Abstract
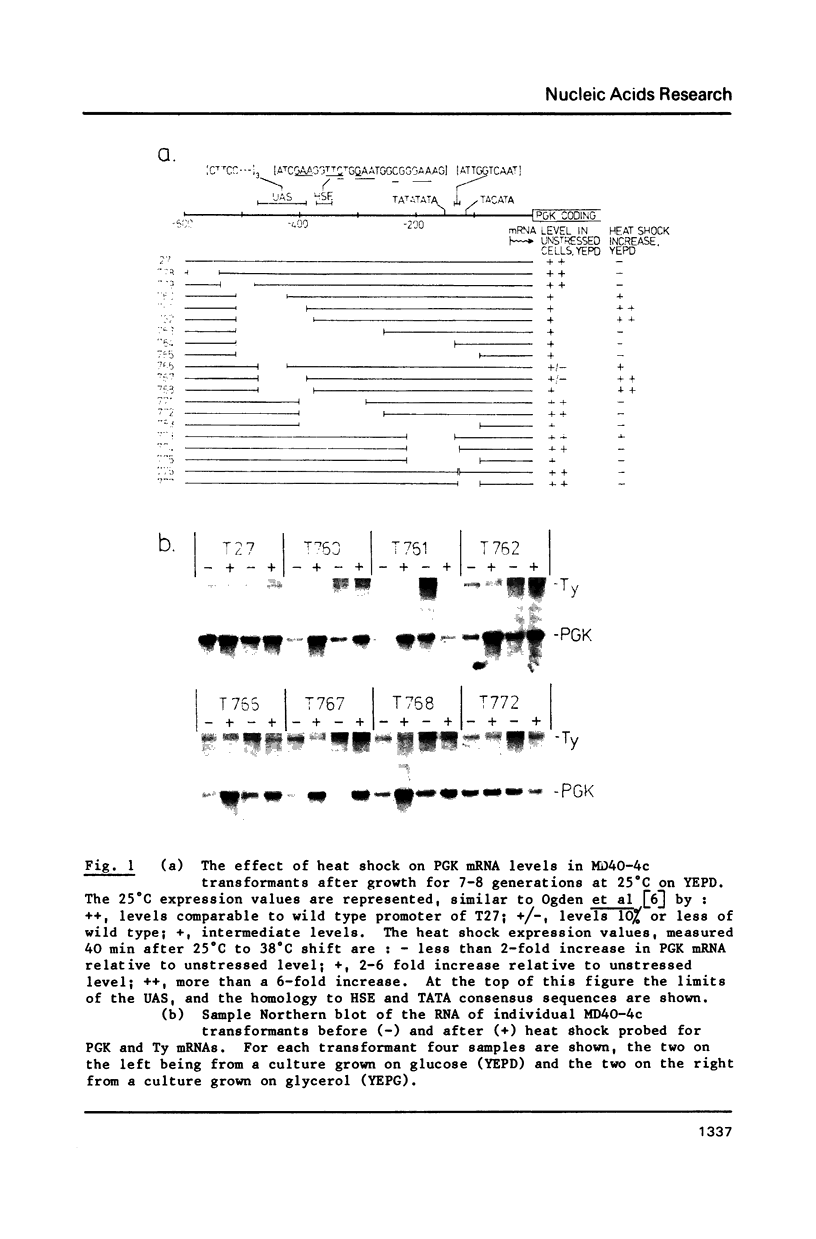
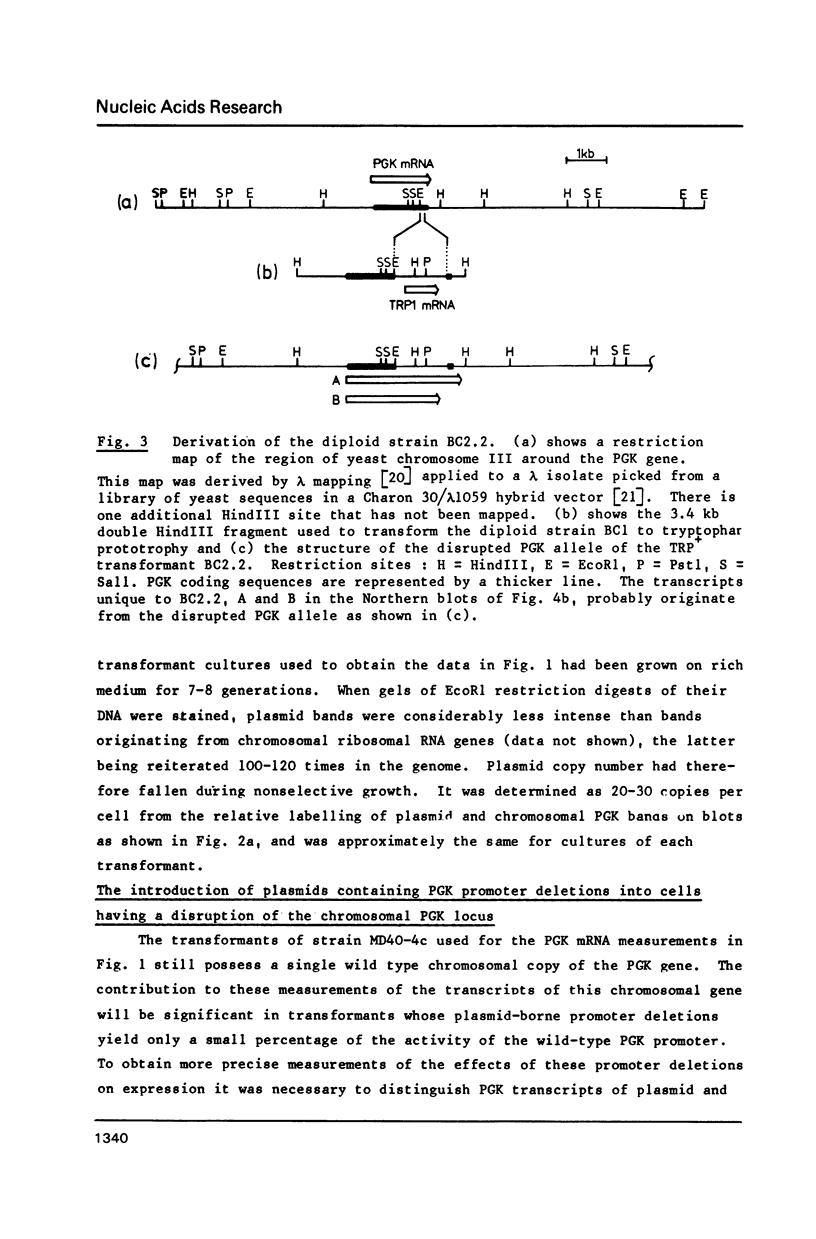
The phosphoglycerate kinase (PGK) promoter is often employed in yeast expression vectors due to its very high efficiency. Its activity in unstressed cells has been shown to be due to an upstream activator site (UASPGK) at -402 to -479. Since levels of PGK mRNA can sometimes be elevated by heat shock of yeast cultures this investigation determined how specific deletions of PGK promoter sequences effect levels of PGK mRNA both before and after heat shock. A series of PGK promoter deletions was inserted on a high copy plasmid into cells having a TRP1 gene disruption of the solitary chromosomal PGK locus. This enabled PGK transcripts of plasmid and chromosomal origin to be distinguished by virtue of their different sizes. Certain deletions lacking UASPGK displayed activities that were very low in unstressed cells, but which increased fifty to one-hundred fold after heat shock. With UASPGK present heat shock had only a relatively small or negligible effect on PGK mRNA levels. Heat shock activation was abolished when the -256 to -377 region with homology to the heat shock element consensus of eukaryotes was deleted in addition to UASPGK, but was unaffected by the deletion of regions further downstream containing TATA- and CAAT- sequence motifs. This is the first demonstration of a heat shock element, an activator site normally found upstream of eukaryotic heat shock protein genes, as a natural constituent of a high efficiency glycolytic promoter. It is proposed that PGK may be one member of a small subset of yeast genes that are highly expressed in unstressed cells yet possess a heat shock element to ensure their continued transcription after heat shock.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodeur G. M., Sandmeyer S. B., Olson M. V. Consistent association between sigma elements and tRNA genes in yeast. