Abstract
Polyglutamine sequences of unknown normal function are present in a significant number of proteins, and their repeat expansion is associated with a number of genetic neurodegenerative diseases. Polyglutamine solution structure and properties are not only important because of the normal and abnormal biology associated with these sequences, but also because they represent an interesting case of a biologically relevant homopolymer. As the common thread in the expanded polyglutamine repeat diseases, it is important to understand the structure and properties of simple polyglutamine sequences. At the same time, experience has shown that sequences attached to polyglutamine, whether in artificial constructs or in disease proteins, can influence structure and properties. The two major contenders for the molecular source of the neurotoxicity implicit in polyglutamine expansion within disease proteins are a populated toxic conformation in the monomer ensemble and a toxic aggregated species. This review summarizes experimental and computational studies on the solution structure and aggregation properties of both simple and complex polyglutamine sequences, and their repeat-length dependence. As a representative of complex polyglutamine proteins, the behavior of huntingtin N-terminal fragments, such as exon-1, receives special attention.
Although functionally obscure polyglutamine (polyQ) sequences are found in a variety of proteins 1–3, many of which are transcription factors 1,4, and although aggregation-prone plant storage proteins rich in Gln are also well-known 5, 6, it is unlikely that polyQ sequences would ever have become a major focus of research were it not for their pathogenic role in proteins associated with a series of inherited, devastating neurodegenerative diseases 7, 8. At least 10 8, 9 of these expanded CAG repeat diseases have been described, including the most prevalent and most well-known condition, Huntington’s disease (HD) 10. All of them are triggered by inheritance of a polyQ sequence with a repeat length longer than normal, where normal is in the 20–35 range for all but one of these diseases 8. All of them feature the accumulation in particular brain regions of polyQ-containing neuronal aggregates, and all of them appear to be primarily gain-of-function diseases 8. These and other data have suggested that expanded polyQ repeat proteins are defective in some aspect of protein folding, such as a lowered barrier to misfolding within the monomer and/or to aggregate formation.
Because of the great interest in understanding the molecular mechanisms of these diseases and in developing therapies, the biophysical properties of polyQ sequences have received considerable scrutiny in the nearly 20 years since the pathogenic role of polyQ expansion was first realized 11. While the exact folded and aggregated states of the toxic species in these diseases are yet to be worked out, a wealth of important biophysical data has been accumulated. In addition to any implications for mechanisms and therapeutic strategies of neurodegenerative diseases, some of these data have far-reaching implications for general protein solution behavior and aggregation. Experimental biophysical studies on polyQ solution structure and aggregation can also provide data to support computational analyses that have potential relevance to polyQ pathology as well as to fundamental polymer physics. PolyQ studies have provided important information on the solution structure of intrinsically disordered proteins, and on the all-important mechanisms by which amyloid growth is initiated.
This review focuses on in vitro experimental studies of polyQ solution and aggregate structure and on the mechanisms of spontaneous aggregation and its inhibition. Selected results from the wealth of in silico and in vivo studies are also discussed. The reader is referred to a recent review for more thorough coverage of biological aspects 10.
Polyglutamine Solution Structure
The polyQ monomer in water
The observation of ubiquitinylated, polyQ containing aggregates in neuronal nuclei of HD patients 12 and animal models 13, in addition to other factors, led directly to the hypothesis that expanded CAG repeat diseases should be included in the growing family of human pathologies involving protein misfolding and aggregation 14. Since globular protein aggregation 15, including amyloid formation 16–18, is understood to be associated with breakdowns in protein conformational integrity, speculation on the nature and source of expanded polyQ gain-of-function has included not only a proposed leading role for protein aggregation but also the related, but distinct, hypothesis of an important, toxic misfolded state within the monomer ensemble 7 that might also, coincidentally, mediate aggregation. But what is the evidence for the existence of a distinct, highly populated folded state in either benign or pathogenic polyQ sequences? In this section I review what is known about populated folding states of polyQ monomers.
Initial evidence for a predominantly disordered structure for polyQ in solution came from CD analysis of solutions of short polyQ monomers 19. Subsequent CD studies on pathological length polyQ sequences, either chemically synthesized 20 or biosynthetic 21, showed that even long polyQ sequences are largely disordered, and that there is no obvious difference in secondary structure between long and short polyQ. NMR studies 22 subsequently confirmed the disordered nature of polyQ monomers of all repeat lengths, and supported earlier CD studies 23 that showed the absence of detectible, conformationally distinct intermediates in the coil to sheet transition that occurs when polyQs grow into amyloid. X-ray diffraction structures of crystals of a sequence-modified huntingtin N-terminal fragment containing a short polyQ insert showed the intervening polyQ sequence to be in a variety of conformations, including α-helix 24. CD analysis shows that a simple K2Q40K2sequence has about 10% α-helix configurations at 35 °C and takes on an additional 10% on cooling to 5 °C 25. The overall picture from solution phase experiments is that polyQ sequences, regardless of repeat length, are largely disordered chain with transient elements of regular secondary structure. This impression is supported by a number of computational analyses starting from unstructured chains that, while not agreeing completely with each other, suggest that both short or long polyQ sequences generally exhibit mixtures in various proportions of disordered structure, α-helix, β-sheet, PPIIhelix, and β-turns 26–32.
There is an additional aspect to the solution structure of the polyQ sequence that likely plays an important role in its behavior – the tendency of the sequence to collapse, in spite of its apparent hydrophilic nature, in aqueous solution. This behavior has been demonstrated in several biophysical studies. A fluorescence correlation spectroscopy (FCS) analysis of a collection of labeled polyQ molecules of differing repeat lengths 33 demonstrated two important points. First, that polyQ peptides behave in a simple aqueous buffer as a polymer in a poor solvent. That is, the polyQ chain in water is neither fully extended in a statistical coil conformation, as it would be in a good solvent, nor is it somewhat less extended, as occurs under “theta” conditions just short of the point where long-range interactions begin to contribute to polymer conformations. Rather, polyQ in water behaves as a polymer in a bad solvent, in which solvent water is excluded from the collapsed polymer chain. Second, that the compactness of the molecule does not change appreciably as polyQ repeat length increases from Q15 to Q54, that is, through the typical disease threshold of ~35. The relatively compact structure of polyQ in solution was confirmed in two FRET studies 32, 34. Atomic force stretching experiments on polyQ sequences embedded within artificial flanking sequences are also consistent with a collapsed structure in contact with water, suggesting a very high barrier to the unfolding or extension of hydrated polyQ sequences 35. These results are also consistent with computer simulations that predict a collapsed coil structure for monomeric polyQ, likely held together by H-bonding between side chain and main chain amide groups, in preference to H-bonding to water 27, 29, 36, 37. This internal H-bonded structure could well play a role in the expected highly inefficient formation of β-hairpin 30 or other regular structures 37 that might be implicated in aggregation nucleation (see below).
Is there a “misfolded“ state of polyQ?
Inconsistent with the above data on a polyQ repeat-length independent disordered state are antibody binding data that have been interpreted to suggest the existence of populated alternative conformations of monomeric polyQ that become more highly populated at repeat lengths above the typical disease risk threshold of ~35–45. Trottier and colleagues showed that the anti-polyQ monoclonal antibody 1C2 binds considerably better, in Western blots, to longer polyQ sequences compared with short versions 38. This led to the idea that 1C2 recognizes a specific polyQ conformation that is much more highly populated in long polyQ sequences than in short, and therefore to the idea that 1C2 recognizes a toxic conformation populated only in long polyQs. An alternative explanation emerged, however, when the Bjorkman group showed that a similar MAb, MW1, owes its preferential binding to long polyQ peptides to a “linear lattice” effect of the polyQ sequence 39. According to their analysis, the affinity of MW1 for a polyQ sequence is enhanced according to the number of individual epitopes in the polyQ chain, even though each individual epitope might be short (as was, in fact, subsequently shown by an X-ray crystal structure of the complex 40).
More recently, another antibody with a preference for long polyQ sequences, 3B5H10, has been described and its selective binding also interpreted as evidence for a toxic folded conformation in long polyQ sequences 41. While this remains formally possible, antibody binding does not prove that a particular conformation is populated in the absence of the antibody. The thermodynamics of complex formation allows antibodies to recruit and enrich kinetically accessible conformations even if they are only minimally populated in the absence of the antibody 42. Conformational recruitment is an especially attractive possibility when the target molecule is an intrinsically disordered protein like polyQ that is presumably capable of undergoing coupled folding and binding 43, 44 to more structurally organized binding partners.
Although there is no convincing evidence for a populated, pre-existing conformation in expanded polyQ sequences that might be considered a “toxic conformation”, it remains possible, nonetheless, that monomers may yet prove to be the toxic species. This could happen if the toxic event (a binding interaction, for example, to some cellular target) is only favorable with a polyQ sequence of a particular length. Models for such binding phenomena might be (a) the linear lattice 39 type of polydentate interaction, (b) a binding cavity (such as found in some molecular chaperones or proteasomes) satisfied by the compact coil state of a sufficiently large polyQ molecule, or (c) a templated, coupled folding and binding event 43 (such as aggregate elongation, for example) with a thermodynamic requirement for a certain amount of buried surface area that can only be supplied by expanded polyQs. Thus there are a number of mechanisms by which one would expect to observe a correlation between cell toxicity and cellular levels of monomeric expanded polyQs, as has been recently observed 41. Such correlative studies do not prove the existence of a populated, toxic, misfolded conformation in the monomer ensemble, however, and in fact merely formally indicate a pool of molecules that are capable of undergoing a toxic binding event.
Solution structure of complex polyQ sequences
In all expanded CAG repeat proteins, the polyQ sequence is embedded within the protein and therefore has both N- and C-terminal flanking sequences. Sequence algorithms make somewhat contrasting predictions of secondary structure of both the polyQ insert and surrounding sequence, with one program predicting disorder 45 and another predicting the ability to assemble into coiled coils 46. Besides the structural details of a particular peptide in solution, one would also like to know how the flanking sequences influence polyQ structure and properties, and how the polyQ sequence (and its expansion) influences surrounding structure. There are significant technical limitations on addressing such questions experimentally with intrinsically unstructured proteins, requiring specialized spectroscopic approaches 44, 47. Perhaps because of this, a number of groups have looked at polyQ disease protein structure issues through computational approaches.
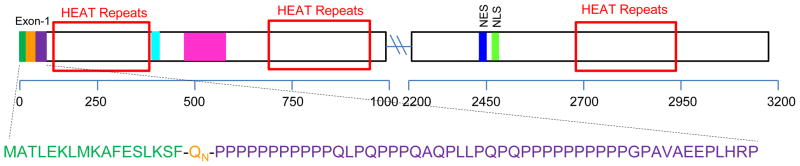
There has been a special emphasis on sequences representing the N-terminal 3% of the huntingtin sequence (Fig. 1), which includes the polyQ segment as well as an N-terminal sequence (httNT) of 17 amino acids and a C-terminal segment rich in proline residues. A hint that flanking sequence can influence polyQ conformation in the monomer ensemble comes from the ability a C-terminal P10 sequence to slow the nucleated growth of polyQ amyloid and to abrogate the ability of a temperature decrease to enhance α-helix formation in a polyQ sequence 25. A series of crystal structures of a significantly altered htt N-terminal fragment containing a short polyQ repeat show the httNTto fold into an α-helix, the C-terminal proline rich sequence to explore PPIIstructures, and the intervening polyQ to occupy a variety of structures, including α-helix 24. The extent to which crystal packing forces dictate any of these results is not clear but certainly must be considered possible, especially for a peptide that is disordered in solution.
Figure 1.
Structure of huntingtin. The 3144 amino acid long human huntingtin protein (numbering based on a Q23 repeat length) showing the N-terminal exon-1 segment and other regions of interest. Boxed in red are three domains rich in ~40 amino acid long HEAT repeats implicated in protein-protein interactions 10, as identified by a neural network analysis 191. Dark green, httNT segment; orange, polyQ; purple, proline-rich; cyan, phosphorylation and acetylation sites; magenta, in vivo caspase and calpain cleavage sites suggesting a disordered region; blue, nuclear export sequence; light green, nuclear import sequence. Based on information reviewed in reference 10.
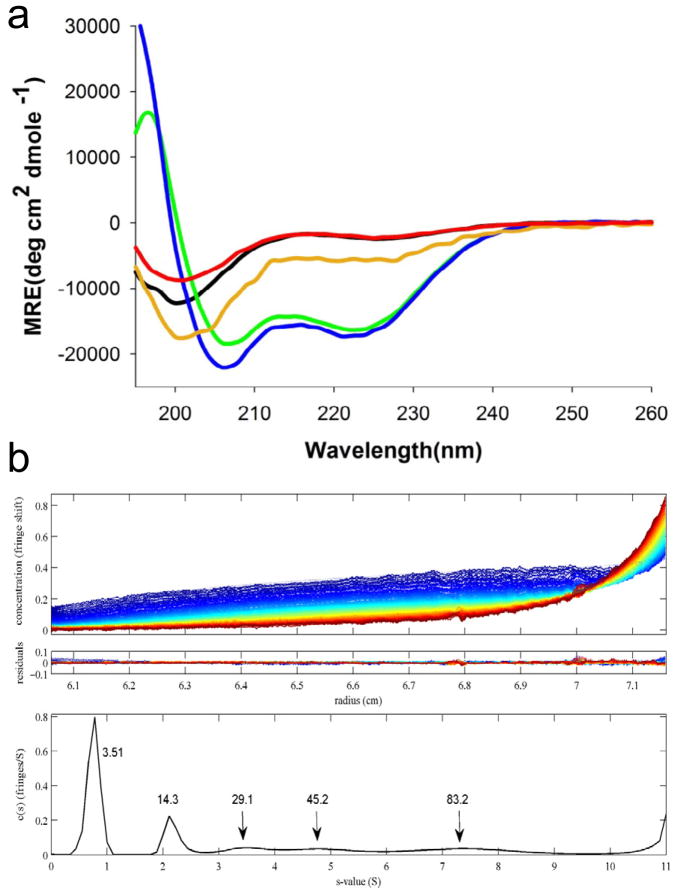
The solution structure of a protein includes its native oligomerization state, and oligomerization can influence secondary structure in profound ways. Recent evidence suggests that htt N-terminal fragments are capable of reversible tetramer formation, almost certainly mediated by the formation of an α-helical bundle centered in the httNT segment. The httNT sequence in isolation as a monomer is disordered 48, exhibiting a slight tendency toward α-helix 48, 49. Recent work shows that the α-helix content of the httNT segment depends on concentration, and that at concentrations of 0.5 mM or higher, the peptide is essentially fully helical 50 (Fig. 2a). These results, coupled with an analytical ultracentrifugation (AUC) analysis suggesting that httNT and httNTQ10K2 molecules exist at modest concentrations as an equilibrium of monomer, tetramer, octomer, and perhaps higher oligomers 50 (Fig. 2b) suggests that htt N-terminal fragments are capable of facile oligomerization through α-helix bundle formation via their httNT segments. Consistent with the AUC data, various fluorescence techniques previously indicated an early assembly in cells of GFP-tagged htt exon-1 proteins into small multimers 51, 52. Small multimers were not observed in striatal neurons expressing htt exon-1, however 41. The httNT sequence has been proposed to be an important regulatory appendage of htt, mediating – with or without post-translational modifications – a variety of targeting, trafficking, and clearance operations in the cell 49, 53–58. In this context, it seems possible that reversible tetramer formation may be a means by which solution exposure of the promiscuous httNT sequence can be itself regulated. Through this or other means, it is possible that tetramer formation by expanded polyQ versions of htt N-terminal fragments may somehow play a direct role in toxicity. The implications of httNT-mediated oligomer formation for the aggregation nucleation mechanism of htt N-terminal fragments is discussed later.
