Abstract
Evolution by natural selection, the unifying theory of all biological sciences, provides a basis for understanding how phenotypic variability is generated at all levels of organization from genes to behavior. However, it is important to distinguish what is the target of selection vs. what is transmitted across generations. Physical traits, behaviors, and the extended phenotype are all selected features of an individual, but genes that covary with different aspects of the targets of selection are inherited. Here we review the variability in cortical organization, morphology, and behavior that have been observed across species and describe similar types of variability within species. We examine sources of variability and the constraints that limit the types of changes that evolution has and can produce. Finally, we underscore the importance of how genes and genetic regulatory networks are deployed and interact within an individual, and their relationship to external, physical forces within the environment that shape the ultimate phenotype.
Evolution is the change in heritable, phenotypic characteristics within a population that occurs over successive generations. The notion that biological life evolves and that animal forms descend from ancient predecessors has been considered for centuries and, in fact, predates Aristotle (1). However, Charles Darwin was the first to articulate a scientific argument based on extensive observations for a theory of evolution through natural selection. Darwin’s theory contains three basic tenets: individuals within a group are variable, variations are heritable, and not all individuals survive (2). Survival is based on selective advantages that particular phenotypic characteristics or behaviors confer to some individuals within a given environmental context. Although in Darwin’s time our understanding of the brain was in its infancy and Mendel’s Laws of Inheritance were little appreciated, Darwin’s assertions regarding evolution through natural selection of adaptive traits, was, and still is, compelling.
Recently our understanding of the mechanisms underlying evolution has become more sophisticated, and we appreciate that slight variations in gene sequence can be correlated with alterations of traits and behaviors within and across species. However, an important but often overlooked distinction is the difference between the targets of selection (i.e., phenotypic variations) vs. what natural selection passes on to the next generation (i.e., genes). Although genes are the heritable part of the equation and have a causal, although not always direct, link with some characteristic of the phenotype, genes are not the targets of selection. Genes are indirectly selected for because they covary with the targets of selection, and if the target of selection is adaptive, then genes or portions of the genome replicate and produce a long line of descendants. The direct target of selection is multilayered but can be thought to center around the individual and the unique phenotypic characteristics and behaviors that it displays. These characteristics include external morphology such as color, size, jaw configuration, digit length, and bone density, to name a few. This physical variability in the phenotype is also accompanied by variability in behavior, such as utilization of individual specialized body parts, as well as more complex whole-animal behavior such as intraspecies communication. Based on the assumption that the gene’s success is due not only to the individual’s success but to its effects on the world, Dawkins (3) proposed the idea of an “extended phenotype,” wherein a gene can find its expression in the body of the next generation or in a created environment that perpetuates its success. For example, bowers built by bower-birds are variable and have variable success in attracting mates. Inasmuch as the structure of the bower is linked to the phenotypic expression of some behavior that has causal links to one or several genes, the bower is part of an extended phenotype of the bower-bird. Thus, phenotypic expression can occur outside of the individual’s body and include inanimate objects used for niche construction and can even include the social niche constructed by differential behaviors of individuals within a population. Because the measure of evolutionary success is reproduction, it follows that the targets of selection must also include covert features of the phenotype that keep the individual alive long enough to reproduce, such as differential resistance to infection or adeptness at reading social cues.
Although our focus is how brains are altered through the course of evolution, brains, like genes, are not the direct targets of selection. Genes are the heritable components that covary with aspects of brain morphology, connectivity, and function, and in this context, provide a scaffold for brain organization. The brain in turn generates behavior. Ultimately, it is the behavior of a phenotypically unique individual along with its extended phenotype that are the direct targets of selection. Thus, although genes (not individuals) replicate themselves through generations, their link to selection is indirect and convoluted. Of course, an important question is how genes and aspects of brain organization covary with each other and with the targets of selection. Associated questions include these: How variable are features of brain organization? How variable is gene expression and gene deployment during development within a population? In addition, what factors contribute to this multilayered variability of the organism?
