Abstract
GPCRs represent the largest family of integral membrane proteins and were first identified as receptor proteins that couple via heterotrimeric G-proteins to regulate a vast variety of effector proteins to modulate cellular function. It is now recognized that GPCRs interact with a myriad of proteins that not only function to attenuate their signalling but also function to couple these receptors to heterotrimeric G-protein-independent signalling pathways. In addition, intracellular and transmembrane proteins associate with GPCRs and regulate their processing in the endoplasmic reticulum, trafficking to the cell surface, compartmentalization to plasma membrane microdomains, endocytosis and trafficking between intracellular membrane compartments. The present review will overview the functional consequence of β-arrestin, receptor activity-modifying proteins (RAMPS), regulators of G-protein signalling (RGS), GPCR-associated sorting proteins (GASPs), Homer, small GTPases, PSD95/Disc Large/Zona Occludens (PDZ), spinophilin, protein phosphatases, calmodulin, optineurin and Src homology 3 (SH3) containing protein interactions with GPCRs.
LINKED ARTICLES
This article is part of a themed section on the Molecular Pharmacology of G Protein-Coupled Receptors (GPCRs). To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-6. To view the 2010 themed section on the same topic visit http://onlinelibrary.wiley.com/doi/10.1111/bph.2010.159.issue-5/issuetoc
Keywords: G-protein-coupled receptors, signalling, trafficking, PDZ proteins, beta arrestin, calmodulin, desensitization, RGS, homer, small G-proteins
Introduction
GPCRs represent the largest family of integral membrane proteins with more than 800 GPCR genes identified in the human genome (Luttrell, 2008). These membrane proteins are comprised of seven transmembrane regions (TM), an extracellular amino-terminus and an intracellular carboxyl-terminal domain. GPCRs not only regulate important physiological processes such as neurotransmission and cardiovascular function, but the dysregulation of GPCR activity also contributes to many different pathophysiological processes (Thompson et al., 2008). The importance of GPCRs for the treatment of disease is highlighted by the fact that at least 30–40% of prescription drugs target these proteins (Overington et al., 2006).
Classically, GPCRs have been thought to transduce the information provided by a diverse assortment of stimuli into intracellular second messengers by functioning as ligand-regulated guanine nucleotide exchange factors for a family of heterotrimeric GTP-binding proteins (G-proteins). In this model, either monomeric or dimeric GPCRs bind agonist and promote the ability of a GPCR to adopt an active conformation that facilitates the exchange of GDP for GTP on the G-protein Gα-subunit, leading to the dissociation of the Gα and Gβγ-subunits (Neer, 1995). Subsequently, the activated G-protein subunits can either positively or negatively regulate a variety of downstream effectors such as phospholipases, adenylyl cyclases and ion channels (Neer, 1995). However, it is now clear that GPCRs can signal via G-protein-independent mechanisms, the best characterized of which is β-arrestin-mediated signalling via Src and mitogen-activated kinase pathways (Ferguson, 2001; Lefkowitz et al., 2006; Luttrell, 2008). However, it is now appreciated that GPCRs also mediate cell signalling by functioning as scaffolds for the recruitment of GPCR interacting proteins (either transmembrane or cytosolic proteins), and through their association with GPCRs, these proteins modulate GPCR function and signal transduction (Bockaert et al., 2010). GPCR interacting proteins play important roles in regulating receptor ligand specificity (e.g. receptor activity modulating proteins), receptor endocytosis (e.g. β-arrestins, Arf6, RalA, phospholipase D2), expression at the cell surface (e.g. post-synaptic density protein 95, PSD95), and receptor recycling (e.g. synapse-associated protein 97, SAP97) (Bhattacharya et al., 2004; Bockaert et al., 2004; 2010; Ferguson, 2007). Thus, by virtue of their ability to modulate the localization of GPCRs to specific intracellular membrane compartments and scaffold multiprotein complexes that have been referred to as ‘signalsomes’, GPCR interacting proteins fine tune GPCR pharmacology and signal transduction. Recent research in the field has also focused extensively on how biased ligands bind to the extracellular face of GPCRs to selectively activate distinct intracellular signal transduction pathways and how receptor oligomerization modulates receptor pharmacology and signal transduction properties and there are several excellent reviews that overview this topic in detail (e.g. Bouvier, 2001; Terrillon and Bouvier, 2004; Galandrin et al., 2007; Kenakin, 2007; Rajagopal et al., 2010). Protein interactions with the intracellular face of GPCRs provide additional strategies for the development of novel therapeutic agents that selectively target the inactivation of specific intracellular signalling pathways downstream of the activation of GPCRs. However, the development of these agents has been hampered due to the complexity of introducing drugs into the cell. The current review will overview the role of GPCR interacting proteins in the regulation of the pharmacology, signalling and subcellular localization of GPCRs and will overview new mechanisms by which GPCR interacting proteins regulate GPCR function that might provide additional prospects for drug development.
GRK/arrestins
The first proteins identified as GPCR interacting proteins involved in the regulation of GPCR/G-protein coupling were the GPCR kinases (GRKs) and arrestins (reviewed in Krupnick and Benovic, 1998; Lefkowitz, 1998; Ferguson, 2001; Penela et al., 2003; Gainetdinov et al., 2004; Lefkowitz and Shenoy, 2005; Premont and Gainetdinov, 2007; Luttrell, 2008; Tobin et al., 2008). GRKs play a central role in regulating (i) the desensitization (uncoupling) of GPCR/G-protein signalling, (ii) the endocytosis of GPCRs to endosomes to allow GPCR dephosphorylation and resensitization, and (iii) GPCR signalling via G-protein-independent mechanisms.
Receptor desensitization
Mammals express seven GRK (GRK1–GRK7) and four arrestin isoforms, of which GRK1, GRK7, arrestin1 and arrestin4 are specifically localized to the visual system (Premont et al., 1995; Pitcher et al., 1998; Ferguson, 2001). Of the remaining GRK and arrestin proteins, GRK2, GRK3, GRK5, GRK6, arrestin2 (β-arrestin1) and arrestin3 (β-arrestin2) are ubiquitously expressed. GRKs are comprised of three function domains, an amino-terminal regulator of G-protein signalling (RGS) homology (RH) domain, a central catalytic domain and carboxyl-terminal membrane targeting domain. GRKs are targeted to the plasma membrane through multiple mechanisms to phosphorylate serine and threonine residues within the third intracellular loop and carboxyl-terminal tail domains of agonist-activated receptors. GRK2 and GRK3 encode pleckstrin homology domains at their carboxyl-termini that allows them to associate with the Gβγ subunit of hetereotrimeric G-proteins, as well as phosphatidylinositol 4,5-bisphosphate (Koch et al., 1993; Touhara et al., 1994; Pitcher et al., 1995). The light-activated association of GRK1 and GRK7 with the plasma membrane is facilitated by the post-translational farnesylation of its carboxyl-terminal CAAX motif (Inglese et al., 1992), whereas GRK4 and GRK6 are associated with the plasma membrane following palmitoylation (Stoffel et al., 1994; 1998; Premont et al., 1996), and GRK5 contains a polybasic domain that allows its association with membrane phospholipids (Kunapuli et al., 1994; Premont et al., 1994). For many GPCRs, GRK-mediated phosphorylation on its own is insufficient to mediate the desensitization of many GPCRs. Instead, the recruitment of arrestin proteins to agonist-activated and GRK-phosphorylated GPCRs facilitates the uncoupling of the receptor from hetereotrimeric G-proteins. However, for Gαq/11-coupled GPCRs, phosphorylation-independent desensitization can be achieved as the consequence of the displacement of Gαq/11 from the receptor complex by the RH domains of GRK2 and GRK3 (reviewed by Dhami and Ferguson, 2006; Ferguson, 2007). Thus, GRKs regulate GPCR desensitization by both phosphorylation-dependent and -independent mechanisms.
Receptor endocytosis
The endocytosis of many GPCRs appears to be mediated by the same mechanism that is required for GPCR desensitization. GRK-mediated phosphorylation promotes the binding of β-arrestins, which function as endocytic adaptor proteins that facilitate the targeting of receptors for clathrin-mediated endocytosis (Ferguson et al., 1996; Goodman et al., 1996) (Figure 1). Both β-arrestin1 and β-arrestin2 specifically bind to both the clathrin heavy chain and the β2-adaptin subunit of the heterotetrameric adaptor complex to facilitate endocytosis (Goodman et al., 1996; Laporte et al., 1998). In addition, Src-mediated phosphorylation of β2-adaptin regulates the dissociation of the β2-adaptin/β-arrestin complex (Zimmerman et al., 2009). The β-arrestin domain involved in clathrin binding is localized to amino acid residues 373–377 in the carboxyl-terminus of β-arrestin2 (Krupnick et al., 1997). Two arginine residues (R394 and R396) in β-arrestin2 mediate binding to β2-adaptin in vitro (Laporte et al., 2000). β-Arrestins also interact with a variety of other protein complexes that have been implicated in the regulation of clathrin-mediated endocytosis including E3 ubiqitin ligases such as mdm2 (Shenoy et al., 2001). Thus, β-arrestins play an essential role in recruiting proteins that are not only essential for the internalization of GPCRs but also for the regulation of the endocytic machinery.
Figure 1.
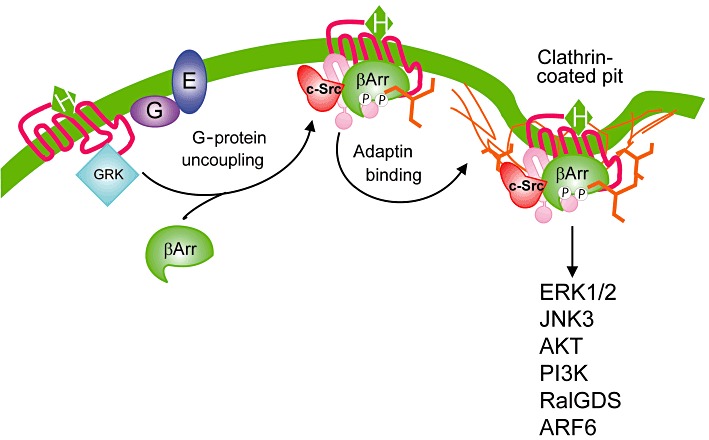
β-Arrestin-dependent endocytosis and signalling of GPCRs. Agonist activation promotes the GRK2-mediated phosphorylation that promotes the translocation and binding of β-arrestins, which serves to uncouple receptors from heterotrimeric G-proteins. β-Arrestins function as adaptor proteins that interact with both clathrin and β2-adaptin promoting the clathrin coated vesicle-mediated endocytosis of many GPCRs. c-Src is recruited to GPCRs as a consequence of its interaction with β-arrestin and couples the receptor to the MAPK pathway. β-Arrestin interactions with a variety of proteins allows for the coupling of GPCRs to a variety of different signal transduction pathways whose activation may be independent of heterotrimeric G-proteins. β-Arr, β-arrestin; P, phosphate.
There are striking differences in the ability of different arrestin isoforms to bind different GPCRs at the plasma membrane (Oakley et al., 2000). Early work indicated that there were two classes of GPCRs: (i) GPCRs that preferentially bind β-arrestin2 with lower affinity and do not internalize as a complex with β-arrestin2 to endosomes and (ii) GPCRs that bind β-arrestins with high affinity and form a stable complex with β-arrestins allowing the receptor to internalize as a complex with β-arrestins to endosomes. This stable association of β-arrestins with many GPCRs is due the phosphorylation of a cluster of serine residues localized a precise distance from the seventh transmembrane domain of the receptor. In general, GPCRs that do not internalize as a complex with β-arrestins are dephosphorylated, resensitized and rapidly recycled to the plasma membrane, whereas receptors that internalize with β-arrestin bound are either inefficiently recycled back to the plasma membrane or are not recycled at all (Ferguson, 2001; Luttrell, 2008). In the case of protease-activated receptors, the carboxyl-terminal tail regulates targeting to lysosomes (Trejo et al., 1998), due to the association of the receptor with sorting nexin 1 (Gullapalli et al., 2006). In addition, the activation of some receptors, such as the 5-HT2AR, corticotropin releasing factor receptor 1 (CRFR1), and CXCR4 results in the redistribution of β-arrestins to vesicular populations that do not contain the receptor proteins themselves (Barlic et al., 2001; Bhatnagar et al., 2001; Holmes et al., 2006). The physiological consequence of this differential localization of β-arrestin and GPCR proteins is unknown, except that β-arrestin redistribution to azruophilic granules is associated with Src family kinase activation and granule release (Barlic et al., 2001).
