Abstract
The RecBCD enzyme is a complex heterotrimeric helicase/nuclease that initiates recombination at double-stranded DNA breaks. In Escherichia coli, its activities are regulated by the octameric recombination hotspot, χ (5′-GCTGGTGG), which is read as a single-stranded DNA sequence while the enzyme is unwinding DNA at over ∼1,000 bp/s. Previous studies implicated the RecC subunit as the “χ-scanning element” in this process. Site-directed mutagenesis and phenotypic analyses identified residues in RecC responsible for χ recognition [Handa N, et al., (2012) Proc Natl Acad Sci USA, 10.1073/pnas.1206076109]. The genetic analyses revealed two classes of mutants. Here we use ensemble and single-molecule criteria to biochemically establish that one class of mutants (type 1) has lost the capacity to recognize χ (lost-recognition), whereas the second class (type 2) has a lowered specificity for recognition (relaxed-specificity). The relaxed-specificity mutants still recognize canonical χ, but they have gained the capacity to precociously recognize single-nucleotide variants of χ. Based on the RecBCD structure, these mutant classes define an α-helix responsible for χ recognition that is allosterically coupled to a structural latch. When opened, we propose that the latch permits access to an alternative exit channel for the single-stranded DNA downstream of χ, thereby avoiding degradation by the nuclease domain. These findings provide a unique perspective into the mechanism by which recognition of a single-stranded DNA sequence switches the translocating RecBCD from a destructive nuclease to a constructive component of recombinational DNA repair.
Keywords: protein–DNA interactions, allosteric switch
In Escherichia coli, homologous recombination is initiated by the RecBCD enzyme (1). RecBCD is a helicase/nuclease that processes linear double-stranded DNA (dsDNA) resulting from dsDNA breaks (2) to produce single-stranded DNA (ssDNA) onto which it loads the RecA protein (3). These RecA-ssDNA filaments are essential to the formation of homologously paired joint molecules that are intermediates of recombinational DNA-break repair.
RecBCD binds to dsDNA ends with high affinity (KM ∼ 0.1–1 nM) (4, 5). It is a rapid and highly processive helicase, unwinding DNA at up to 1,000–1,500 bp/s, translocating about 30,000 bp before dissociating, and using ∼2 ATP molecules per base pair unwound (4–6). The RecBCD enzyme is driven by two motor subunits, RecB and RecD, with opposite translocation polarities, 3′ →5′ and 5′ →3′, respectively (7, 8). As it is unwinding, RecBCD degrades the nascent ssDNA, preferentially on the 3′-teminated strand relative to its DNA entry site (9).
RecBCD is regulated by the recombination hotspot, χ, the octameric DNA sequence (5′-GCTGGTGG-3′) (10), which is recognized from its 3′-side as ssDNA by the translocating enzyme (9, 11, 12). In response to χ recognition, the polarity of RecBCD nucleolytic action is switched: degradation of the 3′-terminated strand is down-regulated, whereas degradation of the 5′-terminated strand is up-regulated (9, 13, 14). Upon interaction with χ, RecBCD also briefly pauses and then resumes translocation, but at approximately half the initial rate (15–17). Because the helicase activity is retained (9), ssDNA is produced with χ at its 3′-terminus, which is the optimal substrate for RecA-promoted invasion of dsDNA (14). Thus, interaction with χ changes RecBCD from its “destructive” mode, digesting DNA as it unwinds, to a “recombinational” mode, preserving the χ-containing ssDNA and loading RecA onto this ssDNA (1, 2); these are two essential biological functions of the RecBCD–χ interaction (18, 19).
The crystal structure of RecBCD bound to a DNA hairpin provided a great deal of information in understanding RecBCD function, in particular with regard to the potential role of RecC in χ recognition (20). Strikingly, RecC displays the fold of a canonical UvrD-like helicase, even though none of the characteristic Superfamily 1 (SF1) helicase motifs are preserved. There are three holes in RecC: a large one flanked by two smaller ones. The large hole serves as the interface between the RecB and RecC subunits. One of the small tunnels feeds the 5′-terminated ssDNA strand to RecD, and the other feeds the 3′-terminated strand from RecB, through RecC, into a nuclease domain immediately “behind” the RecB motor domains. The 3′-strand tunnel in RecC is formed by the helicase-like domains, and the recC* mutants, which alter the efficiency and specificity of χ recognition, map to this region (21–23). Consequently, it was proposed that this tunnel region functions to channel a correctly oriented χ sequence for recognition by RecC.
In the companion article by Handa et al. (24), amino acids within the RecC channel were altered by site-directed mutagenesis to identify the residues responsible for χ recognition. The genetic analyses discovered two types of mutants (Fig. S1). The first category, referred to as type 1, displayed phenotypic characteristics consistent with a complete loss of the χ response; these mutants included L64A, W70A, D133A, D136A, and R186A, and recombination in these cells was not responsive to χ. In contrast, the second category, referred to as type 2, displayed phenotypic characteristics consistent with a promiscuous response to χ: these mutants included Q38A, T40A, L134A, Q137A, R142A, and D705A. To understand the biochemical basis for these complex phenotypes in vivo, six mutant proteins of each type were purified and their in vitro behavior was investigated. We found that the type 1 mutants represent a group of RecBCD enzymes that fail to recognize χ, and that the type 2 mutants represent a group that displays relaxed recognition specificity toward χ. The members of the first group define a recognition helix structure for sequence-specific binding of ssDNA. The members of the second group define an ionic latch structure associated with the recognition helix. Our findings suggest that the latch is a χ-regulated structure responsible for controlling a conformational switch that, we propose, opens a “trap door” to provide a new exit channel for the χ-containing ssDNA, thereby avoiding nucleolytic degradation. Thus, χ recognition initiates a conformational cascade in RecBCD that transforms its biological behavior by a unique mechanism that acts to divert ssDNA to an alternative exit, thereby escaping nucleolytic demise.
Results
RecC-Channel Mutants Possess Approximately Wild-Type Levels of Helicase and Nuclease Activities.