Figure 2.
HttNT-mediated oligomerization. (a) Concentration dependence of the CD spectra of httNTQ: green, 1.3 mM; blue, 0.67 mM; orange, 0.33 mM; black, 0.15 mM; red, 0.10 mM obtained by dilution from a 0.76 mM solution. (b) Sedimentation velocity analysis of 50 μM httNTQ10K2 at 20 °C. Top panel, sedimentation data; middle panel, residuals from the global fit; bottom panel, c(s) profile with peaks labeled (in kDa). Data from reference 50.
Two groups have computationally studied the httNT segment in isolation. Kelley et al. found a high tendency for all or part of the httNTsegment to form α-helix structure 59. Rossetti et al. found a number of conformational families which, except for a small fraction of condensed coil, are dominated by extended, solvated structures with helical segments 60. In general, these findings are in agreement with experimental data that show monomeric httNT to be disordered at low concentrations 48, and to form helical tetramers at higher concentrations 50.
Other groups have conducted simulations on various htt N-terminal fragments. Consistent with the polyproline effect on aggregation mentioned above, Lakhani et al. found that the polyproline region of htt exon-1 decreases the ability of polyQ to engage β-structure elements within the monomer 31. This group also found that polyQ expansion leads to increased β-structure in the httNT segment 31. In contrast, Williamson et al. found some α-helix tendency within the httNT segment of httNT-QN sequences (i.e., without the proline domain), and a tendency to increased disorder in httNT as polyQ repeat length increases 61. Dlugosz and Trylska found an intriguing repeat length dependent conformational change within an htt N-terminal fragment 62. For both long and short polyQ sequence versions, both the httNTand the polyQ were largely α-helical. In the short polyQ molecule, the PPII helix of the proline-rich tail tends to dock to the httNT helix, while in the long polyQ version these elements do not interact and are therefore more solvated 62. The variety of simulation results reflected by the above discussion presumably results from the use of different simulation methods 62.
A number of model studies have been conducted on polyQ fusion proteins, and attempts made to interpret CD or FTIR data to deduce structural consequences. Placing polyQ inserts between secondary structural elements within a folded protein to various extents can destabilize and/or alter the folding or local structure of the host protein 63–67. In a roughly repeat-dependent fashion, polyQ inserts tend to explore disordered and/or β-structure 65, 67. Interestingly, polyQ sequences fused to some intact folded domains tend to acquire additional α-helical character 67,68, although other protein hosts don’t seem to detectibly affect polyQ structural preferences 21. In general, polyQ adjacent to a folded domain does not appreciably affect native structure and/or folding stability of the host protein 66, 67.
What is the normal role of polyQ in protein structure and function?
In some settings, polyQ repeat length correlates inversely with protein activity 69, 70, suggesting the possibility that repeat expansion diseases, while predominantly gain-of-function 8, might also possess some loss-of-function character in some cases 71. As a general rule, however, it is not clear that polyQ segments in proteins actually have important, specific functions. Rather, they might simply have developed over the course of evolution at protein sites where polyQ tracts are tolerated, undergoing relatively cost-free expansion at these sites due to the replicational instability 72 of the CAG repeat sequence. An example is shown in Table 1, a line-up of the polyQ-proximal sequences of various TATA box binding proteins (TBPs) over a wide range of evolution. While the sequences surrounding the polyQ are well-conserved, the polyQ repeat length itself ranges from 4 to 38 (Table 1). Such sequence expansion in an otherwise highly conserved protein suggests a linker between, or adjacent to, structural and/or functional units. At such sites normal protein function might be largely retained even when the polyQ sequence expands to a pathological repeat length. Such a scenario is roughly compatible with the results from model system studies discussed in the preceding paragraph.
Table 1.
PolyQ Repeat Lengths in TATA Box Binding Proteins (TBPs) of Various Speciesa
| Source - GenBank | N-terminal to polyQ | QN | C-terminal to polyQ |
|---|---|---|---|
| Xenopus NP_001089038.1 |
-TYGTGLTPQPVQTTNSLSILEEQQR | 4 | --------TQQSTLQQGNQG-SGQTPQ |
| Zebrafish AAH55549.1 |
-PYGTGLTPQPVQNSNSLSLLEEQQR | 6 | ---------AASQQQGGMVGGSGQTPQ- |
| Viper Q92146.1 |
-PYGTGLTPQPAQSTNSLSILEEQQR | 6 | -------AAAQQSTSQPTQAPSGQTPQ- |
| Chicken NP_990434.1 |
-PYGTGLTPQPVQSTNSLSILEEQQR | 6 | -------AAQSSTSQQATQGTSGQTPQ- |
| Rat AAH81939.1 |
-PYGTGLTPQPVQNTNSLSILEEQQR | 15 | AVATAAASVQQSTSQQPTQGASGQTPQ- |
| Cow AAI13309.1 |
-PYGTGLTPQPIQNTNSLSILEEQQR | 19 | ---AAVAAVQQSTSQQATQGPSGQTPQ- |
| Rabbit XP_002723543.1 |
-PYGTGLTPQPIQNTNSLSILEEQQR | 20 | AVAAAAAAVQQSTSQQAAQGVSGQTPQ- |
| Chimpanzee NP_001098077.1 |
-PYGTGLTPQPIQNTNSLSILEEQQR | 35 | --AVAAAAVQQSTSQQATQGTSGQAPQ- |
| Human AAI10342.1 |
-PYGTGLTPQPIQNTNSLSILEEQQR | 38 | --AVAAAAVQQSTSQQATQGTSGQAPQ- |
Alignments fron Kalign (EMBL).
Aggregation of Simple Polyglutamine Sequences
Because the only apparent common element of the proteins associated with the 10 expanded CAG repeat diseases is the polyQ sequence, the solution and aggregation behavior of simple polyQ sequences has been seen by several groups as a reasonable starting point in studying this disease family 20, 23, 25, 33, 34, 45, 73–79. One common non-Gln element of these sequences has been in inclusion of flanking charged residues 19, 73, 80, 81 which bestow on these peptides a degree of kinetic (i.e., short term) solubility that allows their solution properties, including nucleation of aggregation, to be studied. The presence of flanking charged residues appear to be quite important; a polyQ sequence lacking flanking charged residues is reasonably well-behaved during disaggregation, but precipitates immediately upon adjustment to PBS conditions 81. While it might be argued that a polyQ peptide lacking flanking charged residues is a more appropriate setting in which to study the properties of polyQ sequences in a larger protein context, polyQ fused to a number of disease protein flanking sequences retains the good kinetic solubility of K2QNK2 molecules, in contrast to the poor solution properties of polyQ lacking flanking residues, 25, 45. While charged flanking residues probably introduce some qualitative rate differences, most of the experiments described in this section employ flanking charged residues to make physical experiments and comparisons feasible.
Soon after the observation that expanded polyQ aggregates accumulate in neurons 12, 13, in vitro studies on recombinantly produced exon-1 fragments demonstrated robust aggregation into amyloid-like fragments that is both repeat-length dependent and concentration-dependent 82. Subsequently, in vitro studies on chemically synthesized polyQ peptides in the K2QNK2 background showed similar repeat length dependent aggregation 20 into ordered, amyloid-like 81 aggregates. The repeat length dependence was observed in both a spontaneous aggregation reaction dependent on both nucleation and elongation properties and in a fibril growth assay requiring only elongation of existing seeds 20. A nearly identical repeat length dependence was subsequently demonstrated in a C. elegans model 83. The microtiter-based elongation assay also showed that amyloid-like aggregates of one polyQ repeat length are able to seed elongation of other polyQ repeat lengths 20, supporting recruitment-based mechanisms for aggregate toxicity 84.
Nucleation of simple polyQ aggregation
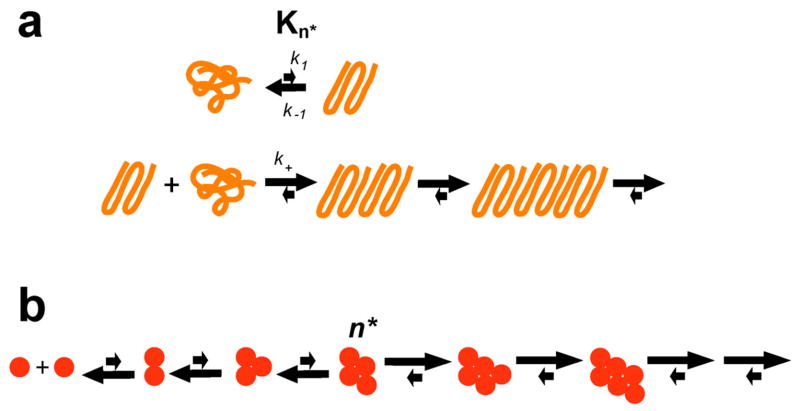
The concentration dependence of an aggregating system is often dictated by the nucleation mechanism 85. Studying the concentration dependence not only reveals clues to the nucleation mechanism, it also provides insight into how the rates observed for relatively high, laboratory concentrations might translate into the lower, steady state concentrations in the cell. An initial investigation of the concentration dependence of spontaneous aggregation for relatively long polyQ peptides in the K2QNK2 series revealed the surprising result of a rather shallow concentration dependence consistent with a critical nucleus (n*) of one 23 (Fig. 3a). Subsequent studies on shorter polyQ sequences in the same series revealed that n* itself is repeat length dependent 45. PolyQ peptides K2Q18K2 and K2Q23K2 follow concentration dependence consistent with n* ≈ 4, while polyQ peptides of K2Q24K2 and K2Q25K2exhibit n* ≈ 2. PolyQ peptides of K 2Q26K2or longer have n* ≈ 1 45. This discovery put the n* ≈ 1 value from earlier work 23 into context, suggesting that all simple polyQ peptides aggregate according to a classical nucleated growth polymerization mechanism.
Figure 3.
Nucleation mechanisms. (a) Mechanism for simple polyQ aggregation nucleation by an energetically unfavorable conformation of the condensed coil polyQ monomer, where k1 and k−1 are the forward and reverse rates of nucleus formation, Kn* is the equilibrium constant for nucleus formation, and k+ is the second order rate constant for elongation of the nucleus 23, 45, 75, 76; (b) classical thermodynamic model for nucleated growth polymerization (from reference 45).
The nucleus for simple polyQ aggregation, regardless of the n* value, can be modeled as a small β-sheet assembly, as expected (although technically not required) for an amyloid growth nucleus. As repeat length increases, folded versions of monomers, themselves containing β-sheet elements, take on enhanced (but still very low) stabilities that allow them to simplify nucleus assembly by requiring the involvement of fewer molecules 45. One attractive model for these transiently populated conformations is the β-turn 45, 74, 86. Indeed, one study showed that polyQ peptides containing L-Pro-Gly pairs compatible with β-hairpins aggregate as rapidly as simple polyQ of the same repeat length, and a similar peptide containing D-pro-Gly pairs that encourage β-hairpin formation aggregate faster than simple polyQ 74. Both peptides exhibit the same n* ≈ 1 as do unbroken polyQs in this repeat length range, consistent with models for nucleus structure invoking folding into a β-sheet assembly 45. A recent study showed a similar rate enhancement for D-Pro-Gly in a short polyQ background 79. The conformational details underlying nucleation and aggregation of polyQ sequences containing Pro-Gly interruptions have been examined by computation analyses 87, 88.
The model for nucleation of aggregation by a long polyQ peptide (Fig. 3a) depicts the monomer ensemble as a condensed coil structure, as suggested by FCS measurements 33, and the nucleus as a specific folded form possessing β-structure. While nucleus formation is therefore depicted as a folding reaction, it is a highly unfavorable folding reaction. This unfavorable folding reaction is a pre-equilibrium, and the nucleus, once it forms, can either collapse back to the condensed coil monomer ensemble or can undergo a productive elongation step by interacting with a condensed coil monomer, forming an elongation intermediate by (presumably) elaborating a β-stand already present in the nucleus. Subsequent folding steps consolidate β-sheet rich, amyloid-like structure in a now-stabilized dimer that is more likely to engage in a new elongation step, and less likely to disintegrate 30. This multi-step elongation process can be thought of as a “dock-and-lock” type of elongation mechanism 89 in which newly added monomers organize at the growth point of the template fibril, rather than encounter the growth point in a pre-organized state.
The ideal behavior of the polyQ nucleation mechanism has allowed a number of studies to elucidate details of the mechanism. The mathematical treatment of the mechanism-based kinetic model yields not only n* but also a complex parameter containing Kn*, the equilibrium constant for nucleus formation, and k+, the second order rate constant for nucleus elongation 23, 85 (Fig. 3a). A value for k+ for a K2Q47K2 peptide of 1.14 × 104 M−1sec−1 was obtained by determining the pseudo-first order kinetics rate constant for a seeded elongation of this peptide, then converting that value into the second order rate constant using experimental estimates of the concentration of fibril growth points 75. Using this second order rate constant, a value for Kn* of 2.6 × 10−9 was obtained 75. ΔGfolding of + 12.2 kcal/mol (compared to the typical negative ΔGfolding for a small protein of −5 to −15 kcal/mol 90), illustrates the rarity of the critical nucleus and the low favorability of nucleus formation.
In the classical nucleated growth mechanism, the efficiency with which the ephemeral critical nucleus elongates is a critical component of the overall efficiency of nucleation. Along with Kn*, the relative rates of nucleus disintegration and nucleus elongation dictate nucleation efficiency (Fig. 3a). Since the rate of nucleus elongation = k+[nucleus][monomer], this rate can be stimulated, without affecting nucleus formation, by adding relatively high concentrations of short polyQ peptides 76. The end result is an increase in nucleation efficiency by short polyQ sequences. This consequence of the promiscuity of polyQ amyloid elongation may have in vivo significance 76.
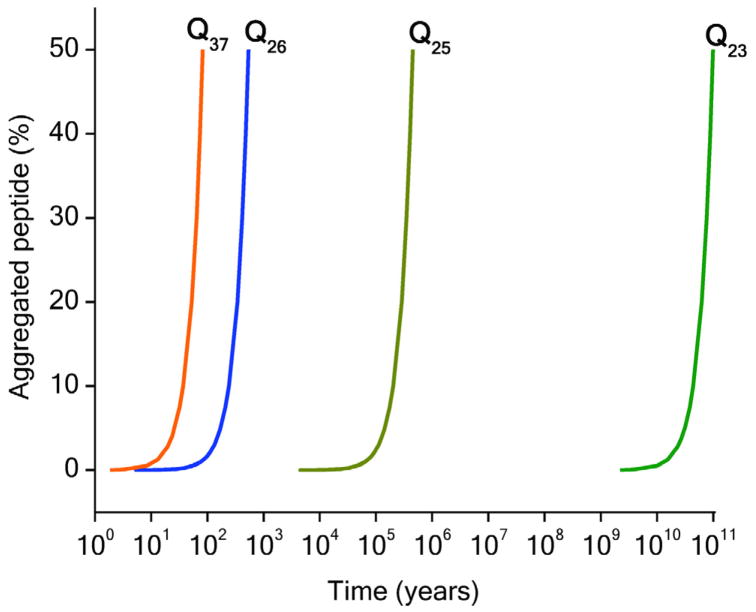
The ability to fit aggregation kinetics to a mechanism-based mathematical model also allows generation of simulated aggregation kinetics, using the parameters from the fit, under conditions where measurement is not possible. Such simulations predict that even very small differences in n* have huge consequences for nucleation efficiency at the low polyQ concentrations of the cell. Thus, projection of aggregation curves for various repeat lengths of K2QNK2 to 1 nM concentration shows onset of aggregation in the 10–100 year span for K2Q37K2 and 10–100 billion years for K2Q23K2 45 (Fig. 4).
