Abstract
Although it is well established that hyperexcitability and/or increased baseline sensitivity of primary sensory neurons can lead to abnormal burst activity associated with pain, the underlying molecular mechanisms are not fully understood. Early studies demonstrated that, after injury to their axons, neurons can display changes in excitability, suggesting increased sodium channel expression, and, in fact, abnormal sodium channel accumulation has been observed at the tips of injured axons. We have used an ensemble of molecular, electrophysiological, and pharmacological techniques to ask: what types of sodium channels underlie hyperexcitability of primary sensory neurons after injury? Our studies demonstrate that multiple sodium channels, with distinct electrophysiological properties, are encoded by distinct mRNAs within small dorsal root ganglion (DRG) neurons, which include nociceptive cells. Moreover, several DRG neuron-specific sodium channels now have been cloned and sequenced. After injury to the axons of DRG neurons, there is a dramatic change in sodium channel expression in these cells, with down-regulation of some sodium channel genes and up-regulation of another, previously silent sodium channel gene. This plasticity in sodium channel gene expression is accompanied by electrophysiological changes that poise these cells to fire spontaneously or at inappropriate high frequencies. Changes in sodium channel gene expression also are observed in experimental models of inflammatory pain. Thus, sodium channel expression in DRG neurons is dynamic, changing significantly after injury. Sodium channels within primary sensory neurons may play an important role in the pathophysiology of pain.
Keywords: dorsal root ganglion neurons, hyperexcitability, ion channels, nerve injury, inflammation
Pain pathways begin with primary sensory neurons [dorsal root ganglion (DRG) neurons; trigeminal neurons]. It is now clear that, in some pain syndromes, hyperexcitability and/or increased baseline sensitivity of these cells leads to abnormal bursting that can produce chronic pain (1–3). The pivotal position of primary sensory neurons as distal sites of impulse generation along the nociceptive pathway, and the experimental and clinical accessibility of these neurons, has resulted in intense interest in mechanisms underlying action potential generation and transmission in them in disease states characterized by pain. Voltage-gated sodium channels, which produce the inward membrane current necessary for regenerative action potential production within the mammalian nervous system, are, of course, expressed in primary sensory neurons and have emerged as important targets in the study of the molecular pathophysiology of pain and in the search for new pain therapies. In this paper we focus on the potential role of sodium channels in the molecular pathophysiology of pain. We will emphasize, in particular, three motifs: first, that DRG neurons express a complex repertoire of multiple distinct sodium channels, encoded by different genes; second, that some of these sodium channels are sensory neuron specific; and third, that sodium channel expression in DRG neurons is highly dynamic, changing substantially not only during development, but also in various disease states, including some that are accompanied by pain.
Hyperexcitability in DRG Cells After Injury
Early studies (4, 5) demonstrated that, after injury to their axons, motor neurons display changes in excitability, suggesting increased sodium channel expression over the cell body and the dendrites, and similar changes were subsequently observed in sensory neurons (6, 7). Abnormal sodium channel accumulation at the tips of injured axons also has been observed (8–10), and both electrophysiological and computer simulation studies have suggested that abnormal increases in sodium conductance can lead to inappropriate, repetitive firing (11–13). Indeed, there is substantial evidence indicating that the abnormal excitability of DRG neurons, after axonal injury, is associated with an increased density of sodium channels (13, 14). These observations, together with experimental and clinical observations on partial efficacy of sodium channel-blocking agents in neuropathic pain (15–18), established a link between sodium channel activity and sensory neuron hyperexcitability producing pain. However, these studies did not examine the crucial question: what type(s) of sodium channels produce inappropriate sensory neuron discharge associated with pain?