The core activities, dsDNA unwinding and nuclease, of the RecBCD mutants were examined; wild-type RecBCD and RecBC (which lacks nuclease activity) were used as the reference enzymes. All of the mutant enzymes catalyzed unwinding of linear dsDNA, seen as disappearance of substrate and appearance of full-length ssDNA (Fig. S2). The mutants also possessed nuclease activity, which was manifest as production of random-sized oligomeric ssDNA and, because of the heterogeneous sizes, apparent loss of DNA. Because the nuclease activity of RecBCD is significantly affected by the free Mg2+ concentration (25), the mutants were also examined at a higher concentration of Mg2+ (Fig. S3). At these conditions, nuclease activity was higher, yielding lower amounts of full-length ssDNA. Thus, the relative unwinding and degradative activities of the mutant enzymes were comparable to wild-type, except for W70A, which produced ∼twofold more full-length ssDNA, and L64A, which produced approximately half of the full-length ssDNA. However, in comparison with the nuclease-deficient RecBC, all of the mutant enzymes display significant levels of nuclease activity.
Type 1 Mutants Produce Trace Amounts of χ-Containing ssDNA, Demonstrating a Loss of χ Response.
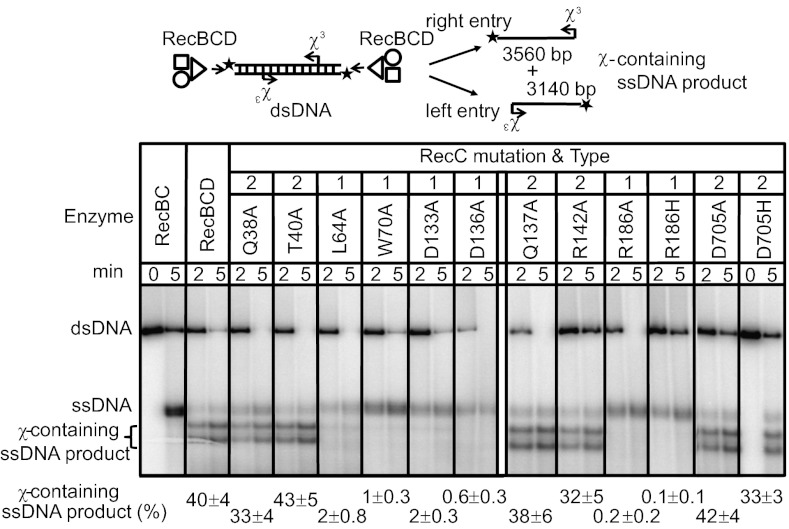
To determine whether the RecC-channel mutant enzymes respond to χ, we measured production of χ-specific ssDNA as a consequence of χ-dependent attenuation of nuclease activity (9). As previously reported, unwinding and degradation of χ-containing dsDNA by the wild-type RecBCD resulted in formation of full-length ssDNA and χ-specific ssDNA fragments (9, 14, 25). RecBCD converted 40 ± 4% of the dsDNA into χ3-containing ssDNA (Fig. 1). In contrast, type 1 mutants (L64A, W70A, D133A, D136A, and R186A) generated barely detectable amounts: only 0.2–2%. Experiments at the higher free-magnesium concentration (Fig. S4) showed that yields of χ3-containing ssDNA were lower (undetectable to 0.5%), but relative behavior was the same. Thus, consistent with their phenotypes, these mutant enzymes lost the ability either to recognize or to be regulated by χ. The inability of type 1 mutants to produce χ-containing ssDNA establishes their designation as “lost-recognition” mutants, a description that we use interchangeably hereafter.
Fig. 1.
Type 1 (lost-recognition) mutants process dsDNA to produce negligible amounts of χ-specific ssDNA, whereas type 2 (relaxed-specificity) mutants display wild-type levels of χ recognition. Enzymes and reaction times are indicated. The substrate was χ3 dsDNA [illustrated; processing produces ssDNA and χ-specific ssDNA fragments (only the χ-containing ssDNA product is shown)]. Final yields (± SD) of χ-containing ssDNA product are from at least three independent experiments.
Type 1 Mutants Have Lost the Ability to Recognize χ.
The lost-recognition mutants failed to either recognize χ or attenuate their nuclease activity in response to χ. To distinguish between these possibilities, we used an assay, reversible inactivation by χ, which is independent of nuclease activity (19, 26). Under conditions of limited Mg2+, RecBCD is stably but reversibly inactivated after an encounter with χ. The enzyme completes unwinding of the DNA molecule to which it is bound, but the inactivated RecBCD is incapable of reinitiating unwinding on a new DNA duplex. However, inactivation is reversed by addition of excess Mg2+, and normal catalytic DNA processing activities are restored to the reactivated enzyme.
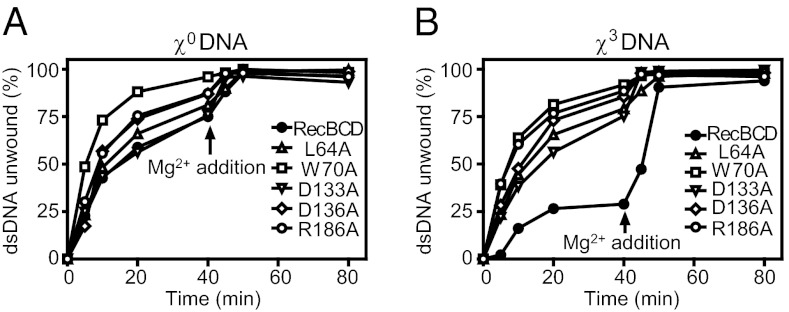
This behavior is shown in Fig. 2. Reactions contained subsaturating amounts of enzyme and either χ0 (Fig. 2A) or χ3 linear dsDNA (Fig. 2B). At the limiting Mg2+ concentration, wild-type RecBCD unwound ∼60% of χ0 dsDNA after 20 min, and nearly all (∼80%) by 40 min; however, for χ3 DNA, only 25% was unwound after 20 min and no further unwinding occurred. The reversibility of this phenomenon was demonstrated by the subsequent unwinding of remaining DNA upon increasing the Mg2+ concentration. In contrast, the behavior of all lost-recognition mutants on either χ0 or χ3 DNA was the same: there was no recognition of χ.
Fig. 2.
Type 1 (lost-recognition) mutants are not reversibly inactivated by χ. Plotted is dsDNA unwound as a function of time. Reactions contained excess [6.25 μM (nucleotides; 1.55 nM ends)] χ0 DNA (A) or χ3 DNA (B), 0.05 nM RecBCD or equivalent of mutant, 1.25 μM SSB, 1 mM Mg(OAc)2, and 5 mM ATP. At 40 min, Mg(OAc)2 was added to 10 mM.
Lost-Recognition Mutants Do Not Promote χ-Dependent Joint Molecule Formation.