Figure 4.
Calculated aggregation kinetics curves for 1 nM of various polyQ peptides using parameters derived from nucleation kinetics analyses. From reference 45.
Deviations from the classical nucleated growth model
In the classical nucleated growth model, the overall aggregation reaction is depicted as a series of homologous equilibria, each of which involves the binding of a ground state monomer to the species An to generate the species An+1 85 (Fig. 3b). Initial binding equilibria (starting with the interaction of two monomers to make A2) are thermodynamically unfavorable, and later equilibria (approaching a limiting, low monomer concentration) are favorable, and the dividing line between these two classes is the critical nucleus An*, the least stable species on the aggregation pathway 85. In the context of this formulation of the classical mechanism, it might be argued that a result of n* = 1 is impossible, since, according to that definition, all species on the assembly pathway are oligomeric. However, what if we slightly alter the definitation of the critical nucleus as a consolidation of structure (i.e., regardless of molecularity) with sufficient stability (and hence lifetime) to sometimes undergo a productive elongation encounter? This does not change the mathematical formalism, while easily accommodating the observation of n* = 1. In fact, it is worth noting that the surprising result of n* = 1 for long polyQ peptides is generated by a data treatment based on a classical model which never anticipated this most simple of solutions. In the next section, the classical nucleated growth model is also found to be too narrow to accommodate an aggregation mechanism for complex polyQ sequences, even though it too features the stochastic formation of a critical species required for amyloid growth.
Other mechanistic models
Based on computational modeling that suggests an ability of polyQ peptides to form dimers 37 and higher oligomers 91 through non-specific interactions, and the observation that such oligomers can form 92, including during fibril formation 77, 93, an alternative nucleation model has been proposed in which amyloid nuclei are generated from within these oligomeric intermediates 77, 94. As further support for this model, an analysis showed that it is compatible with previously reported kinetics data, and could account for the non-integer values that sometimes are reported for n* 94. Models involving non-amyloid intermediates do have a certain attraction, given the ubiquitous appearance of on- or off-pathway oligomeric intermediates in almost all other amyloid systems 95. There are several issues with this alternative model, however, as it is applied to simple polyQ peptides. First, observations of non-amyloid oligomers from simple K2QNK2 peptides are quite possibly due to artifacts in sample preparation (see discussion in aggregate morphology section). Indeed, careful adherence to disaggregation protocols 45, 96 and a variety of tests focused on detecting low levels of oligomers failed to show any evidence for non-amyloid aggregates during amyloid formation by K2QNK2 peptides 45. Furthermore, although fractional n* values are often reported 23, 25, 45, 74–76, 78, there are other theoretical rationalizations for non-integer n* values, and it has also proved technically difficult to determine n* values with accuracies sufficient to show that non-integer values are significantly different than the nearest integer value 45. Finally, direct conversion of amorphous aggregates into amyloid-like aggregates by computer simulations has not been reported, nor have observed non-amyloid oligomeric intermediates been successfully incorporated into kinetic models of aggregation. While it remains possible that very low levels of very small oligomers might be missed in experiments, it has not been necessary to invoke such species in order to account for observed nucleation kinetics 45.
Interestingly, it is possible that an oligomer-mediated mechanism applies to the aggregation of histidine-interruped polyQ peptides in the acid pH-range (see below). And there is a very clear case of oligomer-mediated amyloid nucleation in the case of htt N-terminal fragment aggregation, as discussed later.
PolyQ sequence interruptions
Between the world of simple, unbroken polyQ sequences, as discussed above, and complex polyQ proteins with extensive flanking sequences, as discussed below, are peptides that consist of mostly polyQ but containing sequence interruptions. The limited analysis of these sequences suggests that their aggregation is qualitatively similar to unbroken polyQ but with altered kinetics. Sequences containing Pro or Pro-Gly interruptions 74, 79, 97–99 are discussed elsewhere in this review.
Sequences containing His interruptions are of special interest, since in Spinocerebellar ataxia 1 (SCA1), one of the expanded CAG repeat diseases, repeat expansion is linked to loss of internal His residues. At the protein level, some individuals have expanded polyQ tracts but retain the His residues, and, perhaps because of that, are symptom-free 100. Analysis showed that such His insertions do not appear to alter the disordered state of monomeric peptides 97, and in the neutral pH range do not alter the nucleation mechanism 78. However, His interruptions do substantially reduce aggregation kinetics 78, 97. A proposed model for aggregate structure 97 in which the His residues are placed in reverse turns was supported by a structural analysis of the aggregates 78. Interestingly, His-containing polyQ peptides incubated at pH 6 experience a significantly reduced aggregation rate and exhibit an alternate aggregation reaction profile with clear evidence for a transient non-amyloid intermediate 78. Whether this is on- or off-pathway, however, was not determined. These changes are presumably brought about by protonation of the His residues at the lower pH.
More extreme examples of mixed polyQ sequences should also be mentioned. In a dramatic example, the mixed Ala/Gln sequence K2-(QA)20-K2, derived from the htt interacting protein CA150, forms amyloid fibrils with approximately the same kinetics as a K2Q40K2 sequence 101. Furthermore, amyloid-like aggregates of these two sequences can “cross-seed” the elongation of the other 101, which is typically not a very efficient process for amyloidogenic peptides of widely different sequences 102. These results are especially interesting given the possible involvement of amyloid formation in some polyAla repeat diseases 103. The other mixed sequences discussed in this section, containing His insertions or Pro-Gly insertions, are also very effective in cross-seeding reactions with simple polyQ sequences, suggesting a high degree of structural similarity 74, 78.
Complex Aggregation Mechanisms for Complex PolyQ Sequences
All known expanded CAG repeat disease proteins contain a polyQ sequence surrounded by mixed amino acid sequence peptides. Some of these flanking sequences do not alter the fundamental aggregation nucleation mechanism, although they may affect aggregation rates 25, 45. In other cases, however, flanking sequences can play an enormous role, greatly affecting rates, sometimes by fundamentally changing the aggregation mechanism. This multiplicity of effect is mirrored in studies on artificial polyQ fusion protein model systems. In some cases, producing the polyQ sequence as a fusion protein greatly suppresses amyloid formation, facilitating purification of the protein and allowing control over the start of aggregation via specific protease cleavage sites between fusion partner and polyQ domain 104. Studies on intact fusion proteins give a range of results. Some fusion proteins produce only non-amyloid aggregates 67, some make amyloid via non-amyloid, oligomeric intermediates 105, 106 and some produce amyloid fibrils with little or no evidence for non-amyloid intermediates 66. For those proteins that feature formation of non-amyloid intermediates, an important question is the extent to which polyQ expansion influences the first step, versus the second step, of the two-step mechanism of amyloid formation.
Ataxin-3
An excellent example of the ability of flanking sequence to dramatically alter the aggregation pathway is the role of the “Josephin” domain of the disease protein ataxin-3 (AT3). The Josephin domain by itself or with a short polyQ extension undergoes thermally induced formation of worm-like, SDS-soluble, ThT-positive fibrils 107, 108. While short polyQ versions of AT3 aggregate about as efficiently as the isolated Josephin domain 107, 108, however, longer polyQ versions aggregate more rapidly and do so via a two-step mechanism 108. In this mechanism, the initial product has similar structural features to the wormlike morphologies of the short polyQ AT3 molecules, while the final product is a more stable, amyloid-like fibril 108. There are conflicting data on whether polyQ and its expansion influences the stability of the Josephin domain 107, 109–111, which, if true, could influence the first step of the aggregation mechanism. The lead role played by aggregation of the Josephin domain, and/or by the intervening flexible segment 112 is, however, well documented. Josephin point mutations 113, 114 or binding to chaperones 115 or normal ligands 114 have all been observed to suppress initial aggregation and/or amyloid formation.
Huntingtin N-terminal fragments
Huntingtin, the protein responsible for Huntington’s disease (HD), is an intracellular protein of ~ 3,200 amino acids whose polyQ sequence resides very near the N-terminus (Fig. 1). A variety of studies have implicated one or more key proteolysis events in the disease mechanism, in which release of polyQ-containing N-terminal fragments is required for the emergence of toxicity. Animals expressing expanded polyQ versions of htt N-terminal fragments like exon-1 (Fig. 1) generally exhibit aggressive HD disease features such as neurological symptoms, cellular aggregates, cellular abnormalities, and death 116. In vitro studies on recombinant exon-1 proteins exhibit a parallel polyQ repeat length dependent amyloid formation 82. In contrast with simple polyQ aggregation, however, the aggregation pathway of htt N-terminal fragments in vitro features early formation of aggregates lacking typical amyloid morphologies 117.
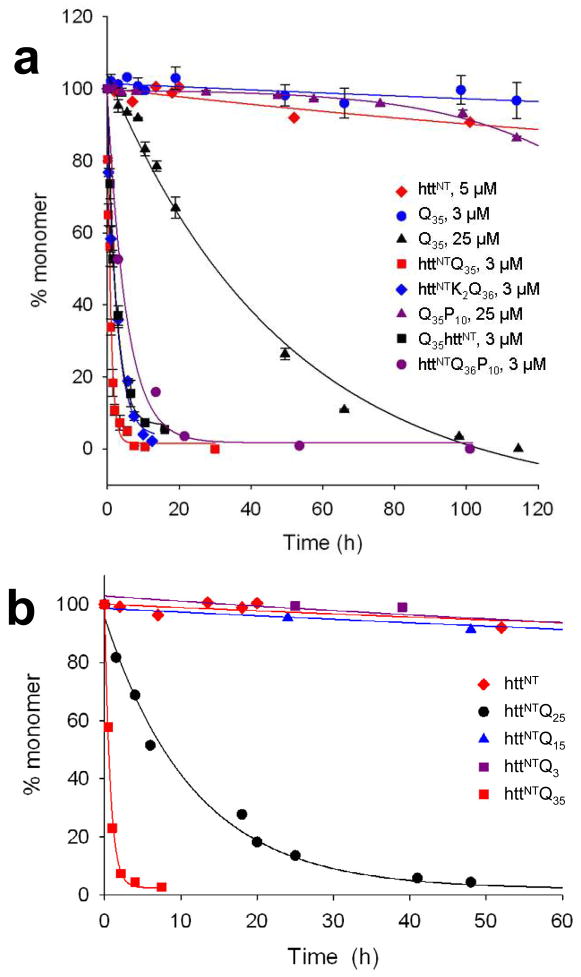
Initial investigation of htt polyQ flanking sequence effects focused on the unusual Pro-rich segment (Fig. 1). In cell models, removal of the Pro-rich sequence from exon-1 significantly enhances aggregate formation and/or toxicity 118, 119. In vitro, the previously discussed ability of a C-terminal polyproline segment to reduce the aggregation rate of a polyQ sequence 25 is also observed in the htt N-terminal fragment context 48 (Fig. 5a). The polyproline effect observed in vitro can be accounted for by its ability to alter the conformational mix in the polyQ component toward less aggregation-prone conformations 25, 120, 121. This conformational induction depends on details of the connection of polyproline to polyQ: a P10 sequence at the polyQ C-terminus inhibits aggregation, but at the polyQ N-terminus does not 25. The situation is more complicated in yeast cells, where independently expressed polyproline sequences can interfere with polyQ aggregation in trans 119.
Figure 5.
Kinetics of spontaneous aggregation of polyQ peptides linked to httNT. (a) Effect of presence and location of httNT; (b) effect of polyQ length on httNTQNK2 aggregation. From reference 48.
The mechanism of the httNT effect on polyQ aggregation
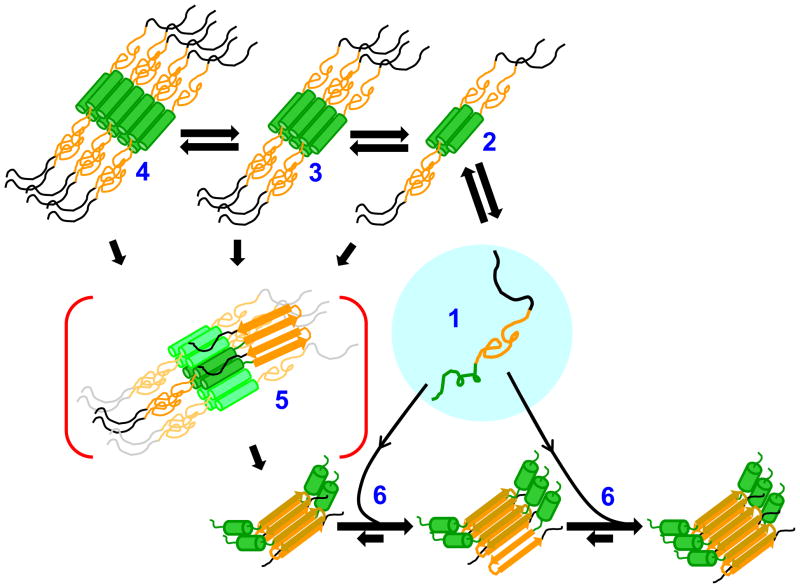
The addition of the 17 amino acid httNT sequence (Fig. 1) to the N-terminus of polyQ produces a dramatic increase in aggregation rate compared with the simple polyQ peptide 48 (Fig. 5a). In contrast with the polyproline effect, httNT has the same rate enhancing effect when placed either on the N- or C-terminus of polyQ 48 (Fig. 5a). In a series of httNTQN peptides, there remains a very strong repeat length dependence 48 (Fig. 5b), consistent with previous observations on htt exon-1 sequences 82, 104. In analogy to the AT3 system (see above), aggregation of httNTQN proteins occurs by a two-step mechanism. Rough features of this two-step mechanism are now fairly well understood (Fig. 6).
Figure 6.
Mechanism of httNT-mediated amyloid formation of htt N-terminal fragments. Disordered htt N-terminal fragment monomers (1; httNT = green; polyQ = orange; polyproline = black) reversibly form α-helix rich tetramers (2) via the httNT domain, and these tetramers can further reversibly assemble into octamers (3), dodecamers (4), and higher order oligomers. Any oligomer has a certain potential to undergo nucleation of amyloid structure (5), supported by the highly concentrated polyQ chains on its periphery; the propensity of any oligomer to undergo nucleation is probably linked to polyQ repeat length and the presence or absence of polyproline. Once polyQ-core amyloid nuclei are formed, amyloid elongation proceeds by monomer addition (6). As monomer concentration decreases due to fibril elongation, oligomers (2,3,4) that have not undergone nucleation dissociate to replenish the monomer pool and continue to support fibril elongation. Adapted from references 50, 143.
After long incubation at relatively high concentration, httNT peptides without any attached polyQ form α-helix rich 50, 122 (Fig. 2a), pelletable 48 aggregates. This is now visualized as a continuation of the ability of httNTto reversibly form α-helix rich tetramers, octomers and dodecamers as part of a native state ensemble (Fig. 2). When polyQ sequences are attached to httNT, pelletable oligomers tend to form more quickly in a polyQ repeat length dependent fashion 48. Once oligomers are formed, they have the ability to stochastically rearrange to form a polyQ-core amyloid nucleus, while retaining the α-helical structure in httNT 50 (Fig. 6). This enhanced polyQ amyloid formation is presumably related to the AT3 nucleation mechanism, being mediated by high local polyQ concentrations within the intermediate aggregates. This second, nucleation step appears to also be polyQ repeat length dependent 48, 50. For example, an httNTQ7K2 peptide oligomer cannot undergo nucleation of polyQ amyloid structure, while an httNTQ8K2 oligomer can 50. While nucleus formation in short polyQ htt N-terminal fragments appears to take place within large, pelletable aggregates 50, it is possible and even likely that long polyQ httNTQN peptides undergo efficient nucleation within tetramers or octomers (Fig. 6). The critical role of α-helical bundle formation in httNT-mediated polyQ aggregation is further supported by inhibitor studies described below. The two-step mechanism involving an initial oligomer held in an α-helix bundle, followed by nucleation of amyloid structure in non-bundled segments, is similar in many respects to a recent proposed mechanism of amyloid nucleation in Aβ and IAPP 123.