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3292–3296. doi: 10.1073/pnas.80.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon R. H. Heat shock and the heat shock proteins. Biochem J. 1986 Dec 1;240(2):313–324. doi: 10.1042/bj2400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Hitzeman R. A. Human, yeast and hybrid 3-phosphoglycerate kinase gene expression in yeast. Nucleic Acids Res. 1987 Jan 26;15(2):643–660. doi: 10.1093/nar/15.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriacy M., Breitenbach I. Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J Bacteriol. 1979 Jul;139(1):152–160. doi: 10.1128/jb.139.1.152-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson M. J., Tuite M. F., Roberts N. A., Kingsman A. J., Kingsman S. M., Perkins R. E., Conroy S. C., Fothergill L. A. Conservation of high efficiency promoter sequences in Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Apr 24;10(8):2625–2637. doi: 10.1093/nar/10.8.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne D C, Mortimer R K. Chromosome Mapping in Saccharomyces: Centromere-Linked Genes. Genetics. 1960 Aug;45(8):1085–1110. doi: 10.1093/genetics/45.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzeman R. A., Clarke L., Carbon J. Isolation and characterization of the yeast 3-phosphoglycerokinase gene (PGK) by an immunological screening technique. J Biol Chem. 1980 Dec 25;255(24):12073–12080. [PubMed] [Google Scholar]
- Kim S., Mellor J., Kingsman A. J., Kingsman S. M. Multiple control elements in the TRP1 promoter of Saccharomyces cerevisiae. Mol Cell Biol. 1986 Dec;6(12):4251–4258. doi: 10.1128/mcb.6.12.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsman S. M., Kingsman A. J., Dobson M. J., Mellor J., Roberts N. A. Heterologous gene expression in Saccharomyces cerevisiae. Biotechnol Genet Eng Rev. 1985;3:377–416. doi: 10.1080/02648725.1985.10647819. