Abstract
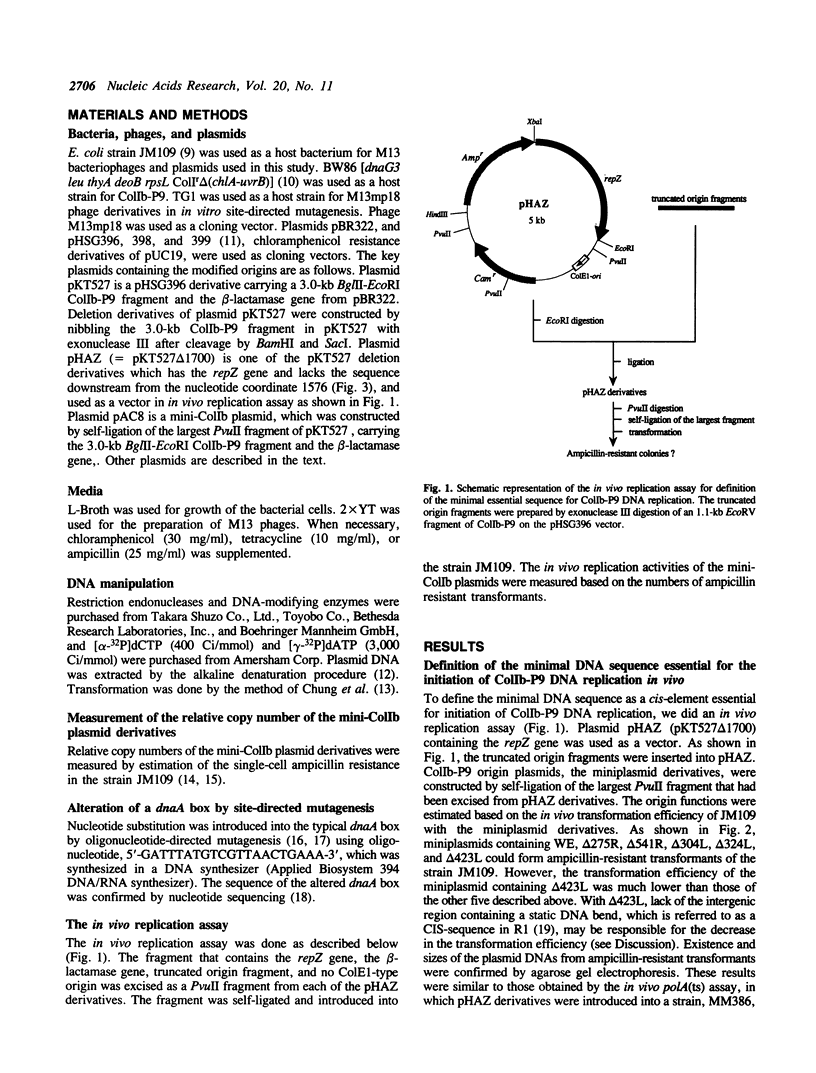
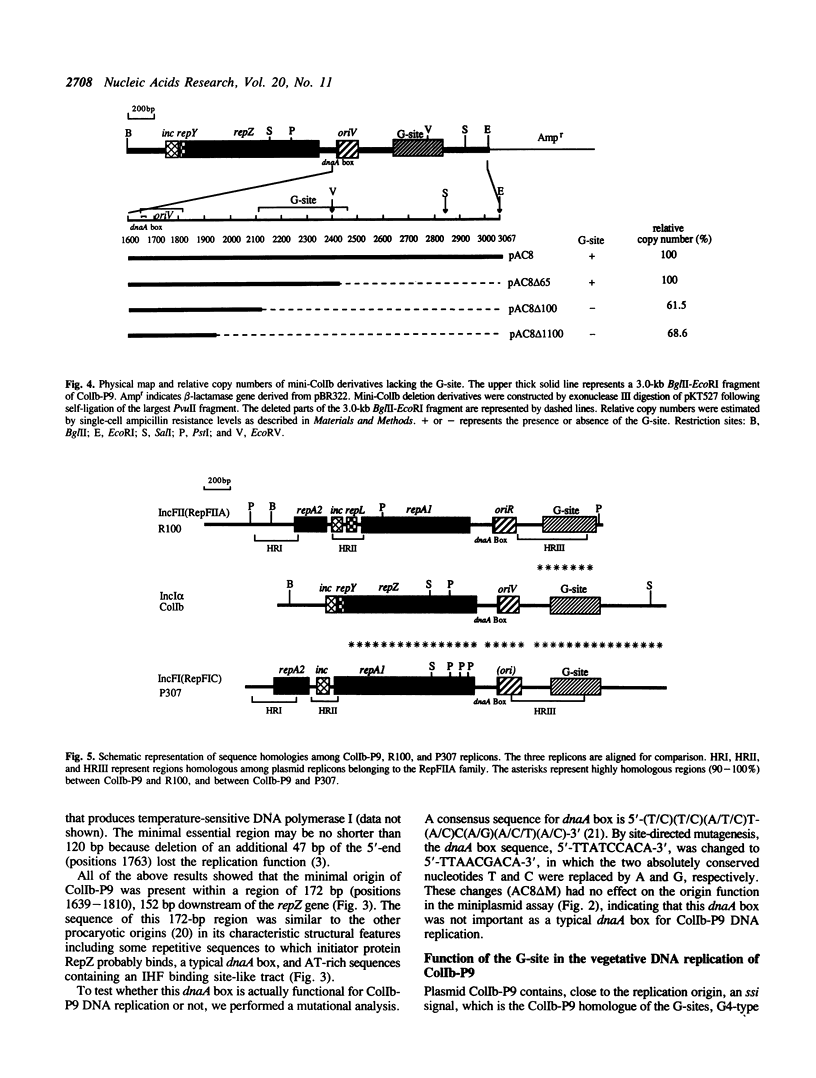
We have structurally and functionally analyzed the cis-elements essential for ColIb-P9 plasmid DNA replication. The putative oriV region encompassed a region of 172 base pairs (bp) located 152 bp downstream of the repZ gene. A typical dnaA box found in this region proved nonessential for the DNA replication of ColIb-P9. The ssi signal of ColIb-P9 is a homologue of the G-sites of R1 and R100 plasmids. Deletion of the G-site led to 1.5-fold reduction of the copy number, suggesting that although this G-site is not essential, it is important for efficient ColIb-P9 DNA replication. In addition, the ColIb-P9 replicon is highly and extensively homologous with the P307 (RepFIC) replicon, and highly homologous with the R100 (RepFIIA) replicon around the G-site region. These facts imply a common ancestry from which the plasmids have evolved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergquist P. L., Saadi S., Maas W. K. Distribution of basic replicons having homology with RepFIA, RepFIB, and RepFIC among IncF group plasmids. Plasmid. 1986 Jan;15(1):19–34. doi: 10.1016/0147-619x(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Chatfield L. K., Orr E., Boulnois G. J., Wilkins B. M. DNA primase of plasmid ColIb is involved in conjugal DnA synthesis in donor and recipient bacteria. J Bacteriol. 1982 Dec;152(3):1188–1195. doi: 10.1128/jb.152.3.1188-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. N., Womble D. D., Rownd R. H. In-vivo studies on the cis-acting replication initiator protein of IncFII plasmid NR1. J Mol Biol. 1988 Aug 5;202(3):495–509. doi: 10.1016/0022-2836(88)90281-1. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Komano T., Nisioka T. Physical and genetic analyses of the Inc-I alpha plasmid R64. J Bacteriol. 1984 Jun;158(3):997–1004. doi: 10.1128/jb.158.3.997-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama C., Takizawa T., Moriwaki H., Mizobuchi K. Role of leader peptide synthesis in repZ gene expression of the ColIb-P9 plasmid. J Biol Chem. 1990 Jun 25;265(18):10666–10673. [PubMed] [Google Scholar]
