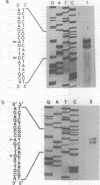
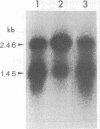
Abstract
The human rod transducin alpha subunit (Tr alpha) gene has been cloned. A cDNA clone, HG14, contained a 1.1 kb insertion when compared with the human Tr alpha cDNA published by Van Dop et al. (1). Based on two overlapping clones isolated from a human genomic library, the human Tr alpha gene is 4.9 kb in length and consists of nine exons interrupted by eight introns. Northern blots of human retina total RNA showed that the gene is transcribed by rod photoreceptors into two species of mRNA, 1.3 kb and 2.4 kb in size. Apparently, this is the result of alternative splicing. Two putative transcription initiation sites were determined by primer extension and S1 nuclease protection assays. The putative promoter regions of the human and mouse Tr alpha genes have an identity of 78.1%. As found in the mouse gene (2), no TATA consensus sequence is present in the human gene.
Full text
PDF

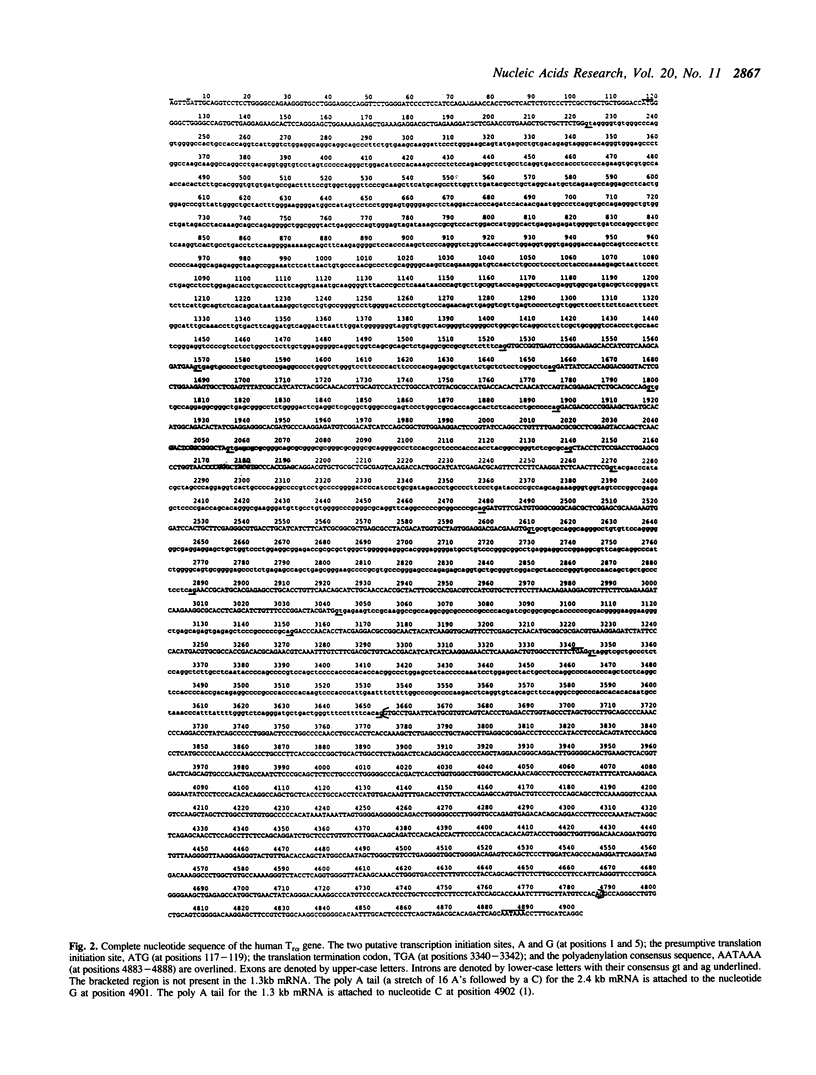
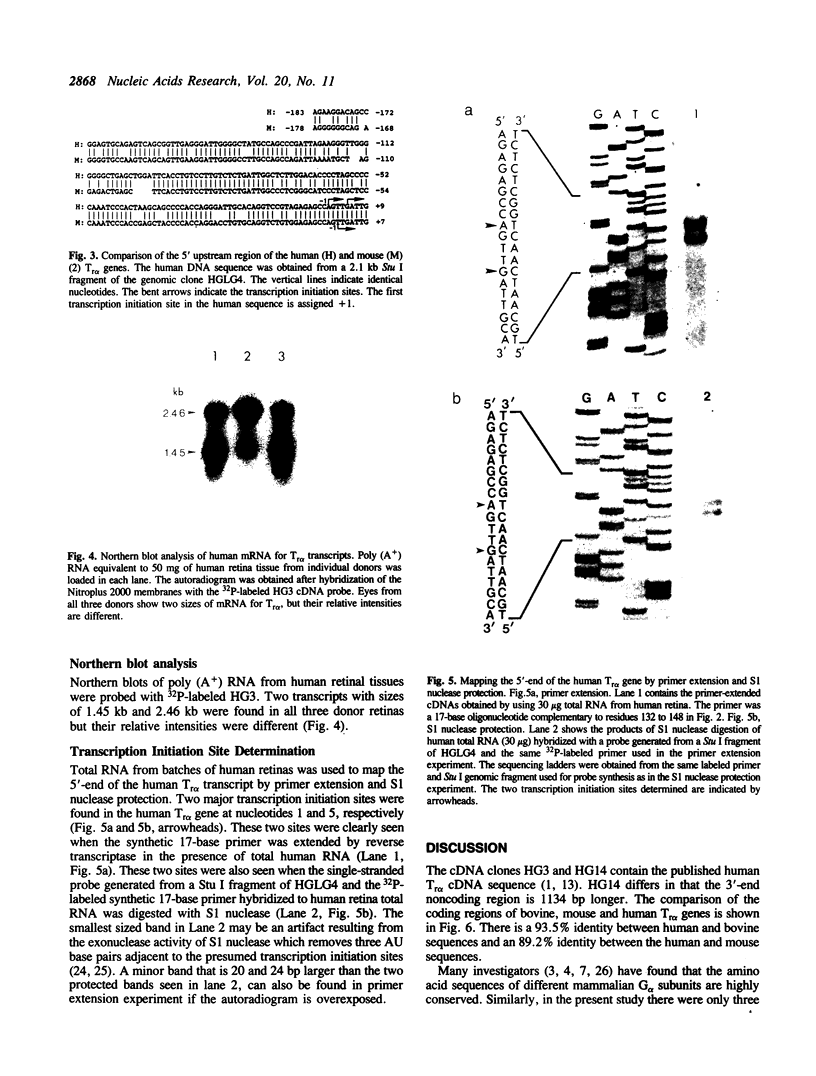

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende J. E. GTP-mediated macromolecular interactions: the common features of different systems. FASEB J. 1988 May;2(8):2356–2367. doi: 10.1096/fasebj.2.8.2452111. [DOI] [PubMed] [Google Scholar]
- Andrisani O. M., Hayes T. E., Roos B., Dixon J. E. Identification of the promoter sequences involved in the cell specific expression of the rat somatostatin gene. Nucleic Acids Res. 1987 Jul 24;15(14):5715–5728. doi: 10.1093/nar/15.14.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Fong S. L., Bridges C. D. Internal quadruplication in the structure of human interstitial retinol-binding protein deduced from its cloned cDNA. J Biol Chem. 1988 Oct 25;263(30):15330–15334. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hawkins J. D. A survey on intron and exon lengths. Nucleic Acids Res. 1988 Nov 11;16(21):9893–9908. doi: 10.1093/nar/16.21.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B. Molecular properties of the cGMP cascade of vertebrate photoreceptors. Annu Rev Physiol. 1987;49:793–812. doi: 10.1146/annurev.ph.49.030187.004045. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- Kroeker W. D., Kowalski D. Gene-sized pieces produced by digestion of linear duplex DNA with mung bean nuclease. Biochemistry. 1978 Aug 8;17(16):3236–3243. doi: 10.1021/bi00609a010. [DOI] [PubMed] [Google Scholar]
- Kroeker W. D., Kowalski D., Laskowski M., Sr Mung bean nuclease I. Terminally directed hydrolysis of native DNA. Biochemistry. 1976 Oct 5;15(20):4463–4467. doi: 10.1021/bi00665a020. [DOI] [PubMed] [Google Scholar]