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Kingsman A. J., Kingsman S. M. A transcriptional activator is located in the coding region of the yeast PGK gene. Nucleic Acids Res. 1987 Aug 11;15(15):6243–6259. doi: 10.1093/nar/15.15.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Kingsman A. J., Kingsman S. M. Factors affecting heterologous gene expression in Saccharomyces cerevisiae. Gene. 1985;33(2):215–226. doi: 10.1016/0378-1119(85)90096-4. [DOI] [PubMed] [Google Scholar]
- Morgan W. D., Williams G. T., Morimoto R. I., Greene J., Kingston R. E., Tjian R. Two transcriptional activators, CCAAT-box-binding transcription factor and heat shock transcription factor, interact with a human hsp70 gene promoter. Mol Cell Biol. 1987 Mar;7(3):1129–1138. doi: 10.1128/mcb.7.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. E., Stanway C., Kim S., Mellor J., Kingsman A. J., Kingsman S. M. Efficient expression of the Saccharomyces cerevisiae PGK gene depends on an upstream activation sequence but does not require TATA sequences. Mol Cell Biol. 1986 Dec;6(12):4335–4343. doi: 10.1128/mcb.6.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. W., Curran B., Davies M. W., Lockheart A., Reid G. Transcription of the phosphoglycerate kinase gene of Saccharomyces cerevisiae increases when fermentative cultures are stressed by heat-shock. Eur J Biochem. 1986 Dec 15;161(3):525–531. doi: 10.1111/j.1432-1033.1986.tb10474.x. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Cloning and expression of a gene encoding hsc73, the major hsp70-like protein in unstressed rat cells. EMBO J. 1987 Apr;6(4):993–998. doi: 10.1002/j.1460-2075.1987.tb04850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway C., Mellor J., Ogden J. E., Kingsman A. J., Kingsman S. M. The UAS of the yeast PGK gene contains functionally distinct domains. Nucleic Acids Res. 1987 Sep 11;15(17):6855–6873. doi: 10.1093/nar/15.17.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]