G-protein-independent signalling
In addition to functioning as adaptor proteins to facilitate the endocytosis of GPCRs, β-arrestins have been demonstrated to scaffold a wide variety of signalling complexes (Reiter and Lefkowitz, 2006; Luttrell, 2008; Luttrell and Gesty-Palmer, 2010; Rajagopal et al., 2010). Initial studies demonstrated that β-arrestins interact with Src family kinases via a polyproline motif and couple the receptor to MAPK ERK1/2 pathways (Luttrell et al., 1999; Barlic et al., 2001). Subsequently, β-arrestins have been demonstrated to bind to a wide variety of kinases, small GTPases, guanine nucleotide exchange factors, E3 ubiqutin ligases, phosphodiesterases and transcription factors (Lefkowtiz and Reiter, 2006; Luttrell, 2008; Getsy-Palmer and Luttrell, 2008; Rajagopal et al., 2010). There is now extensive evidence indicating that ligands that interact with GPCRs can selectively activate G-protein- versus β-arrestin-mediated signalling pathways by facilitating the formation of distinct receptor conformational states required for receptor association with either heterotrimeric G-proteins or β-arrestins. This concept has been extensively reviewed elsewhere (see Reiter and Lefkowitz, 2006; Galandrin et al., 2007; Gesty-Palmer and Luttrell, 2008; Rajagopal et al., 2010).
Receptor activity-modifying proteins (RAMPS)
RAMPS are single transmembrane spanning GPCR accessory proteins that function to modify the expression, and pharmacology of calcitonin receptor and calcitonin-like receptor (CRLR) (McLatchie et al., 1998). The calcitonin receptor and CRLR interact with several cacitonin family peptides including calcitonin, calcitonin gene related peptides (cGRP1 and cGRP2), amylin and adrenomedulin, which have overlapping but diverse physiological functions (Parameswaran and Spielman, 2006). The first RAMP protein was identified by expression cloning in Xenopus oocytes because the heterologous expression of either the calcitonin receptor or CRLR in cell lines failed to recapitulate the calcitonin peptide pharmacology observed in native tissues (McLatchie et al., 1998). RAMP1 is a 148-amino-acid protein that is comprised of a large extracellular domain, a single transmembrane domain and a short intracellular carboxyl-terminus. Database searches revealed the existence of an additional two members of the RAMP family, RAMP2 and RAMP3. RAMPs allosterically interact with the calcitonin receptor and CRLR, and the association of four distinct calcitonin peptides with the calcitonin receptors and RAMPs gives rise to seven distinct receptor phenotypes.
RAMPs appear to allosterically influence the structure of calcitonin family receptors allowing for their terminal glycosylation in the endoplasmic reticulum, thereby facilitating their expression at the cell surface (McLatchie et al., 1998) (Figure 2). In addition, RAMPs influence the pharmacology of calcitonin family peptides by one of three potential mechanism(s). First, RAMPs may allosterically influence the structure of calcitonin family receptors resulting in alterations in receptor/ligand specificity. Second, RAMPs may contribute to the ligand binding site for calcitonin family receptors, and thus, different receptor and RAMP combinations may dictate ligand binding specificity. Finally, RAMP-regulated terminal glycosylation of calcitonin family receptors may influence the specificity of calcitonin peptide binding. RAMP proteins may also influence calcitonin family receptor trafficking via PSD95/Disc large/Zona Occludens (PDZ) protein interactions with a PDZ domain binding motif localized at the end of the carboxyl-terminal tail of RAMP3. Specifically, RAMP3 interacts with N-ethylmaleimide-sensitive factor (NSF) to regulate the agonist-stimulated recycling of the CRLR (Bomberger et al., 2005a; Kuwasako et al., 2006) and Na+/H+ exchanger regulatory factor 1 (NHERF), which is involved in regulating the endocytosis of CRLR (Bomberger et al., 2005b). Thus, RAMP proteins not only regulate the pharmacology of these GPCRs but also the intracellular trafficking and post-translational modification of the receptors that are essential for the regulation of signal transduction via these receptors.
Figure 2.
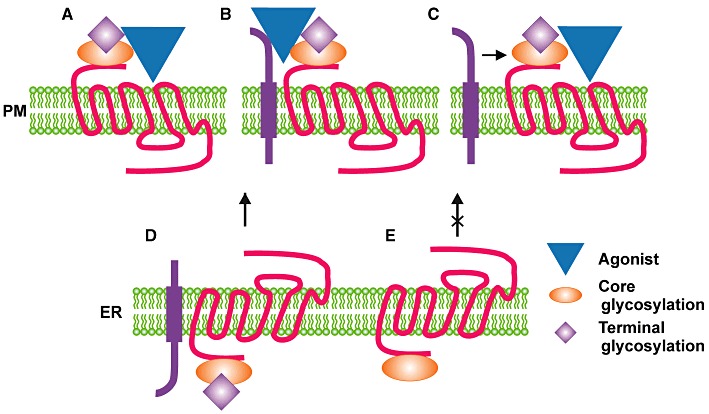
RAMP protein regulation of calcitonin receptor family cell surface expression and pharmacology. (A) RAMP proteins facilitate the terminal glycosylation of calcitonin receptors and glycosylation may contribute to the receptor ligand binding site to regulate ligand binding specificity. (B) RAMP proteins contribute to the calcitonin receptor family ligand binding site to regulate ligand specificity. (C) RAMP proteins interact with and allosterically modulate the ligand binding specificity of calcitonin receptor family members. (D) RAMP proteins facilitate the terminal glycosylation of calcitonin receptors to facilitate their processing in the ER and transport to the cell surface. (E) In the absence of appropriate RAMP protein expression calcitonin receptors are retained in the ER and are not transported to the cell surface.
It is now recognized that the role for RAMPs in the regulation of GPCR signalling may extend beyond the calcitonin receptor family to class C receptors. Bouschet et al. (2005) have demonstrated that the calcium sensing receptor (CaSR) is not transported to the plasma membrane unless either RAMP1 or RAMP3 is co-expressed with the receptor. Similar to what is observed for calcitonin family receptors, RAMP1 and RAMP3 influence the trafficking of the CaSR to the plasma membrane from the endoplasmic reticulum, as well as influences the terminal glycosylation of the receptor. Thus, it is possible that the effect of RAMP proteins on GPCR trafficking and pharmacology may be more diverse than originally envisaged.
Regulators of G-protein signalling (RGS)
RGS proteins comprise a group of proteins that modulates GPCR signalling by acting as GTPase activating proteins (GAPs) and thus accelerates the hydrolysis of GTP bound to the Gα subunit of Gq and Gi/o proteins, causing their inactivation. Therefore, RGS can terminate G-protein signalling after agonist stimulation (reviewed in Nunn et al., 2006). Common to all RGS proteins is a highly conserved region, the RGS box, with around 130 amino acids, which is sufficient and critical for GAP activity. The sequence flanking the RGS box is different for each RGS protein and may contain different motifs enabling a variety of protein–protein interactions (reviewed in Sjögren and Neubig, 2010). The presence of different domains other than the RGS box suggests these proteins have other functions that are not related to GAP activity such as the regulation of protein localization and intracellular trafficking as well as receptor selectivity (reviewed in Sethakorn et al., 2010).
The RGS proteins are divided into four classic groups (R4, R7, R12 and RZ) based on sequence homology (Ross and Wilkie, 2000). The group R4 (RGS 1–5, 8, 13, 16, 18 and 21) is represented by small molecules (except for RGS3) with short N- and C-flanking regions. Nonetheless, these small proteins are able to bind other proteins and seem to present functions beyond the modulation of GAP activity (reviewed in Bansal et al., 2007). RGS proteins in this group present a membrane targeting signal at the N-terminus, which is important to localize them in the proximity of the membrane together with their targets (activated Gα-proteins) (Bernstein et al., 2000). Besides its GAP activity, a sequence in the N-terminus of the RGS2 proteins was shown to bind directly to tubulin, enhancing microtubule polymerization in vitro (Heo et al., 2006). Furthermore, overexpression of RGS2 increased neurite outgrowth in PC-12 cells, suggesting that RGS2 may contribute to cell differentiation by regulating tubulin dynamics. It has been also shown that the amino-terminal sequence of some R4 members, such as RGS4 may regulate GPCR signalling independent of its GAP activity. In fact, there is some evidence showing inhibition of Gq-mediated calcium signalling by the RGS4 amino-terminus sequence (Zeng et al., 1998), and consistent with that, recent studies show direct interaction of RGS proteins to the third intracellular loop of GPCRs (Bernstein et al., 2004; Hague et al., 2005).
The R7 group of RGS proteins consists of four highly homologous proteins, RGS 6, 7, 9 and 11, predominantly expressed in the nervous system (Gold et al., 1997). These proteins can be divided in three domains: the RGS domain, a GGL domain capable of recruiting Gβ5 and a DEP/DHEX domain that binds to membrane anchor proteins R7BP and R9AP (reviewed in Anderson et al., 2009; Sjögren and Neubig, 2010). The RGS domain is located at the C-terminus and is the only catalytic domain (GTP hydrolysis) localized within these proteins. The DEP (Disheveled, Egl-10, Pleckstrin) and DHEX (DEP helical extension) domains are located at the N-terminus of the R7 RGS proteins. The DEP domain is important for protein-protein interactions and anchoring RGS proteins at the membrane near their site of action (Drenan et al., 2006; Jayaraman et al., 2009). It also interacts directly with GPCRs and recruits effectors to the receptor, as well as modulates the nature, duration and specificity of GPCR signalling (Ballon et al., 2006). The GGL domain (G-protein gamma like domain) is homologous to the γ subunit of G-proteins and is able to bind a Gβ subunit (just like a conventional G-protein γ subunit). However, the R7 RGS GGL domain specifically interacts with the Gβ5 subunit (Snow et al., 1998a; Makino et al., 1999). It is known that the R7 RGS–Gβ5 complex is crucial for R7 RGS proteins expression and stability, and Gβ5 knockout animals show total loss of all R7 RGS proteins (Chen et al., 2003). Accumulated data support the idea that these two molecules exist in a complex and function as a single entity to regulate GAP properties of R7 RGS proteins (He et al., 2000; Skiba et al., 2001).
The R12 family is represented by RGS 12 and 14. They contain additional functional domains such as a PDZ binding motif, a GoLoco motif and Rap binding domain at the very C-terminal end of the proteins. At the amino-terminus, these proteins present a PDZ domain and a phosphotyrosine-binding (PTB) domain, both of which promote protein–protein interactions (Ross and Wilkie, 2000; Martin-McCaffrey et al., 2005). The PTB domain can associate with tyrosine-phosphorylated N-type calcium channels, therefore regulating calcium signalling (Schiff et al., 2000; Richman et al., 2005). The amino-terminal PDZ domain is reported to associate with either the interleukin-8 receptor B (CXCR2) or its own C-terminal PDZ binding motif (Snow et al., 1998b). The GoLoco motif has a guanine nucleotide dissociation inhibitor activity and when bound to Gαi1, Gαi2 and Gαi3 leads to inhibition of the activation of G-protein by preventing exchange of GDP for GTP (Kimple et al., 2001; Siderovski and Willard, 2005).
The investigation of RGS protein activity suggests that their functions go beyond GAP activity and accumulating data in the past 15 years show the importance of RGS proteins for regulation of GPCR signalling. Moreover, the amino-termini of these proteins represents potential sites for interaction with both GPCRs and other signal transduction proteins, suggesting the formation of protein complexes that could contribute to the regulation and generation of GPCR-generated signals. The development of drugs targeting those interactions has a great potential and may produce a new class of therapeutic agents likely to be more specific since RGS proteins regulatory mechanisms may be specific for different cellular environments.