There are many details of the nucleation process to work out, but typically this event is associated with a number of ensemble observations. After nucleation, aggregation rate increases dramatically 48, except with very low polyQ repeat lengths 50. Aggregate morphology changes from oligomers and protofibrils to fibrils 48 (Fig. 7). Post-nucleation aggregates (a) respond to ThT, (b) become capable of seeding elongation, (c) exhibit a less solvent exposed httNT sequence, (d) begin to develop β-structure in FTIR spectra, and (e) have polyQ sequences that are no longer recognizable by an anti-polyQ antibody 48, 50. While most of these details were worked out with relatively short htt N-terminal fragments of the general structure httNTQNP6K2 and httNTQNP10K2, recent studies with chemically synthesized htt exon-1 124 show that these short polyproline analogs behave very similarly to htt exon-1 in aggregation rates, mechanism, and response to inhibitors (B. Sahoo, D. Singer, T. Zuchner and R. Wetzel, Ms. in preparation).
Figure 7.
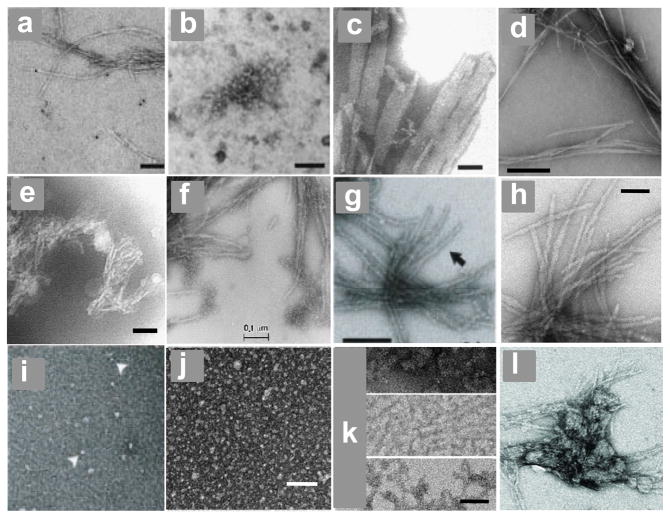
Electron micrographs. (a, b) Htt N-terminal fragment aggregates isolated from tg HD Q150 mouse brains, scale bar = 100 nm 151; (c) PolyQ mature aggregates produced in vitro at 37 °C from K2Q37K2, scale bar = 50 nm 20; (d) Aβ40 amyloid fibrils generated at 37 °C in PBS without agitation, scale bar = 200 nm 192; (e) aggregates of K2Q37K2 generated in PBS solution incubated frozen at −20 °C, scale bar = 50 nm 20; (f - l) aggregates of htt N-terminal fragments generated at 37 °C, as follows: (f) mature fibrils of Q51 exon-1, scale bar = 100 nm 104, (g) mature fibrils of Q44 exon-1, scale bar = 200 nm 117 (h) mature fibrils of httNTQ30P6K2, scale bar = 50 nm 48; (i) initial oligomers of Q44 exon-1 117; (j) initial oligomers of httNTQ30P6K2, scale bar = 50 nm 48; intermediate protofibrillar aggregates of httNTQ30P6K2, scale bar = 50 nm 48; final aggregates of httNTQ37P10K2 (S13D/S16D) 58.
There are some alternative models for the aggregation mechanism of htt N-teminal fragments and how httNT contributes to it. Tam et al. posit that the role of α-helical httNT is to intramolecularly induce a more amyloidogenic conformation in the polyQ segment 125. Others, based on experimental 126 or computational 61 studies, propose that initial oligomer formation by htt N-terminal fragments is mediated primarily by direct polyQ interactions.
Implications of the httNT-dependent aggregation pathway
The role played by the httNT segment in determining the physical properties of htt N-terminal fragments may greatly affect the behavior of such peptides in cells. In addition to the possible regulatory role of httNT-mediated, reversible oligomer formation (see above), the role of httNT in mediating amyloid formation suggests that post-translational modifications within this sequence might influence the rate or extent of pathological aggregation. A potential example is in the phosphorylation of Ser13 and Ser16. A double Ser to Asp phosphomimetic mutation within httNT in expanded polyQ htt in a tg mouse model was found to very effectively block aggregate formation and neurodegeneration 58. In a peptide model system, the same double Ser to Asp mutation in vitro was found to both suppress aggregation rate and to alter final aggregate morphology 58 (Fig. 7l). These data suggest that protein phosphorylation might not always or only play a regulating role in the cell, but might sometimes lead to important cellular consequences simply due to changes in the biophysical properties of the protein substrate 58, 127.
The existence of two radically different aggregation mechanisms for polyQ sequences (i.e., in simple polyQ sequences and in complex sequences like htt fragments) suggests the possibility of mixed mode aggregation under specific conditions. In fact, incubation of an htt N-terminal fragment in the presence of an inhibitor of httNT-mediated aggregation (below) appears to open the door to the alternative aggregation mechanism engaged by simple polyQ sequences (M. Jayaraman, A. K. Thakur, and R. Wetzel, unpublished). This may be the source of temperature-dependent aggregate conformations reported by the Tanaka group 128. In addition to HD-specific issues, the httNT story has more general implications for predictions of amyloidogenic sequences, providing an example of a sequence that greatly enhances amyloid formation rates without ever engaging an amyloid or β-sheet structure 50, 122.
The question of whether non-amyloid, oligomeric intermediates are on-pathway or off-pathway to amyloid formation has stimulated significant work and discussion in the field 95, and there is almost certainly no universal answer that will apply to all amyloid assembly reactions. The assembly mechanism suggested in Figure 6 presents an interesting case, in which α-helix rich oligomers play both an on-pathway role - as the substrate within which nucleation of amyloid structure occurs 129, and an off-pathway role – as a reservoir for the release of monomers 130 to feed amyloid elongation as solution phase monomer is depleted 50.
Inhibitors of Polyglutamine Aggregation
Characterization of aggregation inhibitors is important for several reasons. If accumulation of a particular type of aggregate in cells is responsible for at least some HD symptoms, then understanding inhibitor structure and mechanism of action can contribute directly to the discovery of lead compounds, and indirectly to providing a basis for inhibitor design. Even if the practical aspects of inhibitor delivery complicate an anti-aggregation approach to HD therapy, inhibitors should make excellent tools for determining aggregation mechanism and for understanding the cellular basis of aggregate toxicity. Inhibition of huntingtin aggregation is an especially interesting challenge, since, while the mechanism is quite different from that of simple polyQ amyloid formation, the polyQ core structure of the aggregates appears to be quite similar 122. Although high-throughput screens conducted in cell or animal models are potentially powerful ways of cutting through bioavailability issues, they also have the downside of delivering hits whose mechanisms of action are initially obscure and possibly indirect. Given the focus of this review, cell screening based inhibitors are only described if subsequent characterization indicated a direct polyQ effect.
Inhibitors of simple polyQ aggregation
Directly targeting polyQ aggregation by focusing on simple polyQ is attractive because effective inhibitors might be useful in all 10 expanded CAG repeat diseases. Burke and co-workers identified peptide based inhibitors by using phage display to select short sequences with high affinities to immobilized polyQ sequences 131. One peptide, the Trp-rich sequence QBP1, is particularly effective against polyQ aggregation in vitro 131 with an IC50 in the 5 μM range 132. Subsequent studies characterized structure-activity relationships in this inhibitor 132, 133. This peptide is effective in inhibiting aggregation and/or toxicity in cell 131, 134 and animal 135 models, and is widely used to test the role of polyQ amyloid formation in the aggregation of complex proteins (see, for example reverence 66).
The β-sheet rich structure of the amyloid end products of polyQ aggregation also has provided the basis for several structure-based designs of inhibitors. By inserting periodic Pro-Gly pairs along a polyQ sequence, it is possible to control how that sequence engages an amyloid-like structure 74. By then including additional Pro residues at mid-β-strand locations between the Pro-Gly pairs (i.e., PGQ9P1,2,3, Fig. 8b), inhibitors of polyQ amyloid elongation, with IC50 values in the 1–2 μM range, were obtained that are imagined to work by adding to fibril growing ends while being incapable of consolidating amyloid structure 87, 88, 98 (Fig. 8b). Prolines are anathema to β-sheet formation through a combination of constraining backbone torsion angles, steric clashes with β-sheet geometry, and the absence of an H-bonding N-H group 136. The latter factor has also been used in polyQ aggregation inhibitor design by installing backbone N-methyl groups into oligoGln peptides 137.
Figure 8.
Structure and mechanism-based inhibitors of polyglutamine amyloid formation. (a) dock and lock mechanism of amyloid fibril elongation from an encounter of the growth end of a fibril with a condensed coil polyQ monomer; (b) encounter of the growth end of the fibril with disordered PGQ9P1,2,3 monomer (K2Q9-PG-Q4PQ4-PG-Q4PQ4-PG-Q4PQ4K2; proline, red; glycine, green) showing docking via the single unbroken Q9 repeat, but an inability to rearrange into stable β-sheet in the locking step due to the mid-strand proline residues; (c) hypothetical inhibition complex when httNT (blue) is incubated with an htt N-terminal fragment (color scheme, see Figure 6); hypothetical inhibition complex when httNT-PGQ9P1,2,3K2 (blue httNT and polyQ chain showing only the mid-strand Pro residues in red) is incubated with an htt N-terminal fragment.
Inhibitors of huntingtin N-terminal fragment aggregation
A number of library screening and structure-based design studies have been conducted with huntingtin N-terminal fragments like exon-1 as targets. Using a screening assay built around in situ proteolytic generation of htt exon-1 and a filter-trap aggregation assay 138, Wanker and co-workers have screened several hundred thousand compounds 139, 140. Two classes of inhibitors thus identified are polyphenols 140, 141 and benzothiazoles 139. Polyphenols like EGCG ((−)-epigallocatechin-gallate) appear to work by rapidly directing monomeric htt exon-1 down an alternative aggregation pathway leading to stable, non-amyloid aggregates 140. Two benzothiazoles have been shown to be effective, with EC50 values of 40 μM, at reducing aggregate burden in organotypic brain slice cultures from the R6/2 htt exon-1 tg mouse 142. Unfortunately, at the maximum tolerated dose, one of these compounds, riluzone, is ineffective in the R6/2 mouse, in spite of reaching 100 μM levels in the brain 142.
Another series of inhibitor can be considered to be structure and mechanism based. Peptides related to the isolated httNT sequence prove to be excellent inhibitors of htt N-terminal fragment amyloid formation 143. The mechanism of action appears to be via their ability to make mixed oligomers with the exon-1-like molecules 143. Co-assembly reduces the local concentration of polyQ chains in the oligomers and thereby decreases the frequency of amyloid-like nucleus formation (Fig. 8c). Thus, sequence variants that decrease the ability to fold into an amphipathic helix decrease inhibitory power 143. In general, these inhibitors slow nucleation but do not totally eliminate it, presumably because, statistically, there are always a few oligomers formed that are predominantly composed of htt N-terminal fragments. In an extension of this design, the molecule httNT-PGQ9P1,2,3 – a combination of the httNT segment with the elongation inhibitor PGQ9P1,2,3 (see above) – is an even more effective inhibitor 143. It presumably works by co-assembling with the htt N-terminal fragments, thereby diluting their solvent exposed polyQs with an active elongation inhibitor (Fig. 8d). This mode of inhibition is similar to the proposed model for how Pro-containing variants of islet amyloid polypeptide (IAPP) inhibit WT IAPP amyloid formation 144.
A related mode of inhibition involving the role of httNT is to disallow homo-tetramerization by complexing the httNT moiety of htt N-terminal fragments with another protein. There are several examples. A recent X-ray crystal structure of a complex between httNT and an engineered antibody fragment with anti-aggregation and anti-toxicity activity in cells shows, in a different setting, the tendency of this peptide to adopt α-helix on binding 145. Molecular chaperones have been reported to reduce htt exon-1 aggregation and toxicity in mammalian cells 146, and it now appears that at least some molecular chaperones do so by targeting the httNT sequence to suppress amyloid nucleation. TriC, for example, appears to inhibit htt exon-1 aggregation by binding and sequestering the httNT component 125. It seems possible that the ability of Hsp70/Hsp40 to inhibit htt exon-1 aggregation 126 also relies on sequestration of the httNT segment. This seems especially likely given the reported ability of Hsp40/Hsp70 to reduce non-amyloid oligomer formation 126, as well as the observation that Hsp40/Hsp70 only blocks htt exon-1 aggregation when added during the lag phase 147. The prospects for therapeutic exploitation of these observations are not clear. Although over-expression of Hsp70/Hsp40 suppresses neurodegeneration in a number of cell and animal studies 148, mixed results are obtained in the (very aggressive) R6/2 tg mouse model 148. Especially given the MoRF-like character of the httNT sequence 48, there may be many other cellular proteins, besides chaperones and htt N-terminal fragments themselves, that bind to the httNT sequence during the normal cellular life of htt. These potentially very fluid interactions may play subtle modulating roles of htt aggregation.
Structural Features of Polyglutamine Aggregates
In vivo aggregates
As in other aggregation-associated neurodegenerative diseases, the disperse distribution and relatively light aggregate burden compared with total brain mass makes it difficult to obtain quality data on the underlying structural features of tissue-derived polyQ aggregates. PolyQ protein aggregates appear to be exclusively intracellular. The earliest observation of aggregates placed them in the neuronal nucleus 12, 13. Subsequently it was recognized that aggregates are also often found outside the nucleus in the cell body 149, 150.
There is limited ultra-structural data on in vivo aggregates. High resolution electron micrographs of inclusions in brain slices sometimes reveal a substructure of well-defined fibrils12. In addition, aggregates isolated from the brains of a Huntington’s disease (HD) mouse model exhibit a range of morphologies including a number of filamentous structures similar to those observed in the in vitro assembly of huntingtin fragment aggregates 151 (Fig. 7a,b). At least some of the end-stage aggregates in the brain appear to have amyloid-like structures, since they are capable of specifically and strongly binding biotinylated polyQ in a recruitment stain dependent on the normally highly specific amyloid elongation reaction 152. The lack of specific stains for other types of (non-amyloid) aggregates, such as the oligomeric structures commonly observed in the early stages of the aggregation of huntingtin (htt) exon-1 and similar fragments (see below), makes it difficult to determine if these structures exist in tissue, and, if so, where they are localized and whether they persist.
Many cell models have been described in which polyQ proteins are fused to GFP. In these models, short polyQ sequences give diffuse fluorescence while long polyQs lead to one to several bright puncta either in the nucleus 153 or cytoplasm 118. The limited number of inclusions per cell might be evidence for the rarity of an aggregation nucleation event 154 but could also reflect the ability of the cell to organize aggregates, such as in perinuclear aggresomes 155. Since the assembly of some complex polyQ proteins such as htt exon-1 proceeds with intermediate formation of non-amyloid aggregates (see below), and since different aggregated states may have different toxicities 156, it is of considerable interest whether particular GFP puncta in different experiments represent accumulations of amyloid or other aggregates. AUC of cell contents 157, and fluorescence methods like FRET, FRAP, FLIP, FCS and split-GFP techniques 51, 52, 158–160 may ultimately clarify such issues.
