that VanU represses expression of the qrr genes via an unknown mechanism as well as inducing their expression through the activation of VanO.
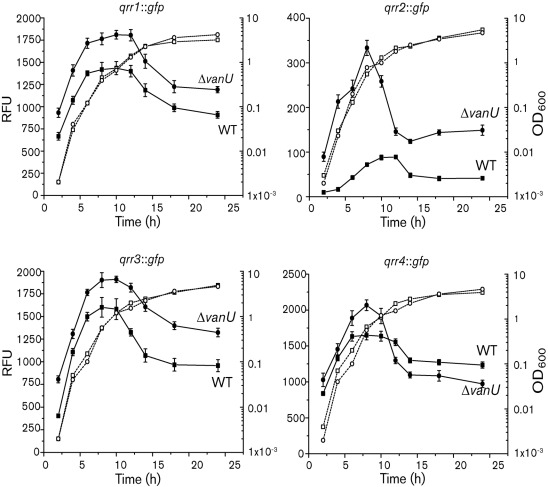
The four qrr : : gfp reporter gene fusions were assayed in a ΔvanU mutant throughout growth and compared with expression in the wild-type (Fig. 9). Expression of all four qrr genes was increased in the ΔvanU mutant while maintaining a cell-density-dependent peak of expression. In particular, expression of qrr2, the least expressed of the qrr genes, was significantly increased in the ΔvanU mutant. Northern analyses (Fig. 3) also showed an increased expression of the qrr3 and qrr4 mRNA levels in the ΔvanU mutant compared with the wild type, confirming that VanU represses expression of these Qrr sRNAs. However, the qrr1 mRNA appeared to be unaffected and this may reflect the variability of the expression of the qrr genes that we have observed in the ΔvanU mutant. These data suggest that VanU represses expression of the qrr genes using a mechanism independent of the quorum-sensing phosphorelay and that VanU may not be required to activate VanO, which is required for expression of the qrr genes. To determine if VanU is needed for a response to signal molecules, the expression of the qrr genes was measured in the ΔvanU mutant at a cell density of 100 bacteria ml−1 in the presence and absence of the C6-HSL signal molecule (Fig. 5, Supplementary Figs S1–S3). Interestingly, in the ΔvanU mutant, the qrr genes were not expressed in either the presence or absence of signal molecules, similar to results in the ΔvanO mutant, suggesting that VanU is needed to activate VanO at this low cell density; however, at higher cell densities, VanO may be phosphorylated by other mechanisms, since the cell-density expression of the qrr genes was unaffected in the vanU mutant.
Fig. 9.
VanU negatively regulates the four qrr genes. The transcriptional gene fusions qrr1 : : gfp, qrr2 : : gfp, qrr3 : : gfp and qrr4 : : gfp (indicated above each graph) were expressed from the chromosome and were measured during growth of the wild type and the vanU mutant. Growth (dotted lines) is indicated as OD600. GFP-ASV expression (solid lines) was measured as fluorescence and is presented as relative fluorescence units (RFU), which equals fluorescence units per 108 cells. Error bars indicate sd.
An increase in Qrr sRNA production in the ΔvanU mutant correlated with a decrease in the half-life of vanT mRNA in the ΔvanU mutant (2–3 min) compared with the wild-type (5 min) further confirming that the Qrr sRNAs aid destabilization of the vanT mRNA (Fig. 6c and d). Consequently, vanT mRNA and VanT protein levels were decreased in the ΔvanU mutant compared with the wild type (Fig. 7). The decrease in VanT expression in the ΔvanU mutant correlated with a decrease in EmpA expression and protease activity (Fig. 8).