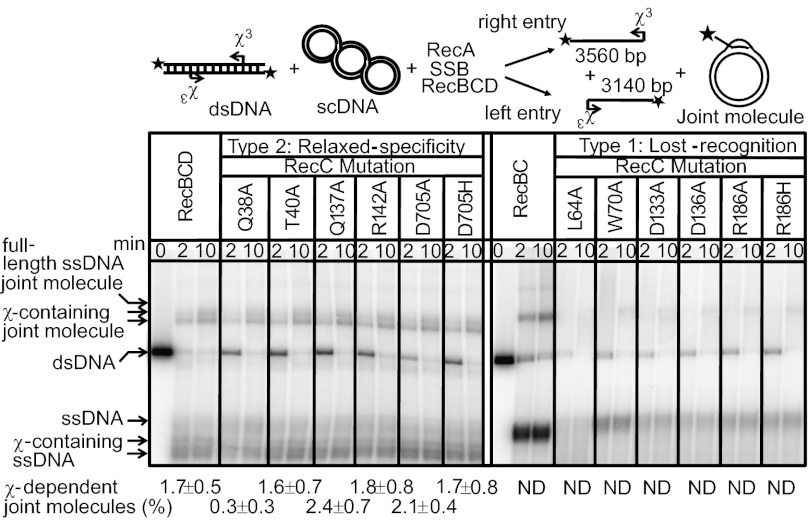
For wild-type RecBCD, interaction with χ not only produces χ-containing ssDNA but results in the preferential loading of RecA onto this product (3). To verify that RecA did not alter our in vitro results, a coupled DNA unwinding and pairing assay was used. In this reaction, RecBCD generates ssDNA, which can be coated by RecA; the resultant RecA nucleoprotein filament invades homologous supercoiled DNA, leading to the formation of joint molecules (Fig. 3). Two types of discrete-sized joint molecules are produced: χ-independent, because of invasion by full-length ssDNA, and χ-dependent, because of invasion by χ-containing ssDNA.
Fig. 3.
Type 2 (relaxed-specificity) mutants stimulate χ-dependent joint molecule formation, whereas type 1 (lost-recognition) mutants do not. Coupled RecABCD reactions (illustrated) were conducted with enzymes for times indicated. Mobilities of full-length ssDNA, χ-specific ssDNA fragments, and joint molecules are indicated. The final yields (± SD) of χ-dependent joint molecules are from at least three independent experiments; ND signifies not detectable (<0.2%).
As shown in Fig. 3, wild-type RecBCD preferentially loads RecA onto the χ-containing ssDNA, which is manifest as an increased yield of χ-dependent joint molecules relative to the χ-independent joint molecules. Processing by wild-type RecBCD resulted in ∼2% of the input dsDNA substrate being incorporated into χ3-specific joint molecules. However, the lost-recognition enzymes did not promote any detectable joint molecule formation and did not increase the yield of χ3-specific ssDNA in the presence of RecA.
Type 2 Mutants Produce χ-Specific ssDNA Fragments.
In contrast to type 1, type 2 mutants (Q38A, T40A, Q137A, R142A, and D705A) behaved in vivo as though they recognized a more frequent variant of the χ sequence, or they switched to the χ-activated state randomly. Fig. 1 also showed that processing of χ3-containing dsDNA by type 2 mutants was distinctly different from lost-recognition mutants. The type 2 mutants behaved similarly to wild-type RecBCD: they recognized χ and produced χ-specific ssDNA products with comparable yields (32–43%).
Type 2 Mutants Stimulate χ-Dependent Joint Molecule Formation.
Type 2 mutant enzymes loaded RecA onto χ-containing ssDNA to produce amounts of χ-specific joint molecules comparable to wild-type RecBCD (Fig. 3). Both wild-type and type 2 mutant enzymes produced ∼10-fold more χ-dependent than χ-independent joint molecules. These data demonstrate that the preferential use of χ-containing ssDNA over full-length ssDNA is retained by type 2 mutants. Thus, type 2 mutants not only respond to χ by attenuating their nuclease activity, but also with the structural changes that enable loading of RecA onto χ-containing ssDNA.
Type 2 Mutations Create RecBCD Mutants with Relaxed-Specificity That Recognize Sequence Variants of χ.
In vivo, type 2 mutants enabled large plaques for both χ0 and χ+ phages, and they showed high recombination frequency even for χ0 phage λ (24). The biochemical data above showed that these mutants recognize and undergo modification of nuclease functions by χ. One possible explanation for their hyper-recombinogenic phenotype is that type 2 mutants acquired the capacity to recognize χ-related sequences other than the canonical χ sequence.
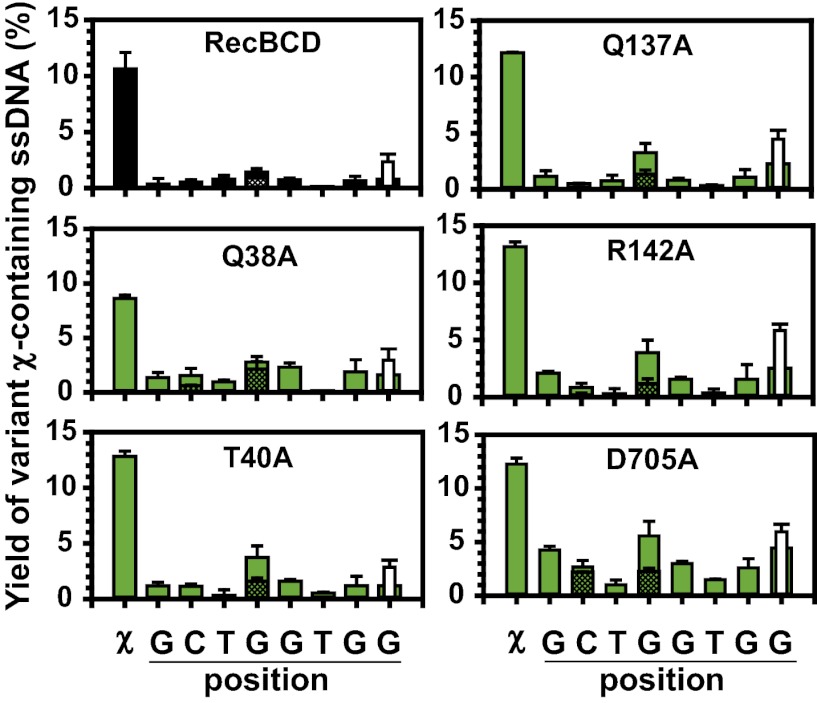
This possibility was tested by using DNA that contained mutant derivatives of the canonical χ sequence, wherein all of the bases of χ were individually altered initially to adenine, and several were changed to thymine or cytosine (Table S1) based on whether they were known to disrupt recognition by wild-type enzyme (27–29). For DNA containing canonical χ, χ-containing ssDNA was produced by wild-type and all type 2 enzymes (Fig. 4, first column in each graph) (9, 14). For wild-type RecBCD, mutation in any position to adenine nearly eliminated χ recognition (7- to 400-fold lower). Interestingly, type 2 mutants responded more promiscuously to the χ variants than wild-type (Fig. 4 and Fig. S5), revealing their behavior as “relaxed-specificity” alterations, a designation that we will use interchangeably with type 2 mutations hereafter. For D705A, recognition of these χ variants is particularly evident: substitution of adenine at any position resulted in downstream χ-variant ssDNA yields as large as ∼25% of the canonical χ response, and recognition of every χ variant was ∼2- to 60-fold greater than for wild-type RecBCD (Fig. S5). The Q38A, T40A, Q137A, and R142A enzymes recognized the same χ variants, with only somewhat lower yields (Fig. 4 and Fig. S5).
