Abstract
The tuberous sclerosis complex (TSC) is caused by mutation in either of 2 tumor suppressor genes, TSC-1 (encodes hamartin) and TSC-2 (encodes tuberin). In humans, deficiency in TSC1/2 is associated with benign tumors in many organs, including renal angiomyolipoma (AML) but rarely renal cell carcinoma (RCC). In contrast, deficiency of TSC function in the Eker rat is associated with RCC. Here, we have investigated the activity of PI 3-K and the expression of PTEN, p53, tuberin, p-mTOR, and p-p70S6K in both Eker rat RCC and human renal AML. Compared to normal tissue, increased PI 3-K activity was detected in RCC of Eker rats but not in human AML tissue. In contrast, PTEN was highly expressed in AML but significantly reduced in the renal tumors of Eker rats. Phosphorylation on Ser2448 of mTOR and Thr389 of p70S6K were significantly increased in both RCC and AML compared to matching control tissue. Total tuberin was significantly decreased in AML while completely lost in RCC of Eker rats. Our data also show that while p53 protein expression is lost in rat RCC, it was highly elevated in AML. These novel data provide evidence that loss of TSC-2, PTEN, and p53 as well as activation of PI 3-K and mTOR is associated with kidney cancer in the Eker rat, while sustained expression of TSC-2, PTEN, and p53 may prevent progression of kidney cancer in TSC patients.
Keywords: tuberin, PTEN, p53, RCC, angiomyolipoma
Introduction
Tuberous sclerosis complex (TSC) is a multisystem disorder characterized by the formation of noninvasive benign tumors in many organs such as the brain, lung, skin, heart, and kidney. Mutations in either TSC-1 or TSC-2 tumor suppressor genes (encoding the proteins hamartin and tuberin, respectively) are responsible for TSC.1 Renal tumors found in TSC in humans are almost always of the benign angiomyolipoma (AML) type and are rarely (2%-3% of all patients) renal cell carcinoma (RCC).1-3 Nevertheless, renal AML and RCC may have a similar underlying genetic basis in the early stages of their genesis and/or progression, specifically in the context of their tuberin deficiency.4 More than half of TSC patients develop AML of the kidney, which contributes to morbidity through destruction of renal parenchyma and hemorrhage.5 Although patients lacking TSC-1 also develop AML, TSC-2 deficiency–associated pathology is more severe.4,5 Also, renal AML associated with TSC tend to be larger, bilateral, and multifocal and present at a younger age compared with sporadic forms. Renal AML are generally composed of smooth muscle, fat, and blood vessels.4-6 In contrast to human TSC, the tumors arising in the Eker rat, heterozygous for TSC-2 and used for many years as a model of TSC, are nearly always of the clear cell RCC type, and the incidence of clear cell RCC in gene carriers approaches 100% by 12 months of age.7-9 Eker rats are heterozygous for an insertion mutation in the TSC-2 tumor suppressor gene. The germline mutation renders heterozygous mutants highly susceptible to renal carcinogens. The pathogenesis of RCC in rats most commonly involves disruption of TSC2, the rat homolog of the human TSC-2 gene.7
The product of the tumor suppressor gene PTEN dephosphorylates the second messenger phosphatidylinositol 3,4,5-tris-phosphate (PIP3) to negatively regulate the PI 3-kinase (PI 3-K) signaling pathway.10,11 Germline mutations in PTEN cause cancer predisposition syndromes such as Cowden disease.12 While PTEN deficiency predisposes to malignancy, such alterations are rare in TSC patients.13 In PTEN-deficient cancer cells, Akt is constitutively active, which results in the phosphorylation and inactivation of tuberin and the activation of mTOR.14,15 Similarly, the disruption or deficiency of TSC-2 also results in significantly increased mTOR activity, and this too may lead to tumorigenesis.6,16,17
Because the renal tumor type associated with TSC-2 heterozygosity differs so significantly between the human and the rat, and because tuberin plays such a central role in the negative regulation of the mTOR pathway, here, we have examined the expression of some of the intermediates in this pathway in Eker rat RCC and in human renal AML to gain insight into their potential roles in promoting benign versus malignant renal tumorigenesis.