Multiple Sodium Channels in Primary Sensory Neurons
Over the past decade, it has become clear that nearly a dozen, molecularly distinct voltage-gated sodium channels are encoded within mammals by different genes. DRG neurons, which had been known to display multiple, distinct sodium currents (19–22), express at least six sodium channel transcripts (23), as illustrated by the in situ hybridizations and reverse transcription–PCR shown in Figs. 1 and 2. These include high levels of expression of the α-I and Na6 channels, also expressed at high levels by other neuronal cell types within the central nervous system, which are known to support tetrodotoxin (TTX)-sensitive sodium currents. In addition, DRG neurons are unique in expressing four additional sodium channel transcripts that are not expressed at significant levels in other neuronal cell types: (i) PN1/hNE, which is expressed preferentially in DRG neurons (24), produces a fast, transient TTX-sensitive sodium current in response to sudden depolarizations and a persistent current elicited by slow depolarizations close to resting membrane potential (25); (ii) SNS/PN3, expressed preferentially in small DRG and trigeminal neurons, encodes a TTX-resistent sodium current (26, 27); (iii) NaN, expressed preferentially in small and trigeminal neurons, exhibits an amino acid sequence that, although only 47% similar to SNS-PN3, predicts that it encodes a TTX-resistant sodium channel (28); and (iv) NaG, another putative sodium channel that was originally cloned from astrocytes and at first thought to be glial specific (29), is also preferentially expressed at high levels within DRG neurons (23) and at low levels within other neurons of neural crest origin but not within other neuronal types (30).
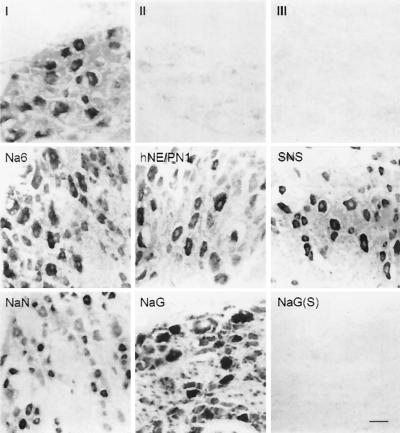
Figure 1.
Sodium channel α-subunit mRNAs visualized in sections from adult rat DRG by in situ hybridization with subtype-specific antisense riboprobes. mRNAs for α-I, Na6, hNE/PN1, SNS, NaN, and NaG are present at moderate to high levels in DRG neurons. Hybridization signal is not present with sense riboprobes, e.g., for NaG (S). (Bar indicates 100 μm.)
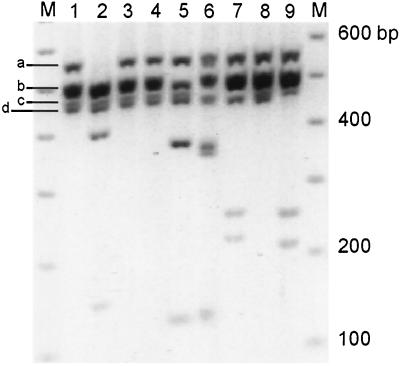
Figure 2.
Restriction enzyme profile analysis of Na channel domain 1 reverse transcription–PCR products from DRG. M lanes contain 100-bp ladder marker. Lane 1 contains the amplification product from DRG cDNA. Lanes 2–9 show the result of cutting this DNA with EcoRV, EcoN1, AvaI, SphI, BamHI, AflII, XbaI, and EcoRI, which are specific to subunits α-I, -II, -III, Na6, PN1, SNS, NaG, and NaN, respectively. Reproduced with permission from ref. 28. (Copyright 1998, National Academy of Sciences, USA).
Preferential expression of SNS/PN3 and NaN within small DRG neurons provides a molecular correlate for the observation (19–22, 32, 33) that these cells express several distinct sodium currents, including TTX-resistant sodium currents. A role for TTX-resistant sodium channels in action potential conduction along small diameter afferent fibers has been postulated (34), and TTX-resistant sodium potentials have, in fact, been recorded from unmyelinated C-fibers (35).
Preferential expression of SNS/PN3 and NaN in small DRG neurons, which include nociceptive cells, and the demonstration of a role of TTX-resistant sodium currents in conduction along their axons, have suggested that these channels may represent unique targets for the pharmacologic treatment of pain. PN1 and NaG also may represent useful molecular targets for the pharmacologic manipulation of DRG neurons because of their preferential expression in these cells.
Sodium Channel Gene Expression Is Altered After Injury to DRG Neurons
The first observations indicating that, in addition to production of excess channels, there is a switch in the type of channels produced after axonal injury were provided by Waxman et al. (36), who found a significant up-regulation of expression of the previously silent α-III sodium channel gene in DRG neurons after axotomy. This finding was followed by demonstration of down-regulation of the SNS/PN3 gene expression, which can persist as long as 210 days after axotomy (37), and of down-regulation of the NaN gene (28). These changes are illustrated in Fig. 3.