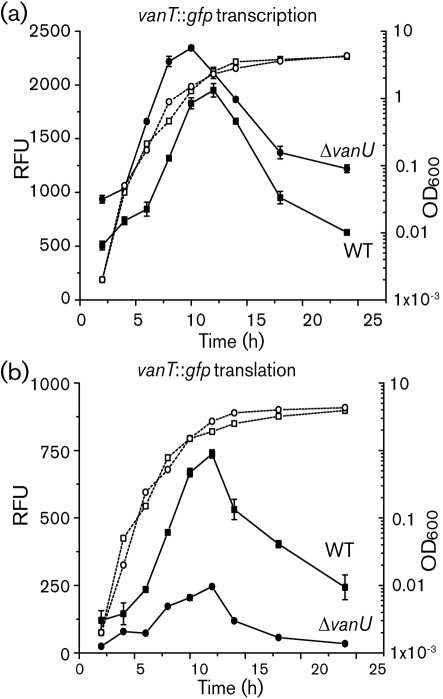
If VanU activates vanT expression indirectly through repressing qrr gene expression, then the effect of VanU on vanT expression may be predicted to occur post-transcriptionally. To test this, vanT : : gfp transcriptional and translational reporter gene fusions were assayed in the ΔvanU mutant throughout growth (Fig. 10). For the translational gene fusion, GFP expression decreased significantly in the ΔvanU mutant compared with the wild type. However, an increase in GFP fluorescence was seen for the transcriptional gene fusion in the mutant compared with the wild type, particularly during late exponential growth when vanT peaks in expression in the wild type (Weber et al., 2008). As discussed above, the increase in vanT transcription is likely due to auto-repression (Croxatto et al., 2004). Taken together, these data show that VanU antagonizes the role of VanO in modulating expression of vanT indirectly by repressing expression of the qrr genes.
Fig. 10.
Expression of vanT during growth of the wild type and the vanU mutant. The transcriptional (a) and translational (b) vanT : : gfp reporter gene fusions were expressed from the chromosome and were measured during growth of the wild type and the vanU mutant. Growth (dotted lines) is indicated as OD600. GFP-ASV expression (solid lines) was measured as fluorescence and is presented as relative fluorescence units (RFU), which equals fluorescence units per 108 cells. Error bars indicate sd.
Discussion
Two-component regulatory systems are used by prokaryotes to sense their environment and to modulate gene expression accordingly (Stock et al., 2000; Mitrophanov & Groisman, 2008). The most common type of two-component signalling systems consist of a histidine sensor kinase, which is an integral inner-membrane protein that senses a specific signal, and a cognate response regulator, whose active state is modulated by the histidine kinase. The histidine kinase responds to a signal and modulates phosphorylation of a response regulator, which in turn modulates gene expression.
Quorum-sensing systems in most vibrios consist of a multi-component phosphorelay signalling system used to sense bacterial populations in an environmental niche (Milton, 2006). These multi-component signalling systems are more complex but are also more versatile versions of the two-component regulatory systems, as they add an additional response regulatory domain and a histidine-containing phosphotransferase domain (Appleby et al., 1996). In vibrios, a common, general theme for the function of these multi-component signalling systems has been established (Ng & Bassler, 2009). The quorum-sensing phosphorelay is initiated via one or more of three hybrid sensor kinases, VanN, VanQ and CqsS (Fig. 1), in the absence of signal molecules at low cell density. The phosphoryl signals transmitted by the sensor kinases converge onto a single phosphotransferease VanU, which phosphorylates and activates the response regulator VanO inducing Qrr sRNA expression. At high cell density, signal molecules accumulate and bind their cognate sensor kinase, inhibiting kinase activity. Phosphatase activity predominates leading to inactivation of VanO and loss of Qrr sRNA expression.
The ancestral qrr1 gene located upstream of vanO, which is found in all sequenced Vibrio and Photobacterium species is also found in V. anguillarum (Weber et al., 2008; Miyashiro et al., 2010). In addition to the ancestral qrr1 gene, many Vibrio species were shown by phylogenetic analysis to contain four or five qrr genes (Miyashiro et al., 2010). In this study, four qrr genes were identified in V. anguillarum. As for other Vibrio species, VanO and σ54 positively regulated all four qrr genes and the Qrr sRNAs together with Hfq destabilized mRNA encoding the global regulator, VanT, which belongs to the HapR family (Lenz et al., 2004; Svenningsen et al., 2009; Tu & Bassler, 2007; Miyashiro et al., 2010). These findings suggest that the multi-component phosphorelay in V. anguillarum would activate and deactivate VanO similarly to that in other vibrios. However, Qrr sRNA expression was induced as the cell numbers increased and was repressed at a low cell density, whereas qrr expression in other vibrios shows a reverse profile (Tu & Bassler, 2007; Svenningsen et al., 2008; Miyashiro et al., 2010).