- Hama C., Takizawa T., Moriwaki H., Urasaki Y., Mizobuchi K. Organization of the replication control region of plasmid ColIb-P9. J Bacteriol. 1990 Apr;172(4):1983–1991. doi: 10.1128/jb.172.4.1983-1991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y., Sakai H., Hiasa H., Tanaka K., Komano T., Bagdasarian M. Functional division and reconstruction of a plasmid replication origin: molecular dissection of the oriV of the broad-host-range plasmid RSF1010. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):179–183. doi: 10.1073/pnas.88.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Kubo A., Kayanuma T., Furuichi T., Nisioka T. Highly mobile DNA segment of IncI alpha plasmid R64: a clustered inversion region. J Bacteriol. 1986 Jan;165(1):94–100. doi: 10.1128/jb.165.1.94-100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Arai K. Leading strand synthesis of R1 plasmid replication in vitro is primed by primase alone at a specific site downstream of oriR. J Biol Chem. 1989 May 15;264(14):8082–8090. [PubMed] [Google Scholar]
- Masai H., Arai K. RepA and DnaA proteins are required for initiation of R1 plasmid replication in vitro and interact with the oriR sequence. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4781–4785. doi: 10.1073/pnas.84.14.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden J. S., Marians K. J. Replication of pBR322 DNA in vitro with purified proteins. Requirement for topoisomerase I in the maintenance of template specificity. J Biol Chem. 1985 Aug 5;260(16):9316–9325. [PubMed] [Google Scholar]
- Molin S., Nordström K. Control of plasmid R1 replication: functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol. 1980 Jan;141(1):111–120. doi: 10.1128/jb.141.1.111-120.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Murakami Y., Imai M. Requirement of the Escherichia coli dnaA gene function for integrative suppression of dnaA mutations by plasmid R 100-1. Mol Gen Genet. 1988 Jul;213(1):163–165. doi: 10.1007/BF00333414. [DOI] [PubMed] [Google Scholar]