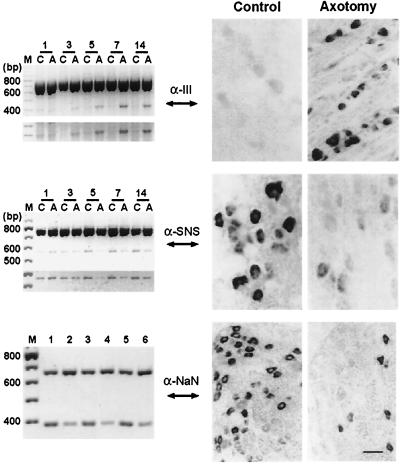
Figure 3.
Transcripts for sodium channel α-III (A) are up-regulated, and transcripts for SNS (B) and NaN (C) are down-regulated, in DRG neurons after transection of their axons within the sciatic nerve. The micrographs (Right) show in situ hybridizations in control DRG, and at 5–7 days postaxotomy. Reverse transcription–PCR (Left) shows products of coamplification of α-III (A) and SNS (B) together with β-actin transcripts in control (C) and axotomized (A) DRG (days postaxotomy indicated above gels in A and B), with computer-enhanced images of amplification products shown below gels. Coamplification of NaN (392 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (606 bp) (C) shows decreased expression of NaN mRNA at 7 days postaxotomy (lanes 2, 4, and 6) compared with controls (lanes 1, 3, and 5). A and B modified from ref. 37; C modified from ref. 28. (Copyright 1998, National Academy of Sciences, USA).
Physiologic Changes Accompany Altered Sodium Gene Expression After DRG Neuron Injury
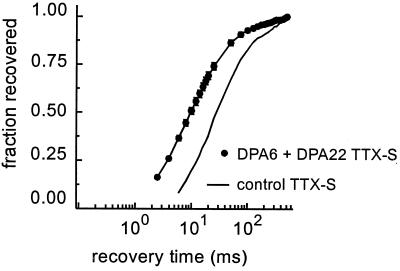
On the basis of the down-regulation of SNS/PN3 and NaN genes in DRG neurons after axonal transection, it would be expected that TTX-resistant sodium currents should be reduced in these cells after axotomy. Patch-clamp studies have demonstrated that, indeed, there is a loss of TTX-resistant sodium currents in DRG neurons after axonal transection (38); this down-regulation persists in small DRG neurons for at least 60 days (39), consistent with the long-lasting changes in gene expression that have been described (37) in these cells (Fig. 4). In addition, as shown in Fig. 5, there is a switch in the properties of the TTX-sensitive sodium currents in these cells after axotomy, with the emergence of a rapidly repriming current (i.e., a current that recovers rapidly from inactivation) (39). Cummins and Waxman (39) have suggested that the type III sodium channel is responsible for the rapidly repriming sodium current, but this conjecture remains to be proven.
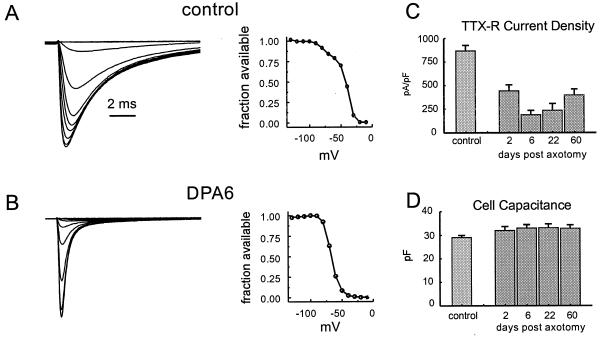
Figure 4.
TTX-resistant sodium currents in small DRG neurons are down-regulated after axotomy. (A and B, Left) Whole-cell patch-clamp recordings from representative control (A) and axotomized (B, 6 days postaxotomy) DRG neurons. Note the loss of the TTX-resistant slowly inactivating component of sodium current after axotomy. Steady-state inactivation curves (A and B, Right) show loss of a component characteristic of TTX-resistant currents. (C) Attenuation of TTX-resistant current persists for at least 60 days postaxotomy. (D) Cell capacitance, which provides a measure of cell size, does not change significantly after axotomy (modified from ref. 39).
Figure 5.
The kinetics of recovery from inactivation in TTX-sensitive sodium currents are different in axotomized DRG neurons. The graph shows recovery of TTX-sensitive sodium current from inactivation as a function of time in DRG neurons after axonal transection (6 and 22 days postaxotomy, results pooled) compared with uninjured controls. Note the leftward shift in the recovery curve. Modified from ref. 39.