GPCR-associated sorting proteins (GASPs)
The GASP family is comprised of 10 proteins with significant sequence similarity, particularly in the C-terminus where they share a conserved 250-amino-acid residue domain. GASP proteins interact with GPCRs. For example, GASP-1 interact with the C tails of more than 30 GPCRs including dopamine D2 and D4, δ opioid and mGluR1a receptors (Whistler et al., 2002; Heydorn et al., 2004; Bartlett et al., 2005). GASPs have also been shown to interact with proteins that are not GPCRs, but the physiological relevance of these interactions is still to be determined. For example, GASP-1 is shown to interact with Period-1, which is one of the proteins involved in the transcription/translation-based auto-regulatory loop of the endogenous master clock (Cermakian and Sassone-Corsi, 2000). This interaction may be relevant, since Period-1 causes translocation of GASP-1 to the nucleus in cultured cells (Matsuki et al., 2001). GASP-2 interacts with huntingtin and four other partners including a nucleocytoplasmatic protein involved in DNA repair and regulation of transcription (Goehler et al., 2004; Irminger-Finger and Jefford, 2006). The first evidence that GASPs regulate GPCR function came from the study of Whistler et al. (2002) showing that GASP-1 is responsible for the rapid degradation of δ opioid, but not µ opioid receptors, following agonist stimulation. This observation suggests that GASPs function to regulate the post-endocytic sorting of GPCRs. The antagonism of D2 dopamine receptor/GASP-1 interactions using neutralizing antibodies results in the attenuation of agonist-stimulated D2 receptor desensitization (Bartlett et al., 2005). These studies suggest that the blockage of GPCR-GASP interactions may lead to increased GPCR signalling in response to sustained agonist stimulation as a consequence of reduced receptor degradation. However, chronic cocaine treatment of a GASP-1 knockout mouse still results in the down-regulation of dopamine and muscarinic receptor responses (Boeuf et al., 2009). This result supports the idea that GASP proteins are involved in post-endocytic sorting, although alternative mechanisms of GPCR sorting in endosomes may be employed in their absence. For example, sorting nexins are likely to play an important role in regulating GPCR trafficking to lysosomes (Marchese et al., 2008). The interactions between these proteins and GPCRs indicate that GPCR trafficking between intracellular compartments is not a passive process but involves a series of protein/protein interactions that can be expected to be highly regulated.
Homer proteins
Homer proteins share a 120-amino-acid amino-terminal domain and are the products of three genes in mammals and are highly expressed in neurons and skeletal muscle (Bockaert et al., 2010). Homer1 exists as three alternative splice variants (Homer1a, b, c), and Homer1a was first identified as an immediate early gene that was up-regulated in response to seizure-induced synaptic activation (Brakeman et al., 1997; Kato et al., 1998; Xiao et al., 1998). The amino-terminus of all Homer proteins encodes an ENA/VASP homology (EVH) domain, which binds to a PP-x-x-F motif found in the carboxyl-terminal tails of both metabotropic glutamate receptors (mGluRs) 1 and 5, as well as the α1D adrenergic receptor (Tu et al., 1998). The long Homer isoforms encode a carboxyl-terminal coiled-coiled domain that is organized into two separate regions, CC1 and CC2 (Hayashi et al., 2006). The Homer EVH domain also interacts with a variety of mGluR effector proteins including inositol 1,4,5 triphosphate receptors, ryanodine receptors, transient receptor channels 1 and 4, P/Q type Ca2+ channels, Shank, phosphoinositide 3-kinase enhancer long, dynamin III, the actin cytoskeleton and activated Rho family GTPases (Tu et al., 1998; 1999; Shiraishi et al., 1999; Kammermeier et al., 2000; Feng et al., 2002; Gray et al., 2003; Rong et al., 2003; Yuan et al., 2003).
The long isoforms of Homer are constitutively expressed and function as scaffolds that regulate their substrates by cross-linking them in the post-synaptic density via the dimerization of the Homer coiled-coiled domains (Hayashi et al., 2006). Homer1a lacks a carboxyl-terminal coiled-coiled domain, and in response to synaptic activity, its up-regulation is thought to disrupt protein complexes that are scaffolded by the Homer long forms (Kammermeier and Worley, 2007). The overexpression of Homer1b leads to the retention of mGluRs in the endoplasmic reticulum and cooperates with Shank to accumulate mGluRs at synapses in a co-cluster with PSD-95 and GKAP (Roche et al., 1999; Tu et al., 1999) (Figure 3). Moreover, Homer/Shank interactions play a role in regulating synaptic spine morphology (Sala et al., 2001). Homer1c also encodes a leucine zipper following the coiled-coiled domains that is involved in the clustering of mGluRs (Tadokoro et al., 1999). Homer1b/c facilitates the localization and clustering of mGluRs in dendrites, whereas overexpression of Homer1a disrupts the clusters and leads to the myslocalization of mGluRs to axons (Ango et al., 2000). Homer1b/c proteins have also been implicated in coupling mGluR5 to extracellular signal-regulated protein kinase cascades in neurons (Mao et al., 2005a). Homer3 is unique in that it binds to the carboxyl-terminal tails of group I mGluRs and functions to antagonize their intrinsic constitutive activity (Ango et al., 2001). Consistent with the role of Homers in regulating the activity and clustering of mGluRs at synapses, disruption of mGluR/Homer complexes is associated with impaired synaptic plasticity and is observed following chronic cocaine administration (Swanson et al., 2001; Szumlinski et al., 2004; Ronesi and Huber, 2008). Consequently, the disruption of mGluR/Homer complexes has been implicated in Fragile X, schizophrenia, anxiety, addiction and epilepsy (Polese et al., 2002; Potschka et al., 2002; Szumlinski et al., 2004; Giuffrida et al., 2005; Ronesi and Huber, 2008; Spellmann et al., 2011).
Figure 3.

Homer proteins function to regulate the association of protein complexes in the post-synaptic density. The Homer Ena/Vasp homology (EVH) domain as a consequence of the dimerization of their coiled-coiled domains couple mGluR1 and mGluR5 to inositol 1,4,5 triphosphate receptors and NMDA receptors via their interaction with SHANK which interacts with a protein complex involving GKAP and PSD95. The AMPA receptor is coupled to PSD95 via an interaction with TARP. The Homer EVH domain also couples mGluRs to the regulation of the actin cytoskeleton.
Small G-proteins
The small GTP-binding protein superfamily contains over 100 members that are generally classified by structural similarity into five subfamilies: Ras family GTPases (e.g. Ras, Rap and Ral), Rho family GTPases (Rho, Rac and cdc42), Arf family GTPases (Arf 1–6, Arl 1–7 and Sar), Rab family GTPases (>60 members, e.g. Rab5) and Ran family GTPases (Takai et al., 2001). Rho family GTPases function as regulators of the actin cytoskeleton and can also influence gene transcription, whereas Rab and Arf family GTPases control the formation, fusion and movement of vesicular traffic between different membrane compartments of the cell. The activation of small GTPases results in their conversion from a GDP-bound ‘inactive’ state to a GTP-bound ‘activated’ state. This conversion requires the dissociation of GDP from the small G-protein a process that is facilitated by guanine nucleotide exchange factors (GEFs).
Arf/PLD2
The first evidence that small GTPases might interact directly with GPCRs comes from a study by Mitchell et al. (1998) where Arf1/3 and Rho were shown to co-immunoprecipitate with m3 muscarinic acetylcholine receptors in response to agonist activation. GPCRs that form a complex with Arf1/3 and Rho exhibit the capacity to activate phospholipase D (PLD) in a manner that is independent of Gq/11 and Gi/o heterotrimeric G-proteins. In addition, Arf1 and Arf6 are implicated in the regulation of GPCR endocytosis (Claing et al., 2001; Koch et al., 2003; Houndolo et al., 2005). Arf6 plays an essential role in regulating the endocytosis of the β2-adrenergic receptor (β2AR), angiotensin II type 1 receptor (AT1R) and vasopression receptor (Claing et al., 2001; Houndolo et al., 2005). The activation of the β2AR has been demonstrated to result in the formation of a complex between β-arrestin, Arf6 and the Arf6 GEF, ARNO, and that the activation of Arf6 is essential for β2AR internalization (Claing et al., 2001). More recently, in studies examining the role of Arf6 in the regulation of AT1R endocytosis, Poupart et al. (2007) demonstrated that Arf6 plays a role in regulating the recruitment of clathrin and β2-adaptin to activated AT1Rs during the endocytic process. Although Arf1 dominant-negative mutants have no effect on the internalization of the β2AR, there is evidence linking Arf1 to the regulation of µ-opioid receptor endocytosis (Koch et al., 2003). Yeast two-hybrid screening with the C-tail of the µ-opioid receptor identified PLD2 as a µ-opioid receptor interacting protein that could also be co-immunoprecipitated with the receptor in a complex with Arf1. µ-Opioid receptor-mediated activation of PLD2 is also dependent on Arf1 activity. PLD2 activation results in the activation of the p38 MAPK pathway, resulting in the phosphorylation of a Rab5 effector, effector early endosome antigen 1 (EEA1), which is essential for µ opioid receptor internalization (Yang et al., 2010a). PLD2 activity also regulates AT1R internalization (Du et al., 2004). Thus, GPCR endocytosis is both mediated and regulated by protein interactions that are both dependent and independent of β-arrestins.
Ral/PLD2
Bhattacharya et al. (2004) have shown that the constitutive endocytosis of mGluR1a and mGluR5 is mediated by a RalA/PLD2-mediated pathway. Group I mGluRs scaffold a constitutive protein complex that contains Ral, Ral guanine nucleotide dissociation stimulator (RalGDS) and PLD2. This complex regulates the constitutive internalization of group I mGluRs in both heterologous cell cultures and primary cortical neurons. Constitutive mGluR internalization is dependent upon PLD2, but not PLD1, activity and requires PLD2-dependent phosphatidic acid formation. Recently, it has been demonstrated that phosphatidic acid plays a regulatory role in clathrin-coated vesicle formation and is involved in receptor mediated endocytosis (Antonescu et al., 2010). RalA has also been shown to interact with the AT1R receptor by bioluminescence energy transfer and provides a novel mechanism for the coupling of this receptor to the activation of phospholipase C (PLC) δ1 (Godin et al., 2010). Thus, PLD and RalA not only contribute to the regulation of GPCR endocytosis, they may also function as GPCR effector proteins.
Rab GTPases
Multiple Rab GTPases, such as Rab1, Rab4, Rab5, Rab7 and Rab11, have been identified to regulate ER–Golgi transport as well as the endocytosis and trafficking of GPCRs between early, late and recycling endosomes and lysosomes (Figure 4). Several groups have investigated the role of Rab GTPases in regulating the endocytosis and recycling of GPCRs, and it is now recognized that many Rab GTPases interact directly with the carboxyl-terminal tails of a number of GPCRs (Seachrist et al., 2002; Hamelin et al., 2005; Parent et al., 2009; Reid et al., 2010; Esseltine et al., 2011). The first indication that Rab GTPases could interact directly with a GPCR came arose from the identification of Rab5a as a protein that interacts with the AT1AR C-tail by yeast two-hybrid (Seachrist et al., 2002). Rab5 plays a role in regulating the formation, trafficking and fusion of clathrin-coated vesicles in the early endosome (Zerial and McBride, 2001). Rab5 can be co-immunoprecipitated with the AT1R and agonist activation leads to the exchange of GDP for GTP on Rab5, suggesting that the receptor can function as a Rab5 GEF. The interaction between Rab5 and the AT1R results in the retention of the receptor in homotypic endosomal vesicles. The AT1R Rab5 binding site is bipartite and involves amino acid residues within both the membrane proximal region of the AT1R carboxyl-terminal tail and the last 10 amino acids of the carboxyl-terminal tail. More recently, it has been reported that Rab4, Rab5, Rab7 and Rab11 all compete for binding to the AT1R, and that proline residue 354 and cysteine residue 355 represent important residues involved in Rab protein binding (Esseltine et al., 2011). Rab4, a Rab GTPase involved in the rapid recycling of vesicles from the endosome to the plasma membrane promotes AT1R dephosphorylation and the overexpression of a constitutively active Rab4 mutant, enhances AT1R resensitization. Several other GPCRs are reported to interact with Rab GTPases (Hamelin et al., 2005; Parent et al., 2009; Reid et al., 2010). However, the residues that are identified to be essential for Rab GTPase binding to the AT1R are not conserved in any of these GPCRs. For example, residues 335–345 within the central region the thromboxane A2 receptor carboxyl-terminal tail are required for Rab11 binding, whereas α-helix 8 at the membrane proximal end of the prostacyclin receptor binds Rab11 (Hamelin et al., 2005; Reid et al., 2010). In contrast, Rab11 binding to the β2AR involves a bipartite binding motif, with arginine 333 and lysine 348 representing the essential amino acid residues mediating Rab11 binding to the receptor (Parent et al., 2009). To date, there is no clearly defined consensus sequence for Rab GTPase binding to GPCRs. Thus, multiple Rab GTPases are able to associate with their cargo GPCRs, and the activity of these receptors can be differentially regulated via their association with these Rab GTPases.
