Abstract
Estrogen deficiency has been considered the seminal mechanism of osteoporosis in both women and men, but epidemiological evidence in humans and recent mechanistic studies in rodents indicate that aging and the associated increase in reactive oxygen species (ROS) are the proximal culprits. ROS greatly influence the generation and survival of osteoclasts, osteoblasts, and osteocytes. Moreover, oxidative defense by the FoxO transcription factors is indispensable for skeletal homeostasis at any age. Loss of estrogens or androgens decreases defense against oxidative stress in bone, and this accounts for the increased bone resorption associated with the acute loss of these hormones. ROS-activated FoxOs in early mesenchymal progenitors also divert ß-catenin away from Wnt signaling, leading to decreased osteoblastogenesis. This latter mechanism may be implicated in the pathogenesis of type 1 and 2 diabetes and ROS-mediated adverse effects of diabetes on bone formation. Attenuation of Wnt signaling by the activation of peroxisome proliferator-activated receptor γ by ligands generated from lipid oxidation also contributes to the age-dependent decrease in bone formation, suggesting a mechanistic explanation for the link between atherosclerosis and osteoporosis. Additionally, increased glucocorticoid production and sensitivity with advancing age decrease skeletal hydration and thereby increase skeletal fragility by attenuating the volume of the bone vasculature and interstitial fluid. This emerging evidence provides a paradigm shift from the “estrogen-centric” account of the pathogenesis of involutional osteoporosis to one in which age-related mechanisms intrinsic to bone and oxidative stress are protagonists and age-related changes in other organs and tissues, such as ovaries, accentuate them.
Increased oxidative stress is strongly implicated in the biology of aging and the pathogenesis of age-related diseases. Recent evidence indicates that oxidative stress is also a fundamental mechanism of the age-dependant decline of bone mass and strength and that loss of estrogens exaggerates the effects of aging on bone by decreasing defense against oxidative stress. Moreover, the balance between the generation of reactive oxygen species versus defense against them by FoxO-activated transcription programs is critical for bone homeostasis throughout life. Attenuation of Wnt signaling by PPARγ activation by oxidized lipids and an increase in endogenous glucocorticoids with age are two additional mechanisms contributing to skeletal involution. This new knowledge provides a paradigm shift from the traditional “estrogen-centric” account of the pathogenesis of osteoporosis to one in which aging per se is inexorably the protagonist.
I. Introduction
II. The Traditional Estrogen-Centric Perspective of the Pathogenesis of Osteoporosis
III. Aging as a Pivotal Determinant of Loss of Bone Mass and Strength
IV. Aging and Oxidative Stress
- V. Defense Mechanisms against Oxidative Stress
- A. Enzymatic
- B. The FoxO transcription factors
- C. β-Catenin as a pivot in the regulation of oxidative stress-induced transcription programs
VI. Organismal Aging, Oxidative Stress, and Skeletal Homeostasis
- VII. The Antiosteoporotic Effects of Estrogens and their Antioxidant Properties
- A. ROS, estrogens, and osteoblasts
- B. ROS, estrogens, and the generation and apoptosis of osteoclasts
VIII. Diabetes, Oxidative Stress, and Osteoporosis
IX. Lipid Oxidation, Oxidative Stress, PPARγ, and the Link between Osteoporosis and Atherosclerosis
- X. Aging, Endogenous Hyperglucocorticoidism, and Bone Strength
- A. Cell autonomous effects of glucocorticoids on bone
- B. Glucocorticoids and bone strength
- C. Angiogenesis and bone
- D. Bone hydration
XI. Oxidative Stress and Nutrient-Dependent Deacetylases (Sirtuins) as Therapeutic Targets
XII. Summary and Conclusions
I. Introduction
I am well aware that scarcely a single point is discussed in this volume on which facts cannot be adduced, often apparently leading to conclusions directly opposite to those at which I have arrived. A fair result can be obtained only by fully stating and balancing the facts and arguments on both sides of each question.”
Charles Darwin, “The Origin of Species by Means of Natural Selection.”
The decline of ovarian function at menopause leads to loss of bone mass, and estrogen replacement prevents such loss (1,2,3). During the last 60 yr, these two seminal clinical observations have shaped the thinking of basic and clinical investigators on the pathogenesis and thereby the treatment of osteoporosis, overwhelmingly more than, and despite, any other clinical observation or experimental discovery. As a result of this estrogen-centric paradigm, the “postmenopausal” or “type I” form of the disease and its treatment with estrogens or estrogen-related compounds preoccupied the field for most of this period. However, advancements in our understanding of the basic biology of the aging process, the effects of aging and age-related oxidative stress (OS) on bone, genetic discoveries in animal models and humans, the demonstration of a similar function of the same genes in different organs, and a better grasp of integrative physiology and pathophysiology along with the serious shortcomings of estrogen-based therapies, strongly dictate the need for a reappraisal of our traditional ideas about the pathogenesis of osteoporosis. The goal of this review is to highlight these new developments and provide a fresh look at the pathogenesis of osteoporosis, not in the traditional context of a single disease entity, but rather as comorbidity with other accompaniments of old age—which often are present in the same patient. In addition, this review aims to provide an understanding of osteoporosis that is reconciled with what is known about the biology of aging and the mechanisms of other degenerative disorders—which inexorably share molecular pathogenetic mechanisms related to aging per se—such as insulin resistance and atherosclerosis.
II. The Traditional Estrogen-Centric Perspective of the Pathogenesis of Osteoporosis
According to the estrogen-centric paradigm of the pathogenesis of osteoporosis, bone mass remains unchanged between the attainment of its peak value in the third decade of life and menopause in women, or the age of 55 in men. Therefore, loss of bone mass in adulthood must be triggered by the loss of sex steroids (3,4). The postmenopausal bone loss is followed within 4–8 yr by a less sharp rate of continuous decline. This latter phase of loss has been traditionally ascribed to “old age,” and it is thought to occur primarily in cortical sites, as opposed to the faster and self-limiting postmenopausal loss that occurs primarily in cancellous sites (3). Men lose cortical and cancellous bone at a rate that is practically identical to that of the “old age”-dependent loss in women, although they do not experience an abrupt decline of sex steroids and the corresponding rapid phase of bone loss (4). Despite these obvious clues for a role of “old age” in osteoporosis, the molecular and cellular mechanisms of the adverse effects of aging on bone and the extent to which sex steroid deficiency contributes to age-related bone loss and to the less sharp rate of skeletal decline in the late postmenopausal years have remained completely unknown—until recently. On the other hand, several lines of evidence have lent credence to the notion that estrogen deficiency is a seminal mechanism of the age-related bone loss not only in females, but also in males (4,5,6). Support for this view has been provided from extrapolation of three types of evidence: 1) genetic evidence from men with estrogen receptor (ER) α or aromatase mutations (7); 2) results of short-term clinical experimentation with administration of aromatase inhibitors (8); and 3) cross-sectional correlations between free serum E2 levels and bone mineral density (BMD) or bone remodeling markers. However, more recent longitudinal studies of the propositus of genetic ERα mutations indicate that these individuals experience a failure of normal bone development and growth, which is of course a different situation than that of the vast majority of patients with osteoporosis who have normal bone development and growth, but who start losing bone after the attainment of peak bone mass (9). Furthermore, it remains unknown whether, and to what extent, small differences of estrogen or androgen levels in the circulation (10) contribute to changes of bone markers or to BMD determinations in elderly males (11), especially because similar small differences do not seem to cause BMD differences in pre- or perimenopausal women (12).
Additionally, it has been suggested that effects of estrogens on peripheral calcium metabolism, specifically the kidney (13) and intestine (14,15), play a role in preventing secondary hyperparathyroidism (and the associated increase in bone resorption) that sometimes accompanies involutional osteoporosis. However, hyperparathyroidism is not a feature of estrogen deficiency in experimental animal models or in humans with primary hypogonadism or estrogen deficiency caused by anorexia nervosa (16,17,18).
III. Aging as a Pivotal Determinant of Loss of Bone Mass and Strength
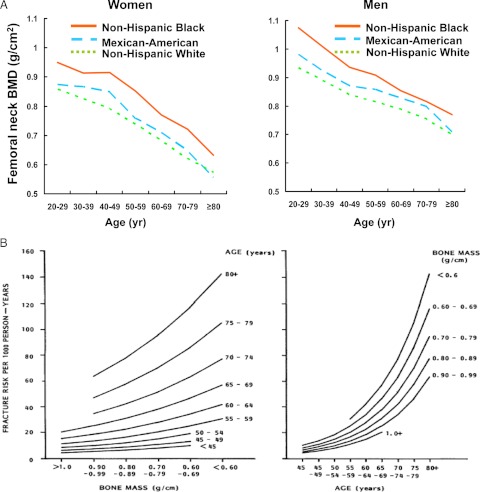
In contrast to the earlier ideas that bone mass remains unchanged between the attainment of its peak and menopause in women or the age of 55 in men (19,20), large epidemiological studies have now clearly established that in both women and men bone loss begins as early as the early part of the third decade—immediately after peak bone mass and long before any change in sex steroid production (21) (Fig. 1). In agreement with the epidemiological data, volumetric BMD analysis at the tibia and spine, on a large age- and sex-stratified population sample using quantitative computed tomography, demonstrates that there is substantial trabecular bone loss in both sexes during sex steroid sufficiency (Table 1) (22). Nonetheless, in women the loss of trabecular bone in the spine accelerates substantially after the menopause, as does the rate of fractures at the wrist, spine, and hip (23), attesting to the adverse role of estrogen deficiency on skeletal homeostasis and its contribution to the acceleration of the age-associated bone loss. Cortical bone loss begins to decline after the age of 50 in both sexes, albeit at a faster rate in women than men.
Figure 1.
A, Bone loss begins in the third decade of life in both sexes. The data are from the Epidemiological Follow-up Study cohort of the First National Health and Nutrition Examination Survey (NHANES), a nationally representative sample of noninstitutionalized civilians who were followed for a maximum of 22 yr. A cohort of 2879 Caucasian men (1437 in the bone density subsample) aged 45–74 yr at baseline (1971–1975) were observed through 1992. [From A. C. Looker et al.: Osteoporosis Int 8:468–489, 1998 (21). Reproduced with permission of the International Bone & Mineral Society and the IBMS BoneKEy where this graphic depiction of the data is provided online.] B, Age is a more critical determinant of fracture risk than bone mass in humans. Data are from a follow-up of 521 Caucasian women over an average of 6.5 yr with repeated bone mass measurements at the radius. A total of 138 nonspinal fractures in 3388 person-years were detected, and the incident fractures were cross-classified by age and bone mass. The incidence of fracture was then fitted to a log-linear model in age and bone mass. [From S. L. Hui et al.: J Clin Invest 81:1804–1809, 1988 (24). Reproduced with permission from The American Society for Clinical Investigation.]
Table 1.
Substantial trabecular bone loss occurs in young adult women and men
| Age groups | Median changes in volumetric BMD (%/yr) |
||||
|---|---|---|---|---|---|
| DR-Trab | DT-Trab | LS-Trab | DR-Cort | DT-Cort | |
| Females | |||||
| 21–49 yr | −0.40 | −0.24 | −1.61 | −0.04 | 0.00 |
| 50+ yr | −0.55 | −0.24 | −2.60 | −0.48, | −0.36, |
| Males | |||||
| 21–49 yr | −0.38 | −0.40 | −0.84 | −0.07 | −0.08 |
| 50+ yr | −0.38 | −0.17 | −1.85 | −0.32 | −0.15 |
Data are from a study of 375 females and 325 males between the ages of 21 and 97 yr, performed by peripheral/central quantitative computed tomography. [From B. L. Riggs et al.: J Bone Miner Res 23:205–214, 2008 (22). Kindly contributed by Dr. Sundeep Khosla and reproduced with the permission of the Journal.] DR, Distal radius; DT, distal tibia; Trab, trabecular; Cort, cortical; LS, lumbar spine.
Difference from zero,
P < 0.05;
P < 0.001.
Sex difference < 0.05.
Notwithstanding the evidence for an age-related decline of bone mass independent of sex steroid status, it is now widely appreciated that osteoporosis (the term used to define decreased bone mass per unit volume of anatomical bone) is not the disease, but rather is one of many factors responsible for the compromised bone strength that predisposes to an increased risk of fracture in the fragility syndrome that has inexactly become synonymous with only one of its features. Indeed, strong evidence for a pivotal contribution of aging to fractures, independent of bone mass, was highlighted almost 20 yr ago by the work of Hui et al. (24), showing that at the same BMD, a 20-yr increase in age is accompanied by a 4-fold increase in fracture risk (Fig. 1). Increased propensity to falls due to age-related decline in neuromuscular function is without doubt a factor for the age-related increase in fracture risk. However, there are also age-related changes in the bone itself, which contribute to the increase in fracture risk for the same BMD with an increase in age (24). For example, type I collagen is structurally complex and can deteriorate as it ages, with changes such as loss of cross-linking between the component chains (25). Collagen can also be damaged by accumulation of advanced glycation end-products (26), another general feature of the aging process. Such changes could account for the age-related decline in cortical bone tensile strength (27). Defective collagen cannot be repaired, so the bone containing it must be replaced by remodeling.
The importance of non-mass factors is demonstrated by several lines of evidence. First, a fracture at any site increases the risk of a subsequent fracture at any other site (28). Second, only a small part of the reduction in fracture incidence in response to anticatabolic therapy can be accounted for by the increase in bone mass (29). Third, many of the genetic effects on bone strength are mediated by factors other than bone mass (30,31). Non-mass factors include disrupted architecture (32), changes in bone mineral and matrix (25), delayed repair of fatigue microdamage (33), excessive turnover (34), and inadequate bone size (35). The most recently appreciated qualitative factor is loss of osteocytes, which makes an independent contribution to vertebral bone strength, both in patients with vertebral fractures (36) and in mice (37). Osteocyte death may disrupt the signals necessary for microdamage repair (38) and also lead to long-term changes in bone hydration, as will be discussed in Section X.D.
Collectively, the above lines of evidence indicate that the estrogen-deficiency paradigm of the pathogenesis of osteoporosis is incomplete. That being said, there is little doubt that loss of bone is due in part to age-related changes in other organs and tissues, such as the ovary (estrogen deficiency), the adrenal gland (glucocorticoid excess or hyperresponsiveness), the kidney [loss of nephrons, reduced synthesis of calcitriol, calcium malabsorption, and secondary hyperparathyroidism (39), which also has other causes], and muscle (sarcopenia, inactivity, reduced mechanical loading). However, the evidence I discussed in this section along with the universality of age-related bone loss indicates that there must be additional age-related mechanisms intrinsic to bone. Excessive accumulation of reactive oxygen species (ROS)—the radical forms of oxygen—contributes to age-related changes in many tissues, and bone is unlikely to be an exception (40,41). In the remainder of this review, I will address some of these mechanisms and highlight evidence for the contribution of OS to the development of osteoporosis.
IV. Aging and Oxidative Stress
To date, more than 50 yr since it was proposed by Harman (42), the most enduring theory of aging stipulates that OS resulting from an increase in intracellular ROS is the major determinant of aging and lifespan (43,44,45), as well as the culprit in several degenerative disorders associated with aging (41). Reactive nitrogen species are also pathogenetic culprits in some of these conditions, but for the sake of space and because very little is known about reactive nitrogen species and osteoporosis, this review will focus on ROS.
Formation of ROS is an inescapable consequence of life in an oxygen-rich environment and occurs primarily in the mitochondria from the escape of electrons passing through the electron transport chain during aerobic metabolism—the process that is fueled by nutrients such as glucose and is responsible for the formation of ATP (43,46). ROS are also generated during fatty acid oxidation or in response to external stimuli, such as inflammatory cytokines, growth factors, environmental toxins, chemotherapeutics, UV light, or ionizing radiation. Free electrons are added to molecular oxygen to generate superoxide (O2·−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH·−). Of these species, H2O2 has the highest oxidative activity, the highest stability, and the highest intracellular molar concentration.
As a consequence of its properties, H2O2 represents a critical signal for the replicative capacity of regenerative cells, apoptosis, and global changes in gene expression leading to aging and aging-related diseases (43). The production of H2O2 is amplified considerably by the adapter protein p66shc, which is released from an inhibitor complex in the inner mitochondrial membrane in response to a variety of proapoptotic stimuli and acts as a redox enzyme catalyzing the reduction of O2 to H2O2 through electron transfer from cytochrome c (47,48,49). H2O2 in turn causes opening of the permeability transition pore, swelling, and apoptosis. Deletion of the p66shc gene enhances cellular resistance to apoptosis induced by H2O2 or UV light, and mice deficient in p66shc not only exhibit increased resistance to OS but also have a 30% increase in lifespan (48). Importantly, p66shc sensitizes cells to proapoptotic stimuli by activating thymoma viral protooncogene 1 (Akt), phosphorylating/inactivating FoxO transcription factors, and preventing the induction of antioxidant/free radical scavenging genes [such as manganese superoxide dismutase (MnSOD)] (50,51).
Until recently, it was assumed that ROS are exclusively harmful by-products of aerobic life that damage proteins, lipids, and DNA leading to cell death, and that it was important for cells to eliminate them. However, extensive new evidence indicates that at levels lower than those that cause OS and damage, ROS, like H2O2, are deliberately produced within cells (and removed) to trigger physiological signaling cascades by causing reversible oxidations of proteins at cysteine residues (52). Such reversible amino acid oxidations regulate ERKs, jun N-terminal kinases (JNKs), p38 MAPK, phosphatidyl inositol 3 kinase (PI3K)/Akt, activator protein-1, p53, tyrosine phosphatases, proteases, molecular adaptors and chaperones, the Wnt/β-catenin signaling pathway, as well as the activity of transcription factors including nuclear factor κB (NF-κB), the glucocorticoid and estrogen receptors, and hypoxia inducible factor-1α (HIF-1α) (52).