These changes may poise DRG neurons to fire spontaneously, or at inappropriately high frequencies, after injury. Increased sodium channel densities, in themselves, will tend to lower threshold (12). In addition, Rizzo et al. (40) have pointed out that the overlap between steady-state activation and inactivation curves, together with weak voltage dependence of TTX-resistant sodium channels may confer instability on the neuronal membrane. Coexpression of abnormal combinations of several types of channels, whose window currents can bracket each other, would be expected to permit subthreshold ocillations in voltage, supported by TTX-resistant channels, to cross-activate other sodium channels, thereby producing spontaneous activity (40). Cummins and Waxman (39) noted that, because the TTX-sensitive sodium current in DRG neurons after axotomy reprimes relatively rapidly, injured neurons would be expected to sustain higher firing frequencies. Moreover, if persistent currents participate in setting the resting potential, as demonstrated in optic nerve axons (41), loss of TTX-resistant currents in DRG neurons after axotomy could produce a hyperpolarizing shift in resting potential, which, by relieving resting inactivation, might increase the amount of TTX-sensitive sodium current available for electrogenesis.
Neurotrophins Modulate Sodium Channel Expression in DRG Neurons
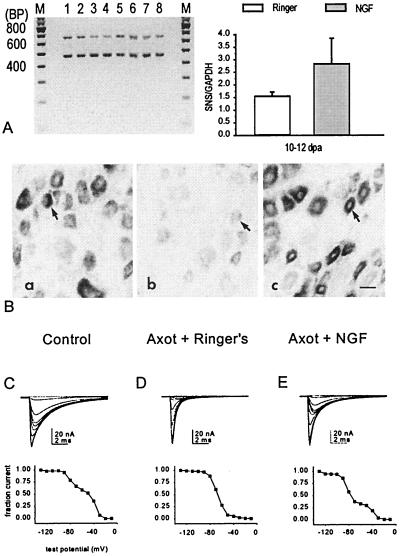
A number of studies have suggested that, in response to nerve or tissue injury, there are changes in synthesis or delivery of various neurotrophins to neurons. Early studies in culture demonstrated that nerve growth factor (NGF) can affect sodium channel expression in DRG neurons (42, 43). Black et al. (44) showed that NGF, delivered directly to DRG cell bodies, acts to down-regulate α-III mRNA and maintain high levels of SNS/PN3 mRNA expression in small DRG neurons in an in vitro model that mimics axotomy. Following up on these observations, Dib-Hajj et al. (45) studied small DRG neurons in vivo after axotomy and demonstrated that administration of exogenous NGF to the proximal nerve stump results in an up-regulation of TTX-resistant sodium current and of SNS/PN3 mRNA levels in small DRG neurons (Fig. 6). These observations suggest that at least some of the changes observed in DRG neurons after axotomy reflect loss of access to peripheral pools of neurotrophic factors.
Figure 6.
Reverse transcription–PCR (A), in situ hybridization (B), and patch-clamp recordings (C), showing partial rescue of SNS mRNA and TTX-resistant sodium currents in axotomized DRG neurons after delivery of NGF to the proximal nerve stump. (A) Coamplification of SNS (479 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (666 bp) products in Ringer’s solution-treated axotomized DRG (lanes 1, 2, 5, and 6) and NGF-treated axotomized DRG (lanes 3, 4, 7, and 8). The graph shows the increase in SNS amplification product in NGF-treated DRG. (B) In situ hybridization showing down-regulation of SNS mRNA in DRG after axotomy (axotomy + Ringer’s solution compared with control), and the partial rescue of SNS mRNA by NGF. (C) Representative patch-clamp recordings showing partial rescue of slowly inactivating TTX-resistant sodium currents in axotomized DRG neurons after exposure to NGF. Corresponding steady-state inactivation curves are shown below the recordings. Modified from ref. 45.
Brain-derived growth factor has been studied and has been found not to alter sodium currents in DRG neurons, although it affects the expression of γ-aminobutyric acid receptor-mediated currents in these cells (46). Glial-derived growth factor has been found to modulate the expression of NaN in a subpopulation of small DRG neurons, which are known to express the ret receptor (53). Multiple neurotrophins and growth factors have effects on DRG neurons, and it is likely that sodium channel expression in these cells reflects combinatorial effects of multiple factors.