Figure 4.

Role of Rab GTPases in regulating GPCR trafficking. Rab5 plays a role in the formation, trafficking and fusion of endocytic vesicles with the early endosome. Rab5 interacts with the AT1R, which regulates Rab5 activity. Rab4 regulates the rapid recycling of vesicles containing GPCRs to the cell surface, whereas Rab11 regulates slow vesicular recycling. Rab7 plays a role in targeting GPCRs to the late endosome and lysosomes. Each of these Rab GTPases exhibits the capcity to interact with GPCR cargo proteins.
PDZ domain protein interactions
PDZ proteins contain multiple PDZ domains that consist of an 80–90 amino acid sequence folded in a globular structure, and PDZ domains bind to specific sequences at the very carboxyl-termini of their interacting protein called PDZ binding motifs. These motifs are constituted of three to four amino acids and represent the minimal sequence that binds to PDZ proteins. The PDZ binding motif can be divided into classes I, II and III depending on their sequence. Class I motifs show a -S/T-x-V/I/L, class II motifs present a -Φ-x-Φ- and class III motifs present a -ψ-x-Φ- sequence where Φ represents an hydrophobic and ψ represents an acidic amino acid (Sheng and Sala, 2001). Many GPCRs contain a PDZ binding motif at their C-terminus enabling PDZ proteins to associate and scaffold multiprotein complexes, which can modulate different receptor properties such as trafficking, signalling, receptor stability and cell distribution (Table 1).
Table 1.
Functional role of known PDZ protein interactions with GPCRs
| PDZ protein | GPCR | Reported effect on GPCR | Reference |
|---|---|---|---|
| CAL | β1AR | ↓ membrane expression | He et al., 2004 |
| (PIST, GOPC, FIG) | mGluR1a | Agonist-induced intracellular co-localization with receptor, ↓ ERK activation | Zhang et al., 2008 |
| mGluR5a | ↑ mGluR5a protein expression, ↓ receptor ubiquitination | Cheng et al., 2010 | |
| SSTR5 | Targets receptor to Golgi apparatus (agonist-independent), ↓ membrane-targeting/recycling | Wente et al., 2005 | |
| GIPC | β1AR | ↓ ERK activation, no effect on cAMP signalling | Hu et al., 2003 |
| (TIP-2, Synectin) | D2 | Targets receptor to Golgi region | Jeanneteau et al., 2004 |
| D3 | ↓ signal transduction through Gi, targets receptor to Golgi region, ↓ D3 degradation | Jeanneteau et al., 2004 | |
| hLHR | Maintains receptor cell surface expression during hormone internalization | Hirakawa et al., 2003 | |
| MAGI-2 | β1AR | ↑ agonist-induced internalization, no effect on cAMP signalling | Xu et al., 2001 |
| (S-SCAM, ARIP-1) | VPAC1 | ↓ cAMP signalling, ↓ agonist-induced internalization | Gee et al., 2009 |
| MAGI-3 | β1AR | ↓ ERK activation | He et al., 2006 |
| β2AR | ↓ ERK activation | Yang et al., 2010b | |
| LPA2 | ↑ ERK activation, ↑ RhoA activation | Zhang et al., 2007 | |
| MPP3 | 5-HT2c | ↑ membrane stability, ↓desensitization | Gavarini et al., 2006 |
| MUPP-1 | 5-HT2a | ↑ localization to cell surface | Jones et al., 2009 |
| GABAB | ↑ Ca2+ signalling | Balasubramanian et al., 2007 | |
| MT1 | ↑ coupling with Gi protein, ↑ inhibition of adenylyl cyclase activity | Guillaume et al., 2008 | |
| OR2AG1 | Modulates Ca2+ signalling | Dooley et al., 2009 | |
| NHERF1 | 5-HT4a | Targets receptor to microvilli to interact with ezrin (potential role in cytoskeletal remodelling) | Joubert et al., 2004 |
| (EBP50) | β2AR | Agonist-induced co-localization with receptor | Hall et al., 1998 |
| β2AR | Regulates receptor sorting through ERM-binding domain interactions | Cao et al., 1999 | |
| hKOR | ↓ receptor down-regulation, ↑ recycling | Li et al., 2002 | |
| PTH1R | ↓ agonist-induced internalization, negligible effect on recycling and cAMP signalling | Wang et al., 2007 | |
| PTH1R | ↑ coupling to, and activation of, Gq | Wang et al., 2010 | |
| PTH1R | ↓ interaction with β-arrestin-2, ↓ desensitization | Wang et al., 2009 | |
| PTH1R | Targets receptor to membrane regions in close proximity to cytoskeleton, ↑ cAMP signalling | Wheeler et al., 2008 | |
| TPβ | ↓ internalization | Rochdi and Parent, 2003 | |
| NHERF2 | LPA2 | ↑ interaction with PLCβ3 | Choi et al., 2010 |
| (E3KARP, SIP-1, TKA-1) | LPA2 | ↑ IP3 signalling, ↑ ERK activation | Oh et al., 2004 |
| mGluR5a | ↑ agonist-induced Ca2+ signalling | Paquet et al., 2006 | |
| P2Y1R | ↑ interaction with PLCβ, ↑ agonist-induced Ca2+ signalling | Fam et al., 2005 | |
| PTH1R | ↑ interaction with, and activates, PLCβ | Mahon et al., 2002 | |
| PTH1R | ↓ adenylyl cyclase activity through stimulation of inhibitory G-proteins (Gi/o proteins). | Mahon et al., 2002 | |
| PTH1R | ↑ coupling to, and activation of, Gq and Gi; ↓ coupling to, and activation of, Gs | Wang et al., 2010 | |
| PAR-3 | BK | ↑ interaction with PLCβ1 | Choi et al., 2010 |
| PDZ-GEF | β1AR | Required for agonist-induced Ras activation via Gs-mediated cAMP signalling | Pak et al., 2002 |
| (CNrasGEF, RA-GEF, Rap GEP) | |||
| PICK1 | mGluR7a | ↓ PKCα-mediated receptor phosphorylation | Dev et al., 2000 |
| PrRP (GPR10) | ↑ intracellular clustering of receptor | Lin et al., 2001 | |
| PSD-95 | 5-HT2a | ↓ agonist-induced internalization, ↑ IP3 signalling | Xia et al., 2003b |
| (DLG4, SAP90) | 5-HT2c | ↑ constitutive and agonist-induced internalization, ↑ desensitization | Gavarini et al., 2006 |
| β1AR | ↓ agonist-induced internalization, no effect on cAMP signalling | Hu et al., 2000 | |
| D1 | ↑ constitutive internalization, ↓ cAMP signalling | Zhang et al., 2007 | |
| D1 | ↑ recycling, ↑ resensitization, no effect on Gs activation or cAMP signalling | Sun et al., 2009 | |
| SAP97 | β1AR | ↑ PKA-mediated receptor phosphorylation, ↑ recycling | Gardner et al., 2007 |
| (DLG1) | |||
| Tamalin | mGluR1a | ↑ membrane expression, ↑ complex formation with cytohesin-2 (GEF) | Kitano et al., 2002 |
| (GRASP) |
PDZ proteins and GPCR signalling
GABAB receptors can associate with the PDZ protein MUPP1, and this interaction enhances receptor signalling either by affecting G-protein coupling or by affecting the receptor interaction with other proteins (Balasubramanian et al., 2007). MUPP1 association with GABAB receptors results in fine tuning of receptor signalling with potential relevance to diseases such as epilepsy. MUPP1 also binds to the melatonin (MT)1 receptor, destabilizing receptor-Gi protein interaction, and abolishing Gi-mediated signalling of MT1 receptor (Guillaume et al., 2008). It has also been shown that PLC-β can interact with PDZ proteins, and NHERF2 physically connects the lipophosphatidic acid receptor 2 to the activation of PLC-β3, while PAR-3 connects bradykinin receptor to PLC-β1 to generate specific and efficient signalling (Oh et al., 2004; Choi et al., 2010). These findings suggest that each subtype of PLC-β can be selectively coupled to a receptor through a PDZ protein in a given cell type and lead to a specific signalling pathway. The PDZ protein NHERF-2 also interacts with mGluR5 to regulate the calcium signalling of this receptor (Paquet et al., 2006). Both mGluR5 and NHERF-2 are localized in populations of astrocytes and neurons in the CNS, and this interaction may be relevant for the regulation of cellular responses to glutamate. It has also been demonstrated that P2Y1 purinergic receptors (P2Y1Rs) can bind NHERF-2, and this association prolongs P2Y1R-mediated calcium signalling (Fam et al., 2005). The β1-adrenergic receptor (β1AR) is known to bind to several PDZ proteins such as CAL, PSD-95, MAGI-2, GIPC and MAGI-3 (He et al., 2006). The interaction of this receptor with MAGI-3 abolished β1-AR-mediated ERK1/2 activation with no effect on agonist dependent receptor internalization or agonist-stimulated cAMP formation.
PDZ proteins and GPCR trafficking
The β2AR interacts with the PDZ protein NHERF/EBP50 via its C-terminus PDZ binding motif, and this interaction is required for efficient plasma membrane recycling of the receptor (Hall et al., 1998; Cao et al., 1999). However, receptor recycling can be mediated by multiple PDZ domain containing proteins and is not unique to a single receptor as a β2AR chimera containing the β1AR C-tail, which contains a PDZ binding motif that does not bind to NHERF, still effectively recycles (Gage et al., 2005). Thus, modulation of GPCR trafficking by PDZ proteins may be a general mechanism of PDZ protein interactions. In contrast, Wente et al. (2005) have demonstrated that the somatostatin receptor subtype 5 (SSTR5) associates with the PDZ protein cystic fibrosis transmembrane conductance regulator-associated ligand (CAL), and this interaction inhibited SSTR5 recycling. In addition, NHERF1 inhibits type I parathyroid hormone receptor (PTH1R) desensitization, and knock down of NHERF1 with shRNA restores normal receptor desensitization (Wang et al., 2009). The serotonin 2C receptor (5-HT2CR) contains a carboxyl-terminal PDZ binding motif class I (SSV) and binds to MUPP1 (MUlti PDZ domain Protein 1). This interaction seems to facilitate receptor phosphorylation and resensitization (Ullmer et al., 1998; Becamel et al., 2001; Parker et al., 2003). CAL is a Golgi-associated PDZ protein, and its binding to β1AR results in the retention of the receptor within the cell (He et al., 2004). PSD-95 can compete with CAL for the binding to β1AR and function to both promote receptor trafficking to the cell surface and antagonize β1R endocytosis (Hu et al., 2000; He et al., 2004). β1AR also strongly binds MAGI-2, and this interaction promotes the internalization of the receptor (Xu et al., 2001).