In vivo aggregates are often found to be associated with other cellular proteins. In some cases this might reflect the operation of cellular mechanisms directed at protein misfolding or aggregation 161. Due to the promiscuity of polyQ amyloid elongation 20, polyQ aggregates can also effectively recruit other polyQ-containing proteins 84. Recruitment and sequestration 162, and/or recruitment-mediated elimination 163, of important cellular proteins such as transcription factors represent attractive mechanisms for how amyloid-like polyQ aggregates might be toxic. Such recruitment mechanisms may even sometimes contribute to identification of polyQ repeat-length dependent protein interaction partners 164, 165. Although normally interpreted as evidence for specific binding partners for monomeric polyQ proteins, in at least some cases identification of binding partners may reflect the binding of proteins to already aggregated polyQ bait proteins166.
Structures and properties of simple polyQ aggregates assembled in vitro
Mirroring the fine-structures of some polyQ aggregates formed in vivo, aggregates of simple polyQ peptides grown in aqueous buffers from disaggregated peptides are amyloid-like, exhibiting filamentous morphologies in EM images (Fig. 7c) 20, 81. In spite of their filamentous morphologies, most aggregates grown in vitro from simple polyQ peptides do not exhibit the classical twisted fibril morphology seen in EM images, as typified, for example, by Aβ40 fibrils (Fig. 7d). Other features of amyloid are well-replicated, however. Simple polyQ aggregates exhibit β-sheet features in circular dichroism 23, FTIR 48, X-ray diffraction 73, 167–169, and solid state NMR 122, 170. Although the signal is unusually low, polyQ aggregates also bind thioflavin T (ThT) and exhibit the ThT fluorescence spectral shift characteristic amyloid fibrils 81. Overall, there is no evidence that the polyQ component of simple or complex polyQ sequences in mature fibrillar aggregates exists in anything other than β-sheet rich, amyloid-like structure, in contrast to a recent proposal that α-helix-rich coiled coil might be the structural basis of such fibrils 46.
The absence of a 10 Å reflection in the X-ray powder diffraction of polyQ aggregates led Perutz to suggest a β-helical structure for these aggregates 167, and a number of computational analyses have been found to support the feasibility of such structures in polyQ sequences 26, 32, 91, 171–173. Subsequent X-ray diffraction analyses, however, suggested a more conventional β-sheet structure for polyQ aggregates, although also finding, in contrast to many peptide-based amyloid fibrils 174, an anti-parallel β-sheet arrangement 168, 169 featuring β-hairpins 169. Certain features of solid state NMR 122, 170 and CD (J. Kardos, personal communication) spectra are also consistent with an anti-parallel β-sheet structure in polyQ aggregates. The ability of polyQ sequences interspersed at regular intervals with Pro-Gly pairs to form aggregates with kinetics, mechanistic features, and morphologies similar to unbroken polyQ has also been interpreted as indicating an anti-parallel β-sheet arrangement 74. Several anti-parallel four stranded sheet models have been studied in silico 30, 31, 36, 73, 175, 176. While there is a lot of physical evidence (see above) that such structures are not measurably populated in the monomeric state, these would appear to be reasonable models for aggregation nuclei or for the fundamental repeat unit of polyQ amyloid fibrils.
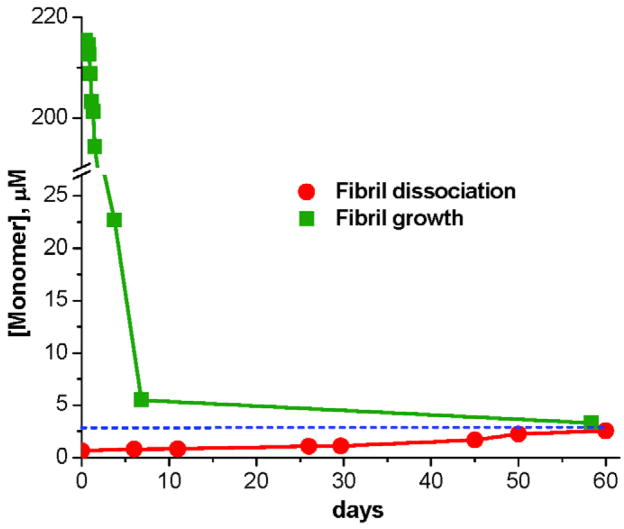
Like other amyloid fibrils, simple polyQ aggregates exhibit a characteristic concentration of monomer when the fibril formation reaction reaches equilibrium 45, 81. This monomer concentration can often be approached both in the growth direction and the dissociation direction 45, 177 (Fig. 9), and is formally equivalent to the critical concentration, Cr, for aggregation (the concentration below which spontaneous fibril formation cannot be initiated). Even for less stable aggregates of short polyQ peptides, the dissociation of fibrils to the equilibrium position takes a very long time 45, 81 (Fig. 9). Since the Cr is related to the elongation equilibrium constant for fibril growth 177, the Cr is an indicator of fibril stability. Not surprisingly, the Cr decreases as polyQ chain length increases, with a value of ~ 3 μM for K2Q23K2 45 and 1 μM or less for longer sequences 81. This trend suggests that aggregates of long polyQ peptides are more stable than those of short polyQs, and may have implications for the thermodynamic probability of aggregate formation in the cell.
Figure 9.
Confirmation, for K2Q23K2, of a dynamic equilibrium for amyloid fibril elongation with a characteristic end-point concentration of monomer equivalent to the Cr. From reference 45.
There are many unresolved questions about amyloid-like polyQ aggregate structure. Although experiments with Pro-Gly interrupted polyQ sequences suggested that polyQ amyloid can be manufactured with β-sheet widths of 7–9 Gln residues 74, it is possible that unbroken polyQ might favor significantly wider sheets 170. There are several chain connectivity patterns possible in an anti-parallel β-sheet 174, and it remains to be seen whether the pattern in polyQ amyloids involves β-hairpins or longer loops and whether it is uniform or variable. The structural role of the Gln sidechains has also not been established with certainty. Diffraction- or ssNMR-based models of fibrillar aggregates 122, 168–170, 178 or microcrystals 179, 180 of polyQ 168, 169 or Gln/Asn-rich 178–180 peptides, as well as AFM studies of Gln-rich peptides 86, exhibit different structural resolutions and degrees of Gln side-chain interdigitation.
Amyloid-like aggregates formed in frozen solutions
Simple polyQ peptides have the unusual ability to highly efficiently make amyloid-like aggregates in frozen solutions in PBS 20,81. These aggregates share many properties of aggregates formed from the same peptides at 37 °C, but exhibit an atypical monofilament morphology (Fig. 7e). Perhaps because of this, compared with 37 °C aggregates, these assemblies are sometimes found to be much better seeds for elongating polyQ monomers 181 and are much more efficiently taken up by mammalian cells in a novel cell model for polyQ aggregate toxicity 182 and elongation 183. Since aggregate formation depends on the amount of time incubated at −20 °C 81, this process seems to occur in the frozen solution and not as a consequence of the freezing and/or thawing processes. It is presumably brought about by the process of “freeze concentration” 184, 185, whereby solutes, at freezing temperatures above their eutectic points, accumulate in liquid fissures between ice lattices resulting in high solute concentrations. The atypical monofilamentous structures of these aggregates may reflect the confined environment in which aggregation takes place or a deviant β-sheet structure within the amyloid protofilaments. These aggregates have a number of potential uses for studies of polyQ phenomena.
Non-amyloid oligomers of simple polyQ peptides
There is a lively discussion in the literature as to whether simple polyQ peptides in the class K2QNK2 are capable of forming non-amyloid aggregates, and whether such aggregates play a role in the mechanism of spontaneous amyloid growth. Some computer simulations predict the ability of polyQ sequences to make such structures 37, 91, and aggregates described as non-amyloid oligomers have been observed in light scattering, EM and AFM measurements 77, 93. These reports are inconsistent with other experiments, however, where oligomeric aggregates are looked for but not observed 23, 45. These divergent results likely stem from differences in sample preparation. The required disaggregation protocol for synthetic polyQ peptides 45, 96, 186, 187 includes transient exposure to the volatile solvent hexamethylisopropanol (HFIP). This solvent is well known to dramatically modify assembly of some amyloidogenic peptides at 2–4% concentrations 188, 189, and thus must be rigorously removed under vacuum as part of the disaggregation protocol. In fact, it is possible to drive the formation of homogeneous K2QNK2 oligomers in aqueous HFIP 92. These data are consistent with the hypothesis that non-amyloid K2QNK2 oligomers arise due to incomplete HFIP removal. This question of non-amyloid oligomer formation is an important issue for several reasons as discussed elsewhere in this review.
In vitro aggregates of complex polyQ polypeptides
Limited available data suggests that the amyloid cores of mature aggregates of simple polyQ peptides and polyQ peptides with flanking sequences are essentially identical. In ss NMR analysis, the spectral features of this core in fibrils of an htt N-terminal fragment are identical to those of a K2QNK2 peptide of similar repeat length 122. Analogous FTIR spectra are also very similar 48. EM images of mature aggregates of htt N-terminal fragments 48, 104, 117, 151 show differences compared to aggregates of simple polyQ peptides, with filaments of greater thickness and rough edges that probably reflect the presence of coats of alternatively structured flanking sequences (Fig. 7f–h). For example, while it was expected that polyQ amyloid structure might propagate into the httNT flanking sequence at some point during amyloid growth, ss NMR showed the surprising result of a stable element of α-helix in a portion of the httNT sequence in mature fibrils 122. This reflects the strong tendency of this sequence to form α-helix in various associated states, even though it is disordered in the monomeric state 48, 50.
In contrast with simple polyQ peptides, oligomer formation in the early stages of htt N-terminal fragment aggregation appears to be universally observed and has a fairly well-understood structural basis, as described in the section on complex polyQ peptide aggregation mechanisms. Similar small oligomers are formed from recombinant (Fig. 7i) or chemically synthesized (Fig. 7j) htt N-terminal fragments. In vitro produced protofibril-like structures (Fig. 7k) intermediate between spherical oligomers and mature fibrils resemble some aggregates isolated from tg HD mouse brains (Fig. 7b)
Conclusions
Expanded polyQ repeat diseases are often referred to as misfolding diseases, where misfolding can be meant to refer to the generation of a wide range of species, from alternative folded states of monomers to abnormal pathological self-assembled states ranging from non-amyloid oligomers to amyloid fibrils to the further self-associated inclusions seen in cell and animal models. The biophysical basis of these hypothesized states has been examined in this review.
How close are we to identifying the toxic species? Evidence for a stable or meta-stable specific folded state of expanded polyQ monomers, either in isolation or as part of a disease protein, remains elusive. At the same time, fundamental consideration of the intrinsically disordered nature of the polyQ sequence suggests that important complexes might be formed in the cell that do not depend on the existence of any populated, pre-existing, misfolded, “toxic” monomer. Thus, intrinsically disordered proteins (IDPs) are understood to be capable of highly specific coupled folding and binding 190 to cellular targets whose nature would never be apparent from only a knowledge of the especially rugged folding landscape of the IDP in isolation. In the context of this hypothetical cellular target, long polyQs might make stable complexes, and short polyQs unstable complexes, simply by virtue of the amount of buried surface area, H-bonds, etc., formed in the complex. Such a model positing that “long polyQs are toxic because they are long” may be scientifically unsatisfying, but is nonetheless consistent with the intrinsically disordered character of the polyQ sequence. The definitive test of the “toxic monomer hypothesis” will be to unequivocally demonstrate molecular events and structures associated with one of more early pathological cellular consequences. In contrast to misfolded polyQ monomers, there is no shortage of demonstrated polyQ aggregates found both in vitro and in vivo. However, the same standard applies to the “toxic aggregate hypothesis” as to the toxic monomer hypothesis. Bad aggregates will have to be caught in the act; the circumstantial evidence of correlation does not provide enough evidence to lead to a conviction.
The physical chemistry of polyQ aggregation is perhaps more clear than its disease role, but is nonetheless not without controversy. PolyQ sequences are most thermodynamically stable in an aggregated state, and there are apparently a number of kinetically accessible paths, with varying efficiencies, to aggregate formation. Mechanisms for aggregation nucleation in vitro can be simple or ornate, but are ultimately driven by the high stability of the amyloid fold. Deciphering some of these mechanisms has allowed the design of structure and mechanism-based aggregation inhibitors which may allow tests of the aggregation hypothesis for expanded polyQ diseases, and ultimately perhaps an entrée into therapeutic design.
An important recent discovery is that at least one disease protein, the N-terminal fragment of huntingtin, is capable of a regular, reversible tetramerization through its httNT N-terminus. While this equilibrium might very well play a normal role in the cellular biology of huntingtin, it also offers a middle ground between monomers and higher-order aggregates as an expanded polyQ species which, in spite of its possible ubiquitous presence and useful functions, may take on an additional negative, and ultimately toxic, set of activities in longer polyQ molecules.
Belying the tedium of their amino acid sequences, the properties of polyglutamine segments are far from monotonous, offering continued surprises in the study of their biophysical behaviors and the search for molecular mechanisms of disease.