In V. harveyi, AHL signal molecules bind LuxN and thus inhibit kinase activity of the sensor kinase domain allowing phosphatase activity to predominate (Timmen et al., 2006; Swem et al., 2008; Neiditch et al., 2005, 2006). VanN has high homology to LuxN (Milton et al., 2001). If we assume that VanN functions similarly to LuxN in the presence of AHLs, the signal molecules would be predicted to repress qrr expression in V. anguillarum. However, AHL signal molecules induced expression of the Qrr sRNAs. Moreover, VanU negatively regulated the qrr genes in a cell-density-independent manner antagonizing activation of the qrr genes via VanO and stabilizing vanT mRNA. If VanN acts as a phosphatase in the presence of signal molecules as LuxN does, then how might VanU repress expression of the Qrr sRNAs while VanO activates expression? One possibility that would explain the data so far is that VanU interacts with a second response regulator that inhibits expression of the Qrr sRNAs and that the second response regulator is part of a distinct, independent two-component system (Fig. 1). Cross regulation such as this between two independent regulatory pathways may provide a physiological benefit to the organism (Laub & Goulian, 2007). In this scenario, VanU becomes a branching point in the phosphorelay signalling system and has the potential to either activate or repress expression of the Qrr sRNAs, possibly in response to various signal molecules, including those for quorum sensing. Such a dual role would also explain the variability we observed in the expression of the qrr genes in the ΔvanU mutant.
How might VanU work through two response regulators with antagonistic activities to modulate VanT expression? VanU is a detached histidine phosphotransferase (HPt) domain, which may provide an additional point of regulation within the quorum-sensing phosphorelay signalling systems (Croxatto et al., 2004). A detached HPt domain, a common feature amongst eukaryotic but not prokaryotic phosphorelay systems, allows the domain to interact with multiple kinases and response regulators providing greater flexibility in signal transfer (Thomason & Kay, 2000). In addition, evidence from both eukaryotic and prokaryotic systems suggests that HPt domains may have additional functions that provide other means of regulation besides that of transferring a phosphoryl group from one response regulatory domain to another. The HPt domains of the BvgS system in Bordetella pertussis and the EvgS system in E. coli function to provide specificity between the kinase domains and the cognate response regulatory domains in these signalling pathways (Perraud et al., 1998). Response regulatory proteins are most often activated upon phosphorylation and the cellular response that is controlled by the response regulator is determined by the length of the phosphorylation state (Mitrophanov & Groisman, 2008). Histidine phosphotransferase domains have been shown to aid decay or prolongation of the phosphorylation state, as shown for the ArcB sensor kinase in E. coli and YPD1 from Saccharomyces cerevisiae, respectively (Georgellis et al., 1998; Janiak-Spens et al., 2000).
YPD1 is the best understood detached HPt domain protein. YPD1 functions to mediate phosphorylation transfer from the histidine sensor kinase SLN1 to two downstream response regulators SSK1 and SKN7 (Li et al., 1998). Thus, YPD1 is a branching point coordinating osmotic stress responses (SSK1) and cell wall biosynthesis and cell cycle control (SKN7). Under normal osmotic conditions, YPD1 has a stronger affinity for interaction with the response regulator domain of SSK1 than that of SKN7. Consequently, YPD1 forms a more stable complex with SSK1 prolonging the phosphorylation state (Janiak-Spens et al., 2000; Porter & West, 2005). When the osmolyte concentrations increase, the YPD1-SSK1~P complex is disrupted leading to dephosphorylation of SSK1 and activation of the osmotic stress response (Kaserer et al., 2009).
As VanU is a detached HPt domain protein, it may interact with VanO and a putative second response regulator to repress qrr gene expression. A complex may form between VanU and the second response regulator altering the phosphorylation state of the regulator, which is modulated via an independent sensor kinase that responds to signals that are not cell density related, since VanU represses qrr expression in a cell-density-independent manner. One possibility is that VanU may prolong the phosphorylated active state of the second response regulator, leading to a prolongation of the repression of qrr gene expression. This model would explain why a vanU mutant showed a derepressed qrr gene expression and shorter half-life of vanT mRNA leading to a decreased VanT expression. This model also suggests that AHL signal molecules, which block the phosphoryl transfers due to kinase activity of VanN, are needed to dephosphorylate VanU and the second response regulator leading to an induced expression of the qrr genes. Furthermore, the data suggest that at higher cell numbers, VanO is not dependent on VanU for activation since the qrr genes are expressed in a vanU mutant. This is not unlikely since in a previous study, VanO was suggested to maintain its activated state in the absence of VanU (Croxatto et al., 2004). The mechanism for how VanU represses qrr expression to activate VanT expression is not yet clear. However, the possible involvement of VanU in cross-regulation with an independent pathway that regulates qrr expression introduces a new mechanism for modulating, with precision, the expression of VanT, a regulator of stress response in V. anguillarum.