PDZ proteins and GPCR subcellular distribution
In the mammalian cerebral cortex, 5-HT2ARs are enriched in pyramidal neurons and preferentially distributed in dendrites instead of axons (Jakab and Goldman-Rakic, 1998). Disruption of PDZ binding motif on 5-HT2AR receptor greatly decreases targeting of those receptors to dendrites, indicating that the PDZ binding motif is a critical signal for the sorting of 5-HT2AR to dendrites in pyramidal neurons (Xia et al., 2003a). The 5-HT2AR is present in a subset of dendritic spines and shafts and is colocalized with MUPP1 (Jones et al., 2009). 5-HT2AR agonist treatment induces a transient increase in dendritic spine size and phosphorylation of p21-activated kinase (PAK), which is downstream of the neuronal Rac guanine nucleotide exchange factor (RacGEF) Kalirin-7. Kalirin-7 is involved in dendritic spine morphogenesis. And its activity is regulated via its association with PDZ proteins such as PSD-95 and afadin/AF-6. Kalirin-7 interference peptides prevent 5-HT2AR-mediated PAK phosphorylation and spine morphogenesis, indicating that 5-HT2AR/PDZ protein interactions contribute to the modulation of spine morphogenesis. PSD-95 is also crucial for 5-HT2AR and 5-HT2CR cell surface expression, and PSD-95 promotes 5-HT2R sorting to apical dendritic spines and stabilizes receptor turnover in vivo (Abbas et al., 2009). Similarly, Tamalin (GRASP) increases the cell surface expression of mGluR1 (Kitano et al., 2002). The interaction of the α1DAR with the PDZ domain containing protein α-syntrophin also regulates α1DAR protein stability, indicating that multiple PDZ domain containing proteins may regulate the life span of GPCRs (Chen et al., 2006). Pick1 interactions with mGluR7 are essential for the pre-synaptic clustering of the receptor (Boudin et al., 2000). Taken together, these observations indicate that PDZ interactions play an important role in the subcellular localization of GPCRs to membrane microdomains.
Specificity of GPCR/PDZ protein interactions
5-HT2AR and 5-HT2CR exhibit very similar pharmacological properties and signalling pathways, and both express very similar class I PDZ binding motif at their carboxyl-termini (Bécamel et al., 2002; 2004). However, using a proteomic approach, Bécamel et al. (2002; 2004) demonstrated that these receptors interact with an overlapping, yet distinct, set of PDZ proteins. The 5-HT2CR, but not the 5-HT2AR, forms a complex with Veli3–CASK–MINT1 complex and to SAP102, whereas both receptors bind to PSD-95, MAGUK and p55 subfamily member 3 (MPP3). In contrast, 5-HT2AR effectively interacts with channel-interacting PDZ protein (CIPP), whereas 5-HT2CR does not appear to associate with CIPP. Thus, despite similarities in the PDZ binding motifs for 5-HT2AR and 5-HT2CR, these proteins exhibit differences in their substrate specificity, suggesting that amino acid residues upstream of the S/T-x-V/I/L motif may influence the selectivity of PDZ domain interactions.
Many interesting concepts regarding the physiological importance of GPCR/PDZ protein interaction arise within the studies described above. It is well known that a single GPCR has the capacity to associate with multiple different PDZ proteins, and that each of these proteins may modulate different intracellular events that often have opposing functions with regards to trafficking and signalling. However, how the physiological context and cell type favour interactions between specific GPCRs and specific PDZ proteins remains unknown. It is tempting to speculate potential scenarios. It is likely that the association PDZ domain proteins with other accessory proteins may drive the specificity of GPCR/PDZ domain interactions. Alternatively, the activation of GPCRs may modulate changes in the transcription regulation of specific PDZ proteins and may stimulate post-translational modifications of either the GPCR or PDZ domain-containing protein that may dictate the specificity of interaction. Finally, it is possible that different GPCR conformational states might contribute to the regulation of PDZ protein interactions with the receptor. However, most studies regarding PDZ protein interactions with GPCRs are performed in over-expression systems, and the assessment of endogenous proteins with GPCRs will be required to determine how interaction specificity is determined.
Physiological role of GPCR/PDZ protein interactions –‘heterologous GPCR sensitization’
Recently, Magalhaes et al. (2010) demonstrated that the activation of the CRFR1 results in the ‘heterologous sensitization’ of 5-HT2R signalling in both heterologous cell cultures and primary prefrontal cortical neurons. Specifically, pre-stimulation of the CRFR1 for 30 min leads to a significant potentiation of 5-HT2R signal transduction. This CRFR1-mediated sensitization of 5-HT2R signalling is dependent upon CRFR1 endocytosis and receptor recycling via Rab4 positive endosomes that results in the recruitment of an intracellular pool of 5-HT2R to the cell surface (Figure 5). Moreover, the CRFR1-mediated sensitization of 5-HT2R signalling and increase in 5-HT2R cell surface expression are dependent upon intact PDZ protein interactions with both CRFR1 and 5-HT2R. However, the identity of the PDZ protein mediating the interaction remains unknown. Both CRFR1 and 5-HT2Rs are associated with stress and anxiety responses, and the pre-administration of corticotrophin-releasing factor (CRF) into the prefrontal cortex of mice enhanced 5-HT2R-mediated anxiety behaviours in response to 2,5-dimethoxy-4-iodoamphetamine. Thus, PDZ protein interactions with GPCRs may serve to regulate functional crosstalk between GPCRs.
Figure 5.

Schema depicting proposed mechanism of CRFR1-mediated sensitization of 5-HT2R signaling. (A) Agonist activation of CRFR1 leads to the β-arrestin and clathrin-dependent endocytosis of CRFR1. (B) CRFR1 is internalized to Rab5-positive endosomes, where it is colocalized with constitutively internalized 5-HT2R. (C) The PDZ binding motifs of both CRFR1 and 5-HT2Rs are required for the Rab4-positive rapid recycling endosome-dependent and CRFR1-facilitated recycling of internalized 5-HT2R to the cell surface via a putative interaction with a PDZ domain containing protein. (D) Due to increased 5-HT2R cell surface expression mediated by CRFR1 activation 5-HT2R signalling is enhanced. H, hormone.
Other GPCR interacting proteins
Spinophilin
Spinophilin is a modular protein that is comprised of two F-actin domains, three putative Src homology 3 (SH3) domains, a receptor- and PP1-binding domain, a PDZ domain, three coiled-coiled domains and a potential leucine/isoleucine zipper motif (Sarrouilhe et al., 2006). Spinophilin has been demonstrated to interact with more than 30 different proteins including GPCRs and ion channels. Spinophilin also interacts with and regulates the activity of protein phosphatase (PP1). Spinophilin interacts with several GPCRs including the IL3 of the D2 dopamine, α2-adrenergic (α2AR) and µ-opioid receptors (Smith et al., 1999; Richman et al., 2001; Charlton et al., 2008). Following agonist treatment, spinophilin competes with GRK2 for binding to the α2AR, which prevents both β-arrestin recruitment and α2AR endocytosis (Wang et al., 2004). Spinophilin also regulates α2AR Ca2+ signalling by scaffolding RGS2 in a complex with the receptor (Wang et al., 2005). In contrast, spinophilin promotes the endocytosis of the µ-opioid receptor (Charlton et al., 2008). Surprisingly, no spinophilin PDZ domain interactions with GPCRs have been reported. Moreover, it has not been reported whether GPCR/spinophilin interactions may contribute to the regulation of spine morphology at synapses.
Protein phosphatases
The resensitization of many GPCRs is dependent upon receptor dephosphorylation in endosomes (Pippig et al., 1995; Seachrist et al., 2000). One candidate phosphatase for mediating the dephosphorylation of the β2AR is protein phosphatase 2A (PP2A), which has been demonstrated to co-immunoprecipitate with the receptor (Krueger et al., 1997). In addition, PP2A binds to the carboxyl-terminal tail of mGluR5 and functions to antagonize mGluR5-dependent activation of ERK1/2 phosphorylation (Mao et al., 2005b). PP1γ is also scaffolded on the carboxyl-terminal tail of mGluR1/5 via a PP1γ binding motif (Croci et al., 2003). However, the functional consequence of this interaction remains to be determined. In addition, PP2C binds to the carboxyl-terminal tail of mGluR3 and mediates the dephosphorylation of the receptor (Flajolet et al., 2003). However, our overall understanding of how the dephosphorylation of desensitized GPCRs is regulated by protein phosphatase is limited.
Calmodulin
Calmodulin has been shown to bind to the third intracellular loop of the µ-opioid receptor and reduces both constitutive and agonist-stimulated G-protein coupling (Wang et al., 1999; 2000). Moreover, single nucleotide polymorphisms within the G-protein coupling domain of the µ-opioid receptor are associated with altered calmodulin binding and increase basal µ-opioid receptor activity (Wang et al., 2001). Turner et al. (2004) demonstrated the first evidence that calmodulin interacts with the 5-HT1A receptor in intact living cells by BRET. They identified two calmodulin binding sites in the third intracellular loop of the receptor, which also happen to be a protein kinase C (PKC) binding site. Their results showed that calmodulin and PKC binding to the receptor were antagonistic in vitro, suggesting a potential role for calmodulin binding in the modulation of PKC-dependent (heterologous) desensitization of 5-HT1A receptor. Calmodulin was also shown to bind 5-HT2A receptors at two additional sites, one within the second intracellular loop and the other within the C-terminus (Turner and Raymond, 2005). The calmodulin binding to the 5-HT2A receptor C-terminus also antagonized PKC binding, whereas calmodulin binding to the second intracellular loop attenuates 5-HT2A receptor G-protein coupling. Calmodulin also binds to the C-terminus of the 5-HT2C receptor (Bécamel et al., 2002; Labasque et al., 2008). Data obtained with over-expressed protein system (HEK 293 cells), as well as endogenously expressed receptors in the choroid plexus demonstrated that calmodulin binding to the 5-HT2C receptor stabilized a 5-HT2C/β-arrestin complex leading to G-protein-independent ERK1/2 signalling (Labasque et al., 2008). mGluR5 also presents a calmodulin binding site in the carboxyl-terminal tail that overlaps a PKC phosphorylation site, and PKC-mediated phosphorylation and calmodulin binding appear to be antagonistic with one another (Minakami et al., 1997). Lee et al. (2008) has shown that mGluR5 surface distribution is stabilized by calmodulin binding. The authors showed that PKC dependent phosphorylation of mGluR5 C-terminus abolishes calmodulin binding and causes receptor internalization (desensitization); on the other hand, calmodulin binding prevented receptor phosphorylation and increased cell surface distribution. These data suggest that calmodulin may be a modulator of glutamate receptor-induced synaptic plasticity. Calmodulin also interacts with the carboxyl-terminal tails of several class B receptors and modulates the agonist-stimulated activity of the parathyroid hormone, vasoactive intestinal peptide, pituitary adenylate cyclase activating corticotrophin releasing hormone, calcitonin and glucagon-like peptide receptors (Mahon and Shimada, 2005). Therefore, calmodulin binding to receptors seem to affect both G-protein-dependent and -independent GPCR signalling as well as receptor trafficking.
Optineurin
Optineurin is a protein linked as a causative factor for open angle glaucoma and has been identified as a huntingtin-interacting protein (Rezaie et al., 2002; Harjes and Wanker, 2003). Optineurin binds to the second intracellular loop domain and carboxyl-terminal tail domains of group I mGluRs and functions to inhibit mGluR1/5 signalling (Anborgh et al., 2005). Polyglutamine expanded mutant huntingtin, but not wild-type huntingtin protein functions synergistically with optineurin to further uncouple mGluR1/5 signalling, and mGluR5 signalling is selectively uncoupled in mice that express polyglutamine expanded huntingtin protein (Ribeiro et al., 2010). Thus, additional phosphorylation-independent mechanisms for the antagonism of GPCR signalling exist.