Sodium Channel Expression in Inflammatory Pain Models
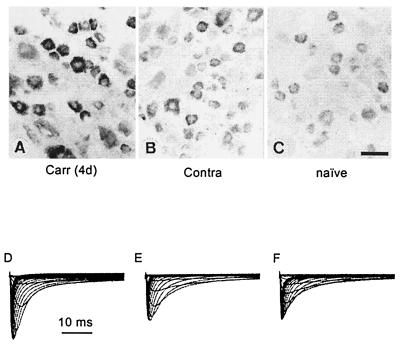
Several studies have demonstrated that inflammatory molecules such as prostaglandins and serotonin can modulate TTX-resistant sodium currents in DRG neurons (47), possibly acting through a cyclic AMP-protein kinase A cascade (48). However, the question, of whether sodium channel gene expression is affected in inflammatory models of pain had not been addressed. To understand the role of sodium channels in inflammatory pain, we have carried out studies in the carageenan inflammatory pain model in the rat (49). In these studies, carried out before our cloning of NaN, we focused on SNS/PN3 because its expression was known to be labile. Based on our previous observation in which we detected peak changes in SNS/PN3 mRNA 5 days after axotomy (37), we studied rats in the subacute phase, 4 days after injection of carageenan into the hind paw. As shown in Fig. 7, these experiments demonstrated significantly increased SNS/PN3 mRNA expression in DRG neurons projecting to the inflamed limb, compared with DRG neurons from the contralateral side or naive (uninjected) controls. Moreover, our patch-clamp recordings demonstrated that the amplitude of the TTX-resistant sodium current in small DRG neurons projecting to the inflamed limb was significantly larger than on the contralateral side 4 days postinjection (31.7 ± 3.3 vs. 20.0 ± 2.1 nA). The TTX-resistant current density was also significantly increased in the carageenan-challenged DRG neurons. Consistent with these results, a persistent increase in sodium channel immunoreactivity is observed in DRG neurons within 24 hr of injection of complete Freund’s adjuvant into their projection field and persists for at least 2 months (50). The mechanism responsible for this inflammation-associated change in sodium channel expression is not known. Interestingly, NGF normally is produced in peripheral target tissues by supporting cells that include fibroblasts, Schwann cells, and keratinocytes; NGF production is stimulated in immune cells, and increased NGF levels have been observed in the local area after treatment with inflammatory agents such as carageenan and Freund’s adjuvant (51, 52), raising the possibility that inflammation may indirectly trigger changes in sodium channel gene expression via changes in neurotrophin levels.
Figure 7.
SNS mRNA levels and TTX-resistant sodium currents are increased 4 days after injection of carrageenan into the projection fields of DRG neurons. (Upper) In situ hybridization showing SNS mRNA in carrageenan-injected (A), contralateral control (B), and naive (C) DRG. Patch-clamp recordings (D–F) do not reveal any change in voltage dependence of activation or steady-state inactivation of TTX-resistant sodium currents after carrageenan injection, but demonstrate an increase in TTX-resistant current amplitude (D) and density. Modified from ref. 49.
Sodium Channels as Molecular Targets in Pain Research
Given what we have learned about sodium channels, where do we go next in the search for better treatments for pain syndromes? The answer to this question is not entirely clear at this time. We can, however, come to a number of conclusions. First, sodium channels are important participants in electrogenesis within primary sensory neurons, including DRG neurons. Second, a multiplicity of sodium channels are present within DRG neurons, where they probably subserve multiple functions (transduction, signal amplification, action potential electrogenesis, etc.) and interact in a complex manner. Third, DRG neurons express a number of sodium channel genes (SNS/PN3, NaN, PN1, and NaG) in a preferential manner, at levels much higher than in any other neuronal cell type. This observation may present a therapeutic opportunity for the selective manipulation of primary sensory neurons in general, or nociceptive neurons in particular. Fourth, sodium channel expression in DRG neurons is highly dynamic, with multiple sodium channel genes (including α-III, SNS/PN3, and NaN) exhibiting up- or down-regulation after various injuries to these cells. Importantly, different injuries may trigger opposing changes of certain sodium channel genes (e.g., down-regulation of SNS/PN3 after axotomy vs. up-regulation in the carageenan inflammation model) in DRG neurons, so that it may be difficult to extrapolate from one model system to another. Nevertheless, we have learned, at a minimum, that sodium channel expression in DRG neurons is dynamic and can change significantly after injury, and that changes in sodium channel expression can substantially alter excitability in these cells.