Fig. 4.
Type 2 (relaxed-specificity) mutants recognize single-base variants of χ. Yield of variant χ-containing ssDNA produced by a mutant enzyme is plotted versus position of the canonical χ sequence. Canonical recognition is the left-most bar; solid, crosshatched, or white bars denote substitution of adenine, thymine, or cytosine at that position, respectively (Table S1). Final yields (± SD) from at least two independent experiments are plotted. There is only one χ sequence in the proper orientation, and because RecBCD can enter from either DNA end, the efficiency of χ recognition is at least twofold higher.
Single-Molecule Visualization Shows That Translocation by a Relaxed-Specificity Mutant is Modified by χ-Like Sequences in a Canonical Manner.
Interaction with χ not only alters the intensity and polarity of the nuclease activity, but it also changes translocation: upon recognition of χ, RecBCD pauses and then continues at approximately one-half of the initial rate (15, 16). Consequently, we were interested in determining whether χ-like sequences behaved as bona fide χ sequences with regard to translocation by relaxed-specificity mutants. The single-molecule approach described (15, 17, 30) was used to directly image translocation of the most promiscuous relaxed-specificity mutant, RecBC(D705A)D. Wild-type λ DNA does not have a canonical χ sequence but, when attached via its cosA end to polystyrene beads, it does contain potential χ variants in the correct orientation relative to the cosB end (Fig. 5A, arrows).
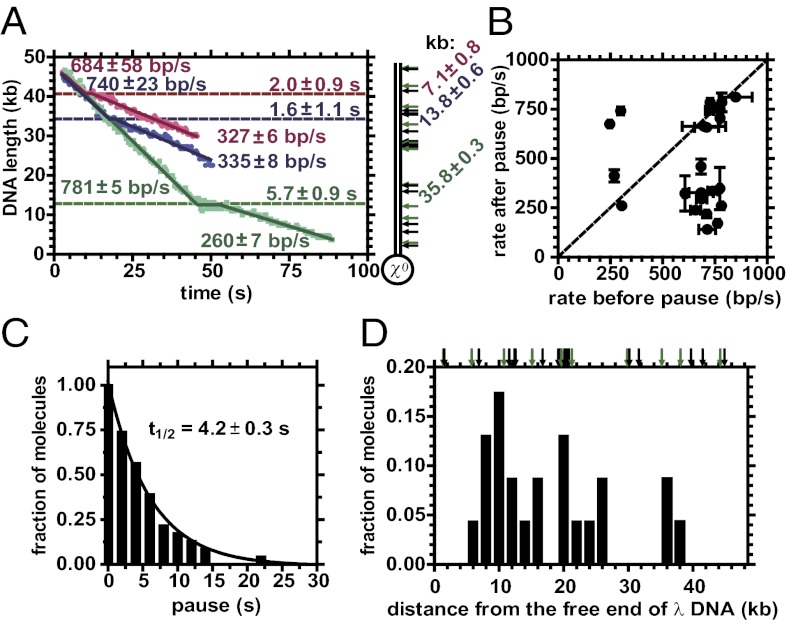
Fig. 5.
Single-molecule visualization shows that translocation by RecBC(D705A)D is modified by encounters with sequences that elicit a χ-like response. (A) Time course for unwinding of three different λ DNA molecules, which lack canonical χ, by individual RecBC(D705A)D enzymes at 29 °C. For each, velocity before and after a pause, pause position, and pause duration are indicated. Arrows represent positions of single-base χ variants; green arrows are variants examined biochemically in Fig. 4 (see SI Materials and Methods for sequences and locations). (B) Dot plot for rate of translocation before pausing versus after pausing. Diagonal line denotes expectation if the rates were identical. Error bars are SEs. (C) Distribution of pause times. Molecules that paused for at least the time indicated were grouped in 2-s bins. The half-time for decay is 4.2 ± 0.3 s. (D) Positions where RecBC(D705A)D paused. Arrows are as in A.
For wild-type RecBCD, unwinding and degradation was linear with time, with neither pauses nor changes in velocity (Table S2) (15, 17, 30). The average rate of unwinding for 24 RecBCD molecules was comprised of two Gaussian populations with mean velocities of 861 ± 99 bp/s and 368 ± 191 bp/s (at 29 °C), and is consistent with earlier measurements (4, 15, 30); the slower population (∼20%), which becomes evident when a large number of molecules was sampled, will not be discussed further herein (31). In contrast, Fig. 5A shows three examples of RecBC(D705A)D translocating on λ DNA lacking the canonical χ sequence. These three enzyme molecules paused and altered velocity; furthermore, they paused at different positions. Out of the 56 RecBC(D705A)D–DNA complexes that were trapped, 23 (41%) of the mutant enzymes were seen to pause (Table S3). In contrast, none of the 24 wild-type enzymes paused. Before pausing, the collection of RecBC(D705A)D molecules displayed a translocation velocity distribution that is nearly the same as wild-type enzyme (710 ± 51 bp/s and 284 ± 44 bp/s) (Fig. S6), within error, verifying that helicase activity is not altered by the D705A mutation. For the 23 RecBC(D705A)D molecules that paused, 17 molecules (74%) changed their translocation velocity (Fig. 5B) (15). For each molecule, the translocation velocity after the pause was constant. Most of the 23 molecules slowed after the pause, except for six enzymes that maintained the same speed and another four that increased velocity, a behavior that can also be observed for wild-type RecBCD (31). The pauses have a half-time of 4.2 ± 0.3 s (Fig. 5C), which is similar (within error) to the half-time (5.0 ± 0.5 s) for wild-type RecBCD elicited by the canonical χ (15).