SH3 domain adaptor proteins
Several SH3 domain proteins have been identified as potential GPCR interacting proteins. The D4 dopamine receptor, as well as other catecholamine receptors, have putative polyproline binding sites for SH3 domain contain proteins (Oldenhof et al., 1998). The SH3 binding motifs within the third intracellular loop domain of the D4 dopamine receptor strongly interact with both Grb2 and Nck. The removal of these SH3 binding motifs results in a D4 dopamine receptor that is unable to activate either adenylyl cyclase or ERK1/2 phosphorylation, but that exhibits normal G-protein interactions. The removal of these motifs also results in a receptor that is constitutively internalized, which may account for the deficit in cell signalling. Similarly, the SH3 domain containing protein endophilin interacts with polyproline motifs localized within the third intracellular loop of the β1AR (Tang et al., 1999). The over-expression of endophilin results in impaired Gs coupling efficacy and enhanced β1AR internalization. In addition, Src can be co-immunoprecipitated with the β3AR in the absence of β-arrestin recruitment and allows the receptor to couple to the activation of the MAPK pathway (Cao et al., 2000). Thus, the interaction of SH3 domain containing proteins with GPCRs not only modulates the G-protein-coupling, and trafficking of GPCRs, but these proteins may function as adaptors coupling GPCRs to signal transduction pathways that are regulated independently of heterotrimeric G-proteins.
Summary
The present review has provided an extensive, but not exhaustive, review of proteins that have been identified to interact with GPCRs. While the prototypic role of GPCRs is G-protein signalling and the generation of second messenger signals that alter the activity of downstream effector enzymes, GPCRs also exhibit the capacity interact with a wide variety of accessory proteins. The interaction of GPCRs with these accessory proteins not only serves to modulate G-protein coupling, desensitization, endocytosis and the subcellular localization of GPCRs, they also allow for the formation of novel signal transduction complexes that have the capacity to alter cellular function. Strategies to either selectively block or promote the formation of GPCR scaffolded complexes may have the potential for the design of novel drugs that target GPCR signalling that is independent of G-protein activation. By targeting specific GPCR interactions, it may be possible to design pharmaceutical agents that are clinically effective but spare the undesired side effects of antagonizing agonist binding to GPCRs. A further understanding of the nature of the protein complexes that are scaffolded by GPCRs and the physiological consequence of these interactions will be required before such an aim is realized.
Acknowledgments
These studies were funded by grants from the Canadian Institutes of Health Research (MA-15506 and MOP 62738) and Heart and Stroke Foundation of Ontario (T5933). SSGF holds a Tier I Canada Research Chair in Molecular Neurobiology and is a Career Investigator of the Heart and Stroke Foundation of Ontario. ACM is the recipient of a fellowship from the Canadian Institutes of Health Research.
Glossary
- 5-HT2AR
serotonin receptor subtype 2
- 5HT2CR
serotonin 2C receptor
- α1DAR
α1D adrenergic receptor
- Arf6
ADP-ribosylation factor 6
- ARNO
ARF nucleotide-binding site opener
- AT1R
angiotensin II type 1 receptor
- β2AR
β2-adrenergic receptor
- CAL
cystic fibrosis transmembrane conductance regulator-associated ligand
- CASK
calcium/calmodulin-dependent serine protein kinase
- CaSR
calcium sensing receptor
- cGRP1/cGRP2
calcitonin gene-related peptides
- CIPP
channel-interacting PDZ protein
- CRF
corticotrophin-releasing factor
- CRFR1
corticotropin releasing factor receptor 1
- CRLR
calcitonin-like receptor
- CXCR2
IL-8 receptor B
- CXCR4
chemokine receptor type 4
- EBP50
ERM-binding phosphoprotein 50
- EEA1
effector early endosome antigen 1
- EVH
ENA/VASP homology domain
- GABAB
GABA type B receptor
- GAPs
GTPase activating proteins
- GASP
GPCR-associated sorting protein
- GDP
guanosine diphosphate
- GEFs
guanine nucleotide exchange factors
- GIPC
GIPC PDZ domain containing family member 1
- GRB2
growth factor receptor-bound protein 2
- GRKs
GPCR kinases
- MAGI
membrane-associated guanylate kinase inverted
- MAGUK
membrane-associated guanylate kinase
- mGluRs
metabotropic glutamate receptors
- MINT1
Munc-18-interacting protein 1
- MPP3
p55 subfamily member 3
- MT1
melatonin type 1 receptor
- MUPP1
multi-PDZ-domain protein
- Nck
non-catalytic region of tyrosine kinase adaptor protein
- NHERF
Na+/H+ exchanger regulatory factor 1
- NSF
N-ethylmaleimide-sensitive factor
- P2Y1
P2Y1 purinergic receptors
- PAK
p21-activated kinase
- PAR3
Protease activated receptor 3
- PDZ
PSD95/Disc Large/Zona Occludens
- Pick1
protein interacting with PRKCA 1
- PKC
protein kinase C
- PLC
phospholipase C
- PLD
phospholipase D
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
- PTB
phosphotyrosine-binding
- RacGEF
Rac guanine nucleotide exchange factor
- RalGDS
Ral guanine nucleotide dissociation stimulators or exchange factors
- RAMPS
receptor activity-modifying proteins
- Ras
Rat sarcoma
- RGS
regulators of G-protein signalling
- Rho
Ras-homologous
- SAP97
synapse-associated protein 97
- SH3
SRC homology 3 domain
- SSTR5
somatostatin receptor subtype 5
- TM
transmembrane regions
- VELI3
vertebrate lin-7 homolog 3
Conflict of interest
The authors declare no competing financial interests.
References
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, et al. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anborgh PH, Godin C, Pampillo M, Dhami GK, Dale LB, Cregan SP, et al. Inhibition of metabotropic glutamate receptor signaling by the huntingtin-binding protein optineurin. J Biol Chem. 2005;280:34840–34848. doi: 10.1074/jbc.M504508200. [DOI] [PubMed] [Google Scholar]
- Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009;54:33–46. doi: 10.1007/s12013-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, et al. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, et al. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- Antonescu CN, Danuser G, Schmid SL. Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol Biol Cell. 2010;21:2944–2952. doi: 10.1091/mbc.E10-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Fam SR, Hall RA. GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J Biol Chem. 2007;282:4162–4171. doi: 10.1074/jbc.M607695200. [DOI] [PubMed] [Google Scholar]
- Ballon DR, Flanary PL, Gladue DP, Konopka JB, Dohlman HG, Thorner J. DEP-domain-mediated regulation of GPCR signaling responses. Cell. 2006;126:1079–1093. doi: 10.1016/j.cell.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116:473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlic J, Andrews JD, Kelvin AA, Bosinger SE, DeVries ME, Xu L, et al. Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol. 2001;1:227–233. doi: 10.1038/79767. [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, et al. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–12982. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- Bécamel C, Alonso G, Galéotti N, Demey E, Jouin P, Ullmer C, et al. Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J. 2002;21:2332–2342. doi: 10.1093/emboj/21.10.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bécamel C, Gavarini S, Chanrion B, Alonso G, Galéotti N, Dumuis A, et al. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- Bernstein LS, Grillo AA, Loranger SS, Linder ME. RGS4 binds to membranes through an amphipathic alpha -helix. J Biol Chem. 2000;275:18520–18526. doi: 10.1074/jbc.M000618200. [DOI] [PubMed] [Google Scholar]
- Bernstein LS, Ramineni S, Hague C, Cladman W, Chidiac P, Levey AI, et al. RGS2 binds directly and selectively to the M1 muscarinic acetylcholine receptor third intracellular loop to modulate Gq/11alpha signaling. J Biol Chem. 2004;279:21248–21256. doi: 10.1074/jbc.M312407200. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem. 2001;276:8269–8277. doi: 10.1074/jbc.M006968200. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Babwah AV, Ferguson SSG. Small G protein-coupled receptors. Biochem Soc Trans. 2004;32:1040–1044. doi: 10.1042/BST0321040. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Perroy J, Bécamel C, Marin P, Fagni L. GPCR interacting proteins (GIPs) in the nervous system: roles in physiology and pathologies. Annu Rev Pharmacol Toxicol. 2010;50:89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [DOI] [PubMed] [Google Scholar]
- Boeuf J, Trigo JM, Moreau PH, Lecourtier L, Vogel E, Cassel JC, et al. Attenuated behavioural responses to acute and chronic cocaine in GASP-1-deficient mice. Eur J Neurosci. 2009;30:860–868. doi: 10.1111/j.1460-9568.2009.06865.x. [DOI] [PubMed] [Google Scholar]
- Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS. Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J Biol Chem. 2005a;280:9297–9307. doi: 10.1074/jbc.M413786200. [DOI] [PubMed] [Google Scholar]
- Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N. Receptor activity-modifying protein (RAMP) isoform-specific regulation of adrenomedullin receptor trafficking by NHERF-1. J Biol Chem. 2005b;280:23926–23935. doi: 10.1074/jbc.M501751200. [DOI] [PubMed] [Google Scholar]
- Boudin H, Doan A, Xia J, Shigemoto R, Huganir RL, Worley P, et al. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–497. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118:4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM, et al. Direct binding of activated c-Src to the beta 3-adrenergic receptor is required for MAP kinase activation. J Biol Chem. 2000;275:38131–38134. doi: 10.1074/jbc.C000592200. [DOI] [PubMed] [Google Scholar]
- Cermakian N, Sassone-Corsi P. Multilevel regulation of the circadian clock. Nat Rev Mol Cell Biol. 2000;1:59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- Charlton JJ, Allen PB, Psifogeorgou K, Chakravarty S, Gomes I, Neve RL, et al. Multiple actions of spinophilin regulate mu opioid receptor function. Neuron. 2008;58:238–247. doi: 10.1016/j.neuron.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Eversole-Cire P, Zhang HK, Mancino V, Chen YJ, He W, et al. Instability of GGL domain-containing RGS proteins in mice lacking the G protein β-subunit Gβ5. Proc Natl Acad Sci U S A. 2003;100:6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hague C, Hall RA, Minneman KP. Syntrophins regulate alpha1D-adrenergic receptors through a PDZ domain-mediated interaction. J Biol Chem. 2006;281:12414–12420. doi: 10.1074/jbc.M508651200. [DOI] [PubMed] [Google Scholar]
- Cheng S, Zhang J, Zhu P, Ma Y, Xiong Y, Sun L, et al. The PDZ domain protein CAL interacts with mGluR5a and modulates receptor expression. J Neurochem. 2010;112:588–598. doi: 10.1111/j.1471-4159.2009.06454.x. [DOI] [PubMed] [Google Scholar]
- Choi JW, Lim S, Oh YS, Kim EK, Kim SH, Kim YH, et al. Subtype-specific role of phospholipase C-beta in bradykinin and LPA signaling through differential binding of different PDZ scaffold proteins. Cell Signal. 2010;22:1153–1161. doi: 10.1016/j.cellsig.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, et al. beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J Biol Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- Croci C, Sticht H, Brandstätter JH, Enz R. Group I metabotropic glutamate receptors bind to protein phosphatase 1C. Mapping and modeling of interacting sequences. J Biol Chem. 2003;278:50682–50690. doi: 10.1074/jbc.M305764200. [DOI] [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S. PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J Neurosci. 2000;20:7252–7257. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther. 2006;111:260–271. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dooley R, Baumgart S, Rasche S, Hatt H, Neuhaus EM. Olfactory receptor signaling is regulated by the post-synaptic density 95, drosophila discs large, zona-occludens 1 (PDZ) scaffold multi-PDZ domain protein 1. FEBS J. 2009;276:7279–7290. doi: 10.1111/j.1742-4658.2009.07435.x. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Doupnik CA, Jayaraman M, Buchwalter AL, Kaltenbronn KM, Huettner JE, et al. R7BP augments the function of RGS7*Gbeta5 complexes by a plasma membrane-targeting mechanism. J Biol Chem. 2006;281:28222–28231. doi: 10.1074/jbc.M604428200. [DOI] [PubMed] [Google Scholar]
- Du G, Huang P, Liang BT, Frohman MA. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell. 2004;15:1024–1030. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseltine JL, Dale LB, Ferguson SS. Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Mol Pharmacol. 2011;79:175–184. doi: 10.1124/mol.110.068379. [DOI] [PubMed] [Google Scholar]