Results
PI 3-K is highly active in RCC but not in AML
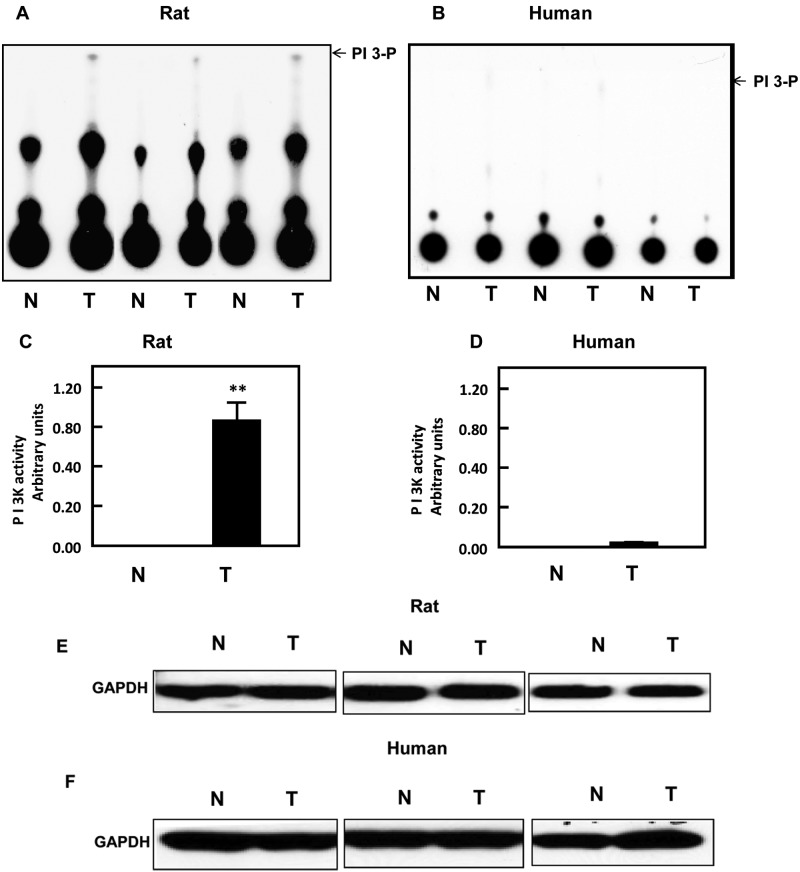
The activation of PI 3-K is an important step in coordinating a number of different signaling pathways, particularly those that involve the kinase Akt and the mTOR complexes. PI 3-K assays were carried out on normal and tumor renal tissues of the Eker rat and on normal and AML human tissues obtained from the Brain and Tissue Bank for Development Disorders (University of Maryland, Baltimore, MD). Substantial kinase activity was observed in the rat tumor tissue, but there was little activity in the normal rat kidney or in the human normal and AML tissues (Fig. 1A and 1B). Quantitation of the PI 3-K spots by liquid scintillation counting confirmed the high activity of PI 3-K in renal tumor tissue of Eker rats relative to the low activity in normal and AML tissues (Fig. 1C and 1D). GAPDH was used to confirm equal amounts of protein in each PI 3-K assay (Fig. 1E and 1F). These data suggest a marked upregulation of the Akt/mTOR pathway in the rat RCC tissue that is absent in the human renal AML tissue.
Figure 1.
PI 3-K is only active in kidney cancer. PI 3-K activity was measured in tissue homogenates of (A) normal (N) and tumor (T) kidney tissue of Eker rats and of (B) normal (N) and tumor (T) kidney tissue of humans using immunoprecipitation assay.
(A, B) These sets represent different samples from the normal and tumor kidney tissue of animals as well as from human kidney tissues. (C, D) Quantitation of PI 3-K activity was measured by liquid scintillation counting, and histograms represent means of the normal and tumor tissues ± SE. Significant difference from normal tissues is indicated by **P < 0.01. (E, F) GAPDH was used as a loading control to confirm equal amounts of protein in each PI 3-K assay.
PTEN is highly expressed in AML but not in RCC
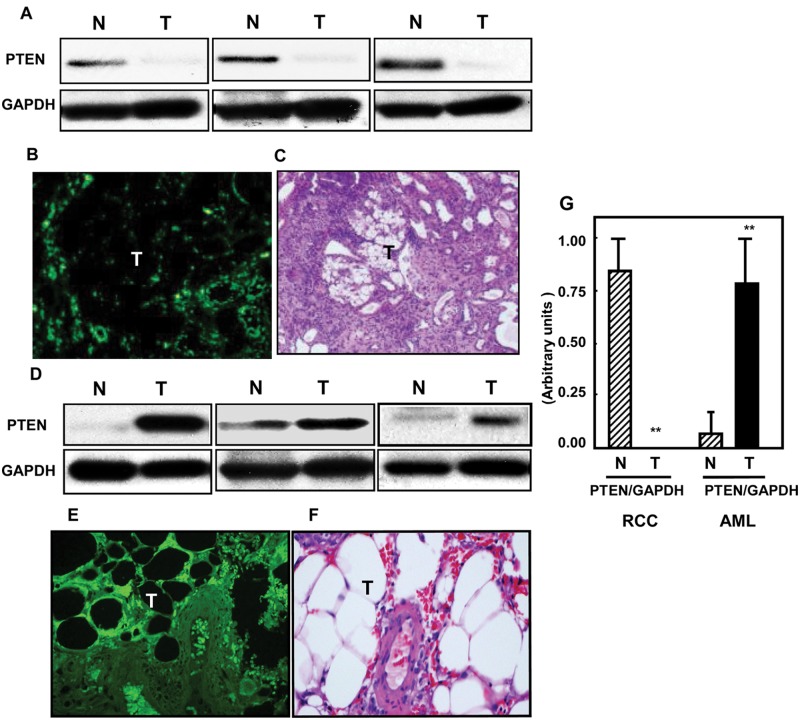
PTEN dephosphorylates PIP3 and is therefore a natural antagonist of PI 3-K and its downstream targets. Because we observed elevated levels of PI 3-K activity in the renal tumors of the Eker rat, but not in the AML tissues, we also investigated the levels of PTEN protein expression in these same tissues using immunoblot analysis. Consistent with our observation of the high PI 3-K activity in rat RCC, PTEN protein expression could be detected in normal rat kidney tissue, whereas none was detected in the rat renal tumors (Fig. 2A). This observation was supported by immunofluorescent studies, which demonstrated strong PTEN immunostaining in the normal tissue surrounding a renal tumor but none in the tumor mass itself (Fig. 2B; H&E staining of a corresponding section is shown in Fig. 2C). In marked contrast and consistent with the PI 3-K data, levels of PTEN in the AML tissues were highly elevated compared to the corresponding normal renal tissues as determined by Western blot (Fig. 2D) or by immunofluorescent staining of tissue (Fig. 2E; H&E staining of a corresponding section is shown in Fig. 2F). Thus, in the rat tumors, signaling through PI 3-K is probably greatly enhanced by a combination of the high activity of the PI 3-K itself together with an almost total absence of PTEN. In contrast, the opposite is true in human AML, where a lack of PI 3-K activity together with elevated PTEN levels would result in much reduced PI 3-K–dependent signaling.