V. Defense Mechanisms against Oxidative Stress
A. Enzymatic
To protect against oxidative insults, organisms ranging from prokaryotes to mammals utilize a network of overlapping mechanisms that involve both enzymatic reactions and altered gene transcription. OS occurs when the rate of generation of ROS exceeds the capacity of the cell to detoxify them. OS increases with advancing age, in part because the ability of cells to scavenge ROS decreases progressively with lifespan (44,53). Of the most important antioxidant enzymes, various forms of SOD catalyze the conversion of O2·− to H2O2, and catalases convert H2O2 to water and oxygen. Alternative mechanisms of ROS detoxification involve reactions with thiol-containing oligopeptides with redox-active sulfhydryl moieties, the most abundant of which are glutathione (GSH) and thioredoxin (Trx). GSH peroxidase (Gpx) converts peroxides to harmless alcohols in a reaction in which it oxidizes GSH to the disulfide GSSH; and GSH reductase (GSR) converts GSSH back to GSH (54). The peroxiredoxin family of enzymes provides a similar defense mechanism using Trx as substrate.
B. The FoxO transcription factors
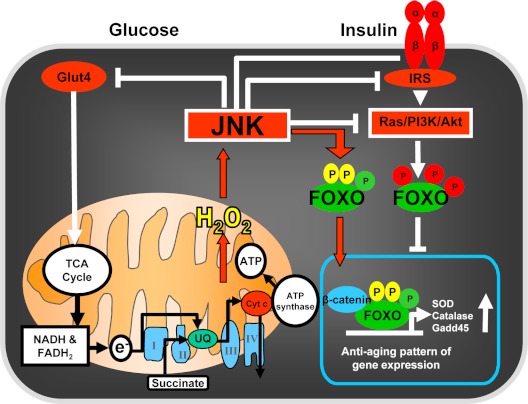
FoxOs, a subclass of a large family of forkhead proteins characterized by the presence of a winged-helix DNA binding domain called Forkhead box, represent another major cell defense mechanism against oxidative insults. In mammals, this subclass comprises four members: FoxO1 (or FKHR), FoxO3 (or FKHRL1), FoxO4 (also called AFX), and FoxO6 (55). FoxO1, -3, and -4 show broad, overlapping patterns of expression in developing and adult tissues, whereas FoxO6 is restricted to specific structures of the developing brain. FoxOs shuttle between the cytoplasm and the nucleus depending on the phosphorylation of specific sites by distinct sets of kinases (Fig. 2). In the setting of stimulation by growth factors, such as insulin and IGFs, FoxO1, -3, and -4 are subject to Akt-mediated phosphorylation, which results in their nuclear export and inhibition of FoxO-mediated transcription (56,57,58,59,60). As will be discussed in Section VIII, exclusion of FoxOs from the nucleus is essential for glucose homeostasis because it is critical for the ability of insulin and other growth factors to stimulate β-cell proliferation and survival and thereby the expansion of β-cell mass in the pancreas (61,62,63).
Figure 2.
Schematic illustration of the shuttling of FoxOs between the cytoplasm (in the setting of growth factor stimulation, e.g., insulin) and the nucleus in response to oxidative stress (exemplified by H2O2). Note the distinct site of phosphorylation and cytoplasmic retention of FoxOs by the insulin- initiated Ras/PI3K/Akt cascade, as opposed to the sites of phosphorylation and nuclear localization of FoxOs in response to the H2O2-induced JNK activation cascade. The structure in the bottom left depicts a mitochondrion and its electron transport chain along with the tricarboxylic acid cycle that is responsible for ATP synthase activation and the generation of ATP.
On the other hand, OS promotes the translocation of FoxOs into the nucleus by a mechanism that involves activation of the JNK or macrophage stimulating 1 kinases by ROS and the phosphorylation of FoxOs in different sites than those activated by Akt. OS also promotes other posttranslational modifications of FoxOs, including ubiquitylation and acetylation (64). These changes in turn lead to the activation of FoxOs and thereby to FoxO-mediated OS responses by regulating the transcription of antioxidant enzymes such as MnSOD and catalase as well as genes involved in cell cycle, DNA repair, and lifespan (64,65). Through these effects, FoxOs promote mammalian cell survival by inducing cell cycle arrest and quiescence in response to OS, and they also regulate longevity in model organisms (66,67,68). Importantly, stress stimuli override the sequestration of FoxO in the cytoplasm by growth factors, both in mammalian cells and in Drosophila (69,70). FoxOs can also induce apoptosis through FoxO-mediated regulation of several proapoptotic genes (56,71). The role of FoxOs in cell death seems counterintuitive considering their role in the protection against OS. However, elimination of damaged or abnormal cells by FoxO-induced apoptosis evidently plays an important role in the ability of these transcription factors to promote tumor suppression and longevity (72).
The importance of FoxOs in mammalian biology has been recently highlighted by the profound effects of the deletion of individual FoxOs or the combined deletion of FoxO1, -3, and -4 in mice. FoxO1 is indispensable during development because FoxO1-null mice die at embryonic d 10.5 due to defects in angiogenesis (73,74). FoxO3-null mice become infertile due to premature activation of the ovarian follicles and subsequent oocyte exhaustion (75). These mice also develop lymphoproliferative disorders, consistent with a role of FoxO3 in the promotion of cell cycle arrest (76). In addition, they exhibit defects in glucose uptake in line with a role for FoxO family members in glucose metabolism (77). Studies of FoxO-deficient mice have revealed that FoxOs are also indispensable for normal erythropoiesis and hematopoietic stem cell quiescence and survival, because of their ability to prevent excessive accumulation of ROS (78,79). Specifically, FoxO3 null mice die rapidly when exposed to OS because of failed erythropoiesis (79). Moreover, combined conditional somatic deletion of FoxO1, -3, and -4 using the Mx-Cre+ transgene results in increased ROS levels in hematopoietic stem cells (HSCs). Increased ROS enforces cell fate decisions, driving HSCs into the cell cycle and terminal differentiation at the expense of self-renewal (78). This phenotype was completely reversed by administration of the antioxidant N-acetyl cysteine (NAC), indicating that FoxOs maintain HSC quiescence and survival by handling free radicals constantly generated in HSCs.
Extensive evidence also indicates that FoxOs restrict angiogenesis. This fact has been convincingly demonstrated by the striking overproduction of new blood vessels in mice with conditional deletion of the three major FoxO genes (80). The suppressive effect of FoxOs on angiogenesis results from their ability to suppress endothelial cell proliferation and survival (80). One of the potential mechanisms of this effect is direct binding of FoxOs to HIF-1α on some target genes and prevention of HIF-1α interaction with p300, a required transcriptional coactivator (81). In addition, FoxOs stimulate the expression of CITED2, a factor that also inhibits HIF-1α and p300 interaction (82). As will be discussed in Section X.D, these molecular mechanisms may be directly relevant to the suppression of angiogenesis and bone formation by old age and glucocorticoid excess because both of these conditions increase OS and FoxO activity.
C. β-Catenin as a pivot in the regulation of oxidative stress-induced transcription programs
During the last few years, Wnts, a large family of secreted glycoproteins, and their signaling pathway have received major attention by bone biologists because of genetic evidence that loss or gain of function mutations of the low-density lipoprotein receptor-related protein 5 or 6 (LRP5 or LRP6), the coreceptors for Wnts, are responsible for the dramatic decrease or increase of bone mass associated with the osteoporosis pseudoglioma syndrome and the hereditary high-bone-mass trait in humans, respectively (83,84,85,86). In line with evidence for a major role of Wnts in the regulation of bone mass, increased Wnt/β-catenin signaling is evidently a normal physiological response of bone to changes in mechanical load (87). Furthermore, osteocytes—former osteoblasts that are entombed in the mineralized matrix and are responsible for sensing and adapting bone to changes in mechanical needs—exert their potent influence on bone mass by controlling Wnt/β-catenin signaling thanks to their ability to produce and secrete sclerostin, a potent Wnt/β-catenin inhibitor (88,89,90). Loss of function mutations of SOST—the gene encoding sclerostin—cause the high bone mass disorders Van Buchem’s disease and sclerosteosis (91,92), whereas mice overexpressing SOST exhibit low bone mass (88). Moreover, a neutralizing antisclerostin antibody dramatically increases bone formation in rodents and humans and is currently under development as an anabolic bone therapy for patients with osteoporosis (93). The ability of Wnts to regulate bone mass results from a critical role of β-catenin in the commitment of multipotential mesenchymal progenitors to the osteoblastic lineage and from the prevention of the apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades (94,95). Unexpectedly, the Wnt coreceptor LRP6 is also a key element of the PTH signaling that regulates osteoblast activity (96). Thus, binding of PTH to its receptor PTH1R induces the association of LRP6 with PTH1R and stabilization of β-catenin. And, an increase in the amount of β-catenin in osteoblasts in response to PTH is evidently responsible for the ability of the hormone to increase bone formation in the rat model. This evidence notwithstanding, the effect of the activating LRP5 mutation on bone formation was recently shown to result not from direct effects on bone cells, but indirectly through the inhibition of serotonin synthesis in the duodenum (97).
Besides bone formation, the Wnt/β-catenin signaling pathway affects many other biological processes ranging from embryonic development, patterning, and postembryonic stem cell fate to insulin secretion in adulthood and cancer (98). β-Catenin mediates canonical Wnt signaling by binding to and activating members of the T cell factor (TCF)/lymphoid enhancer factor transcription factor family (99). β-Catenin also plays a very important role in the defense against OS, by virtue of the fact that in addition to its important role in mediating TCF/lymphoid enhancer factor transcription, β-catenin is an essential coactivator of FoxOs (100,101,102,103,104). OS, as opposed to insulin and growth factor signaling, promotes FoxO binding to β-catenin and activation of FoxO transcription. The interaction between β-catenin and FoxOs is evolutionarily conserved as evidenced by the fact that in Caenorhabditis elegans the β-catenin ortholog, BAR-1, is required for OS-induced expression of the FoxO ortholog DAF-16 target gene sod-3 and for resistance to oxidative damage (100).
VI. Organismal Aging, Oxidative Stress, and Skeletal Homeostasis
The histological hallmark of age-related bone loss in humans and animals is a decline in mean wall thickness—an index of the amount of bone made by each team of osteoblasts during bone remodeling (105,106). This is due mainly to a deficit in the number of osteoblasts rather than their biosynthetic capacity, and it is thought to result from decreased osteoblastogenesis or increased apoptosis (1,107,108). Despite this important histological insight, the molecular and cellular mechanisms of this adverse effect of aging on bone have until fairly recently remained elusive.
In agreement with the clinical and epidemiological evidence that aging per se is a pivotal mechanism of the decline of bone mass and strength, several lines of recent evidence, primarily from the mouse model, strongly suggest that the OS that underlies physiological organismal aging is an important pathogenetic mechanism of the age-related bone loss and strength. It is important to note here that in a difference from humans, mice do not undergo acute loss of estrogen in middle life, making them an invaluable experimental tool for dissecting the effects of old age from the effects of sex steroid deficiency.
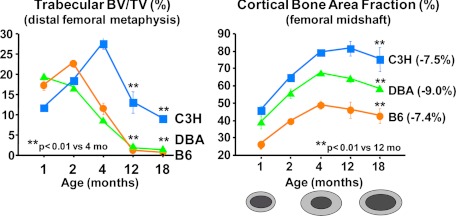
Despite the fact that rodents do not experience acute estrogen loss, a decline of BMD starting as early as 2–3 months of age has been established in several strains of mice (109,110), as well as in rats (111). As shown in Fig. 3, loss of bone mass with age is present in all strains of mice examined and involves both the cancellous and cortical compartment, as determined by micro-computed tomography. However, cortical bone loss occurs later in life than trabecular bone loss. Importantly, C3H, one of the strains depicted in Fig. 3, is resistant to ovariectomy-induced loss of cortical bone (112), but not to the aging-associated loss, highlighting the overriding importance of the age-related mechanisms.
Figure 3.
Both trabecular and cortical bone mass decrease with age in mice. Micro-computed tomography analysis at the distal femoral metaphysis (left) and the femoral midshaft (right) of virgin female mice (n = 6 to 10 per group) was performed at the indicated time points. At the bottom of the right graph is a schematic representation of the changes of the femoral cortex with age, depicting the enlargement and thickening during the growth period (from 1 to 4 months) and the thinning after the attainment of peak bone mass due to endosteal resorption. BV, Bone volume; TV, total volume. [Unpublished data from M. Bouxsein and V. Glatt, Beth Israel Deaconess Medical Center, Harvard Medical School, generously provided for the purpose of this review article.]
Studies from our group have shown that progressive loss of bone strength and mass with age in sex steroid-sufficient female or male C57BL/6 mice is temporally associated with decreased osteoblast and osteoclast numbers and decreased bone formation rate as well as increased osteoblast and osteocyte apoptosis (113). Most importantly, the age-dependent decline of bone mass and strength is temporally linked with increased ROS levels and decreased GSR activity in the bone marrow, as well as a corresponding increase in the phosphorylation of p53 and p66shc—the adapter protein that amplifies mitochondrial ROS generation and influences apoptosis and lifespan in mice (47). In agreement with our findings, studies by others have determined that both osteoblast numbers and bone formation are decreased in mice treated with an inhibitor of GSH (114). Moreover, murine models of premature aging and signs of oxidative damage exhibit osteoporotic features (115,116). Furthermore, as in mice, an association between OS and a decrease in BMD (117,118,119,120,121,122), as well as an effect of antioxidants on bone resorption (123,124), has been noted in several human clinical studies.
As discussed in Section V.B, OS activates FoxO transcription factors, which in turn combat OS by activating genes involved in free radical scavenging, DNA repair, and apoptosis; and β-catenin—a factor required for osteoblast differentiation (125)—is essential for ROS-induced FoxO activation (100). In C57BL/6 mice, the increase in OS and the decreased bone formation with increasing age are associated with increased FoxO-target gene expression and a decrease in β-catenin/TCF-target gene expression on bone. Moreover, in osteoblastic cell models, OS induces the association of FoxOs with β-catenin and promotes FoxO-mediated transcription at the expense of β-catenin/TCF-mediated transcription and osteoblast differentiation (101). The negative effect of ROS on TCF transcription is abrogated by raising the level of β-catenin, suggesting that a limited pool of active β-catenin is diverted from TCF to FoxO transcription under stress conditions (102,104). In line with these ideas, mice with targeted expression of Wnt10b in the bone marrow or mice carrying the LRP5 G171V activating mutation in osteoblasts have increased bone mass and no evidence of age-related loss of bone mass or strength, respectively (126,127). Hence, diversion of the limited pool of β-catenin from TCF- to FoxO-mediated transcription in osteoblastic cells represents a cell-autonomous mechanism of β-catenin/TCF antagonism. FoxOs are also known to suppress the expression and transcriptional activity of peroxisome proliferator-activated receptor (PPAR) γ, which is a potent repressor of osteoblastogenesis (128,129,130). Together, these observations suggest that FoxOs play an important role in bone biology by enabling the maintenance of a physiologically appropriate lifespan of mature osteoblasts through their oxidative defense activities. In addition, FoxOs may control the generation of new osteoblasts from their mesenchymal stem cell progenitors by modulating their proliferation and/or differentiation through their antioxidant properties or via modulating the activity of other transcription factors such as β-catenin or PPARγ.
Based on the evidence that FoxOs translate environmental stimuli, such as OS and hormonal changes, into dynamic gene expression programs involved in many physiological as well as pathological processes (64,65,131), and that decreased defense against OS is responsible, at least in part, for the adverse effects of aging on the murine skeleton, we have investigated in my laboratory the impact of the genetic manipulation of FoxOs on skeletal homeostasis in mice (132). Specifically, we hypothesized that if one were to remove an important defense mechanism against OS from bone cells, one may recapitulate at least some of, the adverse effects of aging on bone in young mice. We found that conditional deletion of the three broadly expressed genes, FoxO1, -3, and -4, in young adult mice or targeted overexpression of FoxO3 in osteoblasts leads to significant changes in bone mass. Specifically, loss of FoxO function in 3-month-old mice for a period of 5 wk results in increased OS in bone as well as increased osteoblast and osteocyte apoptosis and an osteoporotic phenotype characterized by decreased bone mass at both cancellous and cortical sites. The increased osteoblast apoptosis following the triple FoxO deletion is cell autonomous and is the result of increased OS because the rate of osteoblast apoptosis of the FoxO-deficient osteoblasts in ex vivo cultures can be reverted to normal by the addition of the antioxidant NAC. In sharp contrast to the adverse skeletal effects of the loss of FoxO function, overexpression of FoxO3 in mature osteoblasts leads to opposite effects: decreased OS and osteoblast apoptosis, and increased osteoblast number, bone formation rate, and vertebral bone mass. In agreement with findings from this model, the adverse effects of aging on cancellous and cortical bone can be reproduced in young mice by the administration of the prooxidant paraquat (M. Almeida, E. Ambrogini, S. C. Manolagas, unpublished data).
Osteoblast generation in ex vivo cultures of osteoblastic progenitors derived from the bone marrow of FoxO-deficient mice is attenuated compared with the control mice. This could result from either an increase in the apoptosis of these progenitors or attenuation of their differentiation. In support of the latter explanation, deletion of FoxOs causes an increase of PPARγ, the nuclear receptor that stimulates adipogenesis (133), whereas it represses osteoblastogenesis (130) and is tonically suppressed by FoxOs (128,129).
The evidence from the genetic analysis of the role of FoxOs in bone provides strong support for the view that ROS are seminal signals for the fate of osteoblasts and that inappropriate increase of ROS adversely affects bone formation. The FoxO family of transcription factors defends against such an increase by constantly up-regulating free radical scavenging and DNA-repair enzymes, thereby representing an indispensable homeostatic mechanism for skeletal health. In addition, FoxOs may control the generation of new osteoblasts from their mesenchymal stem cell progenitors by modulating their proliferation and/or differentiation through their antioxidant properties or via modulating the activity of other transcription factors such as β-catenin or PPARγ, but this topic is discussed further in Section I.X.
