Abstract
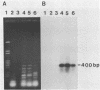
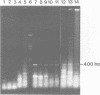
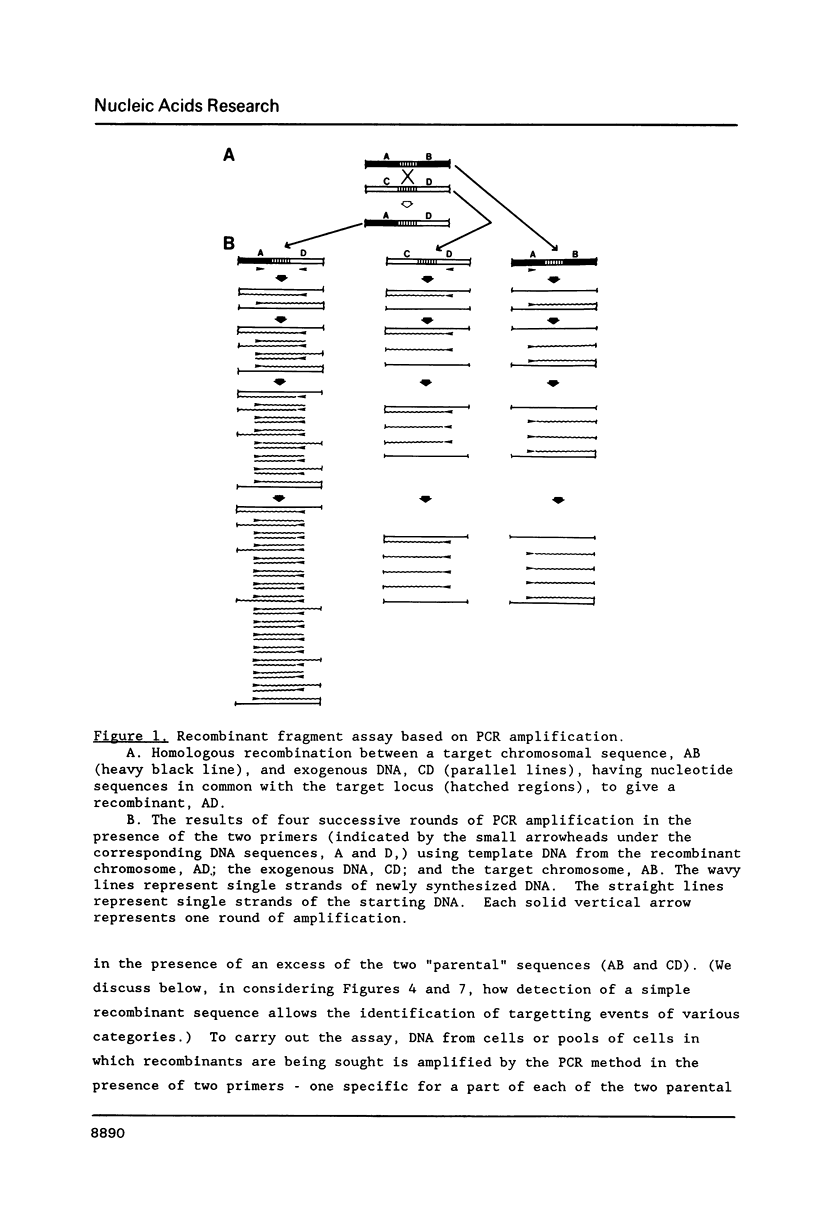
The modification of chromosomal genes by homologous recombination between exogenous DNA and a target locus provides a powerful approach to the study of gene function. One of the current limitations of this gene targetting is the difficulty of identifying cells containing the correctly modified target locus when the modified gene is not amenable to either direct or indirect selection. We here describe a procedure for identifying correctly modified cells that depends on amplifying by the polymerase chain reaction (PCR) predictable fragments of DNA present only in the desired recombinants. This recombinant fragment assay can detect targetted modification using only a few cells, either alone or mixed with tens of thousands of unmodified cells; it does not depend on the phenotype of the modified gene, or on its expression in the target cells. The PCR amplification needed for the procedure is carried out with a heat stable polymerase and a simple automatic temperature-shift apparatus which is described.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987 Dec 10;330(6148):576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Folger K., Thomas K., Capecchi M. R. Analysis of homologous recombination in cultured mammalian cells. Cold Spring Harb Symp Quant Biol. 1984;49:123–138. doi: 10.1101/sqb.1984.049.01.016. [DOI] [PubMed] [Google Scholar]
- Gregg R. G., Smithies O. Targeted modification of human chromosomal genes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1093–1099. doi: 10.1101/sqb.1986.051.01.127. [DOI] [PubMed] [Google Scholar]
- Kaledin A. S., Sliusarenko A. G., Gorodetskii S. I. Vydelenie i svoistva DNK-polimerazy is ekstremal'no-termofil'noi bakterii Thermus Aquaticus YT1. Biokhimiia. 1980 Apr;45(4):644–651. [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Chang K. S., Cabanillas F., Freireich E. J., Trujillo J. M., Stass S. A. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987 Jul 10;237(4811):175–178. doi: 10.1126/science.3110950. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Homologous recombination in mouse L cells. Cold Spring Harb Symp Quant Biol. 1984;49:139–149. doi: 10.1101/sqb.1984.049.01.017. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Recombination in mouse L cells between DNA introduced into cells and homologous chromosomal sequences. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1391–1395. doi: 10.1073/pnas.82.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Rollo F., Amici A., Salvi R. A simple and low cost DNA amplifier. Nucleic Acids Res. 1988 Apr 11;16(7):3105–3106. doi: 10.1093/nar/16.7.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. Extrachromosomal and chromosomal gene conversion in mammalian cells. Mol Cell Biol. 1986 May;6(5):1608–1614. doi: 10.1128/mcb.6.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Berg P. Homologous recombination between defective neo genes in mouse 3T6 cells. Cold Spring Harb Symp Quant Biol. 1984;49:171–181. doi: 10.1101/sqb.1984.049.01.020. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Smithies O., Koralewski M. A., Song K. Y., Kucherlapati R. S. Homologous recombination with DNA introduced into mammalian cells. Cold Spring Harb Symp Quant Biol. 1984;49:161–170. doi: 10.1101/sqb.1984.049.01.019. [DOI] [PubMed] [Google Scholar]
- Song K. Y., Schwartz F., Maeda N., Smithies O., Kucherlapati R. Accurate modification of a chromosomal plasmid by homologous recombination in human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6820–6824. doi: 10.1073/pnas.84.19.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]