- Fam SR, Paquet M, Castleberry AM, Oller H, Lee CJ, Traynelis SF, et al. P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci U S A. 2005;102:8042–8047. doi: 10.1073/pnas.0408818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Tu J, Yang T, Vernon PS, Allen PD, Worley PF, et al. Homer regulates gain of ryanodine receptor type 1 channel complex. J Biol Chem. 2002;277:44722–44730. doi: 10.1074/jbc.M207675200. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ferguson SS. Phosphorylation-independent attenuation of GPCR signalling. Trends Pharmacol Sci. 2007;28:173–179. doi: 10.1016/j.tips.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Zhang J, Barak LS, Caron MG. G-protein-coupled receptor kinases and arrestins: regulators of G-protein-coupled receptor sequestration. Biochem Soc Trans. 1996;24:953–959. doi: 10.1042/bst0240953. [DOI] [PubMed] [Google Scholar]
- Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, Nairn AC, et al. Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3. Proc Natl Acad Sci U S A. 2003;100:16006–16011. doi: 10.1073/pnas.2136600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G Protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J Biol Chem. 2005;280:3305–3313. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gardner LA, Naren AP, Bahouth SW. Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem. 2007;282:5085–5099. doi: 10.1074/jbc.M608871200. [DOI] [PubMed] [Google Scholar]
- Gavarini S, Becamel C, Altier C, Lory P, Poncet J, Wijnholds J, et al. Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol Biol Cell. 2006;17:4619–4631. doi: 10.1091/mbc.E06-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee HY, Kim YW, Jo MJ, Namkung W, Kim JY, Park HW, et al. Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology. 2009;137:607–617. doi: 10.1053/j.gastro.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Luttrell LM. Heptahelical terpsichory. Who calls the tune? J Recept Signal Transduct Res. 2008;28:39–58. doi: 10.1080/10799890801941921. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D'Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, et al. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin CM, Ferreira LT, Dale LB, Gros R, Cregan SP, Ferguson SS. The small GTPase Ral couples the angiotensin II type 1 receptor to the activation of phospholipase C-delta 1. Mol Pharmacol. 2010;77:388–395. doi: 10.1124/mol.109.061069. [DOI] [PubMed] [Google Scholar]
- Goehler H, Lalowski M, Stelzl U, Waelter S, Stroedicke M, Worm U, et al. A protein interaction network links GIT1, an enhancer of huntingtin aggregation, to Huntington's disease. Mol Cell. 2004;15:853–865. doi: 10.1016/j.molcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, Oswald BJ, et al. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Guillaume JL, Daulat AM, Maurice P, Levoye A, Migaud M, Brydon L, et al. The PDZ protein mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J Biol Chem. 2008;283:16762–16771. doi: 10.1074/jbc.M802069200. [DOI] [PubMed] [Google Scholar]
- Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell. 2006;17:1228–1238. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague C, Bernstein LS, Ramineni S, Chen Z, Minneman KP, Hepler JR. Selective inhibition of alpha1Adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem. 2005;280:27289–27295. doi: 10.1074/jbc.M502365200. [DOI] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- Hamelin E, Thériault C, Laroche G, Parent JL. The intracellular trafficking of the G protein-coupled receptor TPβ depends on a direct interaction with Rab11. J Biol Chem. 2005;280:36195–36205. doi: 10.1074/jbc.M503438200. [DOI] [PubMed] [Google Scholar]
- Harjes P, Wanker EE. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem Sci. 2003;28:425–433. doi: 10.1016/S0968-0004(03)00168-3. [DOI] [PubMed] [Google Scholar]
- Hayashi MK, Ames HM, Hayashi Y. Tetrameric hub structure of postsynaptic scaffolding protein homer. J Neurosci. 2006;26:8492–8501. doi: 10.1523/JNEUROSCI.2731-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bellini M, Xu J, Castleberry AM, Hall RA. Interaction with cystic fibrosis transmembrane conductance regulator-associated ligand (CAL) inhibits beta1-adrenergic receptor surface expression. J Biol Chem. 2004;279:50190–50196. doi: 10.1074/jbc.M404876200. [DOI] [PubMed] [Google Scholar]
- He J, Bellini M, Inuzuka H, Xu J, Xiong Y, Yang X, et al. Proteomic analysis of beta1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem. 2006;281:2820–2827. doi: 10.1074/jbc.M509503200. [DOI] [PubMed] [Google Scholar]
- He W, Lu LS, Zhang X, El Hodiri HM, Chen CK, Slep KC, et al. Modules in the photoreceptor RGS9-1.Gβ5L GTPase-accelerating protein complex control effector coupling, GTPase acceleration, protein folding, and stability. J Biol Chem. 2000;275:37093–37100. doi: 10.1074/jbc.M006982200. [DOI] [PubMed] [Google Scholar]
- Heo K, Ha SH, Chae YC, Lee S, Oh YS, Kim YH, et al. RGS2 promotes formation of neurites by stimulating microtubule polymerization. Cell Signal. 2006;18:2182–2192. doi: 10.1016/j.cellsig.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, et al. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide- sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279:54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Galet C, Kishi M, Ascoli M. GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J Biol Chem. 2003;278:49348–49357. doi: 10.1074/jbc.M306557200. [DOI] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Houndolo T, Boulay PL, Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J Biol Chem. 2005;280:5598–5604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, et al. beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275:38659–38666. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, Lefkowitz RJ. GIPC interacts with the beta1-adrenergic receptor and regulates beta1-adrenergic receptor-mediated ERK activation. J Biol Chem. 2003;278:26295–26301. doi: 10.1074/jbc.M212352200. [DOI] [PubMed] [Google Scholar]
- Inglese J, Koch WJ, Caron MG, Lefkowitz RJ. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature. 1992;359:147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- Irminger-Finger I, Jefford CE. Is there more to BARD1 than BRCA1? Nat Rev Cancer. 2006;6:382–391. doi: 10.1038/nrc1878. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman M, Zhou H, Jia L, Cain MD, Blumer KJ. R9AP and R7BP: traffic cops for the RGS7 family in phototransduction and neuronal GPCR signaling. Trends Pharmacol Sci. 2009;30:17–24. doi: 10.1016/j.tips.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Diaz J, Sokoloff P, Griffon N. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Biol Cell. 2004;15:696–705. doi: 10.1091/mbc.E03-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A. 2009;106:19575–19580. doi: 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, et al. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci. 2004;117:5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci U S A. 2007;104:6055–6060. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- Kimple RJ, De Vries L, Tronche're H, Behe CI, Morris RA, Gist Farquhar M, et al. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor Activity. J Biol Chem. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, et al. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci. 2002;22:1280–1289. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Hollt V. ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J Biol Chem. 2003;278:9979–9985. doi: 10.1074/jbc.M206709200. [DOI] [PubMed] [Google Scholar]
- Koch WJ, Inglese J, Stone WC, Lefkowitz RJ. The binding site for the beta gamma subunits of heterotrimeric G proteins on the beta-adrenergic receptor kinase. J Biol Chem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Goodman OB, Jr, Keen JH, Benovic JL. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- Kunapuli P, Gurevich VV, Benovic JL. Phospholipid-stimulated autophosphorylation activates the G protein-coupled receptor kinase GRK5. J Biol Chem. 1994;269:10209–10212. [PubMed] [Google Scholar]
- Kuwasako K, Cao YN, Chu CP, Iwatsubo S, Eto T, Kitamura K. Functions of the cytoplasmic tails of the human receptor activity-modifying protein components of calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem. 2006;281:7205–7213. doi: 10.1074/jbc.M511147200. [DOI] [PubMed] [Google Scholar]
- Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell. 2008;19:4640–4650. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte SA, Roy SF, Escher E, Guillemette G, Leduc R. Essential role of leucine222 in mediating signal transduction of the human angiotensin II type 1 receptor. Receptors Channels. 1998;5:103–112. [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee J, Choi KY, Hepp R, Lee JY, Lim MK, et al. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci U S A. 2008;105:12575–12580. doi: 10.1073/pnas.0712033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Li JG, Chen C, Liu-Chen LY. Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/ NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem. 2002;277:27545–27552. doi: 10.1074/jbc.M200058200. [DOI] [PubMed] [Google Scholar]
- Lin SHS, Arai AC, Wang Z, Nothacker HP, Civelli O. The carboxyl terminus of the prolactin-releasing peptide receptor interacts with PDZ domain proteins involved in alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor clustering. Mol Pharmacol. 2001;60:916–924. doi: 10.1124/mol.60.5.916. [DOI] [PubMed] [Google Scholar]
- Luttrell LM. Reviews in molecular biology and biotechnology: transmembrane signalling by G protein-coupled receptors. Mol Biotechnol. 2008;39:239–264. doi: 10.1007/s12033-008-9031-1. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, et al. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon MJ, Shimada M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G-protein coupled receptors. FEBS Lett. 2005;579:803–807. doi: 10.1016/j.febslet.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Mahon MJ, Donowitz M, Yun CC, Segre GV. Na(+)/H(+) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature. 2002;417:858–861. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- Makino ER, Handy JW, Li TS, Arshavsky VY. The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein β subunit. Proc Natl Acad Sci U S A. 1999;96:1947–1952. doi: 10.1073/pnas.96.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005a;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, et al. Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons. J Biol Chem. 2005b;280:12602–12610. doi: 10.1074/jbc.M411709200. [DOI] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-McCaffrey L, Hains MD, Pritchard GA, Pajak A, Dagnino L, Siderovski DP, et al. Differential expression of regulator of G-protein signaling R12 subfamily members during mouse development. Dev Dyn. 2005;234:438–444. doi: 10.1002/dvdy.20555. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Kiyama A, Kawabuchi M, Okada M, Nagai K. A novel protein interacts with a clock-related protein, rPer1. Brain Res. 2001;916:1–10. doi: 10.1016/s0006-8993(01)02857-8. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Minakami R, Jinnai N, Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J Biol Chem. 1997;272:20291–20298. doi: 10.1074/jbc.272.32.20291. [DOI] [PubMed] [Google Scholar]
- Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, et al. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Nunn C, Mao H, Chidiac P, Albert PR. RGS17/RGSZ2 and the RZ/A family of regulators of G-protein signaling. Semin Cell Dev Biol. 2006;17:390–399. doi: 10.1016/j.semcdb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Oh YS, Jo NW, Choi JW, Kim HS, Seo SW, Kang KO, et al. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Mol Cell Biol. 2004;24:5069–5079. doi: 10.1128/MCB.24.11.5069-5079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, et al. SH3 binding domains in the dopamine D4 receptor. Biochemistry. 1998;37:15726–15736. doi: 10.1021/bi981634+. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Pak Y, Pham N, Rotin D. Direct binding of the beta1 adrenergic receptor to the cyclic AMP-dependent guanine nucleotide exchange factor CNrasGEF leads to ras activation. Mol Cell Biol. 2002;22:7942–7952. doi: 10.1128/MCB.22.22.7942-7952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M, Asay MJ, Fam SR, Inuzuka H, Castleberry AM, Oller H, et al. The PDZ scaffold NHERF-2 interacts with mGluR5 and regulates receptor activity. J Biol Chem. 2006;281:29949–29961. doi: 10.1074/jbc.M602262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N, Spielman WS. RAMPs: the past, present and future. Trends Biochem Sci. 2006;31:631–638. doi: 10.1016/j.tibs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Parent A, Hamelin E, Germain P, Parent JL. Rab11 regulates the recycling of the β2-adrenergic receptor through a direct interaction. Biochem J. 2009;418:163–172. doi: 10.1042/BJ20080867. [DOI] [PubMed] [Google Scholar]
- Parker LL, Backstrom JR, Sanders-Bush E, Shieh BH. Agonist-induced phosphorylation of the serotonin 5-HT2C receptor regulates its interaction with multiple PDZ protein 1. J Biol Chem. 2003;278:21576–21583. doi: 10.1074/jbc.M210973200. [DOI] [PubMed] [Google Scholar]