We address these questions from a comparative perspective. First we examine aspects of the cortical phenotype that are ubiquitous across species because of inheritance from a common ancestor (homology). We then describe how these characteristics vary across species. We contend that the ways in which homologous features vary provide an important insight into the more subtle variations that might be present in individuals within a population. Finally we discuss the external and internal mechanisms that give rise to cross-species and within-species variation and the constraints these forces exert on evolution.
Phenotypic Similarity and Variability Across Species
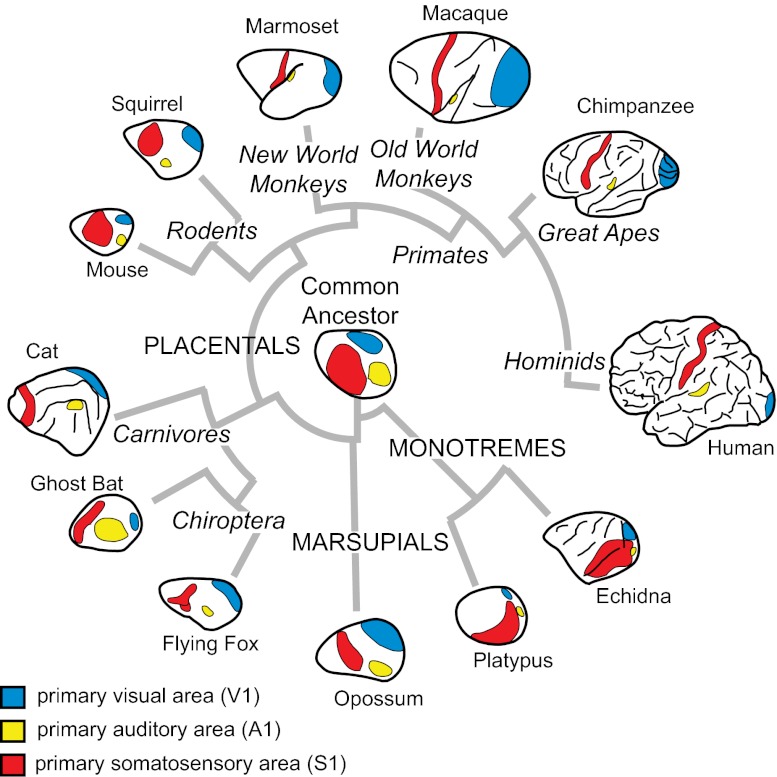
There is a general plan of neocortical organization that has been observed in all mammals investigated. This includes a constellation of cortical fields involved in sensory processing, such as primary visual (V1), somatosensory (S1), and auditory (A1) areas (Fig. 1; Table S1 for abbreviations) (4). These homologous fields share similar patterns of connectivity from both the thalamus and other cortical fields, a common architectonic appearance, and neurons within these fields have similar properties (5). These observed similarities allow us to infer the cortical organization of the common ancestor of all mammals (Fig. 1) and underscore the constraints imposed on the evolving nervous system. For example, the visual system in blind mole rats is used only for circadian functions, and not for visual discrimination. Yet, V1 is still present, as are geniculocortical connections (6, 7). However, V1 is greatly reduced in size, neurons in V1 respond to auditory stimulation, and subcortical connections of auditory pathways have been rerouted to the lateral geniculate (8–10). Comparative studies also allow us to appreciate deviations from this organization that have occurred over evolution.
Fig. 1.
Cladogram of phylogenetic relationships for the major subclasses of mammals. All species examined have a constellation of cortical fields that includes primary somatosensory, visual, and auditory areas (see color codes). However, their relative size and location has been altered in different species.