- Lerea C. L., Bunt-Milam A. H., Hurley J. B. Alpha transducin is present in blue-, green-, and red-sensitive cone photoreceptors in the human retina. Neuron. 1989 Sep;3(3):367–376. doi: 10.1016/0896-6273(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Lerea C. L., Somers D. E., Hurley J. B., Klock I. B., Bunt-Milam A. H. Identification of specific transducin alpha subunits in retinal rod and cone photoreceptors. Science. 1986 Oct 3;234(4772):77–80. doi: 10.1126/science.3529395. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Lee R. H. Cyclic GMP and photoreceptor function. FASEB J. 1990 Sep;4(12):3001–3008. doi: 10.1096/fasebj.4.12.1697545. [DOI] [PubMed] [Google Scholar]
- Medynski D. C., Sullivan K., Smith D., Van Dop C., Chang F. H., Fung B. K., Seeburg P. H., Bourne H. R. Amino acid sequence of the alpha subunit of transducin deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Raport C. J., Dere B., Hurley J. B. Characterization of the mouse rod transducin alpha subunit gene. J Biol Chem. 1989 May 5;264(13):7122–7128. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Strathmann M., Wilkie T. M., Simon M. I. Diversity of the G-protein family: sequences from five additional alpha subunits in the mouse. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7407–7409. doi: 10.1073/pnas.86.19.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Tanabe T., Nukada T., Nishikawa Y., Sugimoto K., Suzuki H., Takahashi H., Noda M., Haga T., Ichiyama A., Kangawa K. Primary structure of the alpha-subunit of transducin and its relationship to ras proteins. Nature. 1985 May 16;315(6016):242–245. doi: 10.1038/315242a0. [DOI] [PubMed] [Google Scholar]
- Traut T. W. Do exons code for structural or functional units in proteins? Proc Natl Acad Sci U S A. 1988 May;85(9):2944–2948. doi: 10.1073/pnas.85.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dop C., Medynski D. C., Apone L. M. Nucleotide sequence for a cDNA encoding the alpha subunit of retinal transducin (GNAT1) isolated from the human eye. Nucleic Acids Res. 1989 Jun 26;17(12):4887–4887. doi: 10.1093/nar/17.12.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. R., Kelleher D. J., Woon C. W., Soparkar S., Osawa S., Heasley L. E., Johnson G. L. Receptor activation of G proteins. FASEB J. 1988 Oct;2(13):2841–2848. doi: 10.1096/fasebj.2.13.3139484. [DOI] [PubMed] [Google Scholar]
- Yatsunami K., Khorana H. G. GTPase of bovine rod outer segments: the amino acid sequence of the alpha subunit as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4316–4320. doi: 10.1073/pnas.82.13.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]