Figure 2.
Loss of PTEN in kidney tumors of the rat compared to higher expression of PTEN in AML human tissues. PTEN expression was measured in (A) normal (N) and tumor (T) kidney tissues of rats and in (D) normal (N) and tumor (T) kidney human tissue homogenates by Western blot analysis. These sets of Western blots represent different samples from the normal and tumor kidney tissue of animals as well as from human kidney tissues. GAPDH was used as a loading control. Immunohistochemistry staining shows similar expression in kidney sections from (B) Eker rat and (E) AML tissues. FITC for PTEN (green color) was detected with excitation wavelengths at 450 to 490 nm. H&E staining of kidney sections of Eker rats (C) and (F) AML tissues shows tumors versus normal adjacent tissues. (G) Histograms represent means of the normal and tumor samples ± SE. Significant difference from normal tissues is indicated by **P < 0.01.
p53 is highly expressed in AML but is greatly reduced or absent in RCC
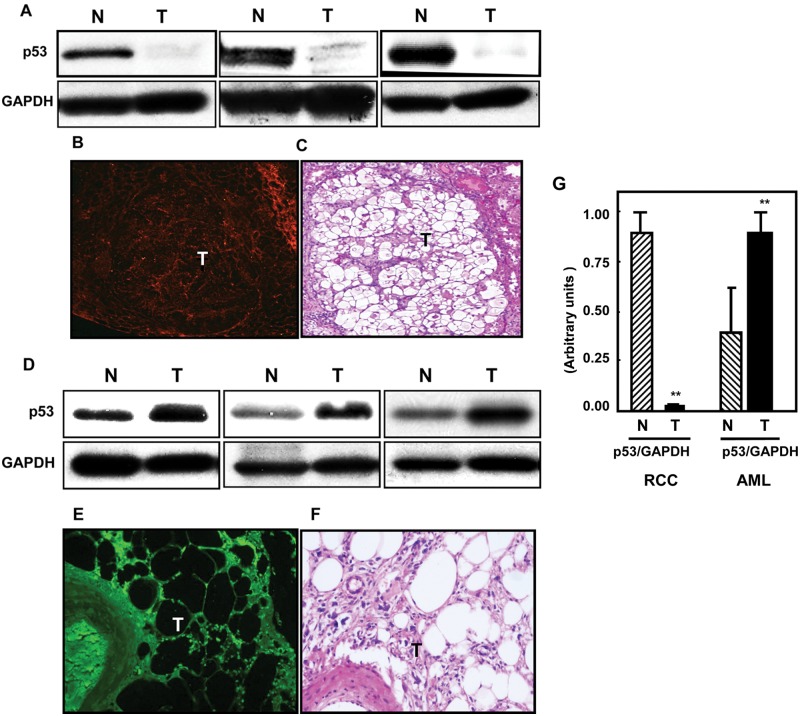
The p53 tumor suppressor gene plays a critical role in cell survival. In response to a wide variety of DNA damaging or stress signals, p53 is activated and regulates a number of genes that can result in cell cycle arrest, apoptosis, or cell senescence. Loss of p53 protein is associated with malignant tumorigenesis, but importantly in the context of our study, there is growing evidence of an interaction between p53 and the mTOR/Akt pathways. We investigated the expression levels of p53 in both rat RCC and in AML tissues. In the Eker rat renal tumors, p53 was barely detectable, if at all, when compared with normal tissue by either Western blot (Fig. 3A) or by immunofluorescent staining of tissue (Fig. 3B; H&E staining of a corresponding section is shown in Fig. 3C). In contrast, p53 expression was elevated in the AML tissues (Fig. 3D and 3E; H&E staining of a corresponding section is shown in Fig. 3F). Histograms represent means of the normal and tumor tissue of rats and humans (Fig. 3G). It is unclear at the moment whether p53 is totally absent or greatly reduced in the rat renal tumors, as immunoblotting studies were carried out on whole tumors, which therefore include normal vascular tissue and extravascular cells within the tumor mass, and these may have been responsible for the faint p53 signal seen. The effective loss or major reduction of p53 in the rat RCC may be one of the more significant reasons for the development of malignant tumors in the kidney.