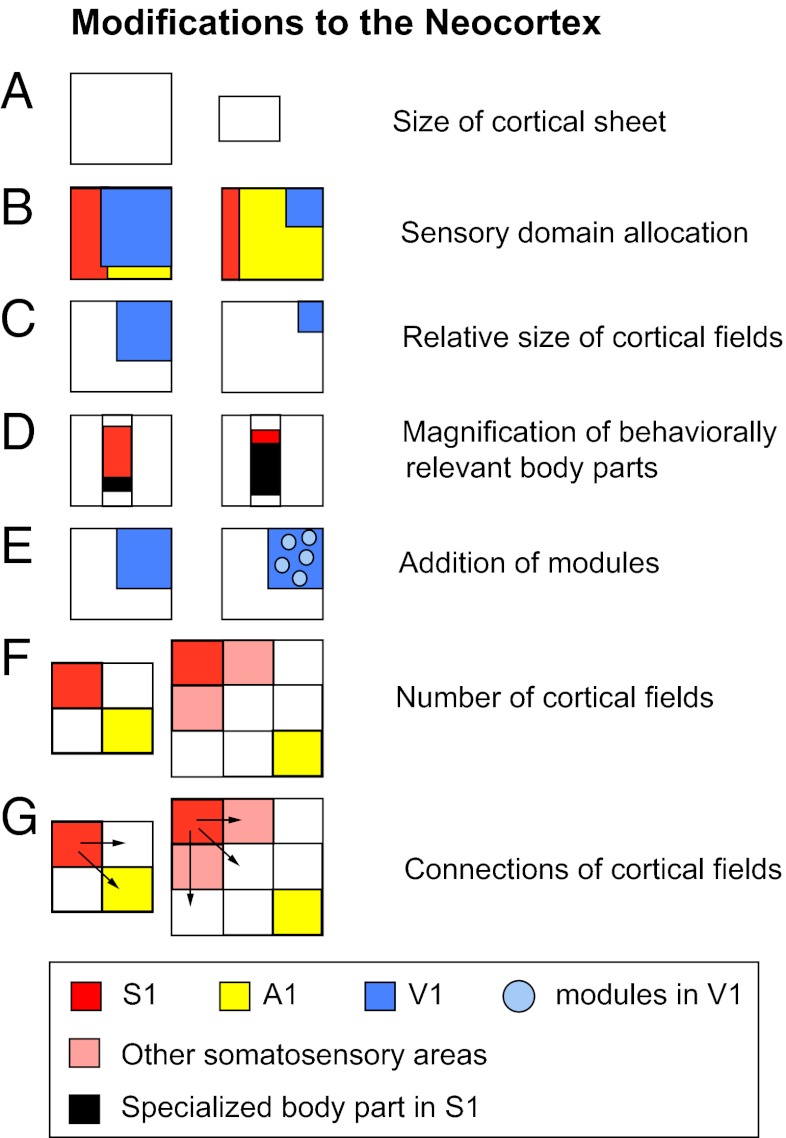
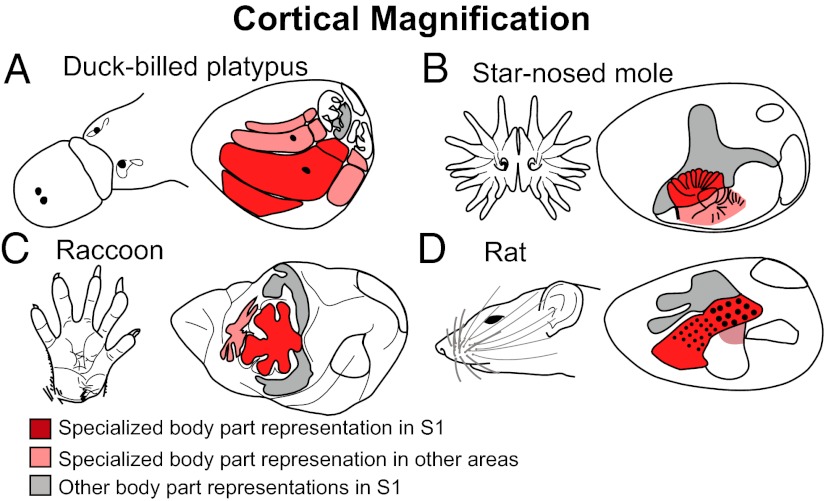
Surprisingly, the systems-level alterations to the mammalian neocortex are limited (Fig. 2). One among these is a change in sensory domain allocation. This specialization begins in the periphery with a relative increase in the innervation of a sensory effector organ, followed by an increase in the size of subcortical structures that receive inputs from this effector organ, an increase in the amount of thalamic territory to which these structures project, and ultimately an expansion in the amount of neocortex devoted to processing inputs from a particular sensory system (11–13). Cortical fields within a sensory domain can also vary, both in their overall size and in the size of the representation (or cortical magnification) of specialized morphological features, such as the nose of a star-nosed mole or the bill of a platypus (Fig. 3). Cortical fields can vary in connectivity with cortical and subcortical structures, and the number of cortical fields varies across species. The persistence of both a common plan of organization, even in the absence of use, and the limited ways in which this plan has been independently altered suggest that there are large constraints imposed on evolving nervous systems.