Delineation of the precise role(s) of each sodium channel subtype in the physiology of DRG neurons and the pathophysiology of pain remains to be established, and the utility of selective blockade of each channel subtype as an approach to the treatment of pain will require further careful study. However, the stage has been set for these investigations. It is quite likely, in our opinion, that sodium channel blockade will emerge as a viable strategy for pharmacologic treatment of pain.
Acknowledgments
This work has been supported in part by grants from the National Multiple Sclerosis Society and the Paralyzed Veterans of America/Eastern Paralyzed Veterans Association, and by the Medical Research Service, Department of Veterans Affairs. T.R.C. was supported in part by a fellowship from the Spinal Cord Research Foundation.
ABBREVIATIONS
- DRG
dorsal root ganglia
- TTX
tetrodotoxin
- NGF
nerve growth factor
References
- 1.Ochoa J, Torebjork H E. Brain. 1980;103:835–854. doi: 10.1093/brain/103.4.835. [DOI] [PubMed] [Google Scholar]
- 2.Nordin M, Nystrom B, Wallin U, Hagbarth K-E. Pain. 1984;20:231–245. doi: 10.1016/0304-3959(84)90013-7. [DOI] [PubMed] [Google Scholar]
- 3.Devor M. In: Textbook of Pain. 2nd Ed. Wall P D, Melzack R, editors. Edinburgh: Churchill Livingstone; 1994. pp. 79–101. [Google Scholar]
- 4.Eccles J C, Libet B, Young R R. J Physiol (London) 1958;143:11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuno M, Llinas R. J Physiol (London) 1970;210:807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallego R, Ivorra I, Morales A. J Physiol (London) 1987;391:39–56. doi: 10.1113/jphysiol.1987.sp016724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurtu S, Smith P A. J Neurophysiol. 1988;59:408–423. doi: 10.1152/jn.1988.59.2.408. [DOI] [PubMed] [Google Scholar]
- 8.Devor M, Keller C H, Deerinck T J, Ellisman M H. Neurosci Lett. 1989;102:149–154. doi: 10.1016/0304-3940(89)90070-0. [DOI] [PubMed] [Google Scholar]
- 9.England J D, Gamboni F, Ferguson M A, Levinson S R. Muscle Nerve. 1994;17:593–598. doi: 10.1002/mus.880170605. [DOI] [PubMed] [Google Scholar]
- 10.England J D, Happel L T, Kline D G, Gamboni F, Thouron C L, Liu Z P, Levinson S R. Neurology. 1996;47:272–276. doi: 10.1212/wnl.47.1.272. [DOI] [PubMed] [Google Scholar]
- 11.Waxman S G, Brill M H. J Neurol Neurosurg Psychiatr. 1978;41:408–417. doi: 10.1136/jnnp.41.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matzner O, Devor M. Brain Res. 1992;597:92–98. doi: 10.1016/0006-8993(92)91509-d. [DOI] [PubMed] [Google Scholar]
- 13.Matzner O, Devor M. J Neurophysiol. 1994;72:349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J-M, Donnelly D F, Song X-J, LaMotte R H. J Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]
- 15.Chabal C, Russell L C, Burchiel K J. Pain. 1989;38:333–338. doi: 10.1016/0304-3959(89)90220-0. [DOI] [PubMed] [Google Scholar]
- 16.Devor M, Wall P D, Catalan N. Pain. 1992;48:261–268. doi: 10.1016/0304-3959(92)90067-L. [DOI] [PubMed] [Google Scholar]
- 17.Omana-Zapata I, Khabbaz M A, Hunter J C, Bley K R. Brain Res. 1997;771:228–237. doi: 10.1016/s0006-8993(97)00770-1. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo M A. J Neurol Sci. 1997;152:103–106. doi: 10.1016/s0022-510x(97)00143-3. [DOI] [PubMed] [Google Scholar]
- 19.Kostyuk P G, Veselovsky N S, Tsyandryenko A Y. Neuroscience. 1981;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- 20.Roy M L, Narahashi T. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caffrey J M, Eng D L, Black J A, Waxman S G, Kocsis J D. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- 22.Elliott A A, Elliott J R. J Physiol (London) 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black J A, Dib-Hajj S, McNabola K, Jeste S, Rizzo M A, Kocsis J D, Waxman S G. Mol Brain Res. 1996;43:117–132. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 24.Toledo-Aral J J, Moss B L, He Z-J, Koszowski A G, Whisenand T, Levinson S R, Wolff J J, Silos-Santiago I, Halegoua S, Mandel G. Proc Natl Acad Sci USA. 1997;94:1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummins T R, Howe J R, Waxman S G. J Neurosci. 1999;18:9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akopian A N, Sivilotti L, Wood J N. J Biol Chem. 1996;271:5953–5956. [Google Scholar]