In summary, V. anguillarum encodes four Qrr sRNAs that are positively regulated by VanO, a response regulator activated by the quorum-sensing phosphorelay system at a low bacterial population. Strikingly different to other vibrios, AHL signal molecules induced expression of the Qrr sRNAs and VanU stabilized vanT mRNA by repressing expression of the qrr genes. These data suggest that VanU may interact with a second response regulator that is part of an independent regulatory pathway and that represses expression of the qrr genes antagonizing the positive regulatory effect of VanO. If this is true, the integration of a putative new branch point downstream of VanU leads to a reversed cellular response of the quorum-sensing phosphorelay system in V. anguillarum compared with other vibrio systems. An extra regulatory checkpoint within the phosphorelay may increase the versatility and accuracy of the V. anguillarum quorum-sensing signalling system, preventing inappropriate cellular responses and unnecessary use of cellular resources allowing the bacterium to regulate precisely stress responses to a constantly changing aquatic environment. V. anguillarum, the oldest known fish pathogen, infects over 50 species of fish worldwide, both wild and cultured, tolerates temperatures in the temperate to subtropic climate zones, and survives in seawater for at least 50 months (Pedersen & Larsen, 1998). The intriguing variation in the V. anguillarum quorum-sensing phosphorelay presented in this study may have evolved to give this bacterium the capacity to survive exceedingly well in the highly variable aquatic environment.
Supplementary Material
Acknowledgements
This work was performed within the Umeå Centre for Microbial Research and was supported by funding from the Swedish Council for Environment, Agricultural Sciences and Spatial Planning, from the Swedish Research Council, from the Carl Tryggers Foundation, Sweden, and from the Natural Science Faculty of Umeå University.
Abbreviations:
- AHL
N-acylhomoserine lactone
- C6-HSL
N-hexanoyl-l-homoserine lactone
- qRT-PCR
real-time quantitative RT-PCR
- sRNA
small regulatory RNA
Footnotes
A supplementary table of primer sequences and three supplementary figures are available with the online version of this paper.
References
- Appleby J. L., Parkinson J. S., Bourret R. B. (1996). Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell 86, 845–848. 10.1016/S0092-8674(00)80158-0 [DOI] [PubMed] [Google Scholar]
- Barrios H., Valderrama B., Morett E. (1999). Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res 27, 4305–4313. 10.1093/nar/27.22.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolovas J., Simon B., Sattler M., Stier G. (2009). Screening of fusion partners for high yield expression and purification of bioactive viscotoxins. Protein Expr Purif 64, 16–23. 10.1016/j.pep.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Croxatto A., Chalker V. J., Lauritz J., Jass J., Hardman A., Williams P., Cámara M., Milton D. L. (2002). VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J Bacteriol 184, 1617–1629. 10.1128/JB.184.6.1617-1629.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto A., Pride J., Hardman A., Williams P., Cámara M., Milton D. L. (2004). A distinctive dual-channel quorum-sensing system operates in Vibrio anguillarum. Mol Microbiol 52, 1677–1689. 10.1111/j.1365-2958.2004.04083.x [DOI] [PubMed] [Google Scholar]
- Denkin S. M., Nelson D. R. (2004). Regulation of Vibrio anguillarum empA metalloprotease expression and its role in virulence. Appl Environ Microbiol 70, 4193–4204. 10.1128/AEM.70.7.4193-4204.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. (1984). A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12, 387–395. 10.1093/nar/12.1Part1.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J. A., Bassler B. L. (1999). A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol 31, 665–677. 10.1046/j.1365-2958.1999.01208.x [DOI] [PubMed] [Google Scholar]
- Georgellis D., Kwon O., De Wulf P., Lin E. C. C. (1998). Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem 273, 32864–32869. 10.1074/jbc.273.49.32864 [DOI] [PubMed] [Google Scholar]