Figure 3.
Loss of p53 in kidney tumors of Eker rats compared to elevated expression of p53 in AML kidney tissues. p53 protein expression was measured in kidney tissue homogenates of Eker rats and AML human tissue by Western blot analysis. Loss of p53 protein expression was mainly detected in (A) kidney tumor tissue (T) of Eker rats, while (D) elevated expression of p53 protein was seen in AML kidney tissues of humans compared to (N) normal kidney tissues. These sets of Western blots represent different samples from the normal and tumor kidney tissue of animals as well as from human kidney tissues. GAPDH was used as a loading control. p53 staining was detected using Cy3-labeled donkey anti-rabbit IgG (red signals) in rats and FITC donkey anti-rabbit IgG (green signals) in human tissue as the secondary antibody. (B, E) Immunostaining of p53 shows a significant decrease in expression of p53 staining in kidney tumors of the rat, while higher staining shows in kidney sections of AML tissues. (C, F) H&E staining of kidney sections of Eker rats shows tumors versus normal adjacent tissues. (G) Histograms represent means of the normal and tumor samples ± SE. Significant difference from normal tissues is indicated by **P < 0.01.
Tuberin expression is abolished in RCC
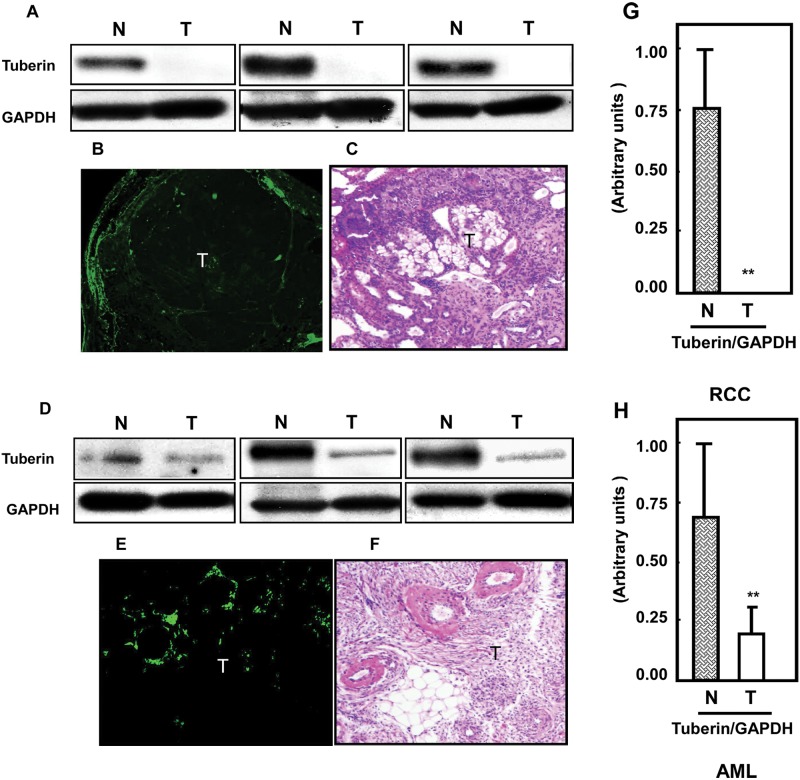
Although Eker rats are heterozygous for TSC-2, the expression of tuberin protein was completely lost in their renal tumor tissue (Fig. 4A) while significantly decreased in AML tissues (Fig. 4D) compared to respective normal tissues. Similar results for tuberin expression were observed by immunostaining of kidney sections of Eker rats and AML tissue (Fig. 4B and 4E). H&E staining of a corresponding section is shown in Figure 4C and 4F. In Figure 4G and 4H, histograms represent means of the normal and tumor tissue of rats and humans. Thus, the development of renal cancers in the Eker rat is associated with complete tuberin deficiency, while in AML, there was a marked reduction.
Figure 4.
Loss of tuberin in kidney tumors of Eker rats compared to partial deficiency of tuberin in AML tissues. Tuberin protein expression was measured in (A) normal (N) and tumor (T) kidney tissues of rats and in (D) AML tissue homogenates by Western blot analysis. These sets of Western blots represent different samples from the normal and tumor kidney tissue of animals as well as from human kidney tissues. GAPDH was used as a loading control. Significant decrease in tuberin expression (B) was also shown in kidney tumor sections from Eker rats by immunohistochemistry. Partial expression of (E) tuberin was also detected in kidney sections of AML tissues by immunostaining. FITC for tuberin (green color) was detected with excitation wavelengths at 550 to 570 nm. H&E staining of kidney sections in rats and humans shows tumors versus normal adjacent tissues. (G, H) Histograms represent means of the normal and tumor samples of rats and humans ± SE. Significant difference from normal tissues is indicated by **P < 0.01.