The antioxidant defense provided by FoxOs is ultimately overwhelmed by high level of OS or OS-activated pathways that interfere with the activity of FoxOs, or both. Thus, extensive evidence indicates that ROS-induced p66shc activation leads to FoxO inactivation via Akt-mediated FoxO phosphorylation (50,51). Moreover, ROS-induced stimulation of NF-κB may lead to inhibitory κB kinase-mediated phosphorylation and inhibition of FoxO3 activity, at least in part by targeting it to ubiquitin-dependent degradation (134). Increased phosphorylation of p66shc as well as increased activity of NF-κB are common features of old age and sex steroid deficiency (113,135,136,137). Furthermore, work from the author’s group which will be discussed in Sections IX and X has elucidated that age-associated increases in lipid oxidation and endogenous glucocorticoid production and sensitivity stimulate osteoblast apoptosis by both ROS-dependent and ROS-independent mechanisms. Hence, a plethora of counteracting mechanisms can account for overwhelming the ability of FoxOs to thwart osteoblast apoptosis in old age.
VII. The Antiosteoporotic Effects of Estrogens and their Antioxidant Properties
In addition to the evidence that the OS that underlies physiological organismal aging is a pivotal pathogenetic mechanism of the age-related bone loss and strength, evidence that will be summarized in this section suggests that the antiosteoporotic effect of estrogens results, at least in part, from their ability to protect against OS. In fact, loss of estrogens or androgens accelerates the effects of aging on bone by decreasing defense against OS.
The hallmark of the acute loss of sex steroids (as in menopause or after ovariectomy of humans and animals) is an increase in the rate of bone remodeling, resulting from an increase in both osteoclastogenesis and osteoblastogenesis and a corresponding increase in bone resorption and formation, albeit the former exceeds the latter, most likely because estrogen deficiency prolongs the lifespan of osteoclasts but decreases the lifespan of osteoblasts. Consistent with this evidence, estrogens protect the adult skeleton against bone loss by slowing the rate of bone remodeling (turnover) and by maintaining a focal balance between bone formation and resorption (1,138,139). Indeed, slowing of bone remodeling is evidently due to the attenuating effects of sex steroids on the birth rate of osteoclast and osteoblast progenitors (140,141). Maintenance of a focal balance between formation and resorption, on the other hand, is explained by the opposite effects of estrogens on the lifespan of osteoclasts and osteoblasts/osteocytes: a proapoptotic effect on osteoclasts and an antiapoptotic effect on osteoblasts and osteocytes (142,143,144). Despite these advances, it has remained unknown whether estrogen deficiency contributes to age-related bone loss—and if so, how.
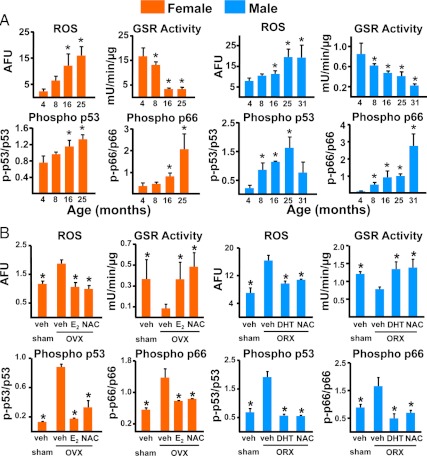
Strikingly, the exact same increases in ROS levels and p53 and p66shc phosphorylation and the decrease of GSR activity observed with advancing age in C57BL/6 mice are caused by the removal of the gonads in 5 month-old female or male mice (Fig. 4). Moreover, all these changes are reversed in the gonadectomized animals by replacement with estrogens or a nonaromatizable androgen or by the administration of the antioxidant NAC (113). More telling, NAC prevents the gonadectomy-induced loss of bone as well as the increase in osteoblast and osteocyte apoptosis as effectively as the replacement with estrogens or androgens. Similar to these findings, Lean et al. (145) have shown earlier that GSH and Trx, the major thiol antioxidants, and GSR and Trx reductase, the enzymes responsible for maintaining them in a reduced state, fall substantially in 2-month-old rat and mouse bone marrow after ovariectomy and are rapidly normalized by exogenous E2. Moreover, administration of NAC, ascorbate, or catalase prevents ovariectomy-induced bone loss in these models, whereas l-buthionine-(S,R)-sulfoximine (BSO), a specific inhibitor of GSH synthesis, causes substantial bone loss. Likewise, Muthusami et al. (146) found that ovariectomy in rats increases H2O2 and lipid peroxidation in the femurs and decreased SOD, Gpx, and GSH S transferase; and H2O2 levels and SOD activity are inversely correlated.
Figure 4.
Advancing age (A) and loss of sex steroids (B) cause similar changes in oxidative stress. ROS and GSR activity were measured in the bone marrow aspirates, and the phosphorylation status of p53 and p66shc was determined by Western blot analysis in vertebral lysates from female or male C57BL6 mice at the indicated ages. Ovariectomy (OVX) or orchidectomy (ORX) was performed at 5 months of age, and analysis was done 6 wk later. Bars indicate mean ± sd; n = 4 mice per group. AFU, Arbitrary fluorescence units; veh, vehicle. *, P < 0.05 compared to 4 months or OVX or ORX + vehicle. [Modified from M. Almeida et al.: J Biol Chem 282:27285–27297, 2007 (113).]
In line with the view that the beneficial effects of estrogens on bone result, at least in part, from their antioxidant properties, beneficial effects of estrogens in several other tissues, such as heart, arteries, central nervous system, lens epithelial cells, fat, liver, and oviducts, are also shown to result from improved defense against OS (147,148,149,150,151,152,153,154,155,156,157,158,159). In fact, practically identical to the evidence in bone, estrogens prevent cardiomyocyte apoptosis and the development of congestive heart failure in mice by increasing the expression of Trx, Trx reductases, and Trx reductase activity in the heart (160). Furthermore, long-term estrogen treatment prevents the activation of apoptosis signal-regulating kinase 1 and its downstream effectors, JNK and p38 MAPK. Estrogen-treated cardiomyocytes are much more resistant to angiotensin II-induced apoptosis; and the antiapoptotic and cardioprotective effects of E2 are blocked by an ER antagonist (ICI 182,780) and by a Trx reductase inhibitor (azelaic acid), indicating that long-term estrogen treatment improves congestive heart failure by antioxidant mechanisms. On the other hand, adverse effects of estrogens on breast cancer, the uterus, and spermatogenesis may be due to increased ROS production or decreased antioxidant defense (161,162,163).
A. ROS, estrogens, and osteoblasts
Earlier work from the author’s group has indicated that estrogens and androgens control osteoblast and osteoclast apoptosis by a mechanism that is distinct from that requiring direct interaction of their receptors with DNA (hormone response element) or protein/protein interaction between the receptor and other transcription factors. Instead, the effect of estrogens on the apoptosis of either cell type is the result of an extranuclear action of the classical receptors that cause activation of cytoplasmic kinases, including ERKs, and kinase-dependent changes in the activity of transcription factors (142,144,164). Additionally, the number of osteoblast progenitors, as measured by colony forming units-osteoblast (CFU-OB), increase after loss of estrogens in mice (165), and this change is partially preserved in mice treated with antiresorptive drugs like bisphosphonates, indicating that bone resorption (and the release of growth factors from the bone matrix) is not required for the increase in osteoblast precursors. Therefore, estrogens must suppress osteoblastogenesis by direct actions on early osteoblast precursors. Furthermore, most murine CFU-OBs are early transit-amplifying progenitors (i.e., dividing cells with limited self-renewal capacity), and their replication is indeed attenuated by estrogens (141).
Prompted by the observations that estrogens diminish OS in bone and bone marrow and attenuate the prevalence of mature osteoblast apoptosis as well as osteoblastogenesis, Almeida et al. (166) have searched for the molecular mechanism of these effects using as tools a mouse model bearing an ERα knock-in mutation that prevents binding to DNA and several osteoblast progenitor cell models expressing the wild-type ERα. The ability of estrogens to diminish the generation of ROS, stimulate the activity of GSR, and to decrease the phosphorylation of p66shc, osteoblastogenesis (in ex vivo bone marrow cultures), and osteoblast number and apoptosis is fully preserved in these mice, indicating that the DNA-binding function of the ERα is dispensable for all these effects. Consistent with the attenuation of osteoblastogenesis in this animal model, E2 attenuates bone morphogenetic protein (BMP)-2- induced gene transcription and osteoblast commitment and differentiation in murine and human osteoblastic cell lines, as well as in primary cultures of calvaria or bone marrow-derived osteoblastic cells from C57BL/6 mice. The inhibitory effect of the hormone on BMP-2 signaling results from an ERα-mediated activation of ERKs and the phosphorylation of Smad1 at the linker region of the protein, which leads to Smad1 proteasomal degradation. In agreement with these findings, the number of CFU-OBs in the bone marrow is attenuated in C57BL/6 mice treated for 28 d with NAC (167). These results indicate that the effects of estrogens on OS and on the birth and death of osteoblasts do not require the binding of ERα to DNA response elements, but instead they result from the activation of cytoplasmic kinases.
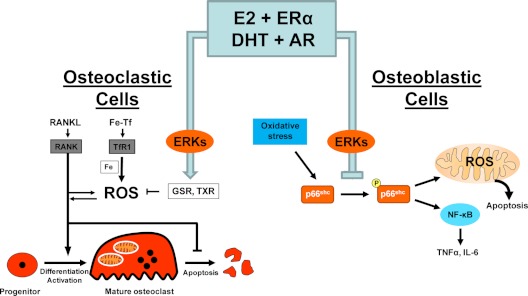
In agreement with the in vivo evidence that estrogens or androgens prevent bone loss and the apoptosis of osteoblasts and osteocytes via antioxidant effects, in both primary bone marrow-derived cell cultures and osteoblastic cell lines, E2 or dihydrotestosterone (DHT) attenuate osteoblasts apoptosis induced by etoposide, H2O2, or TNF and suppress p66shc phosphorylation. These effects are mediated by a mechanism that involves direct antioxidant actions of either class of sex steroids and is dependent on Src and MAPK kinase kinases (113). Similarly, estrogens and selective ER modulators suppress the H2O2-induced apoptosis of the osteocyte-like cell line MLO-Y4 (168). Moreover, estrogens attenuate OS-induced IL-6 and TNFα (two cytokines implicated in estrogen deficiency- induced bone loss) production by stromal/osteoblastic cells by attenuating NF-κB activation (169). In line with this evidence, an increase in ROS, particularly H2O2, or depletion of GSH suppress osteoblastic differentiation of bone marrow cells, also in an ERK-dependent manner (170); and osteoblast apoptosis induced by OS in vitro is attenuated by glutaredoxin 5 (171). Moreover, the progressive increase in OS with age is associated with increased IL-1 and IL-6 mRNA expression in vertebral lysates, as well as an increase in the number of osteoclast progenitors. Importantly, the effects of aging are reproduced in young mice after administration of the GSH inhibitor BSO or the prooxidant agent paraquat (169). Furthermore, administration of the antioxidant NAC to orchidectomized mice decreases osteoclast progenitor numbers as effectively as the administration of DHT or E2. In addition, E2 or DHT potently suppress H2O2-induced p66shc phosphorylation, as well as the phosphorylation of IκB and NF-κB transcriptional activation and also suppress the production of osteoclastogenic cytokines like TNF and IL-6 in two bone marrow-derived stromal cell models (OB6 and UAMS-32) that support osteoclastogenesis. Strikingly, silencing p66shc by short hairpin RNA attenuates the ability of H2O2 to induce IκB phosphorylation and NF-κB transcriptional activity and dramatically reduces the levels of IL-6 mRNA as well as the activation of IL-6 by H2O2. These results demonstrate that p66shc is an essential mediator of the stimulating effects of OS on NF-κB activation, cytokine production, and osteoclastogenesis. The ability of either estrogen or androgen to attenuate the effects of OS on NF-κB activation, cytokine production, and osteoclastogenesis results from their shared ability to suppress p66shc phosphorylation (169) (Fig. 5).
Figure 5.
ROS-activated signals affecting the genesis and lifespan of osteoblasts and osteoclasts and the counter-regulatory actions of sex steroids. In osteoclast precursors, RANKL-induced activation of RANK stimulates ROS production, which is essential for osteoclastogenesis. In addition, mitochondria biogenesis coupled with activation of the transferrin receptor (TIR1) by the iron-transferrin (Fe-Tf) complex stimulates mitochondria respiration and ROS production, which are also essential for osteoclast activation. Estrogens and androgens, acting via their respective receptors (ERα and AR), attenuate both osteoclastogenesis and survival by stimulating GSH and Trx reductase in an ERK-dependant manner (113,145,172,176,177). In osteoblastic cells, p66shc is an essential mediator of the effects of oxidative stress on apoptosis, NF-κB activation, and cytokine production. Estrogens and androgens attenuate these effects by suppressing p66shc phosphorylation in an ERK-dependent manner (113,169).
B. ROS, estrogens, and the generation and apoptosis of osteoclasts
Osteoclasts are acid-secreting polykaryons that have high energy demands and contain abundant mitochondria. Mitochondria and ROS, particularly H2O2, play a crucial role in osteoclast differentiation and function. Indeed, ROS or depletion of GSH increases osteoclast number and resorption in vitro and in vivo by stimulating receptor activator of NF-κB ligand (RANKL) and TNFα expression through ERK and NF-κB activation (172,173,174,175). Moreover, osteoclast formation upon stimulation of bone marrow-derived monocytes/macrophages with the RANKL—a sine qua non osteoclastogenic signal—increases ROS through a cascade involving TNFα, TRAF6, Rac1, and nicotinamide adenine dinucleotide phosphate oxidase (NADPH). The antioxidant NAC and inhibition of NADPH (Nox1), the enzyme that generates ROS, prevent all these effects as well as RANKL-induced JNK, p38, and ERK activation and osteoclastogenesis (176). Similarly, PTH- and IL-1-stimulated bone resorption is inhibited by both natural and recombinant SOD (172). Gpx1, the enzyme primarily responsible for the intracellular degradation of H2O2, is highly expressed in osteoclasts, and its expression is increased in bone marrow macrophages by RANKL and in osteoclasts by E2. Overexpression of Gpx in osteoclast progenitors abolishes osteoclast formation and suppresses RANKL-induced NF-κB activation and increased resistance to oxidation of dihydrodichlorofluorescein by exogenous H2O2 (175). Furthermore, mitochondrial biogenesis is integrated with osteoclast differentiation. Specifically, mitochondrial biogenesis coupled with iron uptake through TfR1 and iron supply to mitochondrial respiratory proteins represent a fundamental pathway linked to osteoclast activation and bone metabolism (177).
Consistent with the requirement of mitochondria-produced ROS in osteoclast generation and the in vivo evidence that estrogens or androgens prevent bone loss via antioxidant effects, E2 or DHT attenuates osteoclastogenesis and stimulates osteoclast apoptosis by a mechanism that involves up-regulation of GSR (113). Similar to the case with their antiapoptotic effects on osteoblasts, the antiosteoclastogenic and proapoptotic effects of E2 or DHT on osteoclasts in vitro are exerted in an Src and MAPK kinase-dependent mechanism. E2 also increases GSH, GSR, and Trx reductase in osteoclast-like cells in vitro (114,145,175). Furthermore, in vitro NAC prevents osteoclast formation and NF-κB activation, whereas BSO and H2O2 have the opposite effect, consistent with the evidence that a certain level of ROS generation is essential for osteoclast generation.
Recently, Nakamura et al. (178) reported that female, but not male, mice in which the ERα has been specifically deleted in mature osteoclasts (ERαΔOc/ΔOc) exhibit decreased cancellous bone due to an increased number of osteoclasts resulting from the loss of a cell autonomous proapoptotic effect of estrogens on osteoclasts, which is mediated by an increase in Fas ligand (FasL) production by osteoclasts. These mice did not lose additional bone after ovariectomy. Based on these results, Nakamura et al. (178) have suggested that the proapoptotic effect of estrogens on osteoclasts is the sole molecular basis of the osteoprotective property of estrogens. In vitro studies by Krum et al. (179) have confirmed the role of FasL in the effect of estrogens on osteoclast apoptosis, but unlike Nakamura et al. (178), Krum et al. have concluded that estrogens increase the transcription of the FasL gene not in osteoclasts, but in osteoblasts, and that FasL must therefore act in a paracrine fashion to stimulate osteoclast apoptosis.
In the author’s laboratory, ERα was deleted in very early osteoclast progenitors including cells of the monocyte/macrophage lineage in mice, using a Lysozyme M promoter (180). These mice exhibited a 2-fold increase in osteoclast progenitors in the marrow and the number of osteoclasts in cancellous bone, along with a decrease in cancellous bone mass. After ovariectomy, these mice failed to exhibit the expected increase in osteoclast progenitors, the number of osteoclasts in bone, and further loss of cancellous bone. However, they lost cortical bone indistinguishably from their littermate controls. Mature osteoclasts lacking ERα were resistant to the proapoptotic effect of E2. However, the effects of estrogens on osteoclasts were unhindered in mice bearing an ERα knock-in mutation that prevented binding to DNA. Moreover, a polymeric form of estrogen that is not capable of stimulating the nuclear-initiated actions of ERα was as effective as E2 in inducing osteoclast apoptosis in cells with the wild-type ERα. These results contradict the conclusions of Nakamura et al. (178) and Krum et al. (179) because they demonstrate that estrogens attenuate osteoclast generation and lifespan via cell autonomous effects mediated by DNA-binding independent actions of ERα. Elimination of these effects is sufficient for loss of bone in the cancellous compartment in which complete perforation of trabeculae by osteoclastic resorption precludes subsequent refilling of the cavities by the bone-forming osteoblasts. However, additional effects of estrogens on osteoblasts, osteocytes, and perhaps other cell types are required for their protective effects on the cortical compartment, which comprises 80% of the skeleton.
Another difference from the results of Nakamura et al. (178) is that we have been unable to elicit a stimulatory effect of E2 on FasL production in primary cultures of murine osteoclasts. Lack of an effect of estrogens on FasL production has also been reported by others in the osteoclast-like cell line RAW 264.7, although estrogens do enhance caspase-3 activity in Fas-induced apoptosis of mature osteoclasts (181). Furthermore, it has been shown that endogenous FasL does not have a role in the apoptosis of mature osteoclasts and has only a minimal effect on the apoptosis of osteoclast progenitors from C57BL/6 mice (182,183). In addition, bone marrow from mice without functional Fas or FasL have similar osteoclastogenic potential as bone marrow from wild-type mice. On the other hand, Wu et al. (184) have shown not only that Fas is expressed in osteoclasts but also that its expression increases during differentiation. Moreover, these workers found that mice lacking Fas have decreased BMD and increased osteoclast number, whereas mice deficient in FasL show no changes in BMD but show a significant increase in osteoclast number.