Fig. 2.
Schematic of the types of cross-species, systems-level modifications that have been observed in the neocortex. The outline of the boxes indicates the entire cortical sheet, and smaller boxes within represent either cortical domains (B), cortical fields (C and E–G), or representations within cortical fields (D). These same types of changes have been observed across individuals within a species, but they are often less dramatic.
Fig. 3.
Examples of cortical magnification for (A) the bill of the platypus, (B) the nose tentacles of the star-nosed mole, (C) the hand of the raccoon, and (D) the whiskers of the rat. The representation of the specialized morphological structures in S1 is red and in other cortical areas is pink. Gray indicates the representation of the rest of the body in S1.
Species also vary in the peripheral morphology of homologous body parts and the use of these structures. A good example is the glabrous hand of humans, the pectoral fin of a dolphin, and the wing of a bat (Fig. 4). The hands of humans have undergone several important changes, including alterations in the size of the distal, middle, and proximal phalanges. The carpal and metacarpal joints, the articulation between the first and second carpals, and the metacarpophalangeal joints underwent significant change, as did the size and position of associated ligaments (14). The distal digit tips also evolved a high concentration of tactile receptors with a high innervation density. These transformations allow for an expanded repertoire of grips, including a precision grip. Although these adaptations are proposed to have evolved for tool use (15), in modern humans the hand is also used for playing instruments and other nontool-related activities.
Fig. 4.
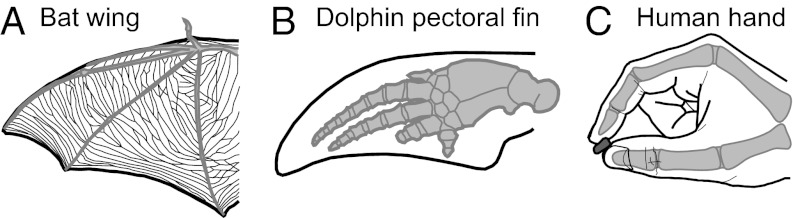
(A) Wing of a bat, (B) pectoral fin of a dolphin, and (C) hand of a human are examples of homologous morphological structures. Although they are used for very different purposes, they are organized around the same basic skeletal frame (in gray).
In dolphins the homolog of the primate hand is the pectoral fin. The fin has undergone several important morphological changes including a transition from bone to soft cartilaginous tissue, elongated digits with additional joints (hyperphalangy), atrophied triceps, immobilization of most of the joints, and lack of most connective tissue structures (16). These alterations to the forelimb allow for different properties and functions associated with locomotion in water, such as increased lift, reduced drag, and the ability of execute turns and braking (17). However, recent studies indicate that fins are also used in “flipper rubbing,” which involves the physical contact between one dolphin’s fin and another dolphin’s body or fin and likely has important social functions (18).
Finally, in bats, the wing is the homolog of the hand and fin. Digits 2–5 form the wing, and digit 1 is unattached from the rest of the wing and used for climbing. Although bats have little to no ability to grip or manipulate objects with this highly derived structure, wings are of course well adapted for self-propelled flight (see ref. 19 for review). Between the elongated digits, elastin-collagen bands or membranes have evolved. These are covered with small, specialized receptor assemblies, termed touch domes, which are exquisitely sensitive to very small changes in air pressure (20). These structures are thought to be used for sensing wing membrane strain during sharp turns, monitoring boundary layer airflow, and locating, tracking, and assisting in the transfer of wing-captured prey to the mouth (19).