Acknowledgments
The author acknowledges NIH R01 AG019322 for financial support and Rakesh Mishra and Robert Fairman for helpful suggestions on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karlin S, Burge C. Trinucleotide repeats and long homopeptides in genes and proteins associated with nervous system disease and development. Proc Natl Acad Sci U S A. 1996;93:1560–5. doi: 10.1073/pnas.93.4.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margolis RL, Abraham MR, Gatchell SB, Li SH, Kidwai AS, Breschel TS, Stine OC, Callahan C, McInnis MG, Ross CA. cDNAs with long CAG trinucleotide repeats from human brain. Hum Genet. 1997;100:114–22. doi: 10.1007/s004390050476. [DOI] [PubMed] [Google Scholar]
- 3.Butland SL, Devon RS, Huang Y, Mead CL, Meynert AM, Neal SJ, Lee SS, Wilkinson A, Yang GS, Yuen MMS, Hayden MR, Holt RA, Leavitt BR, Ouellette BFF. CAG-encoded polyglutamine length polymorphism in the human genome. Bmc Genomics. 2007;8:18. doi: 10.1186/1471-2164-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber HP, Seipel K, Georgiev O, Hofferer M, Hug M, Rusconi S, Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994;263:808–11. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- 5.Wellner N, Belton PS, Tatham AS. Fourier transform IR spectroscopic study of hydration-induced structure changes in the solid state of omega-gliadins. Biochem J. 1996;319(Pt 3):741–7. doi: 10.1042/bj3190741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridgley DM, Ebanks KC, Barone JR. Peptide Mixtures Can Self-Assemble into Large Amyloid Fibers of Varying Size and Morphology. Biomacromolecules. 2011;12:3770–3779. doi: 10.1021/bm201005k. [DOI] [PubMed] [Google Scholar]
- 7.Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–47. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]
- 8.Bates GP, Benn C. The polyglutamine diseases. In: Bates GP, Harper PS, Jones L, editors. Huntington’s Disease. Oxford University Press; Oxford, U.K.: 2002. pp. 429–472. [Google Scholar]
- 9.Wilburn B, Rudnicki DD, Zhao J, Weitz TM, Cheng Y, Gu XF, Greiner E, Park CS, Wang N, Sopher BL, La Spada AR, Osmand A, Margolis RL, Sun YE, Yang XW. An antisense CAG repeat transcript at JPH3 locus mediates expanded polyglutamine protein toxicity in Huntington’s disease-like 2 mice. Neuron. 2011;70:427–440. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev. 2010;90:905–81. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 11.Biancalana V, Serville F, Pommier J, Julien J, Hanauer A, Mandel JL. Moderate instability of the trinucleotide repeat in spino bulbar muscular atrophy. Hum Mol Genet. 1992;1:255–8. doi: 10.1093/hmg/1.4.255. [DOI] [PubMed] [Google Scholar]
- 12.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–3. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 13.Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–48. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 14.Paulson HL. Protein fate in neurodegenerative proteinopathies: polyglutamine diseases join the (mis)fold. Am J Hum Genet. 1999;64:339–45. doi: 10.1086/302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neurath H, Greenstein JP, Putnam FW, Erickson JO. The chemistry of protein denaturation. Chemical Reviews. 1944;34:157–265. [Google Scholar]
- 16.Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–60. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- 17.Hurle MR, Helms LR, Li L, Chan W, Wetzel R. A role for destabilizing amino acid replacements in light chain amyloidosis. Proc Natl Acad Sci (USA) 1994;91:5446–5450. doi: 10.1073/pnas.91.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wetzel R. Mutations and off-pathway aggregation. Trends in Biotechnology. 1994;12:193–198. doi: 10.1016/0167-7799(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 19.Altschuler EL, Hud NV, Mazrimas JA, Rupp B. Random coil conformation for extended polyglutamine stretches in aqueous soluble monomeric peptides. J Pept Res. 1997;50:73–5. doi: 10.1111/j.1399-3011.1997.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Berthelier V, Yang W, Wetzel R. Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J Mol Biol. 2001;311:173–182. doi: 10.1006/jmbi.2001.4850. [DOI] [PubMed] [Google Scholar]
- 21.Masino L, Kelly G, Leonard K, Trottier Y, Pastore A. Solution structure of polyglutamine tracts in GST-polyglutamine fusion proteins. FEBS Lett. 2002;513:267–72. doi: 10.1016/s0014-5793(02)02335-9. [DOI] [PubMed] [Google Scholar]
- 22.Klein FA, Pastore A, Masino L, Zeder-Lutz G, Nierengarten H, Oulad-Abdelghani M, Altschuh D, Mandel JL, Trottier Y. Pathogenic and non-pathogenic polyglutamine tracts have similar structural properties: towards a length-dependent toxicity gradient. J Mol Biol. 2007;371:235–44. doi: 10.1016/j.jmb.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Ferrone F, Wetzel R. Huntington’s Disease age-of-onset linked to polyglutamine aggregation nucleation. Proc Natl Acad Sci USA. 2002;99:11884–11889. doi: 10.1073/pnas.182276099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MW, Chelliah Y, Kim SW, Otwinowski Z, Bezprozvanny I. Secondary structure of Huntingtin amino-terminal region. Structure. 2009;17:1205–12. doi: 10.1016/j.str.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharyya A, Thakur AK, Chellgren VM, Thiagarajan G, Williams AD, Chellgren BW, Creamer TP, Wetzel R. Oligoproline effects on polyglutamine conformation and aggregation. J Mol Biol. 2006;355:524–35. doi: 10.1016/j.jmb.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 26.Khare SD, Ding F, Gwanmesia KN, Dokholyan NV. Molecular origin of polyglutamine aggregation in neurodegenerative diseases. PLoS Comput Biol. 2005;1:230–5. doi: 10.1371/journal.pcbi.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Vitalis A, Wyczalkowski MA, Pappu RV. Characterizing the conformational ensemble of monomeric polyglutamine. Proteins. 2006;63:297–311. doi: 10.1002/prot.20761. [DOI] [PubMed] [Google Scholar]
- 28.Leitgeb B, Kerenyi A, Bogar F, Paragi G, Penke B, Rakhely G. Studying the structural properties of polyalanine and polyglutamine peptides. J Mol Model. 2007;13:1141–50. doi: 10.1007/s00894-007-0241-4. [DOI] [PubMed] [Google Scholar]
- 29.Vitalis A, Wang X, Pappu RV. Quantitative characterization of intrinsic disorder in polyglutamine: insights from analysis based on polymer theories. Biophys J. 2007;93:1923–37. doi: 10.1529/biophysj.107.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano M, Watanabe H, Rothstein SM, Tanaka S. Comparative characterization of short monomeric polyglutamine peptides by replica exchange molecular dynamics simulation. J Phys Chem B. 2010;114:7056–61. doi: 10.1021/jp9122024. [DOI] [PubMed] [Google Scholar]
- 31.Lakhani VV, Ding F, Dokholyan NV. Polyglutamine induced misfolding of huntingtin exon1 is modulated by the flanking sequences. PLoS Comput Biol. 2010;6:e1000772. doi: 10.1371/journal.pcbi.1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laghaei R, Mousseau N. Spontaneous formation of polyglutamine nanotubes with molecular dynamics simulations. Journal of Chemical Physics. 2010;132:8. doi: 10.1063/1.3383244. [DOI] [PubMed] [Google Scholar]
- 33.Crick SL, Jayaraman M, Frieden C, Wetzel R, Pappu RV. Fluorescence correlation spectroscopy shows that monomeric polyglutamine molecules form collapsed structures in aqueous solutions. Proc Natl Acad Sci U S A. 2006;103:16764–9. doi: 10.1073/pnas.0608175103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters RH, Murphy RM. Examining polyglutamine peptide length: a connection between collapsed conformations and increased aggregation. J Mol Biol. 2009;393:978–92. doi: 10.1016/j.jmb.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougan L, Li JY, Badilla CL, Berne BJ, Fernandez JM. Single homopolypeptide chains collapse into mechanically rigid conformations. Proc Natl Acad Sci U S A. 2009;106:12605–12610. doi: 10.1073/pnas.0900678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starikov EB, Lehrach H, Wanker EE. Folding of oligoglutamines: a theoretical approach based upon thermodynamics and molecular mechanics. J Biomol Struct Dyn. 1999;17:409–27. doi: 10.1080/07391102.1999.10508374. [DOI] [PubMed] [Google Scholar]
- 37.Vitalis A, Wang X, Pappu RV. Atomistic simulations of the effects of polyglutamine chain length and solvent quality on conformational equilibria and spontaneous homodimerization. J Mol Biol. 2008;384:279–97. doi: 10.1016/j.jmb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trottier Y, Lutz Y, Stevanin G, Imbert G, Devys D, Cancel G, Saudou F, Weber C, David G, Tora L, et al. Polyglutamine expansion as a pathological epitope in Huntington’s disease and four dominant cerebellar ataxias. Nature. 1995;378:403–6. doi: 10.1038/378403a0. [DOI] [PubMed] [Google Scholar]
- 39.Bennett MJ, Huey-Tubman KE, Herr AB, West AP, Ross SA, Bjorkman PJ. A linear lattice model for poly-glutamine in CAG expansion diseases. Proc Natl Acad Sci U S A. 2002;99:11634–11639. doi: 10.1073/pnas.182393899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Huey-Tubman KE, Gao T, Li X, West AP, Jr, Bennett MJ, Bjorkman PJ. The structure of a polyQ-anti-polyQ complex reveals binding according to a linear lattice model. Nat Struct Mol Biol. 2007;14:381–7. doi: 10.1038/nsmb1234. [DOI] [PubMed] [Google Scholar]
- 41.Miller J, Arrasate M, Brooks E, Libeu CP, Legleiter J, Hatters D, Curtis J, Cheung K, Krishnan P, Mitra S, Widjaja K, Shaby BA, Lotz GP, Newhouse Y, Mitchell EJ, Osmand A, Gray M, Thulasiramin V, Saudou F, Segal M, Yang XW, Masliah E, Thompson LM, Muchowski PJ, Weisgraber KH, Finkbeiner S. Identifying polyglutamine protein species in situ that best predict neurodegeneration. Nat Chem Biol. 2011;7:925–34. doi: 10.1038/nchembio.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pejchal R, Gach JS, Brunel FM, Cardoso RM, Stanfield RL, Dawson PE, Burton DR, Zwick MB, Wilson IA. A conformational switch in human immunodeficiency virus gp41 revealed by the structures of overlapping epitopes recognized by neutralizing antibodies. J Virol. 2009;83:8451–62. doi: 10.1128/JVI.00685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyson HJ, Wright PE. Coupling of folding and binding for unstructured proteins. Curr Opin Struct Biol. 2002;12:54–60. doi: 10.1016/s0959-440x(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 44.Dyson HJ. Expanding the proteome: disordered and alternatively folded proteins. Quarterly Reviews of Biophysics. 2011;44:467–518. doi: 10.1017/S0033583511000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kar K, Jayaraman M, Sahoo B, Kodali R, Wetzel R. Critical nucleus size for disease-related polyglutamine aggregation is repeat-length dependent. Nat Struct Mol Biol. 2011;18:328–36. doi: 10.1038/nsmb.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiumara F, Fioriti L, Kandel ER, Hendrickson WA. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and PolyQ proteins. Cell. 2010;143:1121–35. doi: 10.1016/j.cell.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eliezer D. Biophysical characterization of intrinsically disordered proteins. Current Opinion in Structural Biology. 2009;19:23–30. doi: 10.1016/j.sbi.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thakur AK, Jayaraman M, Mishra R, Thakur M, Chellgren VM, Byeon IJ, Anjum DH, Kodali R, Creamer TP, Conway JF, Gronenborn AM, Wetzel R. Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat Struct Mol Biol. 2009;16:380–9. doi: 10.1038/nsmb.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atwal RS, Desmond CR, Caron N, Maiuri T, Xia JR, Sipione S, Truant R. Kinase inhibitors modulate huntingtin cell localization and toxicity. Nature Chemical Biology. 2011;7:453–460. doi: 10.1038/nchembio.582. [DOI] [PubMed] [Google Scholar]
- 50.Jayaraman M, Kodali R, Sahoo B, Thakur AK, Mayasundari A, Mishra R, Peterson CB, Wetzel R. Slow amyloid nucleation via α-helix-rich oligomeric intermediates in short polyglutamine–containing Huntingtin fragments. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi Y, Okamoto Y, Popiel HA, Fujikake N, Toda T, Kinjo M, Nagai Y. Detection of polyglutamine protein oligomers in cells by fluorescence correlation spectroscopy. J Biol Chem. 2007;282:24039–48. doi: 10.1074/jbc.M704789200. [DOI] [PubMed] [Google Scholar]
- 52.Ossato G, Digman MA, Aiken C, Lukacsovich T, Marsh JL, Gratton E. A two-step path to inclusion formation of huntingtin peptides revealed by number and brightness analysis. Biophys J. 2010;98:3078–85. doi: 10.1016/j.bpj.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. SUMO modification of Huntingtin and Huntington’s disease pathology. Science. 2004;304:100–4. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- 54.Atwal RS, Xia J, Pinchev D, Taylor J, Epand RM, Truant R. Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet. 2007;16:2600–15. doi: 10.1093/hmg/ddm217. [DOI] [PubMed] [Google Scholar]
- 55.Rockabrand E, Slepko N, Pantalone A, Nukala VN, Kazantsev A, Marsh JL, Sullivan PG, Steffan JS, Sensi SL, Thompson LM. The first 17 amino acids of Huntingtin modulate its sub-cellular localization, aggregation and effects on calcium homeostasis. Hum Mol Genet. 2007;16:61–77. doi: 10.1093/hmg/ddl440. [DOI] [PubMed] [Google Scholar]
- 56.Angeli S, Shao J, Diamond MI. F-actin binding regions on the androgen receptor and huntingtin increase aggregation and alter aggregate characteristics. PLoS One. 2010;5:e9053. doi: 10.1371/journal.pone.0009053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson LM, Aiken CT, Kaltenbach LS, Agrawal N, Illes K, Khoshnan A, Martinez-Vincente M, Arrasate M, JG OS-R, Khashwji H, Lukacsovich T, Zhu YZ, Lau AL, Massey A, Hayden MR, Zeitlin SO, Finkbeiner S, Green KN, Laferla FM, Bates G, Huang L, Patterson PH, Lo DC, Cuervo AM, Marsh JL, Steffan JS. IKK phosphorylates Huntingtin and targets it for degradation by the proteasome and lysosome. J Cell Biol. 2009;187:1083–1099. doi: 10.1083/jcb.200909067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu X, Greiner ER, Mishra R, Kodali R, Osmand A, Finkbeiner S, Steffan JS, Thompson LM, Wetzel R, Yang XW. Serines 13 and 16 are critical determinants of full-length human mutant huntingtin induced disease pathogenesis in HD mice. Neuron. 2009;64:828–40. doi: 10.1016/j.neuron.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelley NW, Huang X, Tam S, Spiess C, Frydman J, Pande VS. The predicted structure of the headpiece of the Huntingtin protein and its implications on Huntingtin aggregation. J Mol Biol. 2009;388:919–27. doi: 10.1016/j.jmb.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossetti G, Cossio P, Laio A, Carloni P. Conformations of the Huntingtin N-term in aqueous solution from atomistic simulations. Febs Letters. 2011;585:3086–3089. doi: 10.1016/j.febslet.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Williamson TE, Vitalis A, Crick SL, Pappu RV. Modulation of polyglutamine conformations and dimer formation by the N-terminus of huntingtin. J Mol Biol. 2010;396:1295–309. doi: 10.1016/j.jmb.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dlugosz M, Trylska J. Secondary Structures of Native and Pathogenic Huntingtin N-Terminal Fragments. Journal of Physical Chemistry B. 2011;115:11597–11608. doi: 10.1021/jp206373g. [DOI] [PubMed] [Google Scholar]