Be that as it may, our own work (180) has confirmed the observations of Nakamura et al. (178) that E2 fails to induce the apoptosis of osteoclasts derived from FasL-deficient mice. Strikingly, in our hands these cells are also resistant to the proapoptotic effect of the nonaromatizable androgen DHT. Depending on the cell type, ROS may be required for FasL-induced apoptosis (186) and may play no role (187) or even act as an antiapoptotic signal in Fas-activated cells (188). Taking all the available evidence together, it is unlikely that the ability of estrogens, an estrogen polymer incapable of initiating nuclear actions, and DHT to promote osteoclast apoptosis in a FasL- dependent fashion results from an estrogen response element-dependent stimulation of FasL expression. Instead, it is more likely that FasL provides a tonic stimulatory signal for osteoclast apoptosis that is potentiated by estrogens or androgens via several nontranscriptional mechanisms: 1) attenuation of the osteoclastogenic and antiapoptotic effect of RANKL, secondary to decreasing ROS production by stimulating GSR activity; 2) attenuation of the antiapoptotic effect of NF-κB via protein-protein interaction of the ERα with NF-κB (138,189,190,191,192), which for example will attenuate the ability of NF-κB to inhibit TNF receptor type I-induced apoptosis via augmentation of the synthesis of cellular caspase-8 (FLICE) like inhibitory protein (193,194); and 3) suppression of the transcriptional activity of c-jun (195). Last but not least, the view that stimulation of FasL is unlikely to be the mechanism by which estrogens promote osteoclast apoptosis is supported by the evidence that estrogens have an antiapoptotic effect on osteoblasts (142,143,164), although murine and human osteoblasts do undergo Fas-mediated apoptosis in response to FasL (196,197,198,199,200). This conundrum is particularly incongruent with the contention of Krum et al. (179) that osteoblasts are the source of FasL for the apoptosis of osteoclasts.
If both aging and loss of sex steroids exert their adverse effects on bone by oxidative damage, how can sex steroid deficiency cause an increase in bone turnover associated with increased osteoclastogenesis and osteoblastogenesis, increased osteoclast and osteoblast numbers, and increased resorption and formation—albeit unbalanced—whereas aging mice and elderly individuals (without vitamin D deficiency and secondary hyperthyroidism) exhibit a low rate of bone remodeling associated with a decrease in osteoblast number and bone formation and no increase in osteoclast number? As will be discussed in Sections IX and X, two age-dependent pathogenetic mechanisms that increase OS, namely PPARγ activation by oxidized lipids and endogenous hyperglucocorticoidism, compounded by the loss of the antioxidant protection of estrogens, may over time thwart the effect of the acute loss of estrogens on remodeling and convert the high remodeling state of acute estrogen loss to the low remodeling of old age. In support of these ideas, hyperglucocorticoidism does indeed override the effects of gonadectomy in mice (201). Moreover, cortisol concentration and the rate of bone loss are inversely related in healthy elderly men and women even after adjustments for adiposity, smoking, alcohol consumption, dietary calcium, activity, as well as serum testosterone and E2 levels (202,203). Additionally, polymorphisms of 11β-hydroxysteroid dehydrogenase (11β HSD) type 1 are strongly associated with osteoporosis, independently of sex steroids (204).
VIII. Diabetes, Oxidative Stress, and Osteoporosis
Similar to aging, both type 1 and type 2 diabetes mellitus adversely affect skeletal homeostasis and increase the risk of fractures, primarily by increasing osteoblast apoptosis and suppressing bone formation. Indeed, obese and non-obese diabetic rats and mice exhibit impaired bone formation and enhanced apoptosis of osteoblastic cells (205,206,207,208,209,210). In addition, both streptozotocin-induced diabetic mice, an animal model of type 1 diabetes, and spontaneously diabetic Torii rats, an animal model of type 2 diabetes, have low-turnover osteopenia associated with increased OS; and markers of OS are inversely associated with the histomorphometric parameters of bone formation. Overexpression of Trx-1 in transgenic mice attenuates streptozotocin-induced diabetic osteopenia (211). Moreover, impaired bone formation and decreased serum levels of osteoblast markers, including osteocalcin and bone-specific alkaline phosphatase, are typical features of the bone disease caused by type 1 and 2 diabetes in men and women of all ages (212,213,214,215).
It is now amply documented that OS plays a major role in the development of both type 1 and type 2 diabetes, in part by accelerating the death of pancreatic β-cells (216,217,218). In fact, the pancreatic islet is the tissue least endowed with intrinsic antioxidant defense mechanisms (49). Insulin, glucagon-like peptide-1, or IGF signaling induces PI3K/Akt activation and thereby the exclusion of FoxOs from the nucleus (56,57,58,59,60). Exclusion of FoxOs is critical for the ability of insulin and the other growth factors to stimulate β-cell proliferation and survival and thereby expansion of β-cell mass in the pancreas (Fig. 2) (61,62,63).
Hyperglycemia and insulin resistance, on the other hand, increase ROS production by accelerating mitochondria respiration and activating NADPH (193,216,217,218). Increased production of proinflammatory cytokines, such as TNF and interferon-γ, as well as of free fatty acids in type 2 diabetes also contributes to increased OS (219), as does the formation of increased advanced glycation end-products and glucose autoxidation. As depicted in Fig. 2, OS induces the phosphorylation of JNK, which overrides several effects of insulin including the Akt-induced FoxO phosphorylation, thereby providing a mechanism whereby OS decreases insulin sensitivity (70,220). In line with this, deletion of JNK through genetic knockout or through a JNK-inhibitory peptide improves insulin sensitivity in mice (221,222).
Consistent with the evidence reviewed above, haploinsufficiency of FoxO1 restores insulin sensitivity and rescues the diabetic phenotype in insulin-resistant mice by reducing hepatic expression of glucogenetic genes and increasing adipocyte expression of insulin-sensitizing genes (62). Conversely, a gain of function mutation of FoxO1 targeted to liver and pancreatic β-cells increases glucose production by the liver and impairs β-cell compensation resulting in diabetes. Similarly, enforced expression of FoxO1 in skeletal muscles impairs glycemic control after glucose or insulin administration (223). Furthermore, in line with the critical role of ROS in diabetes, the antioxidant NAC protects against diabetes in diabetic fatty rats and db/db mice and preserves insulin content and insulin gene expression (224,225). And, insulin resistance is prevented or retarded by overexpression of ROS scavenging enzymes such as SOD and catalase (219,226). Genetic deletion of p66shc prevents hyperglycemia-induced endothelial dysfunction (49,227).
Increased production of ROS is the main cause of the development of insulin resistance by TNFα and glucocorticoids (219), two agents that induce rapid bone loss. Moreover, gene expression analysis in human osteoblasts exposed to glucocorticoids reveals that a significant number of transcripts related to OS are altered (228). The proapoptotic action of glucocorticoids and TNFα in cells of the osteoblast lineage is also mediated by ROS (229). Likewise, glucocorticoids and TNFα promote FoxO activity in a ROS-dependent manner and inhibit Wnt-induced osteoblastogenesis. Most importantly, administration of glucocorticoids to mice leads to an increase in ROS levels in the bone marrow, and this effect is prevented by NAC (229).
In addition to its role in bone homeostasis, Wnt/β-catenin signaling plays a critical role in glucose and lipid metabolism as well as atherosclerosis. Thus, a single missense mutation in LRP6, the coreceptor for the Wnt-signaling pathway, has been genetically linked with diabetes and osteoporosis, as well as early coronary artery disease, hyperlipidemia, and hypertension (230). Moreover, TCF4, the partner of β-catenin in the canonical Wnt-signaling pathway has recently emerged as the strongest type 2 diabetes susceptibility gene (231,232,233,234). In agreement with the genetic evidence in humans, β-catenin regulates glucagon-like peptide-1 secretion in mice (235). In addition, overexpression of Wnt10b in two murine models of obesity with marked hyperinsulinemia and insulin resistance (the ob/ob and the lethal yellow agouti) results in improved glucose homeostasis due to improved insulin sensitivity (236). Therefore, as we originally proposed elsewhere (102) and others have since concurred (104,237,238), antagonism of Wnt signaling by OS with increasing age, through the diversion of β-catenin from TCF- to FoxO-mediated transcription, may be a common mechanism that contributes to the development of not only involutional osteoporosis but also other diseases like insulin resistance, hyperlipidemia, coronary artery disease, and neurodegenerative disorders, all of which are more prevalent with advancing age.
Type 2 diabetics have low bone turnover and increased fracture risk, but they generally have normal or unchanged bone mass. This raises the possibility that mechanical strains on bone imposed by the increased bone mass index in such patients counteract the suppressive effects of insulin resistance on bone formation. Abnormal bone quality due to abnormal collagen or other components of the bone matrix, perhaps caused by advanced glycation end-product formation accelerated by hyperglycemia and OS, may contribute to the increased fracture risk in this condition (239).
IX. Lipid Oxidation, Oxidative Stress, PPARγ, and the Link between Osteoporosis and Atherosclerosis
A vast literature dating back to 1979 has established a critical role for lipid oxidation in the development of atherogenesis (240). In addition, epidemiological evidence shows that atherosclerosis and osteoporosis are linked (241,242,243). Thus, bone loss and vascular calcification progress in parallel with advancing age, and aortic calcification is inversely related to bone density and directly related to fractures in postmenopausal women (241). In fact, women in the highest quartile for increased atherosclerosis exhibit four times greater yearly bone loss than women in the lowest quartile.
Aging increases the differentiation of mesenchymal stem cells (MSCs) into adipocytes in many tissues, including the bone marrow (244). Increased marrow adiposity on the other hand, is associated with age-related bone loss (245,246,247). Moreover, extensive evidence accumulated during the last decade shows that one of the mechanisms responsible for reduced osteoblast production during aging is diversion of early MSCs into the adipocyte lineage at the expense of osteoblasts. Lineage commitment of MSCs to adipocytes is strongly dependent on the activation of the nuclear hormone receptor PPARγ by its ligands, which include oxidized lipids, prostaglandin J2, as well as thiazolidinediones (248,249,250,251,252,253,254,255).
The importance of PPARγ in skeletal homeostasis has been recently highlighted by evidence that mice lacking PPARγ in fat or bearing only one copy of the PPARγ gene exhibit increased bone mass associated with increased osteoblastogenesis and decreased adipogenesis (130,256). Therefore, endogenous PPARγ ligands must be acting as negative regulators of bone formation. In line with this view, activation of PPARγ by oxidized lipids or rosiglitazone promotes the development of adipocytes at the expense of osteoblasts in vitro (257). In addition, rosiglitazone induces bone loss in mice and humans, and this effect is associated with increased marrow adiposity, decreased osteoblast number and bone formation rate, and increased osteoblast and osteocyte apoptosis (258,259,260).
Commitment of multipotential progenitors to a specific lineage involves not only activation of a particular differentiation program but also suppression of programmed cell death. Cells actively suppress programmed cell death by continuous synthesis of specific genes, such as the α4 subunit of protein phosphatase 2A, indicating that apoptosis is the default cell fate (261). Consistent with this, antiapoptotic genes such as Mcl-1 and Bcl-2 are required for the survival of hematopoietic and melanocyte stem cells and mediate the effects of cytokines on commitment to a specific lineage (262,263,264). Hence, PPARγ-mediated commitment to the adipocyte lineage may also involve stimulation of apoptosis of the alternative osteoblast lineage. In line with this thinking, thiazolidinediones stimulate the apoptosis of osteoblasts in vitro (265) and in vivo (258).
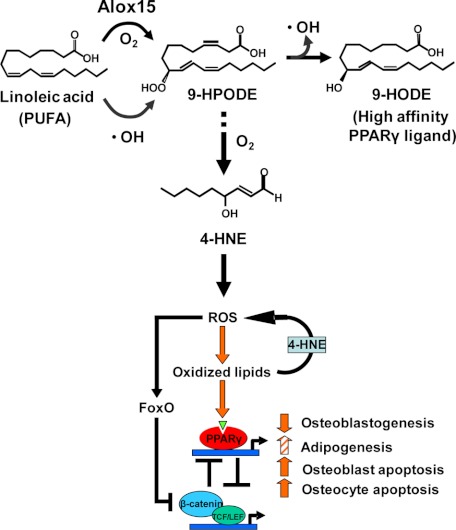
Strong evidence in support of a link between the generation of oxidized lipids and osteoporosis has recently been provided by genetic and biochemical studies of the lipoxygenase Alox15 gene (255,266,267). As depicted in Fig. 6, Alox15 adds oxygen to polyunsaturated fatty acids (PUFAs) and, in the process, the catalytic iron is reduced to the ferrous form. The hydroperoxide product decomposes into a hydroxy derivative to generate a stable oxidation product, which can then bind to PPARγ to exert the skeletal effects. In the process of this decomposition, a hydroxy radical is released (268). Recent work by Almeida et al. (269) in our group indicates that during aging, Alox15 activity increases, as does lipid oxidation, and this probably contributes to the increased osteoblast apoptosis and other cellular changes leading to osteoporosis. Alox15 catalyzes the formation of several ligands of PPARγ, including 9-HODE, 13-HODE, 12-HETE, and 15-HETE (267,270) (Fig. 6). To make matters worse, ROS are generated by Alox15, and ROS in turn further oxidize PUFAs by nonenzymatic means (270). Hence, the chain reaction initiated by ROS has the potential to amplify the original oxidative signal by two to three orders of magnitude, resulting in the generation of large amounts of lipid peroxidation products. To add to this vicious cycle, hydroperoxy-PUFAs generated either enzymatically by lipoxygenases or by the actions of ROS, nonenzymatically decompose into α,β-unsaturated aldehydes, of which 4-hydroxynonenal (4-HNE) is a prototype (271). Such α,β-unsaturated aldehydes indirectly increase ROS by reacting with GSH, thereby depleting cells of this critical component of the coupled GSR/Gpx antioxidant system (272,273).
Figure 6.
Oxidized fatty acids, their degradation products, and bone formation. With advancing age, increased Alox15 expression and increased OS promote lipid peroxidation by adding oxygen to PUFAs, like linoleic acid. The hydroperoxide product of this reaction, 9-HPODE, decomposes into a hydroxy derivative 9-HODE, which binds to and activates PPARγ. Hydroxy radicals generated during this process add to the redox burden of the cell and can further stimulate PUFA peroxidation by nonenzymatic means. Importantly, 9-HPODE is also converted to 4-HNE, itself a potent prooxidant agent. Oxidized PUFAs activate PPARγ and promote its association with β-catenin, resulting in β-catenin degradation. ROS-activated FoxOs divert β-catenin from TCF- to FoxO-mediated transcription and thereby attenuate the restraining effect of β-catenin/TCF on the transcription of PPARγ. The combination of decreased Wnt signaling and increased PPARγ levels as well as PPARγ ligands leads to attenuation of osteoblastogenesis and increased osteoblast/osteocyte apoptosis along with increased adipogenesis (at some sites), thereby suppressing bone formation.
Alox15-null mice exhibit increased bone mass. Conversely, a mouse strain that bears a mutant allele of the Alox15 gene (DBA/2) characterized by 20-fold elevated levels of the enzyme exhibits decreased bone mass compared with C57BL/6 mice or DBA/2 congenic mice bearing a portion of chromosome 11 containing the wild-type allele of C57BL/6. Importantly, E2 decreases oxidized low-density lipoproteins (274), and administration of an Alox15 inhibitor attenuates ovariectomy-induced bone loss and increases bone mass in mice with osteoporosis due to overexpression of IL-4, a stimulator of Alox15 expression (266). These observations strongly suggest that Alox15 generates oxidized lipids that bind to and activate the restraining effect of PPARγ on osteoblast production and bone formation.
Advancing age in C57BL/6 mice causes OS in bone and bone marrow, increases osteoblast apoptosis, and decreases bone formation (113). Advancing age also causes an increase in the expression of Alox15, PPARγ, and 4-HNE (269). Importantly, 4-HNE is a very potent inducer of p66shc phosphorylation and osteoblastic cell apoptosis. Using cultured cell models, Almeida and co-workers (269) have also unraveled an oxidized PUFA-activated ROS/FoxO/PPARγ/β-catenin cascade, which explains how a rise in oxidized lipids caused increased OS, increased PPARγ expression, and reduced canonical Wnt signaling in osteoblasts and osteoblast progenitors (Fig. 6). As depicted in the model, lipid oxidation initiates this cascade by generating 4-HNE, which increases ROS and thereby activates FoxO. This results in diversion of β-catenin from proosteogenic TCF-mediated transcription to antioxidant FoxO-mediated transcription, as previously described for H2O2-induced OS (101). The decrease of β-catenin not only attenuates canonical Wnt signaling, but also unleashes the expression of PPARγ, which is normally suppressed by β-catenin/TCF transcription (275,276,277). The increase in PPARγ levels serves as an additional β-catenin sink by sequestering it and promoting its proteasomal degradation (278). Increased lipoxygenase and PPARγ expression is probably autoamplified, as evidenced by the increased expression of PPARγ, Alox12, and Alox15 in muscle cells stimulated with oxidized PUFAs (94). Considering the facts that canonical Wnt signaling increases (94,95,279,280) whereas Alox15 and PPARγ decrease osteoblast number (130,266), the findings of Almeida et al. (269) strongly suggest that the ROS/FoxO/PPARγ/β-catenin cascade accounts, at least in part, for the osteoblast deficit and the loss of bone with age. As discussed in Section V.C, FoxOs attenuate OS by stimulating the production of antioxidant enzymes, and β-catenin is a partner in this effect. Therefore, a decrease of the absolute amount of β-catenin, secondary to its degradation by PPARγ, should also compromise the ability of osteoblast progenitors to mount an antioxidant response, reducing further the generation of osteoblasts. Such a decrease in osteoblast number coupled with increased production of adipocytes is of course an invariable feature of old age in mice and humans.