- Penela P, Ribas C, Mayor FJ. Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15:973–981. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Daniel K, Puzicha M, Caron MG, Lefkowitz RJ, et al. Sequestration and recycling of β2-adrenergic receptors permit resensitization. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- Pitcher JA, Touhara K, Payne ES, Lefkowitz RJ. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J Biol Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Polese D, de Serpis AA, Ambesi-Impiombato A, Muscettola G, de Bartolomeis A. Homer 1a gene expression modulation by antipsychotic drugs: involvement of the glutamate metabotropic system and effects of d-cycloserine. Neuropsychopharmacology. 2002;27:906–913. doi: 10.1016/S0893-133X(02)00371-8. [DOI] [PubMed] [Google Scholar]
- Potschka H, Krupp E, Ebert U, Gümbel C, Leichtlein C, Lorch B, et al. Kindling-induced overexpression of Homer 1A and its functional implications for epileptogenesis. Eur J Neurosci. 2002;16:2157–2165. doi: 10.1046/j.1460-9568.2002.02265.x. [DOI] [PubMed] [Google Scholar]
- Poupart ME, Fessart D, Cotton M, Laporte SA, Claing A. ARF6 regulates angiotensin II type 1 receptor endocytosis by controlling the recruitment of AP-2 and clathrin. Cell Signal. 2007;19:2370–2378. doi: 10.1016/j.cellsig.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Premont RT, Koch WJ, Inglese J, Lefkowitz RJ. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994;269:6832–6841. [PubMed] [Google Scholar]
- Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, et al. Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem. 1996;271:6403–6410. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid HM, Mulvaney EP, Turner EC, Kinsella BT. Interaction of the human prostacyclin receptor with Rab11: characterization of a novel Rab11 binding domain (RBD) within α-helix 8 that is regulated by palmitoylation. J Biol Chem. 2010;285:18709–18726. doi: 10.1074/jbc.M110.106476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Paquet M, Ferreira LT, Cregan T, Swan P, Cregan SP, et al. Metabotropic glutamate receptor-mediated cell signaling pathways are altered in a mouse model of Huntington's disease. J Neurosci. 2010;30:316–324. doi: 10.1523/JNEUROSCI.4974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated Interaction between alpha2-adrenergic receptors and spinophilin. J Biol Chem. 2001;276:15003–15008. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- Richman RW, Strock J, Hains MD, Cabanilla NJ, Lau KK, Siderovski DP, et al. RGS12 interacts with the SNARE-binding region of the Cav2.2 calcium channel. J Biol Chem. 2005;280:1521–1528. doi: 10.1074/jbc.M406607200. [DOI] [PubMed] [Google Scholar]
- Rochdi MD, Parent JL. Galphaq-coupled receptor internalization specifically induced by galphaq signaling. Regulation by EBP50. J Biol Chem. 2003;278:17827–17837. doi: 10.1074/jbc.M210319200. [DOI] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, et al. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Sala C, Piëch V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Sarrouilhe D, di Tommaso A, Métayé T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–1113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Schiff ML, Siderovski DP, Jordan JD, Brothers G, Snow B, De Vries L, et al. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature. 2000;408:723–727. doi: 10.1038/35047093. [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Anborgh PH, Ferguson SSG. b2-Adrenergic receptor internalization, endosomal sorting and plasma membrane recycling are regulated by Rab GTPases. J Biol Chem. 2000;275:27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Laporte SA, Dale LB, Babwah AV, Caron MG, Anborgh PH, et al. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277:679–685. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- Sethakorn N, Yau DM, Dulin NO. Non-canonical functions of RGS proteins. Cell Signal. 2010;22:1274–1281. doi: 10.1016/j.cellsig.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Bito H, Fujisawa K, Narumiya S, Mikoshiba K, et al. Cupidin, an isoform of Homer/Vesl, interacts with the actin cytoskeleton and activated rho family small GTPases and is expressed in developing mouse cerebellar granule cells. J Neurosci. 1999;19:8389–8400. doi: 10.1523/JNEUROSCI.19-19-08389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B, Neubig RR. Thinking outside of the ‘RGS box’: new approaches to therapeutic targeting of regulators of G protein signaling. Mol Pharmacol. 2010;78:550–557. doi: 10.1124/mol.110.065219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba NP, Martemyanov KA, Elfenbein A, Hopp JA, Bohm A, Simonds WF, et al. RGS9-Gβ5 substrate selectivity in photoreceptors – opposing effects of constituent domains yield high affinity of RGS interaction with the G protein-effector complex. J Biol Chem. 2001;276:37365–37372. doi: 10.1074/jbc.M106431200. [DOI] [PubMed] [Google Scholar]
- Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem. 1999;274:19894–19900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- Snow BE, Krumins AM, Brothers GM, Lee SF, Wall MA, Chung S, et al. A G protein gamma subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proc Natl Acad Sci U S A. 1998a;95:13307–13312. doi: 10.1073/pnas.95.22.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BE, Hall RA, Krumins AM, Brothers GM, Bouchard D, Brothers CA, et al. GTPase activating specificity of RGS12 and binding specificity of an alternatively spliced PDZ (PSD-95/Dlg/ZO-1) domain. J Biol Chem. 1998b;273:17749–17755. doi: 10.1074/jbc.273.28.17749. [DOI] [PubMed] [Google Scholar]
- Spellmann I, Rujescu D, Musil R, Mayr A, Giegling I, Genius J, et al. Homer-1 polymorphisms are associated with psychopathology and response to treatment in schizophrenic patients. J Psychiatr Res. 2011;45:234–241. doi: 10.1016/j.jpsychires.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Stoffel RH, Randall RR, Premont RT, Lefkowitz RJ, Inglese J. Palmitoylation of G protein-coupled receptor kinase, GRK6. Lipid modification diversity in the GRK family. J Biol Chem. 1994;269:27791–27794. [PubMed] [Google Scholar]
- Stoffel RH, Inglese J, Macrae AD, Lefkowitz RJ, Premont RT. Palmitoylation increases the kinase activity of the G protein-coupled receptor kinase, GRK6. Biochemistry. 1998;37:16053–16059. doi: 10.1021/bi981432d. [DOI] [PubMed] [Google Scholar]
- Sun P, Wang J, Gu W, Cheng W, Jin GZ, Friedman E, et al. PSD-95 regulates D1 dopamine receptor resensitization, but not receptor-mediated gs-protein activation. Cell Res. 2009;19:612–624. doi: 10.1038/cr.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Tachibana T, Imanaka T, Nishida W, Sobue K. Involvement of unique leucine-zipper motif of PSD-Zip45 (Homer 1c/vesl-1L) in group 1 metabotropic glutamate receptor clustering. Proc Natl Acad Sci U S A. 1999;96:13801–13806. doi: 10.1073/pnas.96.24.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tang Y, Hu LA, Miller WE, Ringstad N, Hall RA, Pitcher JA, et al. Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the beta1-adrenergic receptor. Proc Natl Acad Sci U S A. 1999;96:12559–12564. doi: 10.1073/pnas.96.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MD, Cole DE, Jose PA. Pharmacogenomics of G protein-coupled receptor signaling: insights from health and disease. Methods Mol Biol. 2008;448:77–107. doi: 10.1007/978-1-59745-205-2_6. [DOI] [PubMed] [Google Scholar]
- Tobin AB, Butcher AJ, Kong KC. Location, location, location . . . site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29:413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Inglese J, Pitcher JA, Shaw G, Lefkowitz RJ. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J Biol Chem. 1994;269:10217–10220. [PubMed] [Google Scholar]
- Trejo J, Hammes SR, Coughlin SR. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc Natl Acad Sci U S A. 1998;95:13698–13702. doi: 10.1073/pnas.95.23.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Turner JH, Raymond JR. Interaction of calmodulin with the serotonin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J Biol Chem. 2005;280:30741–30750. doi: 10.1074/jbc.M501696200. [DOI] [PubMed] [Google Scholar]
- Turner JH, Gelasco AK, Raymond JR. Calmodulin interacts with the third intracellular loop of the serotonin 5-hydroxytryptamine1A receptor at two distinct sites: putative role in receptor phosphorylation by protein kinase C. J Biol Chem. 2004;279:17027–17037. doi: 10.1074/jbc.M313919200. [DOI] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Figge A, Lübbert H. Cloning and characterization of MUPP1, a novel PDZ domain protein. FEBS Lett. 1998;424:63–68. doi: 10.1016/s0014-5793(98)00141-0. [DOI] [PubMed] [Google Scholar]
- Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem. 2007;282:36214–36222. doi: 10.1074/jbc.M707263200. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang Y, Abou-Samra AB, Friedman PA. NHERF1 regulates parathyroid hormone receptor desensitization: interference with beta-arrestin binding. Mol Pharmacol. 2009;75:1189–1197. doi: 10.1124/mol.108.054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Ardura JA, Romero G, Yang Y, Hall RA, Friedman PA. Na/H exchanger regulatory factors control parathyroid hormone receptor signaling by facilitating differential activation of G(alpha) protein subunits. J Biol Chem. 2010;285:26976–26986. doi: 10.1074/jbc.M110.147785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sadée W, Quillan JM. Calmodulin binding to G protein-coupling domain of opioid receptors. J Biol Chem. 1999;274:22081–22088. doi: 10.1074/jbc.274.31.22081. [DOI] [PubMed] [Google Scholar]
- Wang D, Surratt CK, Sadée W. Calmodulin regulation of basal and agonist-stimulated G protein coupling by the mu-opioid receptor (OP(3)) in morphine-pretreated cell. J Neurochem. 2000;75:763–771. doi: 10.1046/j.1471-4159.2000.0750763.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Quillan JM, Winans K, Lucas JL, Sadée W. Single nucleotide polymorphisms in the human mu opioid receptor gene alter basal G protein coupling and calmodulin binding. J Biol Chem. 2001;276:34624–34630. doi: 10.1074/jbc.M104083200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, et al. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7:405–411. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- Wente W, Stroh T, Beaudet A, Richter D, Kreienkamp HJ. Interactions with PDZ domain proteins PIST/GOPC and PDZK1 regulate intracellular sorting of the somatostatin receptor subtype 5. J Biol Chem. 2005;280:32419–32425. doi: 10.1074/jbc.M507198200. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Garrido JL, Bisello A, Kim YK, Friedman PA, Romero G. Regulation of parathyroid hormone type 1 receptor dynamics, traffic, and signaling by the Na+/H+ exchanger regulatory factor-1 in rat osteosarcoma ROS 17/2.8 cells. Mol Endocrinol. 2008;22:1163–1170. doi: 10.1210/me.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003a;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003b;278:21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xu J, Paquet M, Lau AG, Wood JD, Ross CA, Hall RA. beta 1-adrenergic receptor association with the synaptic scaffolding protein membrane-associated guanylate kinase inverted-2 (MAGI-2). Differential regulation of receptor internalization by MAGI-2 and PSD-95. J Biol Chem. 2001;276:41310–41317. doi: 10.1074/jbc.M107480200. [DOI] [PubMed] [Google Scholar]
- Yang L, Seifert A, Wu D, Wang X, Rankovic V, Schröder H, et al. Role of phospholipase D2/phosphatidic acid signal transduction in micro- and delta-opioid receptor endocytosis. Mol Pharmacol. 2010a;78:105–113. doi: 10.1124/mol.109.063107. [DOI] [PubMed] [Google Scholar]
- Yang X, Zheng J, Xiong Y, Shen H, Sun L, Huang Y, et al. Beta-2 adrenergic receptor mediated ERK activation is regulated by interaction with MAGI-3. FEBS Lett. 2010b;584:2207–2212. doi: 10.1016/j.febslet.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zeng W, Xu X, Popov S, Mukhopadhyay S, Chidiac P, Swistok J, et al. The N-terminal domain of RGS4 confers receptor-selective inhibition of G protein signaling. J Biol Chem. 1998;273:34687–34690. doi: 10.1074/jbc.273.52.34687. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SG, Miller GM, et al. Inhibition of the dopamine D1 receptor signaling by PSD-95. J Biol Chem. 2007;282:15778–15789. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cheng S, Xiong Y, Ma Y, Luo D, Jeromin A, et al. A novel association of mGluR1a with the PDZ scaffold protein CAL modulates receptor activity. FEBS Lett. 2008;582:4117–4124. doi: 10.1016/j.febslet.2008.10.054. [DOI] [PubMed] [Google Scholar]
- Zimmerman B, Simaan M, Lee MH, Luttrell LM, Laporte SA. c-Src-mediated phosphorylation of AP-2 reveals a general mechanism for receptors internalizing through the clathrin pathway. Cell Signal. 2009;21:103–110. doi: 10.1016/j.cellsig.2008.09.013. [DOI] [PubMed] [Google Scholar]