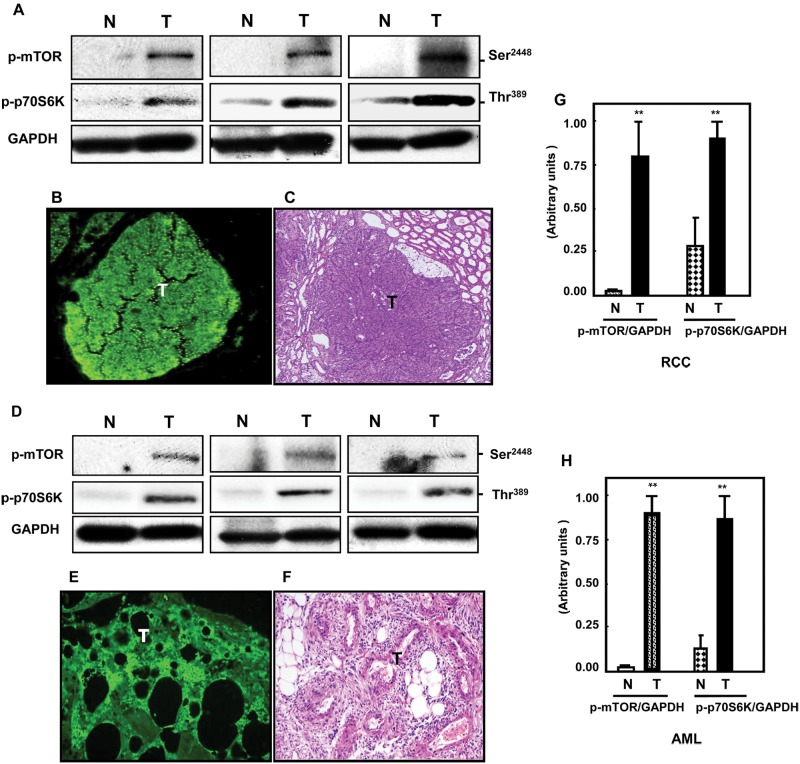
mTOR is highly active in both AML and kidney cancer
To determine the activation status of mTOR and its downstream substrate p70S6K, immunoblots were carried out using antibodies specific for phosphorylation of mTOR on Ser2448 and phosphorylation of p70S6K on Thr389. Both kinases were significantly increased in both RCC from Eker rats (Fig. 5A) and in AML tissues (Fig. 5D) compared to normal tissues. Immunostaining of p-p70S6K also showed higher activity in both tumors (Fig. 5B and 5E) compared to normal kidney tissues. H&E staining of a corresponding section is shown in Figure 5C and 5F. In Figure 5G and 5H, histograms represent means of the normal and tumor samples of rats and humans. Taken together, these data suggest that the high activity of PI 3-K coupled with a deficiency in PTEN, p53, and tuberin is the major mechanism in the development of malignant RCC. In contrast, in benign AML, the levels of PI 3-K were found to be low, with elevated expression of PTEN and p53 perhaps suppressing the development of malignancy. The increased mTOR activity in AML, despite the elevation of PTEN and p53, may be more a consequence of the relative loss of tuberin expression in these tissues.
Figure 5.
Activation of mTOR is associated with increased phosphorylation of p70S6K in kidney tumors of Eker rats and AML tissues of humans. (A, D) Immunoblot data show that mTOR phosphorylation at Ser2448 and p70S6K phosphorylation at Thr389 were highly expressed in both kidney tumors (T) of rats and humans compared to normal (N) tissues. These sets of Western blots represent different samples from the normal and tumor kidney tissue of animals as well as from human kidney tissues. GAPDH was used as a loading control. Staining of p-p70S6K was also shown in tumor kidney sections of (B, E) both tissues compared to normal tissues. FITC for p-p70S6K (green color) was detected with excitation wavelengths at 550 to 570 nm. (C, F) H&E staining of kidney sections in rats and humans shows tumors versus normal adjacent tissues. (G, H) Histograms represent means of the normal and tumor tissues ± SE. Significant difference from normal tissues is indicated by **P < 0.01.
Discussion
The types of renal tumors that arise in TSC patients differ from those seen in the Eker rat model of TSC. Whereas in humans, the tumors tend to be benign AML, in the Eker rat, they are all of the malignant RCC type. Because tuberin regulates the PI 3-K/Akt/mTOR pathway, we investigated the expression and activity of some of these intermediates in both rat RCC and human AML tissues. Here, we show that the expression or activation status of these intermediates may have an important bearing on the type of renal tumor that develops, malignant (RCC) versus benign (AML), in relation to TSC diseases. Malignant renal tumors from Eker rats expressed higher levels of PI 3-K activity and had lost the expression of PTEN, tuberin, and p53 proteins. In contrast, in benign renal AML from TSC patients, very little PI 3-K could be detected, and there was an elevated expression of PTEN and p53 and a significant reduction in tuberin expression compared to normal tissues. Nevertheless, both malignant and benign kidney tissues showed higher activation of mTOR and p70S6K compared to control tissues.