In species in which the neocortex has been explored and related to such extraordinary morphological specializations, corresponding alterations have been noted, including cortical magnification within sensory areas (e.g. refs. 21–23), and in some instances an extreme magnification in higher-order cortical areas, such as area 5 in macaque monkeys (see Fig. 6B) (24). Alterations in neural response properties [e.g., rapidly and slowly adapting direction selectivity (20, 25, 26)], architectonic appearance (e.g., ref. 27), and connectivity have also been observed. Thus, changes in aspects of cortical organization covary with alterations in peripheral morphology and the very unique behaviors associated with this morphology.
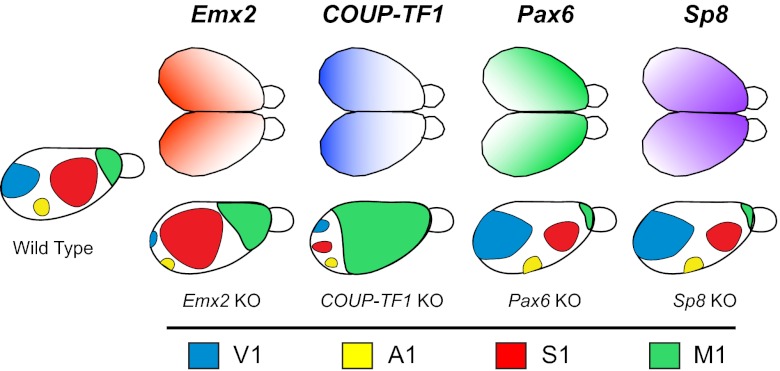
Fig. 6.
Graded patterns of expression of transcription factors (Upper) involved in aspects of arealization such as location and size of cortical fields. Knockout (KO; Lower) of these transcription factors generates radically different sizes and positions of cortical fields compared with wild-type mice (Left). Cortical fields are color-coded (see key at bottom). Adapted from ref. 55.
One can also compare body parts that are analogous, or have the same function. In human and nonhuman primates the hand is one of the main effector organs used to explore nearby objects or space. Other species use different effector organs for exploration, such as the platypus’s bill, the rat’s vibrissae, and the nose of the star-nosed mole. Although these structures may not be homologous they have a similar function, and in turn they share similar features of organization of the neocortex, which have emerged independently. In addition to cortical magnification of the main effector organ in different sensory areas (Fig. 3), similar but independently evolved patterns of connectivity have emerged between motor cortex and posterior parietal cortex, despite the differences in body parts used to explore the immediate environment.
Perhaps the most compelling example of this phenomenon is the independent evolution of an opposable thumb and precision grip in Old World monkeys and only one New World monkey, the cebus monkey. A repertoire of behaviors associated with this hand morphology includes complex manipulation of objects and tool use in the wild. In terms of neural organization, cebus monkeys have independently evolved a relatively larger cortical sheet, such that their encephalization (28, 29) resembles that of distantly related Old World monkeys rather than their closely related sister groups, New World monkeys. In addition, they have independently evolved direct corticospinal projections to the ventral horn motor neurons that project to muscles of the digits (30) and have also independently evolved a cortical field, area 2, associated with processing proprioceptive inputs (31). This example illustrates two important points. First, hand morphology associated with specialized use covaries with cortical sheet size, cortical field addition, and corticospinal connections. Second, the independent evolution of these striking features of the morphological, behavioral, and cortical phenotype suggests that there are strong constraints on how complex brains and behaviors evolve.
The types of cross-species comparisons described above inform us about what types of phenotypic changes have occurred, how homologous aspects of brain organization vary across species, and clearly indicate that evolution of brain, morphology, and behavior is constrained. However, they do not tell us how these phenotypic transitions occur and what factors contribute to or constrain phenotype diversity. Because cross-species variability had to begin as within-species variability, we can understand the process of speciation by looking at individual variability.
Within-Species Variability
Phenotypic variability within a population is the cornerstone of evolution by natural selection, yet most studies of neural organization and connectivity underscore the similarities across individuals within a group rather than their differences. As a result, there are few studies that directly examine and quantify naturally occurring differences in features of nervous system organization within a species. As noted in our introduction, we reasoned that the most likely place to observe measurable within-species differences is in the features of organization that demonstrate dramatic variability across species, like cortical field size and sensory domain allocation, and that are related to or covary with the targets of selection.