In summary, the evidence reviewed in this section indicates that oxidized fatty acids and their degradation products in bone increase with advancing age secondary to elevated levels of Alox15 and ROS. This leads to activation of PPARγ, which increases adipogenesis at the expense of osteoblastogenesis. In addition, oxidized fatty acids and their degradation products stimulate apoptosis of osteoblasts and osteoblast progenitors via PPARγ-dependent and -independent mechanisms. Lipid radicals are detoxified by the Gpx\GSR and Trx peroxidase\reductase enzyme systems to form hydroxy fatty acid derivatives, and in the process of handling these radicals overall oxidative defense is lowered. As discussed in Sections VI and VII, both estrogen deficiency and aging decrease the activity of antioxidant enzymes, such as GSR and Trx reductase. Therefore, estrogen deficiency may potentiate the adverse effects of aging not only on bone but also on lipid metabolism as a result of decreased antioxidant defense and releasing the suppressive effect of estrogens on low-density lipoprotein production (281). In support of these ideas, increased generation of oxidized lipids by Alox15 has been linked with several age-related diseases including atherosclerosis and Alzheimer’s disease (282,283,284,285). In addition, lipid peroxidation in atherosclerotic plaques increases with age in humans in association with both nonenzymatic (ROS) and enzymatic (lipoxygenase) mechanisms (285).
X. Aging, Endogenous Hyperglucocorticoidism, and Bone Strength
A. Cell autonomous effects of glucocorticoids on bone
As opposed to earlier ideas that the adverse effects of glucocorticoids on bone are secondary to effects on calcium handling and the production of PTH or gonadal steroids, it is now quite clear that the deleterious effects of glucocorticoid excess on bone result from direct effects on osteoblasts (and their progenitors), osteocytes, and osteoclasts (286,287,288,289,290). Indeed, glucocorticoid excess suppresses osteoblastogenesis, strongly and rapidly stimulates osteoblast and osteocyte apoptosis, and prolongs the lifespan of osteoclasts. Evidently, the proapoptotic effect of glucocorticoids on osteocytes is initiated by detachment of their cellular processes from the lacunar-canalicular walls as a result of disengagement of integrins from proteins of the extracellular matrix (291). Glucocorticoids cause this detachment-induced apoptosis, also known as anoikis, via a glucocorticoid receptor-mediated rapid inside-out signaling leading to activation of the Pyk2 kinases that oppose the prosurvival signals of the association of integrins with focal adhesion kinases.
To address the contribution of the direct effects of glucocorticoids on osteoblasts and osteocytes to the adverse effects of these agents on bone mass and strength, O’Brien et al. (37) from our group have generated transgenic mice in which osteoblasts and osteocytes are protected from the direct effects of glucocorticoids, by virtue of the fact that they overexpress 11β-HSD type 2, an enzyme that inactivates glucocorticoids. Short-term administration of glucocorticoids to these mice induced equivalent bone loss to that observed in wild-type mice. However, the increase in osteoblast apoptosis that occurs in wild-type mice is prevented in the transgenic mice. Consistent with this, osteoblasts, osteoid area, and bone formation rate are significantly higher in glucocorticoid-treated transgenic mice compared with glucocorticoid-treated wild-type mice. Glucocorticoid-induced osteocyte apoptosis is also prevented in transgenic mice. Strikingly, the loss of vertebral compression strength observed in glucocorticoid-treated wild-type mice was prevented in the transgenic mice, despite equivalent bone loss. These results demonstrate that excess glucocorticoids directly affect bone-forming cells in vivo. Furthermore, these results have strongly suggested that glucocorticoid-induced loss of bone strength results in part from increased death of osteocytes, independently of bone loss.
B. Glucocorticoids and bone strength
Glucocorticoid excess, similar to aging, decreases bone strength disproportionately to its adverse effect on bone mass (292). In fact, the decline of bone strength with glucocorticoids precedes the decline in bone mass (292,293,294). Aging in humans blunts glucocorticoid feedback inhibition of ACTH (295), stimulates conversion of inactive to active glucocorticoids (296), and increases endogenous glucocorticoid production (202). Hyperglucocorticoidism in turn induces endothelial OS and vasoconstriction (297). Furthermore, both aging and glucocorticoid excess cause a reduction in blood flow and the volume of water present in the skeleton (298,299,300,301,302,303). Specifically, less water can be evaporated from older human bone (304), and the water fraction of human vertebrae decreases with age (305). Bone water volume decreases with glucocorticoid administration (302), and skeletal blood flow decreases with aging in rats (303). In addition, both aging and glucocorticoid excess decrease vascular endothelial growth factor (VEGF) in osteoblasts (306,307,308).
C. Angiogenesis and bone
Blood vessels are an essential component of the basic multicellular unit (BMU)—the anatomical structure responsible for bone remodeling—and are most likely the conduits by which both osteoclast and osteoblast precursors reach the site that is targeted for remodeling (309,310) (Fig. 7). Furthermore, bone formation almost always takes place in close proximity to capillaries (311). Thus, during development and fracture healing, the differentiation of osteoblasts requires blood vessel development. Recently, Sacchetti et al. (312) demonstrated that the pericytes (also known as mural cells) associated with the abluminal surface of vessels and sinusoids of the bone marrow are the MSC progenitors of osteoblasts. Remarkably, pericytes also participate in the development of blood vessels by producing an extracellular matrix to support endothelial cells and by providing growth factors like TGF-β. This dual functionality may contribute to the close linkage of vascularization and bone formation. Another link may be provided by growth factors produced by endothelial cells, including BMPs and endothelins (311).
Figure 7.
Photomicrograph of a cross-section of a murine, cancellous BMU comprising osteoclasts and osteoblasts along with two capillaries containing erythrocytes [courtesy of Robert S. Weinstein from the University of Arkansas for Medical Sciences]. Osteoclasts are identified by their discrete tartrate-resistant acid phosphatase-positive red granules, and the osteoblasts by their large nuclei with multiple nucleoli and underlying light blue osteoid. An abundance of osteocytes can be seen embedded individually (blue staining cells) within the mineralized bone (green) surrounding the BMU. Methyl green and tartrate-resistant acid phosphatase staining of undecalcified bone viewed with Nomarski differential interference contrast microscopy (X630).
Blood vessels develop during tissue growth, wound healing, and tissue remodeling to deliver oxygen and nutrients and remove toxic metabolites. During neovasculogenesis in the adult, new vessels may arise from precursors or from preexisting vessels by branching. Both functions are stimulated by hypoxia via stabilization of the Von Hippel-Lindau transcription factor that drives the synthesis of VEGF. VEGF, along with angiopoeitin-1, also promotes the maturation of endothelial cells and the recruitment of pericytes to the vessels to promote their maturation. Genetic studies have established that low oxygen tension, which develops as the bone matrix expands, stimulates the expression of VEGF by osteoblasts and initiates a neovasculogenic program (313,314,315). VEGF may also act indirectly by its ability to stimulate the synthesis of Cyr61 by cells of the osteoblast lineage. This protein is deposited into the extracellular matrix where it promotes neovascularization by interacting with integrins on endothelial cells (316). Endothelial growth factor also plays a key role in angiogenesis by stimulating endothelial cell replication, survival, adhesion, and migration. Interestingly, both Wnt signaling and TGF-β act in concert with VEGF to promote neovascularization, and cultured endothelial cells respond to activation of Wnt/β-catenin signaling with increased proliferation and decreased apoptosis (317,318).
D. Bone hydration
Water represents at least 25% of the wet weight of bone (305) and confers to bone much of its unique strength and resilience by reducing bone stresses during dynamic loading and producing a 2.5-fold increase in ultimate strength (319). Conversely, fracture resistance of hard tissues is defective in the absence of water (320,321). Of the total volume of water, approximately 90% resides in the lacunar-canalicular system that surrounds the intricate network of osteocytes (former osteoblasts entombed within the mineral), bone vasculature, and bone marrow (304) (Fig. 7).
Recent work from our group has strongly suggested that angiogenesis is also critical for bone strength (322). Indeed, we have obtained evidence that the vascularity of the skeleton and perhaps, as a consequence, the osteocyte lacunar-canalicular fluid volume and thereby skeletal hydration are critical determinants of bone strength, independently of bone mass. Moreover, in full agreement with the evidence from humans, we found that aging in C57BL/6 mice is associated with an increase in the adrenal production of glucocorticoids and their conversion from inactive to active glucocorticoids, as evidenced by increased corticosterone levels, adrenal weight, and the expression of 11β-HSD1 (the enzyme that converts inactive to active glucocorticoids) in bone (322). Even more strikingly, both aging and exogenous glucocorticoids decrease bone interstitial fluid in the osteocyte lacunar-canalicular network, decrease the volume of the bone vasculature, suppress VEGF production and action, and also suppress angiogenesis. Furthermore, the effects of aging or glucocorticoid administration on bone angiogenesis, interstitial fluid, and vasculature volume are greatly attenuated or absent in the mice in which osteoblasts and osteocytes are protected from the actions of glucocorticoids by means of transgenic overexpression of 11β-HSD2 (322). Collectively, these findings strongly suggest that increased glucocorticoid production and sensitivity with increasing age contribute to the development of skeletal fragility by decreasing skeletal fluid flow and the volume of the vasculature in bone, and thereby skeletal hydration. The molecular underpinning of these effects is decreased angiogenesis resulting from decreased VEGF production by osteoblasts/osteocytes as well as decreased VEGF action. The possibility that these changes represent a chain of interconnected pathogenetic mechanisms is supported by anatomical evidence that the cellular processes of osteocytes are in fact in direct contact with the bone vasculature (Fig. 8) (185). As discussed earlier, similar to OS, glucocorticoids stimulate FoxO-mediated transcription and suppress β-catenin/TCF-mediated transcription in osteoblast progenitors (229). It is, therefore, quite plausible that the decreased production of VEGF by glucocorticoids is caused, at least in part, by increased OS and the corresponding FoxO-mediated inhibition of HIF-1α-induced VEGF expression. I submit that the elucidation of these molecular, cellular, and tissue changes represents an important breakthrough in understanding the phenomenon of increased skeletal fragility with age.
Figure 8.
The connection between osteocytes and blood vessels. Left, Low (upper panel) and high (lower panel) magnifications of electron microscopy images demonstrating reliefs of the osteocytes and their canalicular network following acid-etching of murine bone sections [courtesy of Lynda Bonewald from the University of Missouri and Kansas City Dental School]. Please note multiple attachments of the osteocyte processes to the vessels depicted in the center of these images. Right, Cartoon depicting the same connections. [From M. L. Knothe Tate et al.: Bone 22:107–117, 1998 (366). Reproduced with permission of the Journal © Elsevier.]
XI. Oxidative Stress and Nutrient-Dependent Deacetylases (Sirtuins) as Therapeutic Targets
During the last few years, a class of nutrient-sensing nicotinamide adenine dinucleotide-dependent protein deacetylases, termed sirtuins, has been shown to play a crucial role in the resistance against OS and the promotion of longevity (323,324,325,326,327). The prototype of this gene family in vertebrates is the histone deacetylase silent information regulator T-1 (SIRT-1). Sirtuins 1, 2, and 3 prolong life-span; improve energy expenditure, glucose production, and insulin sensitivity; and protect against high-fat diet-induced metabolic damage via several mechanisms, including the deacetylation of FoxOs and modulation of FoxO-mediated transcription (328). The importance of the latter mechanism has been recently highlighted by evidence that augmentation of FoxO3a-dependent MnSOD and catalase expression in mice by SIRT3 decreased cellular levels of ROS and thereby protected the mouse heart from cardiac hypertrophy (329).
Based on evidence that the polyphenol resveratrol (3,5,4′-trihydroxy-trans-stilbene), abundantly present in red wine, activates SIRTs and mimics the ability of dietary restriction to prolong lifespan in lower organisms, Pearson et al. (330) fed mice with resveratrol and found that it induces gene expression patterns in multiple tissues that parallel those induced by dietary restriction and every-other-day feeding. More important, resveratrol-fed elderly mice showed a marked reduction in signs of aging, including the preservation of BMD. Nonetheless, resveratrol did not influence the longevity of these mice. On the other hand, resveratrol increased longevity in mice fed with a high-fat diet (331). Furthermore, resveratrol prevented ovariectomy-induced bone loss and osteoblast apoptosis (332).
Consistent with the antiosteoporotic efficacy of resveratrol in rodents, several laboratories have recently shown that this polyphenol, probably acting via the activation of SIRT-1 and PPARγ suppression, decreases bone marrow adipogenesis whereas it promotes osteoblast progenitor proliferation and differentiation and prevents osteoclast formation in vitro (333,334,335). More importantly, Edwards et al. (336) have reported that SIRT-1 is required for the maintenance of normal bone mass because mice null for SIRT-1 have decreased bone mass associated with decreased osteoblast differentiation and increased osteoclast activity. These changes are associated with a 27% increase in osteoclast numbers and a 39% decrease in osteoblast numbers compared with wild-type animals. Furthermore, in agreement with evidence that endothelial NO synthase (eNOS) null mice exhibit a similar bone phenotype to that of the SIRT-1 null mice and SIRT-1 stimulates eNOS in other cell types (337), Edwards and co-workers (338) found that resveratrol enhances eNOS mRNA expression and increases the levels of the eNOS product nitric oxide (NO) in osteoblasts. In addition, NO donors enhance BMP-2 mRNA expression, osteoblast differentiation, and bone formation. On the other hand, eNOS-deficient osteoblast progenitors exhibit decreased BMP-2 transcription and osteoblast differentiation and fail to respond to resveratrol. This suggests that resveratrol increases SIRT-1 expression and activity, and in turn eNOS expression and activity, and by this mechanism, stimulates BMP-2 expression, osteoblast differentiation, and bone formation.
To follow up these observations, Edwards et al. (339) have generated mice with cell-specific deletion of SIRT-1 in osteoblasts, osteoclasts, or both cell types. SIRT-1-specific deletion in cells of the osteoblast lineage (using the 2.3 Col1α1 promoter) causes a decrease in bone volume accompanied by a decrease in osteoblast number and the rate of bone formation, but no change in osteoclast number. Similarly, deletion of SIRT-1 in osteoclast precursor cells, including monocytes and macrophages (using the Lysozyme M promoter), results in decreased bone volume; however unlike the osteoblast-specific deletion, the number of osteoclasts is increased compared with control mice. Crossing these models to generate an osteoblast/osteoclast-specific SIRT-1 knockout resulted in mice with up to 29% less bone volume than control animals. These findings strongly support the conclusion that SIRT-1 controls bone mass by independently regulating cells of both the osteoblast and osteoclast lineage and provide yet another molecular link between aging, osteoclast and osteoblast number, and bone loss.
A critical mediator of the effects of sirtuins on energy expenditure is PPARγ coactivator-1α (PGC-1α), a coactivator protein that interacts with several transcription factors, including FoxOs. Like FoxOs, PGC-1α promotes the transcription of antioxidant enzymes in response to oxidative stress (340,341,342,343). SIRT-1, PGC-1α, and FoxOs function together to accommodate metabolic adaptation to fluctuations in nutrient supply (344). Similar to FoxO-deficient mice, PGC-1α-deficient animals exhibit increased levels of ROS and reduced ability to withstand oxidative damage (345). Activation of PGC-1α/FoxO and subsequent reduction of energy expenditure contribute to decreased fat accumulation. Importantly, PGC-1α also promotes mitochondria biogenesis and thereby the production of ROS and OS, and ROS are feeding forward cAMP-responsive element-binding protein to increase PGC-1α expression (346). Caloric restriction, without malnutrition, represents a promising strategy for life extension and the delay in the onset of age-associated pathologies, and it is thought to work through the PGC-1α-induced mitochondria biogenesis program (347,348,349). Nonetheless, whereas the beneficial effects of caloric restriction on glucose homeostasis are well documented, the effect of caloric restriction on bone metabolism, if any, is minor and perhaps negative. Indeed, caloric restriction for 24 wk in young mice decreased cortical bone mass but had no effect on cancellous bone (350). And, caloric restriction in humans either had no effect on BMD or bone mineral content or led to a reduction of bone mass in the spine, total hip, and femoral neck (351,352).
More specific SIRT-1 agonists, such as SRT1720 or SIRT-1 activators unrelated to resveratrol (with 1000-fold higher potency), are very effective in reducing liver lipid accumulation, protecting from diet-induced obesity, and improving glucose and insulin homeostasis in mice (353,354,355). However, the effect of these new agents on bone is currently unknown.
In keeping with the theme that increased defense against OS may be an effective antiosteoporotic therapy, Jilka and co-workers from our group (unpublished data) have investigated the efficacy of intermittent PTH for old age osteoporosis by comparing its effects in young (age, 6 months) and old (age, 26 months) C57BL/6 mice. Similar to observations by others (356), they found that 4 wk of daily PTH administration (100 ng/g) is as effective in stimulating bone formation and increasing bone mass in old mice as it is in young mice. Moreover, after 4 wk of intermittent administration, PTH reduces the level of ROS and increases the level of GSH and GSR in the bone marrow, decreases the phosphorylation of p66shc in vertebral lysates, and decreases the prevalence of osteoblast apoptosis in sections of vertebral cancellous bone. Moreover, PTH also attenuates the age-related increase in expression of the lipoxygenase Alox15, the enzyme known to generate ROS via the production of the prooxidant 4-HNE. As opposed to PTH, the antioxidant NAC suppresses osteoblast differentiation in vitro and reduces the number of osteoblast progenitors in vivo. Moreover, NAC suppresses Wnt-stimulated β-catenin/TCF-mediated transcription, whereas it is well established that PTH stimulates Wnt signaling by suppressing the Wnt antagonist sclerostin (357,358) and by activating LRP6 (96). The ability of antioxidants to prevent sex steroid deficiency, but not old age-induced bone loss, suggests that they are anticatabolic agents that also suppress osteoblast formation—a process that depends on signaling by low levels of ROS. PTH on the other hand can uniquely reverse the age-dependent decline in osteoblastogenesis because of its ability to selectively suppress OS, stimulate Wnt signaling, and attenuate osteoblast apoptosis, and thereby promote de novo bone formation.