The lipid phosphatase activity of the tumor suppressor PTEN dephosphorylates the second messenger PIP3, thus negatively regulating the PI 3-K signaling pathway. Loss of PTEN is common in many tumors, including sporadic glioblastoma, endometrial carcinoma, melanoma, meningioma, and renal, breast, prostate, and small cell lung cancer.18-20 The malignant tumors arising in Eker rats show a significant increase in PI 3-K activity as a result of PTEN loss, while the high expression of PTEN in AML tissues blocks PI 3-K activity, suggesting an important role for PI 3-K in regulating genes that are involved in malignant tumors. Tuberin is also closely linked to the PI 3-K/Akt pathway, acting as a negative regulator of the downstream target mTOR. Previously, it was found that Akt, regulated by both PI 3-K and p53, phosphorylates and inactivates tuberin at Thr1462.15 Phosphorylation of tuberin by Akt affects its function through at least 2 mechanisms: first, phosphorylation decreases the activity of tuberin; and second, phosphorylation destabilizes tuberin by disrupting the complex formation between hamartin and tuberin, resulting in ubiquitination of free tuberin and its degradation by the proteasome.11
Recent studies showed a direct binding of p53 to a site on the PTEN promoter, suggesting that PTEN is transcriptionally regulated by p53.21-23 Loss of PTEN in the kidney tumors of Eker rats is associated with loss of p53 expression, while the increased expression of PTEN in AML kidney tissues is associated with elevated p53 expression, suggesting that PTEN and p53 may act as important tumor suppressor genes in nonmalignant AML tumors compared to RCC. A consequence of the deficiency in PTEN, p53, and tuberin protein is the activation of the mTOR pathway. Several studies have shown that loss of p53 function is a frequent event in tumorigenesis, and the subsequent dysregulation of the p53 target and other genes leads to the development of malignant tumors. Whereas PTEN deficiency predisposes to malignancy, malignant tumors are rare in TSC patients. p53 is infrequently mutated in either human RCC or in rat renal mesenchymal tumors.24 In addition, deficiency and inactivation of p53 may play an important role in the activation of mTOR and its regulated downstream target.25 The mechanisms by which p53 regulates mTOR involve AMP kinase activation and require the TSC1/2 complex. It has been shown that the TSC-2 gene was coordinately induced in V138 cells upon p53 activation. The consequence of PTEN and TSC-2 mRNA and protein induction is the inhibition of mTOR activity. Thus, cross-talk between p53, tuberin, and mTOR signaling pathways may control cell tumorigenesis. Although we did not investigate the mutation status of the p53 gene in kidney tumors of Eker rats, we believe that the loss of tuberin may be involved in the regulation of p53, PTEN, and other genes that leads to the development of RCC in the rat.
A positive correlation exists between the expression of HIF1α and PTEN in renal AML from TSC patients, suggesting that PTEN may safeguard against developing malignant tumors in patients with TSC deficiency.26 The complete loss of tuberin expression that we recently reported in the malignant renal tumors of Eker rats indicates that the loss of both TSC-2 alleles is an important event in malignant tumor development.9 This is supported by our observation that in the benign AML tissues, only partial loss of tuberin expression is observed. AML represent benign tumors with loss of heterozygosity of one or both TSC genes.27 Loss of TSC-2 is also found in sporadic AML.27 More than half of TSC patients develop AML of the kidney, which contribute to morbidity secondary to the destruction of renal parenchyma and hemorrhage. Although patients lacking TSC1 develop AML, TSC-2 deficiency–associated pathology is more severe than TSC1 cases.27,28
mTOR serves a critical role in regulating the translational machinery that affects growth, proliferation, and differentiation, all of which are abnormally manifested in TSC lesions. The importance of the mTOR pathway in human pathology is reflected in the overexpression of p70S6K in a subset of cancers and its correlation with a poor prognosis.29 Activation of S6 kinase and phosphorylation of its target ribosomal protein S6 were demonstrated in cells lacking TSC-2 expression.30 mTOR is activated in lymphangioleiomyomatosis-associated AML through phosphorylation of S6 protein at Ser235/236.31 Recent published data from our laboratory showed that phosphorylation of p70S6K on Thr389 was significantly higher in AML tissues compared to control samples, indicating the increase of mTOR activity in tumor tissues.6 Deficiency or loss of the TSC1/TSC-2 complex appears also to give rise to the constitutive levels of mTORC1 signaling. However, loss of a number of upstream tumor suppressors, which affects specific inputs into the pathway, is likely to lead to more moderate basal increases in mTORC1 signaling. Therefore, dysregulation of the TSC1/TSC-2 complex and subsequent activation of mTORC1 are likely to be a common molecular event in tumorigenesis. Kidney AML in humans with TSC disease are composed primarily of smooth muscle, fat, and blood vessel cells. Increased phosphorylation of mTOR on Ser2448 and p70S6K on Thr389 in AML kidney and in kidney tumors of Eker rats suggest that activation of mTOR and its downstream signals p70S6K are upregulated in both benign and malignant tumors.
In summary, our data provide novel evidence that loss of tuberin, PTEN, and p53 and activation of PI 3-K and mTOR play an important role in the development of malignant kidney tumors, whereas normal or increased expression of PTEN, p53, and tuberin may prevent progression of malignant kidney tumors in TSC patients (Fig. 6). These data will help to identify the appropriate therapeutic agents to target the genes that are involved in the development of malignant tumors versus benign tumors such as AML. Further study to identify the role of TSC genes in regulating other tumor suppressor genes that are involved in the progression of benign or malignant tumors is under investigation.
