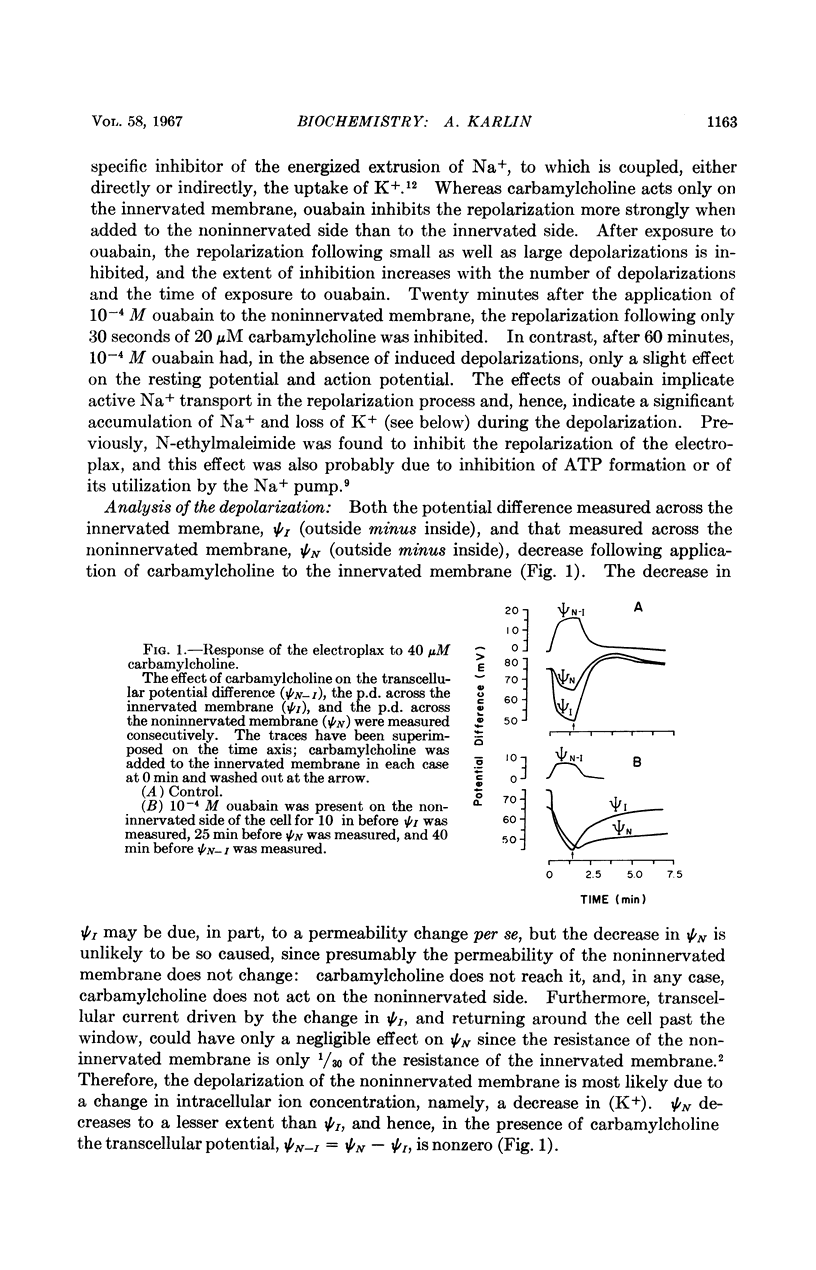
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTAMIRANO M., COATES C. W. Effect of potassium on electroplax of Electrophorus electricus. J Cell Physiol. 1957 Feb;49(1):69–101. doi: 10.1002/jcp.1030490105. [DOI] [PubMed] [Google Scholar]
- ALTAMIRANO M., COATES C. W., GRUNDFEST H., NACHMANSOHN D. Mechanisms of bioelectric activity in electric tissue. I. The response to indirect and direct stimulation of electroplaques of Electrophorus electricus. J Gen Physiol. 1953 Sep;37(1):91–110. doi: 10.1085/jgp.37.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- HIGMAN H. B., PODLESKI T. R., BARTELS E. CORRELATION OF MEMBRANE POTENTIAL AND POTASSIUM FLUX IN THE ELECTROPLAX OF ELECTROPHORUS. Biochim Biophys Acta. 1964 Jan 27;79:138–150. doi: 10.1016/0926-6577(64)90047-6. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., MARTINS-FERREIRA H. Membrane potentials in the electroplates of the electric eel. J Physiol. 1953 Feb 27;119(2-3):315–351. doi: 10.1113/jphysiol.1953.sp004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochim Biophys Acta. 1966 Nov 8;126(3):525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- SCHOFFENIELS E. An isolated single electroplax preparation. II. Improved preparation for studying ion flux. Biochim Biophys Acta. 1957 Dec;26(3):585–596. doi: 10.1016/0006-3002(57)90106-3. [DOI] [PubMed] [Google Scholar]
- SCHOFFENIELS E. Ion movements studied with single isolated electroplax. Ann N Y Acad Sci. 1959 Aug 28;81:285–306. doi: 10.1111/j.1749-6632.1959.tb49314.x. [DOI] [PubMed] [Google Scholar]
- SCHOFFENIELS E., NACHMANSOHN D. An isolated single electroplax preparation. I. New data on the effect of acetylcholine and related compounds. Biochim Biophys Acta. 1957 Oct;26(1):1–15. doi: 10.1016/0006-3002(57)90047-1. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R., GUINNEBAULT M. The effect of blocking electrical activity on the efflux of potassium from electroplax. Biochim Biophys Acta. 1960 Dec 4;45:336–347. doi: 10.1016/0006-3002(60)91456-6. [DOI] [PubMed] [Google Scholar]
- Webb G. D. Affinity of benzoquinonium and ambenonium derivatives for the acetylcholine receptor, tested on the electroplax, and for acetylcholinesterase in solution. Biochim Biophys Acta. 1965 May 25;102(1):172–184. doi: 10.1016/0926-6585(65)90211-6. [DOI] [PubMed] [Google Scholar]