- Nomura N., Masai H., Inuzuka M., Miyazaki C., Ohtsubo E., Itoh T., Sasamoto S., Matsui M., Ishizaki R., Arai K. Identification of eleven single-strand initiation sequences (ssi) for priming of DNA replication in the F, R6K, R100 and ColE2 plasmids. Gene. 1991 Dec 1;108(1):15–22. doi: 10.1016/0378-1119(91)90482-q. [DOI] [PubMed] [Google Scholar]
- Nordström K., Molin S., Aagaard-Hansen H. Partitioning of plasmid R1 in Escherichia coli. II. Incompatibility properties of the partitioning system. Plasmid. 1980 Nov;4(3):332–339. doi: 10.1016/0147-619x(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Ortega S., Lanka E., Diaz R. The involvement of host replication proteins and of specific origin sequences in the in vitro replication of miniplasmid R1 DNA. Nucleic Acids Res. 1986 Jun 25;14(12):4865–4879. doi: 10.1093/nar/14.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees C. E., Bradley D. E., Wilkins B. M. Organization and regulation of the conjugation genes of IncI1 plasmid colIb-P9. Plasmid. 1987 Nov;18(3):223–236. doi: 10.1016/0147-619x(87)90065-5. [DOI] [PubMed] [Google Scholar]
- Saadi S., Maas W. K., Hill D. F., Bergquist P. L. Nucleotide sequence analysis of RepFIC, a basic replicon present in IncFI plasmids P307 and F, and its relation to the RepA replicon of IncFII plasmids. J Bacteriol. 1987 May;169(5):1836–1846. doi: 10.1128/jb.169.5.1836-1846.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C., Messer W. DnaA protein/DNA interaction. Modulation of the recognition sequence. Mol Gen Genet. 1991 Apr;226(1-2):34–40. doi: 10.1007/BF00273584. [DOI] [PubMed] [Google Scholar]
- Shiba K., Mizobuchi K. Posttranscriptional control of plasmid ColIb-P9 repZ gene expression by a small RNA. J Bacteriol. 1990 Apr;172(4):1992–1997. doi: 10.1128/jb.172.4.1992-1997.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sakai T., Honda Y., Hiasa H., Sakai H., Komano T. Plasmid Co1IB contains an ssi signal close to the replication origin. Plasmid. 1991 Mar;25(2):125–130. doi: 10.1016/0147-619x(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Tang X. B., Womble D. D., Rownd R. H. DnaA protein is not essential for replication of IncFII plasmid NR1. J Bacteriol. 1989 Oct;171(10):5290–5295. doi: 10.1128/jb.171.10.5290-5295.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