At a gross morphological level, animals with a large neocortex show variations in the size and configuration of sulcal patterns. Within-species variation is also observed in the size of cortical fields in rats (32), opossums (33), squirrels (34), and both nonhuman (35) and human primates (36). Intraspecies comparisons of the size of V1 in humans and nonhuman primates reveal a high degree of variability, ranging from 13% to 27% with respect to the entire visual cortex (see ref. 37 for review). In rats, Riddle and Purves (32) observed that both the overall size of S1 and the proportion of cortex devoted to different body parts, such as the lip, barrel field, and forepaw, varied significantly across animals and even across hemispheres in the same rat. Our laboratory directly examined intraspecies variability in the primary sensory areas of opossums (Monodelphis domestica) and measured and compared their sizes across hemispheres for each animal and across individuals within a species. We found that the size of primary cortical areas was similar across hemispheres but varied considerably across individuals (33). Based on recent comparative studies in rodents, we propose this variability was mediated by environmental influences. Specifically, wild-caught Rattus norvegicus had a large V1 and a greater amount of variability in cortical field size than their laboratory counterparts (34). Although these studies did not demonstrate large variability in overall cortical sheet size, the amount of cortex that was allocated to individual cortical fields was variable.
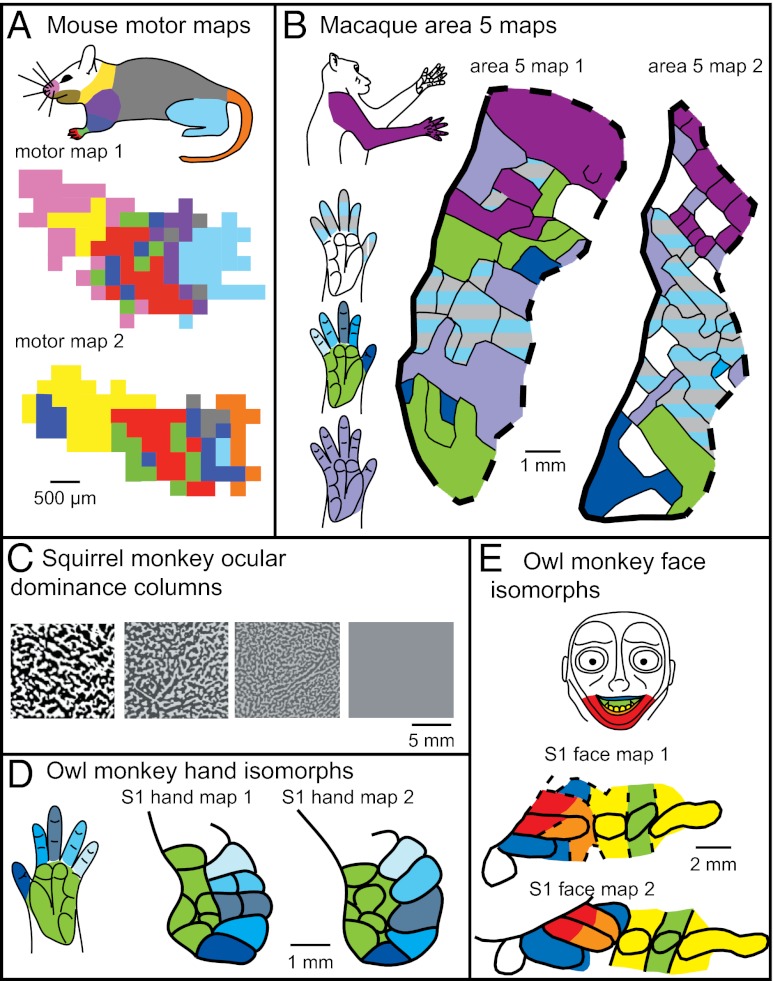
Within-species variability has also been observed in the internal organization of both sensory and motor maps. For example, Albus and Beckman (38) observed notable differences in the visuotopic organization of V2 and V3 in cats. Variability in somatotopic organization has been reported for the hand representation in primates (39). In addition, although not always directly measured or the focus of a study, examination of somatotopic maps generated from functional mapping studies indicates that the representation of different portions of the body in adjacent somatosensory areas, such as 3a, 1, and 2, is variable across individuals within a primate species (e.g., refs. 22 and 40). The differences in the somatotopic organization of these sensory areas are clearly present but not extreme. However, the within-species variability in topographic organization of higher-order areas, such as posterior parietal area 5, is remarkable (Fig. 5B) (e.g., refs. 24 and 40). Finally, when similar microstimulation parameters are used across animals, the functional organization of primary motor cortex (M1) is highly variable within many species, including mice (41) (Fig. 5A), rats (42), squirrels (43), and owl monkeys (44).