Although wild-type λ DNA does not have a canonical χ sequence, it does have 27 singe-base variants of χ (Fig. 5D, arrows), a subset of which were biochemically examined in Fig. 4 (green arrows). Individual RecBC(D705A)D enzymes were seen to pause at many different positions in the λ DNA, ranging from 6 to 38 kb from the free end (Fig. 5D). The spatial resolution of our method does not permit precise mapping of these pause sites, but it is clear that they do not always coincide with any arrows (i.e., variants containing seven of eight bases), suggesting that other novel sequences are being recognized. Furthermore, the observed recognition frequency decreases with distance from the entry site; this phenomenon is consistent with a χ-like behavior because RecBCD is switched at the first productive encounter with χ, and it does not respond to subsequent downstream χ sequences (15, 32). It is clear that the most promiscuous of the relaxed-specificity mutants is responding to many different χ-like sequences in a way that is identical to the behavior induced upon recognition of χ by wild-type RecBCD. Thus, the interaction with χ variants not only modifies the nuclease activities of RecBC(D705A)D, but it also modifies its translocation behavior.
Discussion
To understand the mechanism by which RecBCD is regulated by its interaction with χ, structure-directed mutagenesis was used to alter residues in the channel of RecC through which the χ-containing ssDNA traverses (24). Based on phenotype, these RecC-channel mutants were classified into two categories: type 1 [L64A, W70A, D133A, D136A, and R186A (or R186H)], and type 2 [Q38A, T40A, L134A, Q137A, R142A, and D705A (or D705H)]. Their in vivo behavior suggested that the first class represented mutants that lost the ability to recognize or respond to χ, and the second class likely represented mutants that gained the ability to respond to more frequent χ-like sequences. Here we established, based on biochemical and single-molecule criteria, that type 1 mutations produce enzymes unable to recognize χ, and that type 2 mutations relax the sequence specificity of χ recognition. All mutant enzymes possess helicase and nuclease activities; when normalized for specific helicase activity (4), their nuclease activities are similar (within ∼twofold) to wild-type levels.
The lost-recognition mutants lose the ability to respond to χ and they produce negligible amounts of χ-containing ssDNA. The collective lack of χ response is most consistent with a deficiency in the primary event of χ recognition, not just a failure to communicate the recognition to the required structural changes.
In contrast, relaxed-specificity mutants recognize χ, respond appropriately to produce χ-containing ssDNA, and stimulate DNA pairing by loading RecA onto ssDNA in a χ-dependent manner. Most significantly, relaxed-specificity mutant enzymes recognize variants of χ that alter one of the canonical bases, resulting in a χ-like biochemical response with sequences that contain only seven of the eight bases. In addition, the relaxed-specificity D705A mutant shows precocious χ-like behavior at the single-molecule level, emulating the canonical χ-response at many positions on λ DNA. Thus, type 2 mutants have acquired a relaxed and altered χ-recognition capacity, demonstrating that evolution of novel ssDNA sequence recognition requires only simple point mutation. Because wild-type phage λ has many χ variants, even if less efficient than canonical χ, the seven-base variants will be more frequent and both recombination and repair proficiency of χ0 phage in the relaxed-specificity class of mutant cells is readily understood. Furthermore, frequent recognition of χ variants, particularly before the crossover interval studied in vivo, would preempt any activation by canonical χ, explaining the lack of hotspot activity in the in vivo assays. The relaxed-specificity mutants might also recognize shorter versions (e.g., six nucleotides) of χ; this was not tested here. It is known, for example, that the χ sequence for the Bacillus subtilis RecBCD homolog, AddAB, is five nucleotides: 5′-AGCGG-3′ (33).
The ensemble conclusions are also confirmed by single-molecule analyses. Wild-type λ DNA lacks canonical χ but, nonetheless, both a pause and change in translocation rate (characteristic of bona fide χ recognition) occur at different positions for the relaxed-specificity mutant, RecBC(D705A)D. This finding supports the idea that relaxed-specificity mutants do indeed interact with novel sequences, and that translocation is quantitatively modified in the canonical manner upon recognition of noncanonical sequences. The pause positions are not limited to the sites of χ variants examined here biochemically, implying that RecBC(D705A)D, the most relaxed member of this class, responds to additional undefined χ-like sequences.
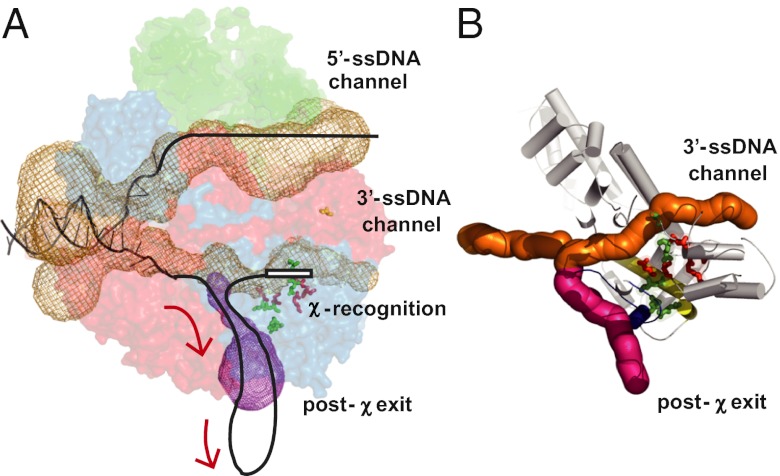
Structurally, RecC has the same tertiary-fold as canonical UvrD-like helicases (20). UvrD-like enzymes bind ssDNA via aromatic stacking interactions with nucleobases and electrostatic contacts with the phosphate backbone. Remarkably, many residues involved in χ recognition are located in regions of RecC that are equivalent to regions of helicases involved in ssDNA binding (34, 35), supporting the notion that this region in RecC provides an ideal protein architecture to function as a scanning site for a correctly oriented χ sequence (20, 23). One relaxed-specificity residue, D705, is found in subdomain 2A and five other relaxed-specificity residues (Q38, T40, L134, Q137, and R142) are located in subdomains 1A, 1B, and 2A. Q38 and T40 are in positions where they could contact the ssDNA backbone (Fig. S1). R142 and D705 form an ion pair latch in a loop at the end of a long helix, on which the relaxed-specificity residues (L134 and Q137) and lost-recognition residues (D133 and D136) are located. Disruption of the ion pair by mutation results in a RecBCD that is more easily activated by effector (χ-like) sequences. It might be that when χ is recognized, via both specific and nonspecific interactions, helix movement results in disruption of the ion pair between R142 and D705. This latch disruption and subsequent structural change would allow RecC to adopt a conformation more typical of an SF1 helicase, opening the existing crevice between RecB and RecC to function as an alternative exit channel for ssDNA away from the nuclease site (Fig. 6A, magenta tunnel), thereby escaping nucleolytic degradation. The stable binding of χ to RecC would encourage use of this post-χ recognition exit, because the binding would obstruct the normal exit as the RecB motor continues to pump ssDNA into RecC at ∼1,000 nt/s (15, 36). Both structural and biochemical work have noted existence of the alternative channel in Fig. 6A (20, 37). A conformational change linked to latch disruption would enable this conformational switch. A similar mechanism of signal transduction along a helix connecting two sites was proposed for an allosteric switch in lactate dehydrogenase (38). For lost-recognition RecBCD mutants, the alteration likely weakens direct interactions with χ to the extent that the binding energy is insufficient to trigger subsequent changes needed to open the latch structure.