Figure 6.
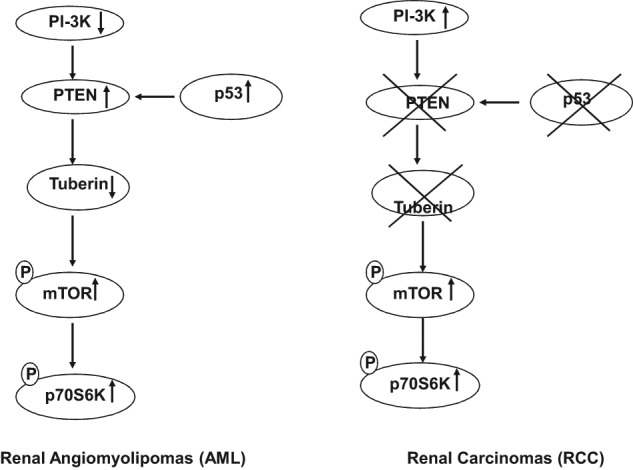
Proposed model of genes that are turned “off” or “on” in TSC-deficient cells to develop RCC versus those genes responsible for renal AML. This may explain why patients with TSC disease mainly develop AML and not RCC.
Materials and Methods
Animals
Mutant (TSC-2 +/−) Long-Evans Eker rats were purchased from a breeding colony maintained at the University of Texas MD Anderson Cancer Center (Smithville, TX). The animals were allowed food and water ad libitum throughout the study. Animals were euthanized for nephrectomy at 12 months, at which age 100% of the rats had developed tumors. The kidneys were dissected longitudinally, half preserved in 10% formalin in PBS, pH 7.4, and the remaining tissue used for biochemical assays. Tumor kidney tissues from Eker rats were also dissected for immunostaining and biochemical analysis. The Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio approved these animal studies. Kidney tissues from 6 animals from each group were used to perform all biochemical analysis.
Human kidney tissues
Kidney AML tissue from 20 TSC patients and 18 unrelated healthy people were obtained from the Brain and Tissue Bank for Development Disorders (University of Maryland, Baltimore, MD) and the San Antonio Cancer Institute Core (San Antonio, TX). The study has been approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio.
PI 3-K assay
Protein concentration was determined in kidney homogenates, and equal amounts of protein were immunoprecipitated with antibody to the p85 (Santa Cruz Biotechnology, Santa Cruz, CA) regulatory subunit of PI 3-K or phosphotyrosine antibody. The immunoprecipitates were used in the PI 3-K assay using PI as substrate in the presence of [γ-32P] ATP, and the products were separated by thin layer chromatography followed by autoradiography.32 PI 3-K products were quantified by collecting the appropriate regions of the silica gel and liquid scintillation counting.
Histology of normal and kidney tumors
Sections (3 µm) of FFPE kidneys were stained with H&E for histological examination by light microscopy.
Protein extraction and immunoblot analysis
Homogenates of kidney tissue from Eker rats and human AML tissues were prepared as described previously.33 Protein concentrations were determined using Bradford reagent34 and bovine serum albumin as a standard. Tuberin (cat# 2971), PTEN (cat# 9559), p53 (cat# 9282), p-p70S6K (cat# 9234), and p-mTOR (cat# 2971) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Immunoblotting for each protein was carried out as described.35 Membranes were then stripped with 0.2 M NaOH for 10 minutes each, blocked with 5% milk for 1 hour, and then incubated with GAPDH (Santa Cruz Biotechnology) as a loading control. Expression of each protein was quantified by densitometry using ImageJ v1.62 software (National Institutes of Health, Bethesda, MD).
Immunostaining of RCC and AML tissues
Localization and expression of PTEN, p-p70S6K, p53, and tuberin were assessed by immunofluorescence histochemistry as previously described.6,8 Acetone-fixed frozen sections (4 µm) were incubated with nonimmune donkey IgG to block nonspecific binding and then incubated with each of the following primary antibodies: tuberin, PTEN, p53, or p-p70S6K (Cell Signaling Technology) followed by FITC- or Cy3-labeled donkey anti-rabbit IgG (Chemicon International Inc., Temecula, CA) as the secondary antibody. Nonimmune rabbit IgG or PBS/BSA in place of primary antibody was used as a control in the above procedure. Sections were imaged and photographed using an Olympus research microscope (Tokyo, Japan) equipped for epifluorescence with excitation and band-pass filters optimal for either FITC or Cy3.
Statistics
Data are presented as mean ± standard error. Statistical differences were determined using ANOVA followed by the Student Dunnett (exp. v. control) test using 1-trial analysis. P values less than 0.01 were considered statistically significant.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants from the American Heart Association and a Merit Review Award from the South Texas Veterans Health Care System (to S.L.H.).