Fig. 5.
Examples of intraspecies variability for (A) motor cortex in mice (adapted from ref. 41), (B) area 5 in macaque monkeys (adapted from ref. 24), (C) ocular dominance columns in squirrel monkeys (adapted from ref. 47), (D) S1 architectonic isomorphs in the owl monkey face representation (adapted from ref. 45), and (E) hand representation (adapted from ref. 46). In mice, motor maps are grossly topographically organized but are locally fractured. A depicts motor maps from two different individual mice. Each small square represents a microstimulation location that evoked a movement of a particular body part, color-coded according to the colored mouse body at top. In macaques (B), maps of posterior parietal area 5 are highly variable and are fractured. Area 5 also demonstrates an extreme magnification of the forelimb. Color codes of the hand and arm correspond to their representations in cortical maps. In squirrel monkeys (C) ocular dominance columns vary from highly distinct (leftmost square) to nonexistent (far right square). Finally, the myeloarchitectonically distinct modules of the face (D) and hand (E) representations in S1 of owl monkeys vary in their specific size and shape between individual animals. Color codes of the hand and face correspond to their representations in cortical maps.
Individual differences have also been observed in smaller units of organization within a cortical field, termed modules. For example, in rats the succinic dehydroxinase-rich barrels and barrel-like structures that represent different body parts vary in size between individuals (32). In owl monkeys and squirrel monkeys, myelin-rich isomorphs associated with the oral structures and digits vary in size (Fig. 5 D and E) (45, 46), as do the digit isomorphs for the digits in macaque monkeys, particularly D1 (27). Ocular dominance columns in V1 of squirrel monkeys can show extreme variability (47). In some monkeys they are discrete, stripe-like bands, in others they are smaller and less distinct, and in some monkeys they are nonexistent (Fig. 5C).
As noted in the previous section, homologous fields vary in their patterns of connectivity across phyla and even across species within an order such as rodents (see ref. 48). Connectional studies of the neocortex in any mammal share two common features. First, if the sources of technical variability are minimized (e.g., placement of injection of anatomical tracer, age, rearing condition), the majority of connections for a given cortical field are similar across individuals. Second, the variability that does exist takes two forms: alterations in the density of common inputs and the presence of novel but sparse connections to some structures or areas in different individuals.
Recent studies also demonstrate that cellular composition varies within a population. For example, within the cortex of primates the total number of neurons varies between individuals by a factor of ∼1.3 (calculated from ref. 49). In another study, wild-caught rats (Rattus norvegicus) were found to have a larger percentage of neurons and a greater density of neurons in V1 compared with laboratory rats of the same species (50).
Some of the within-species variations in cortical organization described above are undoubtedly linked with behavior, although the relationship is often nonlinear and indirect. However, examination of certain aspects of organization, such as the size and cellular composition of the primary visual area, are correlated with diel patterns and lifestyle of an animal. These, in turn, are linked to alterations in the visual system, such as the emergence of two-cone color vision and a highly laminated lateral geniculate nucleus in the highly visual, diurnal squirrel (see ref. 34 for review). These alterations, which cross multiple levels of organization, provide some insight into the relationship between the brain and behavior. Although these brain–behavior relationships are interesting, there have been few studies of within-species variation that examined how sensory-mediated behavior covaries with some measurable aspect of the cortical phenotype. In contrast, studies of variability in behavior within a population abound.