Fig. 6.
Proposed model for the interaction between RecBCD and χ. (A) Location of the χ-binding locus within RecBCD. Red and green residues are type 1 and type 2, respectively. Duplex DNA enters on left and exits at nuclease domain on right. Pre-χ recognition channels are orange; proposed post-χ recognition exit is magenta. The proposed exit path for ssDNA after χ recognition is shown. (B) Proposed alternative exit in RecBCD post-χ recognition. Path of ssDNA before χ recognition is in orange; the proposed alternative exit after χ recognition is shown in rose. Recognition helix is yellow and structural elements (blue) comprising the latch line alternative exit.
The crystal structure of RecBCD with the longest DNA substrate showed how the 3′-tail of DNA interacts with RecB; unfortunately, the ssDNA stopped at the boundary between the RecB and RecC subunits (39). However, an examination of RecC reveals that, in its present conformation (the preinitiation complex before χ recognition), the RecC domains equivalent to the 1A, 1B, and 2A domains of RecB, which interact with ssDNA, are not properly oriented to bind ssDNA in the canonical SF1-helicase manner. Therefore, to gain insight into the possible structural changes required for binding, we used the RecB-ssDNA components from the RecBCD complex (Fig. S7A) to model the hypothetical orientations of the 1A, 1B, and 2A domains of RecC when bound to ssDNA. This process was done by simply superimposing them onto the Cα backbone of equivalent domains of RecB to place them in orientations equivalent to the ssDNA binding mode of RecB. We then highlighted the mutated side chains of each class to see where they were located relative to the ssDNA in this fitted model; for ssDNA, we used the eight bases of the χ sequence. This crude “model” (Fig. S7B) revealed that the lost-recognition mutants were in positions that could interact with the DNA bases themselves and, hence, their interactions with ssDNA would be entirely sequence-dependent (Fig. S7C). Consistent with our experimental observations, this structural approximation shows that alteration of these residues could result in the loss of specific interactions that contribute to the free energy of interaction, leading to an expected loss of recognition. The relaxed-specificity mutants were either in positions that could interact with the DNA backbone or were associated with the latch structure. Alteration of some of these resides could lead to interactions that potentially increased nonspecific interactions with ssDNA, and could lead to a heightened overall free energy of interaction with noncanonical χ sequences, stabilizing this proposed post-χ state. Alternatively, and more probably, alteration could weaken the stability of the latch structure (notably, D705A and R142A), permitting the normally weaker interactions with noncanonical χ sequences to trigger opening of the latch at a lower threshold of binding free energy (i.e., at a lower energetic cost). Interestingly, to allow the movement of the domain required for this simple conformational modeling, the latch structure must be disrupted, which is qualitatively consistent with the mutational interpretations and energetic consideration mentioned above. Although this rough model does not provide an explanation for why D705A shows more promiscuous recognition than mutation of its partner, R142, we suspect that mutation on one side of the latch, but not the other, is detrimental to other local contacts in the vicinity. Finally, although this model appears to give some insights into the likely roles of the mutations, it must be stressed that this is only a crude model and merely serves for illustrative purposes to indicate the likely positions of the mutations in the channel and how ssDNA might run past them (Fig. 6B). It is certainly too imprecise for any detailed analysis beyond that which we present here.
In summary, our genetic, biochemical, single-molecule, and structural analyses reveal both a complexity and elegance to the manner by which RecBCD is regulated by its interaction with the ssDNA sequence, χ, while it is translocating through duplex DNA, and they suggest a model for how that recognition event is transmitted into both structural and functional alterations. We show that χ is recognized by the RecC subunit while it is being pushed through a channel by the RecB motor (40). The lost-recognition (type 1) mutations define the locus for this interaction, and they define an ssDNA-recognition helix that is needed to confer and communicate the specificity of this interaction. The relaxed-specificity (type 2) mutations define the latch as a crucial structural element. We propose that latch opening is triggered via allosteric coupling with the recognition helix that permits conformation switching to the χ-activated structure. This structure is an engineering masterpiece that couples recognition of χ to movement of an α-helix that then snaps open a latch, which then opens an alternative exit for the χ-containing ssDNA to avoid degradation by the nuclease domain. Mutations that weaken this latch structure or that increase nonspecific interaction with ssDNA, facilitate structural unlatching: thus, evolutionarily, it would be a simple matter to tune in the requisite specificity and stability of sequence-specific ssDNA interaction to evolve proteins with a broad range of χ-sequence interaction. We propose that, as a consequence of structural changes that accompany unlatching of the χ-activated state, the combined nuclease and RecA-loading site of RecBCD is then forced to undock from its interaction site on RecC (15). This structural change is both encouraged and stabilized by the stable binding of χ to RecC in the channel (15, 36). This stable binding both prevents the 3′-end of the χ-containing ssDNA from exiting into the nuclease domain and, because RecB continues to pump ssDNA into the channel (17), the requirement for an alternative exit is imposed. Furthermore, undocking of the RecB nuclease and loading domain has two consequences: (i) an attenuation of nuclease activity overall and specifically preventing degradation of the recombinogenic χ-containing ssDNA at the original 3′-exit location (9), and (ii) liberation of the RecB domain responsible for RecA-loading, which is now able to swing on the end of its 70 amino acid tether and finally load RecA onto the χ-containing ssDNA (41). Although elements of this model are conjectural, collectively the existing data underscore an exquisite structural and mechanical coupling in this finely tuned macromolecular machine.