- Gottesman S. (2004). The small RNA regulators of Escherichia coli: roles and mechanisms. Annu Rev Microbiol 58, 303–328. 10.1146/annurev.micro.58.030603.123841 [DOI] [PubMed] [Google Scholar]
- Henke J. M., Bassler B. L. (2004). Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol 186, 6902–6914. 10.1128/JB.186.20.6902-6914.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. A., Pomianek M. E., Kraml C. M., Taylor R. K., Semmelhack M. F., Bassler B. L. (2007). The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 450, 883–886. 10.1038/nature06284 [DOI] [PubMed] [Google Scholar]
- Janiak-Spens F., Sparling D. P., West A. H. (2000). Novel role for an HPt domain in stabilizing the phosphorylated state of a response regulator domain. J Bacteriol 182, 6673–6678. 10.1128/JB.182.23.6673-6678.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaserer A. O., Andi B., Cook P. F., West A. H. (2009). Effects of osmolytes on the SLN1–YPD1–SSK1 phosphorelay system from Saccharomyces cerevisiae. Biochemistry 48, 8044–8050. 10.1021/bi900886g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larionov A., Krause A., Miller W. (2005). A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6, 62. 10.1186/1471-2105-6-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub M. T., Goulian M. (2007). Specificity in two-component signal transduction pathways. Annu Rev Genet 41, 121–145. 10.1146/annurev.genet.41.042007.170548 [DOI] [PubMed] [Google Scholar]
- Lenz D. H., Mok K. C., Lilley B. N., Kulkarni R. V., Wingreen N. S., Bassler B. L. (2004). The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118, 69–82. 10.1016/j.cell.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Li S., Ault A., Malone C. L., Raitt D., Dean S., Johnston L. H., Deschenes R. J., Fassler J. S. (1998). The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J 17, 6952–6962. 10.1093/emboj/17.23.6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N., Vanderpool C. K., Gottesman S. (2005). Bacterial small RNA regulators. Crit Rev Biochem Mol Biol 40, 93–113. 10.1080/10409230590918702 [DOI] [PubMed] [Google Scholar]
- McGee K., Hörstedt P., Milton D. L. (1996). Identification and characterization of additional flagellin genes from Vibrio anguillarum. J Bacteriol 178, 5188–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D. L. (2006). Quorum sensing in vibrios: complexity for diversification. Int J Med Microbiol 296, 61–71. 10.1016/j.ijmm.2006.01.044 [DOI] [PubMed] [Google Scholar]
- Milton D. L., Norqvist A., Wolf-Watz H. (1992). Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol 174, 7235–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D. L., O’Toole R., Hörstedt P., Wolf-Watz H. (1996). Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol 178, 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D. L., Chalker V. J., Kirke D., Hardman A., Cámara M., Williams P. (2001). The LuxM homologue VanM from Vibrio anguillarum directs the synthesis of N-(3-hydroxyhexanoyl)homoserine lactone and N-hexanoylhomoserine lactone. J Bacteriol 183, 3537–3547. 10.1128/JB.183.12.3537-3547.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrophanov A. Y., Groisman E. A. (2008). Signal integration in bacterial two-component regulatory systems. Genes Dev 22, 2601–2611. 10.1101/gad.1700308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T., Wollenberg M. S., Cao X., Oehlert D., Ruby E. G. (2010). A single qrr gene is necessary and sufficient for LuxO-mediated regulation in Vibrio fischeri. Mol Microbiol 77, 1556–1567. 10.1111/j.1365-2958.2010.07309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiditch M. B., Federle M. J., Miller S. T., Bassler B. L., Hughson F. M. (2005). Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell 18, 507–518. 10.1016/j.molcel.2005.04.020 [DOI] [PubMed] [Google Scholar]