- 63.Stott K, Blackburn JM, Butler PJ, Perutz M. Incorporation of glutamine repeats makes protein oligomerize: implications for neurodegenerative diseases. Proc Natl Acad Sci U S A. 1995;92:6509–13. doi: 10.1073/pnas.92.14.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen YW, Stott K, Perutz MF. Crystal structure of a dimeric chymotrypsin inhibitor 2 mutant containing an inserted glutamine repeat. Proc Natl Acad Sci U S A. 1999;96:1257–61. doi: 10.1073/pnas.96.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka M, Morishima I, Akagi T, Hashikawa T, Nukina N. Intra- and intermolecular beta-pleated sheet formation in glutamine-repeat inserted myoglobin as a model for polyglutamine diseases. J Biol Chem. 2001;276:45470–5. doi: 10.1074/jbc.M107502200. [DOI] [PubMed] [Google Scholar]
- 66.Robertson AL, Horne J, Ellisdon AM, Thomas B, Scanlon MJ, Bottomley SP. The structural impact of a polyglutamine tract is location-dependent. Biophys J. 2008;95:5922–30. doi: 10.1529/biophysj.108.138487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tobelmann MD, Murphy RM. Location Trumps Length: Polyglutamine-Mediated Changes in Folding and Aggregation of a Host Protein. Biophysical Journal. 2011;100:2773–2782. doi: 10.1016/j.bpj.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagai Y, Inui T, Popiel HA, Fujikake N, Hasegawa K, Urade Y, Goto Y, Naiki H, Toda T. A toxic monomeric conformer of the polyglutamine protein. Nat Struct Mol Biol. 2007;14:332–40. doi: 10.1038/nsmb1215. [DOI] [PubMed] [Google Scholar]
- 69.Lamb DJ, Puxeddu E, Malik N, Stenoien DL, Nigam R, Saleh GY, Mancini M, Weigel NL, Marcelli M. Molecular analysis of the androgen receptor in ten prostate cancer specimens obtained before and after androgen ablation. J Androl. 2003;24:215–25. doi: 10.1002/j.1939-4640.2003.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 70.Wang Q, Udayakumar TS, Vasaitis TS, Brodie AM, Fondell JD. Mechanistic relationship between androgen receptor polyglutamine tract truncation and androgen-dependent transcriptional hyperactivity in prostate cancer cells. J Biol Chem. 2004;279:17319–28. doi: 10.1074/jbc.M400970200. [DOI] [PubMed] [Google Scholar]
- 71.Cattaneo E, Rigamonti D, Goffredo D, Zuccato C, Squitieri F, Sipione S. Loss of normal huntingtin function: new developments in Huntington’s disease research. Trends Neurosci. 2001;24:182–8. doi: 10.1016/s0166-2236(00)01721-5. [DOI] [PubMed] [Google Scholar]
- 72.Pearson CE, Sinden RR. Trinucleotide repeat DNA structures: dynamic mutations from dynamic DNA. Curr Opin Struct Biol. 1998;8:321–30. doi: 10.1016/s0959-440x(98)80065-1. [DOI] [PubMed] [Google Scholar]
- 73.Perutz MF, Johnson T, Suzuki M, Finch JT. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci U S A. 1994;91:5355–8. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thakur A, Wetzel R. Mutational analysis of the structural organization of polyglutamine aggregates. Proc Natl Acad Sci U S A. 2002;99:17014–17019. doi: 10.1073/pnas.252523899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhattacharyya AM, Thakur AK, Wetzel R. polyglutamine aggregation nucleation: thermodynamics of a highly unfavorable protein folding reaction. Proc Natl Acad Sci U S A. 2005;102:15400–5. doi: 10.1073/pnas.0501651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slepko N, Bhattacharyya AM, Jackson GR, Steffan JS, Marsh JL, Thompson LM, Wetzel R. Normal-repeat-length polyglutamine peptides accelerate aggregation nucleation and cytotoxicity of expanded polyglutamine proteins. Proc Natl Acad Sci U S A. 2006;103:14367–72. doi: 10.1073/pnas.0602348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee CC, Walters RH, Murphy RM. Reconsidering the mechanism of polyglutamine Peptide aggregation. Biochemistry. 2007;46:12810–20. doi: 10.1021/bi700806c. [DOI] [PubMed] [Google Scholar]
- 78.Jayaraman M, Kodali R, Wetzel R. The impact of ataxin-1-like histidine insertions on polyglutamine aggregation. Protein Eng Des Sel. 2009;22:469–78. doi: 10.1093/protein/gzp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walters RH, Murphy RM. Aggregation kinetics of interrupted polyglutamine peptides. J Mol Biol. 2011;412:505–19. doi: 10.1016/j.jmb.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma D, Sharma S, Pasha S, Brahmachari SK. Peptide models for inherited neurodegenerative disorders: conformation and aggregation properties of long polyglutamine peptides with and without interruptions. FEBS Lett. 1999;456:181–5. doi: 10.1016/s0014-5793(99)00933-3. [DOI] [PubMed] [Google Scholar]
- 81.Chen S, Berthelier V, Hamilton JB, O’Nuallain B, Wetzel R. Amyloid-like features of polyglutamine aggregates and their assembly kinetics. Biochemistry. 2002;41:7391–9. doi: 10.1021/bi011772q. [DOI] [PubMed] [Google Scholar]
- 82.Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, Bates GP, Lehrach H, Wanker EE. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc Natl Acad Sci U S A. 1999;96:4604–9. doi: 10.1073/pnas.96.8.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:10417–22. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCampbell A, Fischbeck KH. Polyglutamine and CBP: fatal attraction? Nat Med. 2001;7:528–30. doi: 10.1038/87842. [DOI] [PubMed] [Google Scholar]
- 85.Ferrone F. Analysis of protein aggregation kinetics. Meths Enzymol. 1999;309:256–274. doi: 10.1016/s0076-6879(99)09019-9. [DOI] [PubMed] [Google Scholar]
- 86.Smith MH, Miles TF, Sheehan M, Alfieri KN, Kokona B, Fairman R. Polyglutamine fibrils are formed using a simple designed beta-hairpin model. Proteins. 2010;78:1971–9. doi: 10.1002/prot.22713. [DOI] [PubMed] [Google Scholar]
- 87.VanSchouwen BM, Oblinsky DG, Gordon HL, Rothstein SM. Structure propensities in mutated polyglutamine peptides. Interdiscip Sci. 2011;3:1–16. doi: 10.1007/s12539-011-0058-9. [DOI] [PubMed] [Google Scholar]
- 88.VanSchouwen BMB, Nakano M, Watanabe H, Tanaka S, Gordon HL, Rothstein SM. Molecular mechanics and all-electron fragment molecular orbital calculations on mutated polyglutamine peptides. Journal of Molecular Structure-Theochem. 2010;944:12–20. [Google Scholar]
- 89.Esler WP, Stimson ER, Jennings JM, Vinters HV, Ghilardi JR, Lee JP, Mantyh PW, Maggio JE. Alzheimer’s disease amyloid propagation by a template-dependent dock-lock mechanism. Biochemistry. 2000;39:6288–95. doi: 10.1021/bi992933h. [DOI] [PubMed] [Google Scholar]
- 90.Pfeil W. The problem of the stability globular proteins. Mol Cell Biochem. 1981;40:3–28. doi: 10.1007/BF00230185. [DOI] [PubMed] [Google Scholar]
- 91.Marchut AJ, Hall CK. Effects of chain length on the aggregation of model polyglutamine peptides: molecular dynamics simulations. Proteins. 2007;66:96–109. doi: 10.1002/prot.21132. [DOI] [PubMed] [Google Scholar]
- 92.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 93.Legleiter J, Mitchell E, Lotz GP, Sapp E, Ng C, DiFiglia M, Thompson LM, Muchowski PJ. Mutant huntingtin fragments form oligomers in a polyglutamine length-dependent manner in vitro and in vivo. J Biol Chem. 2010;285:14777–90. doi: 10.1074/jbc.M109.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vitalis A, Pappu RV. Assessing the contribution of heterogeneous distributions of oligomers to aggregation mechanisms of polyglutamine peptides. Biophysical Chemistry. 2011;159:14–23. doi: 10.1016/j.bpc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kodali R, Wetzel R. Polymorphism in the intermediates and products of amyloid assembly. Curr Opin Struct Biol. 2007;17:48–57. doi: 10.1016/j.sbi.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 96.O’Nuallain B, Thakur AK, Williams AD, Bhattacharyya AM, Chen S, Thiagarajan G, Wetzel R. Kinetics and thermodynamics of amyloid assembly using a high-performance liquid chromatography-based sedimentation assay. Methods Enzymol. 2006;413:34–74. doi: 10.1016/S0076-6879(06)13003-7. [DOI] [PubMed] [Google Scholar]
- 97.Sen S, Dash D, Pasha S, Brahmachari SK. Role of histidine interruption in mitigating the pathological effects of long polyglutamine stretches in SCA1: A molecular approach. Protein Sci. 2003;12:953–62. doi: 10.1110/ps.0224403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thakur AK, Yang W, Wetzel R. Inhibition of polyglutamine aggregate cytotoxicity by a structure-based elongation inhibitor. FASEB J. 2004;18:923–5. doi: 10.1096/fj.03-1238fje. [DOI] [PubMed] [Google Scholar]
- 99.Popiel HA, Nagai Y, Onodera O, Inui T, Fujikake N, Urade Y, Strittmatter WJ, Burke JR, Ichikawa A, Toda T. Disruption of the toxic conformation of the expanded polyglutamine stretch leads to suppression of aggregate formation and cytotoxicity. Biochem Biophys Res Commun. 2004;317:1200–6. doi: 10.1016/j.bbrc.2004.03.161. [DOI] [PubMed] [Google Scholar]
- 100.Quan F, Janas J, Popovich BW. A novel CAG repeat configuration in the SCA1 gene: implications for the molecular diagnostics of spinocerebellar ataxia type 1. Hum Mol Genet. 1995;4:2411–3. doi: 10.1093/hmg/4.12.2411. [DOI] [PubMed] [Google Scholar]
- 101.Arango M, Holbert S, Zala D, Brouillet E, Pearson J, Regulier E, Thakur AK, Aebischer P, Wetzel R, Deglon N, Neri C. CA150 expression delays striatal cell death in overexpression and knock-in conditions for mutant huntingtin neurotoxicity. J Neurosci. 2006;26:4649–59. doi: 10.1523/JNEUROSCI.5409-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 103.Scheuermann T, Schulz B, Blume A, Wahle E, Rudolph R, Schwarz E. Trinucleotide expansions leading to an extended poly-L-alanine segment in the poly (A) binding protein PABPN1 cause fibril formation. Protein Sci. 2003;12:2685–92. doi: 10.1110/ps.03214703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–58. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 105.Ignatova Z, Gierasch LM. Extended polyglutamine tracts cause aggregation and structural perturbation of an adjacent beta barrel protein. J Biol Chem. 2006;281:12959–67. doi: 10.1074/jbc.M511523200. [DOI] [PubMed] [Google Scholar]
- 106.Bulone D, Masino L, Thomas DJ, San Biagio PL, Pastore A. The Interplay between PolyQ and Protein Context Delays Aggregation by Forming a Reservoir of Protofibrils. PLoS ONE. 2006;1:e111. doi: 10.1371/journal.pone.0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Masino L, Nicastro G, Menon RP, Dal Piaz F, Calder L, Pastore A. Characterization of the structure and the amyloidogenic properties of the Josephin domain of the polyglutamine-containing protein ataxin-3. J Mol Biol. 2004;344:1021–35. doi: 10.1016/j.jmb.2004.09.065. [DOI] [PubMed] [Google Scholar]
- 108.Ellisdon AM, Thomas B, Bottomley SP. The two-stage pathway of ataxin-3 fibrillogenesis involves a polyglutamine-independent step. J Biol Chem. 2006;281:16888–96. doi: 10.1074/jbc.M601470200. [DOI] [PubMed] [Google Scholar]
- 109.Bevivino AE, Loll PJ. An expanded glutamine repeat destabilizes native ataxin-3 structure and mediates formation of parallel beta -fibrils. Proc Natl Acad Sci U S A. 2001;98:11955–60. doi: 10.1073/pnas.211305198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chow MK, Ellisdon AM, Cabrita LD, Bottomley SP. Polyglutamine expansion in ataxin-3 does not affect protein stability: implications for misfolding and disease. J Biol Chem. 2004;279:47643–51. doi: 10.1074/jbc.M405799200. [DOI] [PubMed] [Google Scholar]
- 111.Chow MK, Paulson HL, Bottomley SP. Destabilization of a non-pathological variant of ataxin-3 results in fibrillogenesis via a partially folded intermediate: a model for misfolding in polyglutamine disease. J Mol Biol. 2004;335:333–41. doi: 10.1016/j.jmb.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 112.Santambrogio C, Frana AM, Natalello A, Papaleo E, Regonesi ME, Doglia SM, Tortora P, Invernizzi G, Grandori R. The role of the central flexible region on the aggregation and conformational properties of human ataxin-3. FEBS J. 2011 doi: 10.1111/j.1742–4658.2011.08438.x. [DOI] [PubMed] [Google Scholar]
- 113.Saunders HM, Gilis D, Rooman M, Dehouck Y, Robertson AL, Bottomley SP. Flanking domain stability modulates the aggregation kinetics of a polyglutamine disease protein. Protein Science. 2011;20:1675–1681. doi: 10.1002/pro.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masino L, Nicastro G, Calder L, Vendruscolo M, Pastore A. Functional interactions as a survival strategy against abnormal aggregation. FASEB J. 2011;25:45–54. doi: 10.1096/fj.10-161208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Robertson AL, Headey SJ, Saunders HM, Ecroyd H, Scanlon MJ, Carver JA, Bottomley SP. Small heat-shock proteins interact with a flanking domain to suppress polyglutamine aggregation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10424–10429. doi: 10.1073/pnas.0914773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walker FO. Huntington’s disease. Lancet. 2007;369:218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 117.Poirier MA, Li H, Macosko J, Cai S, Amzel M, Ross CA. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem. 2002;277:41032–7. doi: 10.1074/jbc.M205809200. [DOI] [PubMed] [Google Scholar]
- 118.Apostol BL, Kazantsev A, Raffioni S, Illes K, Pallos J, Bodai L, Slepko N, Bear JE, Gertler FB, Hersch S, Housman DE, Marsh JL, Thompson LM. A cell-based assay for aggregation inhibitors as therapeutics of polyglutamine-repeat disease and validation in Drosophila. Proc Natl Acad Sci U S A. 2003;100:5950–5. doi: 10.1073/pnas.2628045100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duennwald ML, Jagadish S, Muchowski PJ, Lindquist S. Flanking sequences profoundly alter polyglutamine toxicity in yeast. Proc Natl Acad Sci U S A. 2006;103:11045–50. doi: 10.1073/pnas.0604547103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Darnell G, Orgel JP, Pahl R, Meredith SC. Flanking Polyproline Sequences Inhibit beta-Sheet Structure in Polyglutamine Segments by Inducing PPII-like Helix Structure. J Mol Biol. 2007;374:688–704. doi: 10.1016/j.jmb.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 121.Darnell GD, Derryberry J, Kurutz JW, Meredith SC. Mechanism of Cis-Inhibition of PolyQ Fibrillation by PolyP: PPII Oligomers and the Hydrophobic Effect. Biophysical Journal. 2009;97:2295–2305. doi: 10.1016/j.bpj.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sivanandam VN, Jayaraman M, Hoop CL, Kodali R, Wetzel R, van der Wel PC. The aggregation-enhancing huntingtin N-terminus is helical in amyloid fibrils. J Am Chem Soc. 2011;133:4558–66. doi: 10.1021/ja110715f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abedini A, Raleigh DP. A role for helical intermediates in amyloid formation by natively unfolded polypeptides? Phys Biol. 2009;6:015005. doi: 10.1088/1478-3975/6/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singer D, Zauner T, Genz M, Hoffmann R, Zuchner T. Synthesis of pathological and nonpathological human exon 1 huntingtin. Journal of Peptide Science. 2010;16:358–363. doi: 10.1002/psc.1252. [DOI] [PubMed] [Google Scholar]
- 125.Tam S, Spiess C, Auyeung W, Joachimiak L, Chen B, Poirier MA, Frydman J. The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat Struct Mol Biol. 2009;16:1279–85. doi: 10.1038/nsmb.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol. 2004;11:1215–22. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- 127.Broncel M, Falenski JA, Wagner SC, Hackenberger CP, Koksch B. How post-translational modifications influence amyloid formation: a systematic study of phosphorylation and glycosylation in model peptides. Chemistry. 2010;16:7881–8. doi: 10.1002/chem.200902452. [DOI] [PubMed] [Google Scholar]
- 128.Nekooki-Machida Y, Kurosawa M, Nukina N, Ito K, Oda T, Tanaka M. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:9679–84. doi: 10.1073/pnas.0812083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi JJ, Serpell L, Arnsdorf MF, Lindquist SL. Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science. 2000;289:1317–21. doi: 10.1126/science.289.5483.1317. [DOI] [PubMed] [Google Scholar]