Materials and Methods
Proteins and DNA.
Biotinylated RecBCD enzymes with RecC-channel mutations, RecA, and SSB were purified as detailed in the SI Materials and Methods. Plasmids pBR322 (wild-type, χ0), pBR322 χ(+)−3F3H (referred to as χ3 throughout) (13), and pBR322 containing the χ-variants (Table S1) were purified by cesium chloride density gradient centrifugation. Plasmid DNA was linearized with NdeI and radioactively labeled at the 5′ end.
DNA Unwinding, χ-Specific ssDNA Fragment Production, Joint Molecule Formation, Reversible Inactivation, and Single-Molecule Visualization Assays.
The following assays were performed as described previously: DNA unwinding (4), production of χ-specific ssDNA fragments (9, 14), joint molecule formation (3, 9, 14), and reversible inactivation by χ (26). Unless indicated otherwise, reaction conditions were 25 mM Tris acetate (pH 7.5), 2 mM Mg(OAc)2, 1 mM ATP, 1 mM DTT, 10 μM (nucleotides) linear pBR322 dsDNA (2.25-nM ends), and 2 μM SSB at 37 °C. Other than reversible inactivation, assays contained 0.1 nM RecBCD, equivalent concentrations (helicase units) of RecC-channel mutants, or 10 nM RecBC. The reaction products were separated on a 1% (wt/vol) TAE agarose gel at 600 V·h, visualized, and quantified using an Amersham Biosciences Storm 840 PhosphorImager; product formation was normalized for the extent of DNA unwinding.
The translocation on single molecules of λ DNA was visualized as previously described (15, 17, 30, 42). For the single-molecule assays, the reaction conditions were 45 mM NaHCO3 (pH 8.2), 20% (wt/vol) sucrose, 50 mM DTT, 1 mM ATP, 2 mM magnesium acetate, and 20 nM YOYO-1 at 29 °C. Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the following institutions: grants from the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad, The Naito Foundation, the Kato Memorial Bioscience Foundation, the Takeda Foundation, the Sumitomo Foundation; Grants-in-Aid for Scientific Research from both the Japan Society for the Promotion of Science and Ministry of Education, Culture, Sports, Science and Technology (to N.H.); a Royal Society and Wellcome Trust grant (to M.S.D.); and National Institutes of Health Grant GM41347 (to S.C.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206081109/-/DCSupplemental.
References
- 1.Kowalczykowski SC, Dixon DA, Eggleston AK, Lauder SD, Rehrauer WM. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillingham MS, Kowalczykowski SC. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol Mol Biol Rev. 2008;72:642–671. doi: 10.1128/MMBR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DG, Kowalczykowski SC. The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a χ-regulated manner. Cell. 1997;90:77–86. doi: 10.1016/s0092-8674(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 4.Roman LJ, Kowalczykowski SC. Characterization of the helicase activity of the Escherichia coli RecBCD enzyme using a novel helicase assay. Biochemistry. 1989;28:2863–2873. doi: 10.1021/bi00433a018. [DOI] [PubMed] [Google Scholar]
- 5.Roman LJ, Kowalczykowski SC. Characterization of the adenosinetriphosphatase activity of the Escherichia coli RecBCD enzyme: Relationship of ATP hydrolysis to the unwinding of duplex DNA. Biochemistry. 1989;28:2873–2881. doi: 10.1021/bi00433a019. [DOI] [PubMed] [Google Scholar]
- 6.Roman LJ, Eggleston AK, Kowalczykowski SC. Processivity of the DNA helicase activity of Escherichia coli recBCD enzyme. J Biol Chem. 1992;267:4207–4214. [PubMed] [Google Scholar]
- 7.Dillingham MS, Spies M, Kowalczykowski SC. RecBCD enzyme is a bipolar DNA helicase. Nature. 2003;423:893–897. doi: 10.1038/nature01673. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AF, Smith GR. RecBCD enzyme is a DNA helicase with fast and slow motors of opposite polarity. Nature. 2003;423:889–893. doi: 10.1038/nature01674. [DOI] [PubMed] [Google Scholar]
- 9.Dixon DA, Kowalczykowski SC. The recombination hotspot χ is a regulatory sequence that acts by attenuating the nuclease activity of the E. coli RecBCD enzyme. Cell. 1993;73:87–96. doi: 10.1016/0092-8674(93)90162-j. [DOI] [PubMed] [Google Scholar]
- 10.Lam ST, Stahl MM, McMilin KD, Stahl FW. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974;77:425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianco PR, Kowalczykowski SC. The recombination hotspot Chi is recognized by the translocating RecBCD enzyme as the single strand of DNA containing the sequence 5′-GCTGGTGG-3′. Proc Natl Acad Sci USA. 1997;94:6706–6711. doi: 10.1073/pnas.94.13.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor AF, Schultz DW, Ponticelli AS, Smith GR. RecBC enzyme nicking at Chi sites during DNA unwinding: location and orientation-dependence of the cutting. Cell. 1985;41:153–163. doi: 10.1016/0092-8674(85)90070-4. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DG, Kowalczykowski SC. The recombination hot spot χ is a regulatory element that switches the polarity of DNA degradation by the RecBCD enzyme. Genes Dev. 1997;11:571–581. doi: 10.1101/gad.11.5.571. [DOI] [PubMed] [Google Scholar]
- 14.Dixon DA, Kowalczykowski SC. Homologous pairing in vitro stimulated by the recombination hotspot, Chi. Cell. 1991;66:361–371. doi: 10.1016/0092-8674(91)90625-9. [DOI] [PubMed] [Google Scholar]
- 15.Spies M, et al. A molecular throttle: The recombination hotspot χ controls DNA translocation by the RecBCD helicase. Cell. 2003;114:647–654. doi: 10.1016/s0092-8674(03)00681-0. [DOI] [PubMed] [Google Scholar]