References
- 1. Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC-2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stillwell TJ, Gomez MR, Kelalis PP. Renal lesions in tuberous sclerosis. J Urol. 1987;138:477-81 [DOI] [PubMed] [Google Scholar]
- 3. Al-Saleem T, Wessner LL, Scheithauer BW, et al. Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer. 1998;83:2208-16 [PubMed] [Google Scholar]
- 4. Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int. 2004;66:924-34 [DOI] [PubMed] [Google Scholar]
- 5. Shapiro RA, Skinner DG, Stamley P, et al. Renal tumors associated with tuberous sclerosis: the case for aggressive surgical management. J Urol. 1984;132:1170-4 [DOI] [PubMed] [Google Scholar]
- 6. Habib SL. Insight into mechanism of oxidative DNA damage in angiomyolipoma from TSC patients. Mol Cancer. 2009;8:8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDorman KS, Wolf DC. Use of the spontaneous TSC-2 knockout (Eker) rat model of hereditary renal cell carcinoma for the study of renal carcinogens. Toxicol Pathol. 2002;30:675-80 [DOI] [PubMed] [Google Scholar]
- 8. Eker R, Mossige J. A dominant gene for renal adenomas in the rat. Nature. 1961;189:858-913780066 [Google Scholar]
- 9. Habib SL, Simone S, Barnes JJ, et al. Tuberin haploinsufficiency is associated with the loss of OGG1 in rat kidney tumors. Mol Cancer. 2008;7:10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habib SL, Phan MN, Patel SK, et al. Reduced constitutive 8-oxoguanine-DNA glycosylase expression and impaired induction following oxidative DNA damage in the tuberin deficient Eker rat. Carcinogenesis. 2003;24:573-82 [DOI] [PubMed] [Google Scholar]
- 11. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501 [DOI] [PubMed] [Google Scholar]
- 12. Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355-65 [DOI] [PubMed] [Google Scholar]
- 13. Wang H, Karikomi M, Naidu S, et al. Allele-specific tumor spectrum in PTEN knockin mice. Proc Natl Acad Sci U S A. 2010;107:5142-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29-39 [DOI] [PubMed] [Google Scholar]
- 15. Manning BD, Tee AR, Logsdon MN, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell. 2002;10:151-62 [DOI] [PubMed] [Google Scholar]
- 16. Sun H, Lesche R, Li DM, et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci U S A. 1999;96:6199-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta MS, Vazquez A, Kulkarni DA, et al. Polymorphic variants in TSC1 and TSC-2 and their association with breast cancer phenotypes. Breast Cancer Res Treat. 2011;125:861-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chakraborty S, Mohiyuddin SM, Gopinath KS, et al. Involvement of TSC genes and differential expression of other members of the mTOR signaling pathway in oral squamous cell carcinoma. BMC Cancer. 2008;8:163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cully M, You H, Levine AJ, et al. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184-92 [DOI] [PubMed] [Google Scholar]
- 21. Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943-7 [DOI] [PubMed] [Google Scholar]
- 22. Lee CH, Inoki K, Karbowniczek M, et al. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 2007;26:4812-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stambolic V, MacPherson D, Sas D, et al. Regulation of PTEN transcription by p53. Cancer Cell. 2001;8:317-25 [DOI] [PubMed] [Google Scholar]
- 24. Horesovsky G, Recio L, Everitt J, et al. p53 status in spontaneous and dimethylnitrosamine-induced renal cell tumors from rats. Mol Carcinog. 1995;12:236-40 [DOI] [PubMed] [Google Scholar]
- 25. Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahimainathan L, Ghosh-Choudhury N, Venkatesan B, et al. TSC-2 deficiency increases PTEN via HIF1alpha. J Biol Chem. 2009;284:27790-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henske EP, Scheithauer BW, Short MP, et al. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Genes Chromosomes Cancer. 1995;13:295-8 [DOI] [PubMed] [Google Scholar]
- 28. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345-56 [DOI] [PubMed] [Google Scholar]
- 29. Martignoni G, Pea M, Bonetti F, et al. Carcinomalike monotypic epithelioid angiomyolipoma in patients without evidence of tuberous sclerosis: a clinicopathologic and genetic study. Am J Surg Pathol. 1998;22:663-72 [DOI] [PubMed] [Google Scholar]
- 30. Cheadle JP, Reeve MP, Sampson JR, et al. Molecular genetic advances in tuberous sclerosis. Hum Genet. 2000;107:97-114 [DOI] [PubMed] [Google Scholar]
- 31. Kenerson HL, Aicher LD, True LD, et al. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645-50 [PubMed] [Google Scholar]
- 32. Phillips WA, St Clair F, Munday AD, et al. Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer. 1998;83:41-7 [DOI] [PubMed] [Google Scholar]
- 33. Habib SL, Bhandari BK, Sadek N, et al. Novel mechanism of regulation of the DNA repair enzyme OGG1 in tuberin-deficient cells. Carcinogenesis. 2010;31:2022-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54 [DOI] [PubMed] [Google Scholar]
- 35. Habib SL, Kasinath BS, Arya RR, et al. Novel mechanism of reducing tumourigenesis: upregulation of the DNA repair enzyme OGG1 by rapamycin-mediated AMPK activation and mTOR inhibition. Eur J Cancer. 2010;46:2806-20 [DOI] [PubMed] [Google Scholar]