- 130.Powers ET, Ferrone F. Kinetic models for protein misfolding and association. In: Dobson CM, Kelly JW, Ramirez-Alvarado M, editors. Protein Misfolding Diseases: Current and Emerging Principles and Therapies. Wiley; New York: 2009. pp. 73–92. [Google Scholar]
- 131.Nagai Y, Tucker T, Ren H, Kenan DJ, Henderson BS, Keene JD, Strittmatter WJ, Burke JR. Inhibition of polyglutamine protein aggregation and cell death by novel peptides identified by phage display screening. J Biol Chem. 2000;275:10437–42. doi: 10.1074/jbc.275.14.10437. [DOI] [PubMed] [Google Scholar]
- 132.Tomita K, Popiel HA, Nagai Y, Toda T, Yoshimitsu Y, Ohno H, Oishi S, Fujii N. Structure-activity relationship study on polyglutamine binding peptide QBP1. Bioorg Med Chem. 2009;17:1259–63. doi: 10.1016/j.bmc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 133.Hamuro L, Zhang G, Tucker TJ, Self C, Strittmatter WJ, Burke JR. Optimization of a polyglutamine aggregation inhibitor peptide (QBP1) using a thioflavin T fluorescence assay. Assay Drug Dev Technol. 2007;5:629–36. doi: 10.1089/adt.2007.083. [DOI] [PubMed] [Google Scholar]
- 134.Popiel HA, Nagai Y, Fujikake N, Toda T. Protein transduction domain-mediated delivery of QBP1 suppresses polyglutamine-induced neurodegeneration in vivo. Mol Ther. 2007;15:303–9. doi: 10.1038/sj.mt.6300045. [DOI] [PubMed] [Google Scholar]
- 135.Nagai Y, Fujikake N, Ohno K, Higashiyama H, Popiel HA, Rahadian J, Yamaguchi M, Strittmatter WJ, Burke JR, Toda T. Prevention of polyglutamine oligomerization and neurodegeneration by the peptide inhibitor QBP1 in Drosophila. Hum Mol Genet. 2003;12:1253–9. doi: 10.1093/hmg/ddg144. [DOI] [PubMed] [Google Scholar]
- 136.Richardson JS, Richardson DC. Principles and patterns of protein conformation. In: Fasman GD, editor. Prediction of Protein Structure and the Principles of Protein Conformation. Plenum; New York: 1989. pp. 1–98. [Google Scholar]
- 137.Lanning JD, Hawk AJ, Derryberry J, Meredith SC. Chaperone-like N-methyl peptide inhibitors of polyglutamine aggregation. Biochemistry. 2010;49:7108–18. doi: 10.1021/bi1006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heiser V, Scherzinger E, Boeddrich A, Nordhoff E, Lurz R, Schugardt N, Lehrach H, Wanker EE. Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implications for Huntington’s disease therapy. Proc Natl Acad Sci U S A. 2000;97:6739–44. doi: 10.1073/pnas.110138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heiser V, Engemann S, Brocker W, Dunkel I, Boeddrich A, Waelter S, Nordhoff E, Lurz R, Schugardt N, Rautenberg S, Herhaus C, Barnickel G, Bottcher H, Lehrach H, Wanker EE. Identification of benzothiazoles as potential polyglutamine aggregation inhibitors of Huntington’s disease by using an automated filter retardation assay. Proc Natl Acad Sci U S A. 2002;99(Suppl 4):16400–6. doi: 10.1073/pnas.182426599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Marsh JL, Thompson LM, Lindquist S, Muchowski PJ, Wanker EE. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum Mol Genet. 2006;15:2743–51. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 141.Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15:558–66. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 142.Hockly E, Tse J, Barker AL, Moolman DL, Beunard JL, Revington AP, Holt K, Sunshine S, Moffitt H, Sathasivam K, Woodman B, Wanker EE, Lowden PA, Bates GP. Evaluation of the benzothiazole aggregation inhibitors riluzole and PGL-135 as therapeutics for Huntington’s disease. Neurobiol Dis. 2006;21:228–36. doi: 10.1016/j.nbd.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 143.Mishra R, Jayaraman M, Roland BP, Landrum E, Fullam R, Kodali R, Thakur AK, Arduini I, Wetzel R. Inhibiting nucleation of amyloid structure in a huntingtin fragment by targeting α-helix rich oligomeric intermediates. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cao P, Meng F, Abedini A, Raleigh DP. The ability of rodent islet amyloid polypeptide to inhibit amyloid formation by human islet amyloid polypeptide has important implications for the mechanism of amyloid formation and the design of inhibitors. Biochemistry. 2010;49:872–81. doi: 10.1021/bi901751b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schiefner A, Chatwell L, Korner J, Neumaier I, Colby DW, Volkmer R, Wittrup KD, Skerra A. A Disulfide-Free Single-Domain V(L) Intrabody with Blocking Activity towards Huntingtin Reveals a Novel Mode of Epitope Recognition. J Mol Biol. 2011;414:337–55. doi: 10.1016/j.jmb.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 146.Carmichael J, Chatellier J, Woolfson A, Milstein C, Fersht AR, Rubinsztein DC. Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington’s disease. Proc Natl Acad Sci U S A. 2000;97:9701–5. doi: 10.1073/pnas.170280697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci U S A. 2000;97:7841–6. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hay DG, Sathasivam K, Tobaben S, Stahl B, Marber M, Mestril R, Mahal A, Smith DL, Woodman B, Bates GP. Progressive decrease in chaperone protein levels in a mouse model of Huntington’s disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet. 2004;13:1389–405. doi: 10.1093/hmg/ddh144. [DOI] [PubMed] [Google Scholar]
- 149.Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, Rye D, Ferrante RJ, Hersch SM, Li XJ. Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J Neurosci. 1999;19:2522–34. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li H, Li SH, Cheng AL, Mangiarini L, Bates GP, Li XJ. Ultrastructural localization and progressive formation of neuropil aggregates in Huntington’s disease transgenic mice. Hum Mol Genet. 1999;8:1227–36. doi: 10.1093/hmg/8.7.1227. [DOI] [PubMed] [Google Scholar]
- 151.Sathasivam K, Lane A, Legleiter J, Warley A, Woodman B, Finkbeiner S, Paganetti P, Muchowski PJ, Wilson S, Bates GP. Identical oligomeric and fibrillar structures captured from the brains of R6/2 and knock-in mouse models of Huntington’s disease. Hum Mol Genet. 2010;19:65–78. doi: 10.1093/hmg/ddp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Osmand AP, Berthelier V, Wetzel R. Imaging polyglutamine deposits in brain tissue. Methods Enzymol. 2006;412:106–22. doi: 10.1016/S0076-6879(06)12008-X. [DOI] [PubMed] [Google Scholar]
- 153.Moulder KL, Onodera O, Burke JR, Strittmatter WJ, Johnson EM., Jr Generation of neuronal intranuclear inclusions by polyglutamine-GFP: analysis of inclusion clearance and toxicity as a function of polyglutamine length. J Neurosci. 1999;19:705–15. doi: 10.1523/JNEUROSCI.19-02-00705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Colby DW, Cassady JP, Lin GC, Ingram VM, Wittrup KD. Stochastic kinetics of intracellular huntingtin aggregate formation. Nat Chem Biol. 2006;2:319–23. doi: 10.1038/nchembio792. [DOI] [PubMed] [Google Scholar]
- 155.Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell. 2001;12:1393–407. doi: 10.1091/mbc.12.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ross CA, Poirier MA. Opinion: What is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Biol. 2005;6:891–8. doi: 10.1038/nrm1742. [DOI] [PubMed] [Google Scholar]
- 157.Olshina MA, Angley LM, Ramdzan YM, Tang J, Bailey MF, Hill AF, Hatters DM. Tracking mutant huntingtin aggregation kinetics in cells reveals three major populations that include an invariant oligomer pool. J Biol Chem. 2010;285:21807–16. doi: 10.1074/jbc.M109.084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chai Y, Shao J, Miller VM, Williams A, Paulson HL. Live-cell imaging reveals divergent intracellular dynamics of polyglutamine disease proteins and supports a sequestration model of pathogenesis. Proc Natl Acad Sci U S A. 2002;99:9310–5. doi: 10.1073/pnas.152101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stenoien DL, Mielke M, Mancini MA. Intranuclear ataxin1 inclusions contain both fast- and slow-exchanging components. Nat Cell Biol. 2002;4:806–10. doi: 10.1038/ncb859. [DOI] [PubMed] [Google Scholar]
- 160.Lajoie P, Snapp EL. Formation and Toxicity of Soluble Polyglutamine Oligomers in Living Cells. PLoS ONE. 2010;5:e15245. doi: 10.1371/journal.pone.0015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet. 1998;19:148–54. doi: 10.1038/502. [DOI] [PubMed] [Google Scholar]
- 162.Nucifora FC, Jr, Sasaki M, Peters MF, Huang H, Cooper JK, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson VL, Dawson TM, Ross CA. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–8. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 163.Jiang H, Nucifora FC, Jr, Ross CA, DeFranco DB. Cell death triggered by polyglutamine-expanded huntingtin in a neuronal cell line is associated with degradation of CREB-binding protein. Hum Mol Genet. 2003;12:1–12. doi: 10.1093/hmg/ddg002. [DOI] [PubMed] [Google Scholar]
- 164.Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur ELF, Ross CA. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–12. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- 165.Boutell JM, Thomas P, Neal JW, Weston VJ, Duce J, Harper PS, Jones AL. Aberrant interactions of transcriptional repressor proteins with the Huntington’s disease gene product, huntingtin. Hum Mol Genet. 1999;8:1647–55. doi: 10.1093/hmg/8.9.1647. [DOI] [PubMed] [Google Scholar]
- 166.Davranche A, Aviolat H, Zeder-Lutz G, Busso D, Altschuh D, Trottier Y, Klein FAC. Huntingtin affinity for partners is not changed by polyglutamine length: aggregation itself triggers aberrant interactions. Human Molecular Genetics. 2011;20:2795–2806. doi: 10.1093/hmg/ddr178. [DOI] [PubMed] [Google Scholar]
- 167.Perutz MF, Finch JT, Berriman J, Lesk A. Amyloid fibers are water-filled nanotubes. Proc Natl Acad Sci U S A. 2002;99:5591–5. doi: 10.1073/pnas.042681399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sharma D, Shinchuk LM, Inouye H, Wetzel R, Kirschner DA. Polyglutamine homopolymers having 8–45 residues form slablike beta-crystallite assemblies. Proteins. 2005;61:398–411. doi: 10.1002/prot.20602. [DOI] [PubMed] [Google Scholar]
- 169.Sikorski P, Atkins E. New model for crystalline polyglutamine assemblies and their connection with amyloid fibrils. Biomacromolecules. 2005;6:425–32. doi: 10.1021/bm0494388. [DOI] [PubMed] [Google Scholar]
- 170.Schneider R, Schumacher MC, Mueller H, Nand D, Klaukien V, Heise H, Riedel D, Wolf G, Behrmann E, Raunser S, Seidel R, Engelhard M, Baldus M. Structural characterization of polyglutamine fibrils by solid-state NMR spectroscopy. J Mol Biol. 2011;412:121–36. doi: 10.1016/j.jmb.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 171.Rossetti G, Magistrato A, Pastore A, Persichetti F, Carloni P. Structural Properties of Polyglutamine Aggregates Investigated via Molecular Dynamics Simulations. Journal of Physical Chemistry B. 2008;112:16843–16850. doi: 10.1021/jp806548p. [DOI] [PubMed] [Google Scholar]
- 172.Ogawa H, Nakano M, Watanabe H, Starikov EB, Rothstein SM, Tanaka S. Molecular dynamics simulation study on the structural stabilities of polyglutamine peptides. Comput Biol Chem. 2008;32:102–10. doi: 10.1016/j.compbiolchem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 173.Zhou ZL, Zhao JH, Liu HL, Wu JW, Liu KT, Chuang CK, Tsai WB, Ho Y. The Possible Structural Models for Polyglutamine Aggregation: A Molecular Dynamics Simulations Study. Journal of Biomolecular Structure & Dynamics. 2011;28:743–758. doi: 10.1080/07391102.2011.10508603. [DOI] [PubMed] [Google Scholar]
- 174.Kajava AV, Baxa U, Steven AC. Beta arcades: recurring motifs in naturally occurring and disease-related amyloid fibrils. FASEB J. 2010;24:1311–9. doi: 10.1096/fj.09-145979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Esposito L, Paladino A, Pedone C, Vitagliano L. Insights into structure, stability, and toxicity of monomeric and aggregated polyglutamine models from molecular dynamics simulations. Biophys J. 2008;94:4031–40. doi: 10.1529/biophysj.107.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang QC, Yeh TL, Leyva A, Frank LG, Miller J, Kim YE, Langen R, Finkbeiner S, Amzel ML, Ross CA, Poirier MA. A compact beta model of huntingtin toxicity. J Biol Chem. 2011;286:8188–96. doi: 10.1074/jbc.M110.192013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O’Nuallain B, Shivaprasad S, Kheterpal I, Wetzel R. Thermodynamics of abeta(1–40) amyloid fibril elongation. Biochemistry. 2005;44:12709–18. doi: 10.1021/bi050927h. [DOI] [PubMed] [Google Scholar]
- 178.van der Wel PC, Lewandowski JR, Griffin RG. Solid-state NMR study of amyloid nanocrystals and fibrils formed by the peptide GNNQQNY from yeast prion protein Sup35p. J Am Chem Soc. 2007;129:5117–30. doi: 10.1021/ja068633m. [DOI] [PubMed] [Google Scholar]
- 179.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435:773–8. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJ, McFarlane HT, Madsen AO, Riekel C, Eisenberg D. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447:453–7. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 181.Berthelier V, Hamilton JB, Chen S, Wetzel R. A microtiter plate assay for polyglutamine aggregate extension. Anal Biochem. 2001;295:227–36. doi: 10.1006/abio.2001.5217. [DOI] [PubMed] [Google Scholar]
- 182.Yang W, Dunlap JR, Andrews RB, Wetzel R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet. 2002;11:2905–2917. doi: 10.1093/hmg/11.23.2905. [DOI] [PubMed] [Google Scholar]
- 183.Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nature Cell Biology. 2009;11:219–U232. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Franks F. Biophysics and biochemistry at low temperatures. Cambridge University Press; Cambridge: 1985. [Google Scholar]
- 185.Franks F. Storage stabilization of proteins. In: Franks F, editor. Protein Biotechnology: Isolation, Characterization and Stabilization. Humana Press; New York: 1993. pp. 489–531. [Google Scholar]
- 186.Chen S, Wetzel R. Solubilization and disaggregation of polyglutamine peptides. Protein Science. 2001;10:887–891. doi: 10.1110/ps.42301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jayaraman M, Thakur AK, Kar K, Kodali R, Wetzel R. Assays for studying nucleated aggregation of polyglutamine proteins. Methods. 2011;53:246–54. doi: 10.1016/j.ymeth.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Padrick SB, Miranker AD. Islet amyloid: phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry. 2002;41:4694–703. doi: 10.1021/bi0160462. [DOI] [PubMed] [Google Scholar]
- 189.Nichols MR, Moss MA, Reed DK, Cratic-McDaniel S, Hoh JH, Rosenberry TL. Amyloid-beta protofibrils differ from amyloid-beta aggregates induced in dilute hexafluoroisopropanol in stability and morphology. J Biol Chem. 2005;280:2471–80. doi: 10.1074/jbc.M410553200. [DOI] [PubMed] [Google Scholar]
- 190.Sugase K, Dyson HJ, Wright PE. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature. 2007;447:1021–5. doi: 10.1038/nature05858. [DOI] [PubMed] [Google Scholar]
- 191.Palidwor GA, Shcherbinin S, Huska MR, Rasko T, Stelzl U, Arumughan A, Foulle R, Porras P, Sanchez-Pulido L, Wanker EE, Andrade-Navarro MA. Detection of alpha-rod protein repeats using a neural network and application to huntingtin. PLoS Comput Biol. 2009;5:e1000304. doi: 10.1371/journal.pcbi.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Williams AD, Portelius E, Kheterpal I, Guo JT, Cook KD, Xu Y, Wetzel R. Mapping abeta amyloid fibril secondary structure using scanning proline mutagenesis. J Mol Biol. 2004;335:833–42. doi: 10.1016/j.jmb.2003.11.008. [DOI] [PubMed] [Google Scholar]