- 27.Sangameswaran L, Delgado S G, Fish L M, Koch B D, Jakeman L B, Stewart G R, Sze P, Hunter J C, Eglen R M, Herman R C. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 28.Dib-Hajj S D, Tyrrell L, Black J A, Waxman S G. Proc Natl Acad Sci USA. 1998;95:8963–8969. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautron S, Dos Santos G, Pinto-Henrique D, Koulkoff A, Gros F, Berwald-Netter Y. Proc Natl Acad Sci USA. 1992;89:7272–7276. doi: 10.1073/pnas.89.15.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felts P A, Black J A, Dib-Hajj S D, Waxman S G. Glia. 1997;21:269–277. doi: 10.1002/(sici)1098-1136(199711)21:3<269::aid-glia2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Rizzo M A, Kocsis J D, Waxman S G. J Neurophysiol. 1995;72:2796–2816. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rush A M, Brau M E, Elliott A A, Elliott J R. J Physiol (London) 1998;511:771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholz A, Appel N, Vogel W. Eur J Neurosci. 1998;10:2547–2556. [PubMed] [Google Scholar]
- 34.Jeftinija S. Brain Res. 1994;639:125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- 35.Quasthoff S, Grosskreutz J, Schroder J M, Schneider U, Grafe P. Neuroscience. 1995;69:955–965. doi: 10.1016/0306-4522(95)00307-5. [DOI] [PubMed] [Google Scholar]
- 36.Waxman S G, Kocsis J K, Black J A. J Neurophysiol. 1994;72:466–471. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dib-Hajj S, Black J A, Felts P, Waxman S G. Proc Natl Acad Sci USA. 1996;93:14950–14954. doi: 10.1073/pnas.93.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzo M A, Kocsis J D, Waxman S G. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 39.Cummins T R, Waxman S G. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzo M A, Kocsis J D, Waxman S G. Eur Neurol. 1996;36:3–12. doi: 10.1159/000117192. [DOI] [PubMed] [Google Scholar]
- 41.Stys P K, Ransom B R, Waxman S G. Proc Natl Acad Sci USA. 1993;90:6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguayo L G, White G. Brain Res. 1992;570:61–67. doi: 10.1016/0006-8993(92)90564-p. [DOI] [PubMed] [Google Scholar]
- 43.Zur K B, Oh Y, Waxman S G, Black J A. Mol Brain Res. 1995;30:97–103. doi: 10.1016/0169-328x(94)00283-k. [DOI] [PubMed] [Google Scholar]
- 44.Black J A, Langworthy K, Hinson A W, Dib-Hajj S D, Waxman S G. NeuroReport. 1997;8:2331–2335. doi: 10.1097/00001756-199707070-00046. [DOI] [PubMed] [Google Scholar]
- 45.Dib-Hajj S D, Black J A, Cummins T R, Kenney A M, Kocsis J D, Waxman S G. J Neurophysiol. 1998;79:2668–2678. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- 46.Oyelese A A, Rizzo M A, Waxman S G, Kocsis J D. J Neurophysiol. 1997;78:31–42. doi: 10.1152/jn.1997.78.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold M S, Reichling D B, Shuster M J, Levine J D. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.England S, Bevan S, Docherty R J. J Physiol (London) 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka M, Cummins T R, Ishikawa K, Dib-Hajj S D, Black J A, Waxman S G. NeuroReport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- 50.Gould H J, III, England J D, Liu Z P, Levinson S R. Brain Res. 1998;802:69–74. doi: 10.1016/s0006-8993(98)00568-x. [DOI] [PubMed] [Google Scholar]
- 51.Woolf C J, Safieh-Garabedian B, Ma Q-P, Crilly P, Winter J. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 52.Weskamp G, Otten U. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- 53.Fzell J, Cummins P R, Dib-Hajj S D, Fried K, Black J A, Waxman S G. Mol Brain Res. 1999;67:267–282. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]