- 16.Handa N, Bianco PR, Baskin RJ, Kowalczykowski SC. Direct visualization of RecBCD movement reveals cotranslocation of the RecD motor after χ recognition. Mol Cell. 2005;17:745–750. doi: 10.1016/j.molcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Spies M, Amitani I, Baskin RJ, Kowalczykowski SC. RecBCD enzyme switches lead motor subunits in response to χ recognition. Cell. 2007;131:694–705. doi: 10.1016/j.cell.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold DA, Kowalczykowski SC. Facilitated loading of RecA protein is essential to recombination by RecBCD enzyme. J Biol Chem. 2000;275:12261–12265. doi: 10.1074/jbc.275.16.12261. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DG, Churchill JJ, Kowalczykowski SC. A single mutation, RecB(D1080A,) eliminates RecA protein loading but not Chi recognition by RecBCD enzyme. J Biol Chem. 1999;274:27139–27144. doi: 10.1074/jbc.274.38.27139. [DOI] [PubMed] [Google Scholar]
- 20.Singleton MR, Dillingham MS, Gaudier M, Kowalczykowski SC, Wigley DB. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature. 2004;432:187–193. doi: 10.1038/nature02988. [DOI] [PubMed] [Google Scholar]
- 21.Schultz DW, Taylor AF, Smith GR. Escherichia coli RecBC pseudorevertants lacking chi recombinational hotspot activity. J Bacteriol. 1983;155:664–680. doi: 10.1128/jb.155.2.664-680.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handa N, Ohashi S, Kobayashi I. Clustering of chi sequence in Escherichia coli genome. Microb Comp Genomics. 1997;2:287–298. doi: 10.1089/omi.1.1997.2.287. [DOI] [PubMed] [Google Scholar]
- 23.Arnold DA, Handa N, Kobayashi I, Kowalczykowski SC. A novel, 11 nucleotide variant of χ, χ*: One of a class of sequences defining the Escherichia coli recombination hotspot χ. J Mol Biol. 2000;300:469–479. doi: 10.1006/jmbi.2000.3861. [DOI] [PubMed] [Google Scholar]
- 24.Handa N, et al. Molecular determinants responsible for recognition of the single-stranded DNA regulatory sequence, χ, by RecBCD enzyme. Proc Natl Acad USA. 2012;109:8901–8906. doi: 10.1073/pnas.1206076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon DA, Kowalczykowski SC. Role of the Escherichia coli recombination hotspot, χ, in RecABCD-dependent homologous pairing. J Biol Chem. 1995;270:16360–16370. doi: 10.1074/jbc.270.27.16360. [DOI] [PubMed] [Google Scholar]
- 26.Dixon DA, Churchill JJ, Kowalczykowski SC. Reversible inactivation of the Escherichia coli RecBCD enzyme by the recombination hotspot χ in vitro: Evidence for functional inactivation or loss of the RecD subunit. Proc Natl Acad Sci USA. 1994;91:2980–2984. doi: 10.1073/pnas.91.8.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng KC, Smith GR. Recombinational hotspot activity of Chi-like sequences. J Mol Biol. 1984;180:371–377. doi: 10.1016/s0022-2836(84)80009-1. [DOI] [PubMed] [Google Scholar]
- 28.Cheng KC, Smith GR. Cutting of chi-like sequences by the RecBCD enzyme of Escherichia coli. J Mol Biol. 1987;194:747–750. doi: 10.1016/0022-2836(87)90252-x. [DOI] [PubMed] [Google Scholar]
- 29.Schultz DW, Swindle J, Smith GR. Clustering of mutations inactivating a Chi recombinational hotspot. J Mol Biol. 1981;146:275–286. doi: 10.1016/0022-2836(81)90388-0. [DOI] [PubMed] [Google Scholar]
- 30.Bianco PR, et al. Processive translocation and DNA unwinding by individual RecBCD enzyme molecules. Nature. 2001;409:374–378. doi: 10.1038/35053131. [DOI] [PubMed] [Google Scholar]
- 31.Liu B. Davis: Univ of California; 2010. Single molecule study on the mechanism of χ-regulation of RecBCD activities. PhD thesis. [Google Scholar]
- 32.Taylor AF, Smith GR. RecBCD enzyme is altered upon cutting DNA at a chi recombination hotspot. Proc Natl Acad Sci USA. 1992;89:5226–5230. doi: 10.1073/pnas.89.12.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chédin F, Ehrlich SD, Kowalczykowski SC. The Bacillus subtilis AddAB helicase/nuclease is regulated by its cognate Chi sequence in vitro. J Mol Biol. 2000;298:7–20. doi: 10.1006/jmbi.2000.3556. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Yang W. UvrD helicase unwinds DNA one base pair at a time by a two-part power stroke. Cell. 2006;127:1349–1360. doi: 10.1016/j.cell.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velankar SS, Soultanas P, Dillingham MS, Subramanya HS, Wigley DB. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 36.Chédin F, Handa N, Dillingham MS, Kowalczykowski SC. The AddAB helicase/nuclease forms a stable complex with its cognate χ sequence during translocation. J Biol Chem. 2006;281:18610–18617. doi: 10.1074/jbc.M600882200. [DOI] [PubMed] [Google Scholar]
- 37.Wong CJ, Rice RL, Baker NA, Ju T, Lohman TM. Probing 3′-ssDNA loop formation in E. coli RecBCD/RecBC-DNA complexes using non-natural DNA: A model for “Chi” recognition complexes. J Mol Biol. 2006;362:26–43. doi: 10.1016/j.jmb.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Iwata S, Kamata K, Yoshida S, Minowa T, Ohta T. T and R states in the crystals of bacterial L-lactate dehydrogenase reveal the mechanism for allosteric control. Nat Struct Biol. 1994;1:176–185. doi: 10.1038/nsb0394-176. [DOI] [PubMed] [Google Scholar]
- 39.Saikrishnan K, Griffiths SP, Cook N, Court R, Wigley DB. DNA binding to RecD: Role of the 1B domain in SF1B helicase activity. EMBO J. 2008;27:2222–2229. doi: 10.1038/emboj.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spies M, Dillingham MS, Kowalczykowski SC. Translocation by the RecB motor is an absolute requirement for χ-recognition and RecA protein loading by RecBCD enzyme. J Biol Chem. 2005;280:37078–37087. doi: 10.1074/jbc.M505521200. [DOI] [PubMed] [Google Scholar]
- 41.Spies M, Kowalczykowski SC. The RecA binding locus of RecBCD is a general domain for recruitment of DNA strand exchange proteins. Mol Cell. 2006;21:573–580. doi: 10.1016/j.molcel.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Amitani I, Liu B, Dombrowski CC, Baskin RJ, Kowalczykowski SC. Watching individual proteins acting on single molecules of DNA. Methods Enzymol. 2010;472:261–291. doi: 10.1016/S0076-6879(10)72007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.