- Neiditch M. B., Federle M. J., Pompeani A. J., Kelly R. C., Swem D. L., Jeffrey P. D., Bassler B. L., Hughson F. M. (2006). Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell 126, 1095–1108. 10.1016/j.cell.2006.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. L., Bassler B. L. (2009). Bacterial quorum-sensing network architectures. Annu Rev Genet 43, 197–222. 10.1146/annurev-genet-102108-134304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norqvist A., Hagström A., Wolf-Watz H. (1989). Protection of rainbow trout against vibriosis and furunculosis by the use of attenuated strains of Vibrio anguillarum. Appl Environ Microbiol 55, 1400–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole R., Milton D. L., Hörstedt P., Wolf-Watz H. (1997). RpoN of the fish pathogen Vibrio (Listonella) anguillarum is essential for flagellum production and virulence by the water-borne but not intraperitoneal route of inoculation. Microbiology 143, 3849–3859. 10.1099/00221287-143-12-3849 [DOI] [PubMed] [Google Scholar]
- Pedersen K., Larsen J. L. (1998). Characterization and typing methods for the fish pathogen Vibrio anguillarum. Recent Res Dev Microbiol 2, 17–93. [Google Scholar]
- Perraud A.-L., Kimmel B., Weiss V., Gross R. (1998). Specificity of the BvgAS and EvgAS phosphorelay is mediated by the C-terminal HPt domains of the sensor proteins. Mol Microbiol 27, 875–887. 10.1046/j.1365-2958.1998.00716.x [DOI] [PubMed] [Google Scholar]
- Porter S. W., West A. H. (2005). A common docking site for response regulators on the yeast phosphorelay protein YPD1. Biochim Biophys Acta 1748, 138–145. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. (1989). Molecular Cloning: A Laboratory Manual, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Semsey S., Geanacopoulos M., Lewis D. E., Adhya S. (2002). Operator-bound GalR dimers close DNA loops by direct interaction: tetramerization and inducer binding. EMBO J 21, 4349–4356. 10.1093/emboj/cdf431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A. M., Robinson V. L., Goudreau P. N. (2000). Two-component signal transduction. Annu Rev Biochem 69, 183–215. 10.1146/annurev.biochem.69.1.183 [DOI] [PubMed] [Google Scholar]
- Svenningsen S. L., Waters C. M., Bassler B. L. (2008). A negative feedback loop involving small RNAs accelerates Vibrio cholerae’s transition out of quorum-sensing mode. Genes Dev 22, 226–238. 10.1101/gad.1629908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsen S. L., Tu K. C., Bassler B. L. (2009). Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. EMBO J 28, 429–439. 10.1038/emboj.2008.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem L. R., Swem D. L., Wingreen N. S., Bassler B. L. (2008). Deducing receptor signaling parameters from in vivo analysis: LuxN/AI-1 quorum sensing in Vibrio harveyi. Cell 134, 461–473. 10.1016/j.cell.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Tejero R., Zimmerman D. E., Celda B., Nilsson B., Montelione G. T. (1997). High-resolution solution NMR structure of the Z domain of staphylococcal protein A. J Mol Biol 272, 573–590. 10.1006/jmbi.1997.1265 [DOI] [PubMed] [Google Scholar]
- Thomason P., Kay R. (2000). Eukaryotic signal transduction via histidine-aspartate phosphorelay. J Cell Sci 113, 3141–3150. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. (1994). clustal w: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22, 4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmen M., Bassler B. L., Jung K. (2006). AI-1 influences the kinase activity but not the phosphatase activity of LuxN of Vibrio harveyi. J Biol Chem 281, 24398–24404. 10.1074/jbc.M604108200 [DOI] [PubMed] [Google Scholar]
- Tu K. C., Bassler B. L. (2007). Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev 21, 221–233. 10.1101/gad.1502407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-Y., Lauritz J., Jass J., Milton D. L. (2002). A ToxR homolog from Vibrio anguillarum serotype O1 regulates its own production, bile resistance, and biofilm formation. J Bacteriol 184, 1630–1639. 10.1128/JB.184.6.1630-1639.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Croxatto A., Chen C., Milton D. L. (2008). RpoS induces expression of the Vibrio anguillarum quorum-sensing regulator VanT. Microbiology 154, 767–780. 10.1099/mic.0.2007/014167-0 [DOI] [PubMed] [Google Scholar]
- Weber B., Hasic M., Chen C., Wai S. N., Milton D. L. (2009). Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ Microbiol 11, 3018–3028. 10.1111/j.1462-2920.2009.02005.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.