XII. Summary and Conclusions
Epidemiological evidence indicates that in both women and men bone loss begins as early as the early part of the third decade—long before any change in sex steroid production. In addition, evidence primarily from mechanistic studies in rodents shows that advancing age, and specifically increased OS, is a fundamental mechanism of the loss of bone mass and strength. ROS are continuously generated as normal by-products of aerobic metabolism and can cause protein damage and DNA lesions leading to cell death, but they also serve as signaling molecules at lower concentrations to control cell proliferation and differentiation in a plethora of cell types. Consistent with this, mitochondria biogenesis and ROS play a very important role in the generation and survival of osteoclasts, osteoblasts, and osteocytes. Loss of estrogens or androgens decreases defense against OS, and this accounts for the increased bone resorption associated with acute sex steroid deficiency as well as the effects of either class of sex steroids on osteoclastogenesis and the apoptosis of osteoclasts and osteoblasts.
Genetic analysis of the role of the FoxO family of transcription factors in bone indicates that FoxO-dependent oxidative defense provides a mechanism to handle the oxygen free radicals constantly generated by the aerobic metabolism of osteoblasts and is therefore indispensable for bone mass homeostasis. FoxO activation in early mesenchymal progenitors also diverts ß-catenin away from Wnt signaling, leading to decreased osteoblastogenesis. The importance of this latter mechanism is supported by evidence that OS and FoxO activation are implicated not only in the pathogenesis of type 1 and 2 diabetes but also in the adverse effects of diabetes on bone formation. Similarly, attenuation of Wnt signaling by the activation of PPARγ by ligands generated from free fatty oxidation and a ROS/FoxO/PPARγ/β-catenin cascade contributes to the age-dependent decrease in bone formation, suggesting a mechanistic explanation for the link between atherosclerosis and osteoporosis. Lastly, increased glucocorticoid production and sensitivity with increasing age contribute to the suppression of bone formation. These age-dependent changes also contribute to development of skeletal fragility by decreasing skeletal fluid flow and the volume of the vasculature in bone, and thereby skeletal hydration. The molecular underpinning of these effects is decreased angiogenesis resulting from decreased VEGF production by osteoblasts/osteocytes as well as decreased VEGF action.
Elucidation of these mechanisms provides a paradigm shift from the “estrogen-centric” account of the pathogenesis of involutional osteoporosis to one in which age-related mechanisms intrinsic to bone and OS are protagonists and age-related changes in other organs and tissues, such as ovaries, accentuate them (Table 2). It also provides an understanding of osteoporosis that is well aligned with what is known about the biology of aging and the mechanisms of several other degenerative disorders—which often are present in the same patient and inexorably share molecular pathogenetic mechanisms related to aging per se. Elucidation of determinants of bone strength independent of bone mass not only provides fresh insights into the decline of bone strength with age and may explain the apparent mismatch between bone strength and bone mass that characterizes aging and glucocorticoid excess in animals and humans alike, but also suggests novel therapeutic opportunities for preventing it—in distinction from the current alternatives that aim at altering bone mass. Lastly, the evidence for the effectiveness of sirtuin ligands in both insulin resistance and osteoporosis in rodents (330) raises expectations that in the near future this and other classes of pathogenesis of aging-tailored drugs may provide a one-drug unified treatment for several degenerative diseases.
Table 2.
Pathogenetic mechanisms of involutional osteoporosis
| 1. OS |
| 2. Decreased defense against OS, resulting from sex steroid deficiency |
| 3. Increased lipid oxidation/PPARγ activation (partially through OS) |
| 4. Endogenous hyperglucocorticoidism (partially through OS) |
Almost 10 yr ago, I had reviewed in this journal the cellular and molecular mechanisms of osteoporosis and highlighted the overriding importance of osteoclast, osteoblast, and osteocyte numbers for bone homeostasis, as well as the paramount importance of the aberrations of the rate of birth and death of these cells in the pathogenesis of osteoporosis (1). In the present article, I have dealt with evidence accumulated in the interim that helps to unravel the complexities of the mechanisms whereby cell number can be dysregulated as a function of increasing age and how bone strength can decline independently from the decline of bone mass. At present, major advances in the field of OS and cell death indicate that enzymatic and transcriptional defenses preventing damage to intracellular components by free radicals (the subject of this article) are not enough to avoid cellular injury. A second line of defense, termed autophagy, is necessary for the repair and removal of damaged components of the cell in response to mild OS, via the ubiquitin/proteasome system and lysosomes (359,360,361). I strongly suspect that such mechanisms will be critical for the response of osteoclast and osteoblast precursors and their progeny to various levels of mild OS. Osteocytes, the bone cells with by far the most extensive lifespan, choreograph the process of bone remodeling by the short-lived osteoclasts and osteoblasts (362,363). Autophagy is likely to be critical for the ability of osteocytes to remain alive while sensing, and probably responding to, major changes in the ambient level of oxygen, at the same time as they deal with the ravages of aging, including the nuclear leakiness that accelerates with age in postmitotic cells (364,365). Understanding these events in the context of skeletal aging in the future will most likely dictate yet another revision of our current ideas about the pathogenesis of osteoporosis.
Acknowledgments
The author is grateful to Maria Almeida, Robert Jilka, Robert S. Weinstein, Charles O’Brien, Elena Ambrogini, Marta Martin-Millan, Joe Goellner, A. Michael Parfitt, and the rest of the staff of the Osteoporosis and Metabolic Bone Disease Center of the University of Arkansas for Medical Sciences for endless discussions over many years about the concepts presented here, sharing of their ideas, and the contribution of their latest research findings during the preparation of this article; and to Phil Morgan and Beth Bailey for help with the preparation of the manuscript. Apologies are due to the investigators whose work has not been cited here because of space limitations.
Footnotes
This work was supported by the National Institutes of Health (Grant P01 AG13918), the Department of Veterans Affairs (Merit Review), and Tobacco Settlement funds provided by the University of Arkansas for Medical Sciences.
Disclosure Statement: The author has nothing to disclose.
First Published Online January 5, 2010
Abbreviations: Akt, Thymoma viral protooncogene 1; BMD, bone mineral density; BMP, bone morphogenetic protein; BMU, basic multicellular unit; BSO, l-buthionine-(S,R)-sulfoximine; CFU-OB, colony forming unit-osteoblast; DHT, dihydrotestosterone; E2, 17β-estradiol; eNOS, endothelial NO synthase; ER, estrogen receptor; FasL, Fas ligand; FoxO, Forkhead box O; Gpx, GSH peroxidase; GSH, glutathione; GSR, GSH reductase; HIF, hypoxia inducible factor; 4-HNE, 4-hydroxynonenal; HSC, hematopoietic stem cell; 11β-HSD, 11β-hydroxysteroid dehydrogenase; JNK, jun N-terminal kinase; LRP, low-density lipoprotein receptor-related protein; MnSOD, manganese SOD; MSC, mesenchymal stem cell; NAC, N-acetyl cysteine; NADPH, nicotinamide adenine dinucleotide phosphate oxidase; NF-κB, nuclear factor κB; NO, nitric oxide; OS, oxidative stress; PGC-1α, PPAR γ coactivator-1α; PI3K, phosphatidyl inositol 3 kinase; PPAR, peroxisome proliferator-activated receptor; PUFA, polyunsaturated fatty acid; RANKL, receptor activator of NF-κB ligand; ROS, reactive oxygen species; SIRT, silent information regulator T; SOD, superoxide dismutase; TCF, T cell-specific transcription factor; Trx, thioredoxin; VEGF, vascular endothelial growth factor.
References
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Nordin BEC, Need AG, Prince RL, Horowitz M, Gutteridge DH, Papapoulos SE 1993 Osteoporosis. In: Nordin BEC, Need AG, Morris HA, eds. Metabolic bone and stone disease. Edinburgh, UK: Churchill Livingstone; 1–82 [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 1998 A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 13:763–773 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL 1998 Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- Tuck SP, Scane AC, Fraser WD, Diver MJ, Eastell R, Francis RM 2008 Sex steroids and bone turnover markers in men with symptomatic vertebral fractures. Bone 43:999–1005 [DOI] [PubMed] [Google Scholar]
- Khosla S 2008 Estrogen and bone: insights from estrogen-resistant, aromatase-deficient, and normal men. Bone 43:414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S 2000 Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF, Fedarko NS, Abuzzahab MJ, Frank GR, Cohen RM, Lubahn DB, Korach KS 2008 Impact on bone of an estrogen receptor-α gene loss of function mutation. J Clin Endocrinol Metab 93:3088–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Dolomie-Fagour L, Georges A, Corcuff JB 2008 [What about bioavailable estradiol?]. Ann Biol Clin (Paris) 66:493–497 [DOI] [PubMed] [Google Scholar]
- Leder BZ, Finkelstein JS 2005 Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporos Int 16:1487–1494 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, Ettinger B 2003 Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int 14:191–197 [DOI] [PubMed] [Google Scholar]
- McKane WR, Khosla S, Burritt MF, Kao PC, Wilson DM, Ory SJ, Riggs BL 1995 Mechanism of renal calcium conservation with estrogen replacement therapy in women in early postmenopause—a clinical research center study. J Clin Endocrinol Metab 80:3458–3464 [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF 1979 Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest 64:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari C, Agnusdei D, Nardi P, Civitelli R 1990 Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab 71:1288–1293 [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Nussbaum SR, Herzog DB, Neer RM 1984 Osteoporosis in women with anorexia nervosa. N Engl J Med 311:1601–1606 [DOI] [PubMed] [Google Scholar]
- Saggese G, Federico G, Bertelloni S, Baroncelli GI 1992 Mineral metabolism in Turner’s syndrome: evidence for impaired renal vitamin D metabolism and normal osteoblast function. J Clin Endocrinol Metab 75:998–1001 [DOI] [PubMed] [Google Scholar]
- Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A 1999 The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab 84:4489–4496 [DOI] [PubMed] [Google Scholar]
- Nordin BE, Need AG, Bridges A, Horowitz M 1992 Relative contributions of years since menopause, age, and weight to vertebral density in postmenopausal women. J Clin Endocrinol Metab 74:20–23 [DOI] [PubMed] [Google Scholar]
- Nordin BE, Morris HA 1992 Osteoporosis and vitamin D. J Cell Biochem 49:19–25 [DOI] [PubMed] [Google Scholar]
- Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston Jr CC, Lindsay R 1998 Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S 2008 A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Riggs BL 2005 Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am 34:1015–1030, xi [DOI] [PubMed] [Google Scholar]
- Hui SL, Slemenda CW, Johnston Jr CC 1988 Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 81:1804–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AJ, Knott L 1999 Molecular changes in bone collagen in osteoporosis and osteoarthritis in the elderly. Exp Gerontol 34:337–351 [DOI] [PubMed] [Google Scholar]
- Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP 2001 Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28:195–201 [DOI] [PubMed] [Google Scholar]
- Wall JC, Chatterji SK, Jeffery JW 1979 Age-related changes in the density and tensile strength of human femoral cortical bone. Calcif Tissue Int 27:105–108 [DOI] [PubMed] [Google Scholar]
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott 3rd TA, Berger M 2000 Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM 2002 Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112:281–289 [DOI] [PubMed] [Google Scholar]
- Li X, Masinde G, Gu W, Wergedal J, Mohan S, Baylink DJ 2002 Genetic dissection of femur breaking strength in a large population (MRL/MpJ x SJL/J) of F2 mice: single QTL effects, epistasis, and pleiotropy. Genomics 79:734–740 [DOI] [PubMed] [Google Scholar]
- Wergedal JE, Sheng MH, Ackert-Bicknell CL, Beamer WG, Baylink DJ 2005 Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross-sectional geometry and bone density. Bone 36:111–122 [DOI] [PubMed] [Google Scholar]
- Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM 1985 The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 37:594–597 [DOI] [PubMed] [Google Scholar]
- Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R 1998 Does microdamage accumulation affect the mechanical properties of bone? J Biomech 31:337–345 [DOI] [PubMed] [Google Scholar]
- Heaney RP 2003 Is the paradigm shifting? Bone 33:457–465 [DOI] [PubMed] [Google Scholar]
- Duan Y, Parfitt A, Seeman E 1999 Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res 14:1796–1802 [DOI] [PubMed] [Google Scholar]
- Qiu S, Rao DS, Palnitkar S, Parfitt AM 2003 Reduced iliac cancellous osteocyte density in patients with osteoporotic vertebral fracture. J Bone Miner Res 18:1657–1663 [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS 2004 Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841 [DOI] [PubMed] [Google Scholar]
- Verborgt O, Gibson GJ, Schaffler MB 2000 Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 15:60–67 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 2000 Osteoporosis: 50 years of change, mostly in the right direction. In: Compston JRS, ed. Osteoporosis and bone biology: the state of the art. London: International Medical Press; 1–13 [Google Scholar]
- Finkel T, Holbrook NJ 2000 Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247 [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T 2005 Mitochondria, oxidants, and aging. Cell 120:483–495 [DOI] [PubMed] [Google Scholar]
- Harman D 1956 Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300 [DOI] [PubMed] [Google Scholar]
- Giorgio M, Trinei M, Migliaccio E, Pelicci PG 2007 Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8:722–728 [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR 2007 Endocrine regulation of ageing. Nat Rev Mol Cell Biol 8:681–691 [DOI] [PubMed] [Google Scholar]
- Lu T, Finkel T 2008 Free radicals and senescence. Exp Cell Res 314:1918–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer DD, Ferguson-Miller S 2003 Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112:481–490 [DOI] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG 2005 Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233 [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG 1999 The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313 [DOI] [PubMed] [Google Scholar]
- Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Lüscher TF, Cosentino F 2007 Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA 104:5217–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Finkel T 2002 Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295:2450–2452 [DOI] [PubMed] [Google Scholar]
- Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ, Finkel T, Kusiak JW 2005 Phosphorylation of p66Shc and forkhead proteins mediates Aβ toxicity. J Cell Biol 169:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A 2008 Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA 2007 How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8:703–713 [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ 2002 Glutathione in defense and signaling: lessons from a small thiol. Ann NY Acad Sci 973:488–504 [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A 2005 FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24:7410–7425 [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME 1999 Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868 [DOI] [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM 1999 Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630–634 [DOI] [PubMed] [Google Scholar]
- Biggs 3rd WH, Meisenhelder J, Hunter T, Cavenee WK, Arden KC 1999 Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96:7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ED, Nuñez G, Barr FG, Guan KL 1999 Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 274:16741–16746 [DOI] [PubMed] [Google Scholar]
- Takaishi H, Konishi H, Matsuzaki H, Ono Y, Shirai Y, Saito N, Kitamura T, Ogawa W, Kasuga M, Kikkawa U, Nishizuka Y 1999 Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc Natl Acad Sci USA 96:11836–11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J, Accili D 2007 Regulation of pancreatic β-cell function by the forkhead protein FoxO1. Diabetes Obes Metab 9 (Suppl 2):140–146 [DOI] [PubMed] [Google Scholar]
- Nakae J, Biggs 3rd WH, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D 2002 Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 32:245–253 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs 3rd WH, Wright CV, White MF, Arden KC, Accili D 2002 The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A, Burgering BM 2007 Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 8:440–450 [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A 2008 FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R 1993 A C. elegans mutant that lives twice as long as wild type. Nature 366:461–464 [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C 1997 daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278:1319–1322 [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G 1997 The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389:994–999 [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME 2004 Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015 [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H 2005 JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121:115–125 [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ 2000 Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol 10:1201–1204 [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A 2008 FOXO transcription factors in ageing and cancer. Acta Physiol (Oxf) 192:19–28 [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K, Ikeda K, Motoyama N, Mori N 2004 Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem 279:34741–34749 [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs 3rd WH, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC 2004 Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA 101:2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA 2003 Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215–218 [DOI] [PubMed] [Google Scholar]
- Lin L, Hron JD, Peng SL 2004 Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21:203–213 [DOI] [PubMed] [Google Scholar]
- Accili D, Arden KC 2004 FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426 [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegué E, DePinho RA, Gilliland DG 2007 FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128:325–339 [DOI] [PubMed] [Google Scholar]
- Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S 2007 Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117:2133–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA 2007 FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128:309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS 2008 PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a). Proc Natl Acad Sci USA 105:2622–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker WJ, Harris IS, Mak TW 2007 FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell 28:941–953 [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML 2001 LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523 [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP 2002 High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521 [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML 2002 A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner- Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO 2004 Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res 19:2033–2040 [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ 2006 Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281:31720–31728 [DOI] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA 2003 Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW 2004 Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevetson B, Taylor S, Pan Y 2004 Cbfa1/RUNX2 directs specific expression of the sclerosteosis gene (SOST). J Biol Chem 279:13849–13858 [DOI] [PubMed] [Google Scholar]
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W 2001 Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543 [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J 2001 Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmington K, Morony S, Sarosi I, Gong G, Stephens P, Winkler DG, Sutherland MK, Latham JA, Kirby H, Moore A, Robinson M, Kostenuik PJ, Simonet S, Lacey DL, Paszty C 2004 Sclerostin antagonism in adult rodents, via monoclonal antibody mediated blockade, increases bone mineral density and implicates sclerostin as a key regulator of bone mass during adulthood. J Bone Miner Res 19:S56 (Abstract) [Google Scholar]
- Rodda SJ, McMahon AP 2006 Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231–3244 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S 2005 Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem 280:41342–41351 [DOI] [PubMed] [Google Scholar]
- Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X 2008 Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev 22:2968–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G 2008 Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R 2003 Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development 130:5297–5305 [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H 2003 Armadillo/β-catenin signals in the nucleus—proof beyond a reasonable doubt? Nat Cell Biol 5:179–182 [DOI] [PubMed] [Google Scholar]
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC 2005 Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science 308:1181–1184 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC 2007 Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor-to forkhead box O-mediated transcription. J Biol Chem 282:27298–27305 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Almeida M 2007 Gone with the Wnts: β-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol 21:2605–2614 [DOI] [PubMed] [Google Scholar]
- Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM 2008 Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. J Biol Chem 283:9224–9230 [DOI] [PubMed] [Google Scholar]
- Hoogeboom D, Burgering BM 2009 Should I stay or should I go: β-catenin decides under stress. Biochim Biophys Acta 1796:63–74 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Villanueva AR, Foldes J, Rao DS 1995 Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res 10:466–473 [DOI] [PubMed] [Google Scholar]
- Lips P, Courpron P, Meunier PJ 1978 Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res 26:13–17 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC 1996 Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest 97:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Bellido T, Almeida M, Plotkin LI, O'Brien CA, Weinstein RS, Manolagas SC 2008 Apoptosis and bone cells. In: Bilezikian JP, Raisz LG, Martin T, eds. Principles of bone biology. San Diego: Academic Press; 235–259 [Google Scholar]
- Glatt V, Canalis E, Stadmeyer L, Bouxsein ML 2007 Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 22:1197–1207 [DOI] [PubMed] [Google Scholar]
- Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S 2002 Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res 17:1044–1050 [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Pun S, Liang H 1999 Effects of age, estrogen depletion, and parathyroid hormone treatment on vertebral cancellous wall width in female rats. Bone 25:465–468 [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG 2005 Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res 20:1085–1092 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2007 Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger CJ, Lean JM, Davies JT, Chambers TJ 2005 Tumor necrosis factor-α mediates osteopenia caused by depletion of antioxidants. Endocrinology 146:113–118 [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA 2002 p53 mutant mice that display early ageing-associated phenotypes. Nature 415:45–53 [DOI] [PubMed] [Google Scholar]
- de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH 2002 Premature aging in mice deficient in DNA repair and transcription. Science 296:1276–1279 [DOI] [PubMed] [Google Scholar]
- Basu S, Michaëlsson K, Olofsson H, Johansson S, Melhus H 2001 Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288:275–279 [DOI] [PubMed] [Google Scholar]
- Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A 2003 Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab 88:1523–1527 [DOI] [PubMed] [Google Scholar]
- Oh B, Kim SY, Kim DJ, Lee JY, Lee JK, Kimm K, Park BL, Shin HD, Kim TH, Park EK, Koh JM, Kim GS 2007 Associations of catalase gene polymorphisms with bone mineral density and bone turnover markers in postmenopausal women. J Med Genet 44:e62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez MA, Ruiz-Ramos M, Correa-Muñoz E, Mendoza-Núñez VM 2007 Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord 8:124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altindag O, Erel O, Soran N, Celik H, Selek S 2008 Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol Int 28:317–321 [DOI] [PubMed] [Google Scholar]
- Varanasi SS, Francis RM, Berger CE, Papiha SS, Datta HK 1999 Mitochondrial DNA deletion associated oxidative stress and severe male osteoporosis. Osteoporos Int 10:143–149 [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA 2006 Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health (Larchmt) 15:295–300 [DOI] [PubMed] [Google Scholar]
- Sanders KM, Kotowicz MA, Nicholson GC 2007 Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early post-menopausal women: a pilot study. Transl Res 150:215 [DOI] [PubMed] [Google Scholar]
- Glass 2nd DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G 2005 Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764 [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA 2005 Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102:3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F 2003 High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18:960–974 [DOI] [PubMed] [Google Scholar]
- Dowell P, Otto TC, Adi S, Lane MD 2003 Convergence of peroxisome proliferator-activated receptor γ and Foxo1 signaling pathways. J Biol Chem 278:45485–45491 [DOI] [PubMed] [Google Scholar]
- Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E 2006 FOXO1 represses peroxisome proliferator-activated receptor-γ1 and -γ2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem 281:19881–19891 [DOI] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H 2004 PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansen TB, Burgering BM 2008 Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol 18:421–429 [DOI] [PubMed] [Google Scholar]
- Ambrogini E, Almeida M, Martin-Millan M, Paik J, DePinho RA, Han L, Goellner J, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC, 2 Feb 2010 FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metabolism doi: 10.1016/j.cmet.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM 2008 Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312 [DOI] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC 2004 IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117:225–237 [DOI] [PubMed] [Google Scholar]
- Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD 2005 Crossroads of estrogen receptor and NF-κB signaling. Sci STKE 2005:pe27 [DOI] [PubMed] [Google Scholar]
- Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY 2007 Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev 21:3244–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G 2006 Cross-talk between nuclear receptors and nuclear factor κB. Oncogene 25:6868–6886 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL 2002 Sex steroids and bone. Recent Prog Horm Res 57:385–409 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Chen JR, Schuller M, Plotkin L, Bellido T 2004 Kinase-mediated transcription, activators of nongenotropic estrogen-like signaling (ANGELS), and osteoporosis: a different perspective on the HRT dilemma. Kidney Int Suppl 91:S41–S49 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC 1992 Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91 [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL 2001 Attenuation of the self-renewal of transit amplifying osteoblast progenitors in the murine bone marrow by 17β-estradiol. J Clin Invest 107:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC 2001 Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2002 Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298:843–846 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC 2003 Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 111:1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ 2003 A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 112:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A, Narasimhan S 2005 Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta 360:81–86 [DOI] [PubMed] [Google Scholar]
- Moor AN, Gottipati S, Mallet RT, Sun J, Giblin FJ, Roque R, Cammarata PR 2004 A putative mitochondrial mechanism for antioxidative cytoprotection by 17β-estradiol. Exp Eye Res 78:933–944 [DOI] [PubMed] [Google Scholar]
- Chiang K, Parthasarathy S, Santanam N 2004 Estrogen, neutrophils and oxidation. Life Sci 75:2425–2438 [DOI] [PubMed] [Google Scholar]
- Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF 2002 Estradiol alters nitric oxide production in the mouse aorta through the α-, but not β-, estrogen receptor. Circ Res 90:413–419 [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Muñoz FJ, Metcalfe MJ, Hitschfeld M, Olivares G, Godoy JA, Inestrosa NC 2005 Trolox and 17β-estradiol protect against amyloid β-peptide neurotoxicity by a mechanism that involves modulation of the Wnt signaling pathway. J Biol Chem 280:11615–11625 [DOI] [PubMed] [Google Scholar]
- Sack MN, Rader DJ, Cannon 3rd RO 1994 Oestrogen and inhibition of oxidation of low-density lipoproteins in postmenopausal women. Lancet 343:269–270 [DOI] [PubMed] [Google Scholar]
- Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, Yamazaki I, Yoshizumi M, Eto M, Ouchi Y 2001 Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation 103:724–729 [DOI] [PubMed] [Google Scholar]
- Arnal JF, Clamens S, Pechet C, Negre-Salvayre A, Allera C, Girolami JP, Salvayre R, Bayard F 1996 Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc Natl Acad Sci USA 93:4108–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Honda K, Nakanishi M, Akaike A, Shimohama S 2000 Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. FASEB J 14:1202–1214 [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW 2005 Dissecting the basis of nongenomic activation of endothelial nitric oxide synthase by estradiol: role of ERα domains with known nuclear functions. Mol Endocrinol 19:277–289 [DOI] [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH 2004 Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc Natl Acad Sci USA 101:17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Shimizu T, Suzuki Y, Ogawara M, Isono K, Koseki H, Kurosawa H, Shirasawa T 2005 Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J Biol Chem 280:16417–16426 [DOI] [PubMed] [Google Scholar]
- Lapointe J, Kimmins S, Maclaren LA, Bilodeau JF 2005 Estrogen selectively up-regulates the phospholipid hydroperoxide glutathione peroxidase in the oviducts. Endocrinology 146:2583–2592 [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP 2008 Vascular actions of estrogens: functional implications. Pharmacol Rev 60:210–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn 2nd GW, Liao JK 2007 Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation 115:3197–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurh YJ, Chen ZH, Na HK, Han SY, Surh YJ 2004 2- Hydroxyestradiol induces oxidative DNA damage and apoptosis in human mammary epithelial cells. J Toxicol Environ Health A 67:1939–1953 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Hewitt SC, Peddada SD, Korach KS 2004 Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology 145:5485–5492 [DOI] [PubMed] [Google Scholar]
- Mishra DP, Shaha C 2005 Estrogen-induced spermatogenic cell apoptosis occurs via the mitochondrial pathway: role of superoxide and nitric oxide. J Biol Chem 280:6181–6196 [DOI] [PubMed] [Google Scholar]
- Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC 2005 Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem 280:4632–4638 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC 1998 Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow: evidence for autonomy from factors released during bone resorption. J Clin Invest 101:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Martin-Millan M, Ambrogini E, Han L, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC, 12 October 2009 Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA binding-independent actions of the ERα. J Bone Miner Res 24 doi: 10.1359/jbmr.091017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Martin-Millan M, Han L, Warren A, Shelton RS, Goellner JJ, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC 2008 Overexpression of glutathione reductase in osteoblasts decreases bone formation and partially prevents ovariectomy-induced bone loss. J Bone Miner Res 23:S88 (Abstract) [Google Scholar]
- Mann V, Huber C, Kogianni G, Collins F, Noble B 2007 The antioxidant effect of estrogen and selective estrogen receptor modulators in the inhibition of osteocyte apoptosis in vitro. Bone 40:674–684 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Ambrogini E, Martin-Millan M, Vyas K, Warren A, Shelton R, O'Brien CA, Jilka RL, Manolagas SC, 15 August 2009 Estrogens and androgens attenuate oxidative stress-induced NF-κB activation, cytokine production, and osteoclast progenitors by decreasing p66shc phosphorylation. J Bone Miner Res 24 (Suppl 1). Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=dc75038f-f228-40c7-8e4e- f9cb9f21dd62 [Google Scholar]
- Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ 2004 Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem Biophys Res Commun 314:197–207 [DOI] [PubMed] [Google Scholar]
- Linares GR, Xing W, Govoni KE, Chen ST, Mohan S 2009 Glutaredoxin 5 regulates osteoblast apoptosis by protecting against oxidative stress. Bone 44:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR 1990 Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur R, Barrios R, Elefteriou F, Glass 2nd DA, Lieberman MW, Karsenty G 2003 Reversible skeletal abnormalities in γ-glutamyl transpeptidase-deficient mice. Endocrinology 144:2761–2764 [DOI] [PubMed] [Google Scholar]
- Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ 2005 Reactive oxygen species stimulates receptor activator of NF-κB ligand expression in osteoblast. J Biol Chem 280:17497–17506 [DOI] [PubMed] [Google Scholar]
- Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ 2005 Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146:728–735 [DOI] [PubMed] [Google Scholar]
- Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY 2005 A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859 [DOI] [PubMed] [Google Scholar]
- Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K 2009 Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15:259–266 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S 2007 Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M 2008 Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien C, Manolagas SC 2009 The estrogen receptor α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24:323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saintier D, Khanine V, Uzan B, Ea HK, de Vernejoul MC, Cohen-Solal ME 2006 Estradiol inhibits adhesion and promotes apoptosis in murine osteoclasts in vitro. J Steroid Biochem Mol Biol 99:165–173 [DOI] [PubMed] [Google Scholar]
- Park H, Jung YK, Park OJ, Lee YJ, Choi JY, Choi Y 2005 Interaction of Fas ligand and Fas expressed on osteoclast precursors increases osteoclastogenesis. J Immunol 175:7193–7201 [DOI] [PubMed] [Google Scholar]
- Kovaciæ N, Lukiæ IK, Grceviæ D, Kataviæ V, Croucher P, Marusiæ A 2007 The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol 178:3379–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM 2003 Osteoclast apoptosis: the role of Fas in vivo and in vitro. Endocrinology 144:5545–5555 [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Niederer P, Knothe U 1998 In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22:107–117 [DOI] [PubMed] [Google Scholar]
- Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang BH, Rojanasakul Y 2008 The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J Immunol 180:3072–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug H, Enari M, Nagata S 1994 No requirement of reactive oxygen intermediates in Fas-mediated apoptosis. FEBS Lett 351:311–313 [DOI] [PubMed] [Google Scholar]
- Aronis A, Melendez JA, Golan O, Shilo S, Dicter N, Tirosh O 2003 Potentiation of Fas-mediated apoptosis by attenuated production of mitochondria-derived reactive oxygen species. Cell Death Differ 10:335–344 [DOI] [PubMed] [Google Scholar]
- Galien R, Evans HF, Garcia T 1996 Involvement of CCAAT/enhancer-binding protein and nuclear factor-κB binding sites in interleukin-6 promoter inhibition by estrogens. Mol Endocrinol 10:713–722 [DOI] [PubMed] [Google Scholar]
- Galien R, Garcia T 1997 Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Res 25:2424–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B, Yang MX 1995 Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-κB and C/EBP β. Mol Cell Biol 15:4971–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell DP, Norris JD 1997 Analysis of the molecular pharmacology of estrogen receptor agonists and antagonists provides insights into the mechanism of action of estrogen in bone. Osteoporos Int 7(Suppl 1):S29–S34 [DOI] [PubMed] [Google Scholar]
- Temkin V, Karin M 2007 From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev 220:8–21 [DOI] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M 2006 The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell 124:601–613 [DOI] [PubMed] [Google Scholar]
- Shevde NK, Bendixen AC, Dienger KM, Pike JW 2000 Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA 97: 7829–7834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC 1998 Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 13:793–802 [DOI] [PubMed] [Google Scholar]
- Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Koji T, Urayama S, Fujiyama K, Kiriyama T, Nakashima T, Nakane PK, Nagataki S 1997 Fas and Fas ligand interaction is necessary for human osteoblast apoptosis. J Bone Miner Res 12:1637–1646 [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nakashima T, Tsuboi M, Urayama S, Matsuoka N, Ida H, Kawabe Y, Sakai H, Migita K, Aoyagi T, Nakashima M, Maeda K, Eguchi K 1998 Insulin-like growth factor I stimulates proliferation and Fas-mediated apoptosis of human osteoblasts. Biochem Biophys Res Commun 247:46–51 [DOI] [PubMed] [Google Scholar]
- Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, Noble B 2004 Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci 75:2879–2895 [DOI] [PubMed] [Google Scholar]
- Duque G, El Abdaimi K, Henderson JE, Lomri A, Kremer R 2004 Vitamin D inhibits Fas ligand-induced apoptosis in human osteoblasts by regulating components of both the mitochondrial and Fas-related pathways. Bone 35:57–64 [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, Manolagas SC 2004 The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology 145:1980–1987 [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Dennison EM, Walker BR, Syddall HE, Wood PJ, Andrew R, Phillips DI, Cooper C 2005 Cortisol secretion and rate of bone loss in a population-based cohort of elderly men and women. Calcif Tissue Int 77:134–138 [DOI] [PubMed] [Google Scholar]
- Dennison E, Hindmarsh P, Fall C, Kellingray S, Barker D, Phillips D, Cooper C 1999 Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J Clin Endocrinol Metab 84:3058–3063 [DOI] [PubMed] [Google Scholar]
- Cooper MS 2008 11β-Hydroxysteroid dehydrogenase: a regulator of glucocorticoid response in osteoporosis. J Endocrinol Invest 31(Suppl 7):16–21 [PubMed] [Google Scholar]
- Lu H, Kraut D, Gerstenfeld LC, Graves DT 2003 Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144:346–352 [DOI] [PubMed] [Google Scholar]
- He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT 2004 Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology 145:447–452 [DOI] [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, Krothapalli N, Alyassi M, Luan Q, Graves DT 2006 Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res 85:510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M 2007 Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone 40:1408–1414 [DOI] [PubMed] [Google Scholar]
- Liu Z, Aronson J, Wahl EC, Liu L, Perrien DS, Kern PA, Fowlkes JL, Thrailkill KM, Bunn RC, Cockrell GE, Skinner RA, Lumpkin Jr CK 2007 A novel rat model for the study of deficits in bone formation in type-2 diabetes. Acta Orthop 78:46–55 [DOI] [PubMed] [Google Scholar]
- Fujii H, Hamada Y, Fukagawa M 2008 Bone formation in spontaneously diabetic Torii-newly established model of non-obese type 2 diabetes rats. Bone 42:372–379 [DOI] [PubMed] [Google Scholar]
- Hamada Y, Fujii H, Kitazawa R, Yodoi J, Kitazawa S, Fukagawa M 2009 Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin-induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone 44:936–941 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Bex M, Van Herck E, Laureys J, Dooms L, Lesaffre E, Ravussin E 1995 Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab 80:1194–1202 [DOI] [PubMed] [Google Scholar]
- Achemlal L, Tellal S, Rkiouak F, Nouijai A, Bezza A, Derouiche el M, Ghafir D, El Maghraoui A 2005 Bone metabolism in male patients with type 2 diabetes. Clin Rheumatol 24:493–496 [DOI] [PubMed] [Google Scholar]
- Oz SG, Guven GS, Kilicarslan A, Calik N, Beyazit Y, Sozen T 2006 Evaluation of bone metabolism and bone mass in patients with type-2 diabetes mellitus. J Natl Med Assoc 98:1598–1604 [PMC free article] [PubMed] [Google Scholar]
- Hofbauer LC, Brueck CC, Singh SK, Dobnig H 2007 Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 22:1317–1328 [DOI] [PubMed] [Google Scholar]
- Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS 2007 NF-κB prevents β cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci USA 104:1913–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL 2005 Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- Brownlee M 2001 Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES 2006 Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948 [DOI] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM 2004 FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS 2002 A central role for JNK in obesity and insulin resistance. Nature 420:333–336 [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M 2004 Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med 10:1128–1132 [DOI] [PubMed] [Google Scholar]
- Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O 2004 Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279:41114–41123 [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M 1999 Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes 48:2398–2406 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP 1999 Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA 96:10857–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM 2004 Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res 95:1075–1081 [DOI] [PubMed] [Google Scholar]
- Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G 2006 Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 55:1642–1650 [DOI] [PubMed] [Google Scholar]
- Hurson CJ, Butler JS, Keating DT, Murray DW, Sadlier DM, O'Byrne JM, Doran PP 2007 Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet Disord 8:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Martin-Millan M, Han L, Warren A, Shelton R, Plotkin L, Bellido T, O'Brien CA, Jilka RL, Weinstein RS, Manolagas SC 2008 Induction of oxidative stress and diversion of b-catenin from TCF- to FOXO-mediated transcription by glucocorticoids or TNFa in osteoblastic cells. J Bone Miner Res 23:S170 (Abstract) [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP 2007 LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315:1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U 2007 TCF7L2 and type 2 diabetes—we WNT to know. Diabetologia 50:5–7 [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K 2006 Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38:320–323 [DOI] [PubMed] [Google Scholar]
- Owen KR, McCarthy MI 2007 Genetics of type 2 diabetes. Curr Opin Genet Dev 17:239–244 [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M 2007 A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Brubaker PL, Jin T 2005 TCF-4 mediates cell type-specific regulation of proglucagon gene expression by β-catenin and glycogen synthase kinase-3β. J Biol Chem 280:1457–1464 [DOI] [PubMed] [Google Scholar]
- Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, Susulic VS, MacDougald OA 2007 Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes 56:295–303 [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Wharton Jr KA, Eisch AJ 2008 Which way does the Wnt blow? Exploring the duality of canonical Wnt signaling on cellular aging. BioEssays 30:102–106 [DOI] [PubMed] [Google Scholar]
- Jin T 2008 The WNT signalling pathway and diabetes mellitus. Diabetologia 51:1771–1780 [DOI] [PubMed] [Google Scholar]
- Hamada Y, Fujii H, Fukagawa M 2009 Role of oxidative stress in diabetic bone disorder. Bone 45:S35–S38 [DOI] [PubMed] [Google Scholar]
- Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM 2004 The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res 45:993–1007 [DOI] [PubMed] [Google Scholar]
- Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V 2004 Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89:4246–4253 [DOI] [PubMed] [Google Scholar]
- Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K 2006 Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health Across the Nation. J Bone Miner Res 21:1839–1846 [DOI] [PubMed] [Google Scholar]
- Marcovitz PA, Tran HH, Franklin BA, O'Neill WW, Yerkey M, Boura J, Kleerekoper M, Dickinson CZ 2005 Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol 96:1059–1063 [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I 2002 Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol 37:757–767 [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M 2001 Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171 [DOI] [PubMed] [Google Scholar]
- Meunier P, Aaron J, Edouard C, Vignon G 1971 Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop 80:147–154 [DOI] [PubMed] [Google Scholar]
- Kajkenova O, Lecka-Czernik B, Gubrij I, Hauser SP, Takahashi K, Parfitt AM, Jilka RL, Manolagas SC, Lipschitz DA 1997 Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res 12:1772–1779 [DOI] [PubMed] [Google Scholar]
- Knouff C, Auwerx J 2004 Peroxisome proliferator-activated receptor-γ calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev 25:899–918 [DOI] [PubMed] [Google Scholar]
- Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS 2002 PPARγ knockdown by engineered transcription factors: exogenous PPARγ 2 but not PPARγ 1 reactivates adipogenesis. Genes Dev 16:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL 1999 Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem 74:357–371 [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM 1995 15-Deoxy-δ 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR γ. Cell 83:803–812 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM 1997 Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci USA 94:4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA 1995 An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPAR γ). J Biol Chem 270:12953–12956 [DOI] [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK 1999 Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 400:378–382 [DOI] [PubMed] [Google Scholar]
- Kuhn H, Walther M, Kuban RJ 2002 Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat 68–69:263–290 [DOI] [PubMed] [Google Scholar]
- Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J 2004 Enhanced bone formation in lipodystrophic PPARγ(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep 5:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL 2002 Divergent effects of selective peroxisome proliferator-activated receptor-γ 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143:2376–2384 [DOI] [PubMed] [Google Scholar]
- Sorocéanu MA, Miao D, Bai XY, Su H, Goltzman D, Karaplis AC 2004 Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol 183:203–216 [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka- Czernik B 2004 Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL 2005 Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 146:1226–1235 [DOI] [PubMed] [Google Scholar]
- Kong M, Fox CJ, Mu J, Solt L, Xu A, Cinalli RM, Birnbaum MJ, Lindsten T, Thompson CB 2004 The PP2A-associated protein α4 is an essential inhibitor of apoptosis. Science 306:695–698 [DOI] [PubMed] [Google Scholar]
- Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ 2005 Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307:1101–1104 [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE 2005 Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307:720–724 [DOI] [PubMed] [Google Scholar]
- Townsend KJ, Zhou P, Qian L, Bieszczad CK, Lowrey CH, Yen A, Craig RW 1999 Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J Biol Chem 274:1801–1813 [DOI] [PubMed] [Google Scholar]
- Kim SH, Yoo CI, Kim HT, Park JY, Kwon CH, Kim YK 2006 Activation of peroxisome proliferator-activated receptor-γ (PPARγ) induces cell death through MAPK- dependent mechanism in osteoblastic cells. Toxicol Appl Pharmacol 215:198–207 [DOI] [PubMed] [Google Scholar]
- Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES 2004 Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science 303:229–232 [DOI] [PubMed] [Google Scholar]
- Kühn H, Borchert A 2002 Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic Biol Med 33:154–172 [DOI] [PubMed] [Google Scholar]
- Schewe T 2002 15-Lipoxygenase-1: a prooxidant enzyme. Biol Chem 383:365–374 [DOI] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL 2009 Increased lipid oxidation causes oxidative stress, increased PPARγ expression and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem 284:27438–27448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteller G 2001 Peroxidation of linoleic acid and its relation to aging and age dependent diseases. Mech Ageing Dev 122:617–657 [DOI] [PubMed] [Google Scholar]
- Schneider C, Porter NA, Brash AR 2008 Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem 283:15539–15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T 1999 Activation of stress signaling pathways by the end product of lipid peroxidation. 4-Hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem 274:2234–2242 [DOI] [PubMed] [Google Scholar]
- Lee JY, Jung GY, Heo HJ, Yun MR, Park JY, Bae SS, Hong KW, Lee WS, Kim CD 2006 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett 166:212–221 [DOI] [PubMed] [Google Scholar]
- Yen CH, Hsieh CC, Chou SY, Lau YT 2001 17β-Estradiol inhibits oxidized low density lipoprotein-induced generation of reactive oxygen species in endothelial cells. Life Sci 70:403–413 [DOI] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA 2007 Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J Biol Chem 282:14515–14524 [DOI] [PubMed] [Google Scholar]
- Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A 2009 Adipogenesis and WNT signalling. Trends Endocrinol Metab 20:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne TF, Hamakubo T, Ito S, Aburatani H, Yanagisawa M, Kodama T, Sakai J 2009 COUP-TFII acts downstream of Wnt/β-catenin signal to silence PPARγ gene expression and repress adipogenesis. Proc Natl Acad Sci USA 106:5819–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Pradeep A, Wong L, Rana A, Rana B 2004 Peroxisome proliferator-activated receptor γ activation can regulate β-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. J Biol Chem 279:35583–35594 [DOI] [PubMed] [Google Scholar]
- Glass 2nd DA, Karsenty G 2006 Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Curr Top Dev Biol 73:43–84 [DOI] [PubMed] [Google Scholar]
- Bodine PV 2008 Wnt signaling control of bone cell apoptosis. Cell Res 18:248–253 [DOI] [PubMed] [Google Scholar]
- Karim R, Mack WJ, Lobo RA, Hwang J, Liu CR, Liu CH, Sevanian A, Hodis HN 2005 Determinants of the effect of estrogen on the progression of subclinical atherosclerosis: Estrogen in the Prevention of Atherosclerosis Trial. Menopause 12:366–373 [DOI] [PubMed] [Google Scholar]
- Stocker R, Keaney Jr JF 2004 Role of oxidative modifications in atherosclerosis. Physiol Rev 84:1381–1478 [DOI] [PubMed] [Google Scholar]
- Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, Vandenhoff G, Pei H, Natarajan R, Nadler JL, Hedrick CC 2004 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem 279:9440–9450 [DOI] [PubMed] [Google Scholar]
- Praticò D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM 2004 12/15-Lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol 164:1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folcik VA, Nivar-Aristy RA, Krajewski LP, Cathcart MK 1995 Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J Clin Invest 96:504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC 1998 Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Nicholas RW, Manolagas SC 2000 Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab 85:2907–2912 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Weinstein RS 1999 New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res 14:1061–1066 [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC 2002 Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest 109:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS 2006 Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology 147:5592–5599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Manolagas SC, Bellido T 2007 Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival: evidence for inside-out signaling leading to anoikis. J Biol Chem 282:24120–24130 [DOI] [PubMed] [Google Scholar]
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C 2003 Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48:3224–3229 [DOI] [PubMed] [Google Scholar]
- Manolagas SC 2000 Corticosteroids and fractures: a close encounter of the third cell kind [editorial]. J Bone Miner Res 15:1001–1005 [DOI] [PubMed] [Google Scholar]
- Peel NF, Moore DJ, Barrington NA, Bax DE, Eastell R 1995 Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann Rheum Dis 54:801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CW, Petrie EC, Murray SR, Colasurdo EA, Raskind MA, Peskind ER 2001 Human glucocorticoid feedback inhibition is reduced in older individuals: evening study. J Clin Endocrinol Metab 86:545–550 [DOI] [PubMed] [Google Scholar]
- Sandeep TC, Walker BR 2001 Pathophysiology of modulation of local glucocorticoid levels by 11β-hydroxysteroid dehydrogenases. Trends Endocrinol Metab 12:446–453 [DOI] [PubMed] [Google Scholar]
- Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, Matsumoto T 2003 Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res 92:81–87 [DOI] [PubMed] [Google Scholar]
- Chen WT, Shih TT, Chen RC, Lo SY, Chou CT, Lee JM, Tu HY 2001 Vertebral bone marrow perfusion evaluated with dynamic contrast-enhanced MR imaging: significance of aging and sex. Radiology 220:213–218 [DOI] [PubMed] [Google Scholar]
- Drescher W, Li H, Qvesel D, Jensen SD, Flo C, Hansen ES, Bünger C 2000 Vertebral blood flow and bone mineral density during long-term corticosteroid treatment: an experimental study in immature pigs. Spine 25:3021–3025 [DOI] [PubMed] [Google Scholar]
- Goans RE, Weiss GH, Abrams SA, Perez MD, Yergey AL 1995 Calcium tracer kinetics show decreased irreversible flow to bone in glucocorticoid treated patients. Calcif Tissue Int 56:533–535 [DOI] [PubMed] [Google Scholar]
- Kita K, Kawai K, Hirohata K 1987 Changes in bone marrow blood flow with aging. J Orthop Res 5:569–575 [DOI] [PubMed] [Google Scholar]
- Lien J, Kaye M 1978 Changes in the red cell, plasma and inulin spaces and in the total water contents of rat femurs in cortisone induced osteoporosis. Calcif Tissue Res 25:245–248 [DOI] [PubMed] [Google Scholar]
- Prisby RD, Ramsey MW, Behnke BJ, Dominguez 2nd JM, Donato AJ, Allen MR, Delp MD 2007 Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res 22:1280–1288 [DOI] [PubMed] [Google Scholar]
- Timmins PA, Wall JC 1977 Bone water. Calcif Tissue Res 23:1–5 [DOI] [PubMed] [Google Scholar]
- Ishijima H, Ishizaka H, Horikoshi H, Sakurai M 1996 Water fraction of lumbar vertebral bone marrow estimated from chemical shift misregistration on MR imaging: normal variations with age and sex. AJR Am J Roentgenol 167:355–358 [DOI] [PubMed] [Google Scholar]
- Harada S, Nagy JA, Sullivan KA, Thomas KA, Endo N, Rodan GA, Rodan SB 1994 Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J Clin Invest 93:2490–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez P, Esbrit P, Rodrigo A, Alvarez-Arroyo MV, Martínez ME 2002 Age-related changes in parathyroid hormone-related protein and vascular endothelial growth factor in human osteoblastic cells. Osteoporos Int 13:874–881 [DOI] [PubMed] [Google Scholar]
- Pufe T, Scholz-Ahrens KE, Franke AT, Petersen W, Mentlein R, Varoga D, Tillmann B, Schrezenmeir J, Glüer CC 2003 The role of vascular endothelial growth factor in glucocorticoid-induced bone loss: evaluation in a minipig model. Bone 33:869–876 [DOI] [PubMed] [Google Scholar]
- Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T 1987 Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8:157–164 [DOI] [PubMed] [Google Scholar]
- Michael Parfitt A 2006 Misconceptions V—activation of osteoclasts is the first step in the bone remodeling cycle. Bone 39:1170–1172 [DOI] [PubMed] [Google Scholar]
- Brandi ML, Collin-Osdoby P 2006 Vascular biology and the skeleton. J Bone Miner Res 21:183–192 [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P 2007 Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131:324–336 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL 2007 The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117:1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, D'Amore PA, Olsen BR 2002 Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129:1893–1904 [DOI] [PubMed] [Google Scholar]
- Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL 2008 Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc Natl Acad Sci USA 105:686–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C, Chavakis T 2007 Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem 282:26746–26753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin M, Julius MA, Kitajewski J 2008 Wnt/Frizzled signaling in angiogenesis. Angiogenesis 11:63–69 [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S 2003 Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100:3299–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW 2006 Three structural roles for water in bone observed by solid-state NMR. Biophys J 90:3722–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschner MA, Keller TS 2005 Hydraulic strengthening affects the stiffness and strength of cortical bone. Ann Biomed Eng 33:26–38 [DOI] [PubMed] [Google Scholar]
- Miserez A, Schneberk T, Sun C, Zok FW, Waite JH 2008 The transition from stiff to compliant materials in squid beaks. Science 319:1816–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R, Almeida M, O'Brien C, Manolagas S, 15 August 2009 Increased endogenous glucocorticoid production and decreased vascularity increase skeletal fragility with age. J Bone Miner Res 24 (Suppl 1). Available at http://www.asmbr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=fdc63d71-ff58-49ef-b9d4-c02b97d6232a [Google Scholar]
- Michan S, Sinclair D 2007 Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R 2009 Recent progress in the biology and physiology of sirtuins. Nature 460:587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L 2006 Sirtuins as potential targets for metabolic syndrome. Nature 444:868–874 [DOI] [PubMed] [Google Scholar]
- Guarente L 2007 Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol 72:483–488 [DOI] [PubMed] [Google Scholar]
- Guarente L 2008 Mitochondria—a nexus for aging, calorie restriction, and sirtuins? Cell 132:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH 2008 Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105:9793–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP 2009 Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119:2758–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R 2008 Resveratrol delays age- related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA 2006 Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JL, Yang CY, Zhao M, Kuo ML, Yen ML 2007 Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J Biol Chem 282:19385–19398 [DOI] [PubMed] [Google Scholar]
- Bäckesjö CM, Li Y, Lindgren U, Haldosén LA 2006 Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res 21:993–1002 [DOI] [PubMed] [Google Scholar]
- Boissy P, Andersen TL, Abdallah BM, Kassem M, Plesner T, Delaissé JM 2005 Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res 65:9943–9952 [DOI] [PubMed] [Google Scholar]
- Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, Xiao Z 2007 Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 14:806–814 [DOI] [PubMed] [Google Scholar]
- Edwards JR, Zainabadi K, Elefteriou E, Connelly L, Yull F, Blackwell TS, Alt F, Guarente L, Mundy GR 2007 The aging associated gene SIRT-1 regulates osteoclast formation and bone mass in vivo. J Bone Miner Res 22:S29 (Abstract) [Google Scholar]
- Armour KE, Armour KJ, Gallagher ME, Gödecke A, Helfrich MH, Reid DM, Ralston SH 2001 Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142:760–766 [DOI] [PubMed] [Google Scholar]
- Zhao M, Ko S, Edwards JR, Garrett IR, Mundy GR 2008 Mechanism of action of the anti-aging agent resveratrol on bone. J Bone Miner Res 23:S39 (Abstract) [Google Scholar]
- Edwards JR, Zainabadi K, Lwin ST, Elefteriou E, Munoz S, Moore MM, Guarente L, Mundy GR 2008 The longevity gene SIRT-1 independently controls both osteoblast and osteoclast function. J Bone Miner Res 23:S28 (Abstract) [Google Scholar]
- Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, Monsalve M 2009 Mutual dependence of Foxo3a and PGC-1α in the induction of oxidative stress genes. J Biol Chem 284:14476–14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P 2008 Metabolic adaptations through the PGC-1 α and SIRT1 pathways. FEBS Lett 582:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW 2009 A PGC-1α-O- GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem 284:5148–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R 2006 Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103:1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J 2009 AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM 2006 Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408 [DOI] [PubMed] [Google Scholar]
- Finkel T 2006 Cell biology: a clean energy programme. Nature 444:151–152 [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E 2003 Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 78:361–369 [DOI] [PubMed] [Google Scholar]
- Frisard M, Ravussin E 2006 Energy metabolism and oxidative stress: impact on the metabolic syndrome and the aging process. Endocrine 29:27–32 [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R 2009 Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM 2008 Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res 23:870–878 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO 2006 Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 166:2502–2510 [DOI] [PubMed] [Google Scholar]
- Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E 2008 Calorie restriction and bone health in young, overweight individuals. Arch Intern Med 168: 1859–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J 2008 Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358 [DOI] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O 2009 Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol 3:31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, Bukhari A, Suzuki H, Senda S, Imanishi S, Hirata K, Ishiki M, Hayashi R, Urakaze M, Nemoto H, Kobayashi M, Tobe K 1 September 2009 Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am J Physiol Endocrinol Metab 297:1179–1186 doi: 10.1152/ajpendo.90997.2008 [DOI] [PubMed] [Google Scholar]
- Knopp E, Troiano N, Bouxsein M, Sun BH, Lostritto K, Gundberg C, Dziura J, Insogna K 2005 The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology 146:1983–1990 [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL 2005 Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583 [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T 2008 Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE 3:e2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz T, Terman A, Brunk UT 2007 Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys 462:220–230 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ 2007 Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937 [DOI] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM 2006 Autophagy as a cell-repair mechanism: activation of chaperone-mediated autophagy during oxidative stress. Mol Aspects Med 27:444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC 2006 Perspective: Choreography from the tomb: an emerging role of dying osteocytes in the purposeful, and perhaps not so purposeful, targeting of bone remodeling. IBMS BoneKey-Osteovision 3:5–14, doi: 10.1138/20060193 [Google Scholar]
- Seeman E, Delmas PD 2006 Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261 [DOI] [PubMed] [Google Scholar]
- D'Angelo MA, Raices M, Panowski SH, Hetzer MW 2009 Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136:284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo MA, Hetzer MW 2008 Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol 18:456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]