Some of the best examples of behavioral/neural/genetic variation are in the field of behavioral neuroendocrinology. For example, numerous studies have demonstrated that GnRh (gonadotropin-releasing hormone) regulates reproduction through a cascade of intermediaries. This begins with regulation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion by the anterior pituitary, which in turn stimulates sex steroid production and gametogenesis. These sexual steroids (estrogen and testosterone) then bind to receptors in the brain in regions that regulate sexual behaviors. Important for this review, the volume and pattern of GnRh secretion varies with external cues, such as photoperiod, food availability, stress, and conflict (51, 52), which in turn generates variable release of LH and FSH by the anterior pituitary and so on. Natural variation in genes that regulate this pathway has also been demonstrated in different individuals within populations of deer mice and white-footed mice (51, 53). Thus, variability in the brain and behavior can be generated through external or internal cues.
Thus far, we have discussed features of the cortex such as cortical field size, connectivity, and cellular composition that vary between and within species and are correlated with, and likely covary with, the targets of selection (i.e., behavior). Given that genes or portions of the genome are linked to these neural phenotypic characteristics, which in turn are linked to behavior, it is not surprising that features such as the location, amount, and time of expression of the same gene or gene network are variable across individuals within a population.
Recent studies demonstrate that this variability is due to differential activation of genetic regulatory networks (54). These networks are composed of transcription factors and genes (nodes) as well as regulatory interactions (edges). The level of differential gene expression can be robust (persistent under perturbation) or stochastic (nondeterministic and flexible) and in turn generate phenotypic characteristics that differ in the extent to which they are variable within a population. Stochasticity of gene expression often results in more variable phenotypic characteristics of the individual, whereas robustness of a gene regulatory network often, but not always, results in less variability of a phenotypic characteristic. Not surprisingly, fundamental biological functions, such as the cell cycle, cell growth, and transcription, are generally governed by robust regulatory networks, suggesting that high variability for these key functions is nonadaptive. It seems likely that the basic, ubiquitous mammalian constellation of cortical fields with its homologous patterns of connections is regulated by robust networks, because these fields persist even in the absence of use. Other aspects of organization that are highly variable within and across species are likely stochastically regulated. In fact it has been suggested that there may be “core” gene regulatory networks that are conserved between species and that differential alterations in the nodes or the edges contribute to species-specific differences (54).
What Factors Contribute to Phenotypic Variability?
There are two important factors that contribute to phenotypic variability: genes and external signals, the latter consisting of the distribution of physical stimuli in a particular environmental context. Genes both intrinsic and extrinsic to the neocortex play an important role in shaping different features of cortical organization. Equally important are the patterns of sensory stimuli that the developing organism is exposed to, and by extension, the patterned activity within and across major effectors such as the retina, skin, and cochlea.
Transcription factors such as Emx2, Pax6, and COUP-TFI regulate patterns of cell adhesion molecules (e.g., cadherins; see ref. 55 for review) and are graded in their expression across the developing cortical sheet (Fig. 6). Numerous studies have shown that transcription factors and their downstream target genes covary with aspects of cortical organization, such as cortical field size, location, and connectivity (see ref. 55 for review), and deletion or overexpression of these factors results in changes in gene expression, contractions and expansions in the sizes of cortical fields, and altered patterns of connectivity from the dorsal thalamus (56) (Fig. 6). As we discussed previously, such genetic changes only indirectly affect behavior, the actual target of selection. The relationship between alterations in transcription factors and changes in the direct targets of selection is complex but has been demonstrated to some degree in the mouse. Overexpression of Emx2 increases the size of V1 but decreases the size of somatosensory and motor areas (57, 58). When these mice were tested on sensorimotor tasks that assessed hindlimb and forelimb coordination, they performed significantly worse than wild-type mice. This study establishes a clear link between genes, cortical field size, and behavior and demonstrates how alterations in patterns of expression of transcription factors and their downstream targets can generate relatively large degrees of phenotypic variability in the cortex, which in turn generates variability in the target of selection.
Genes extrinsic to the neocortex can also affect cortical organization. For example, homeobox genes from the Hox family are highly conserved across animals and are involved in forelimb development (59, 60). Comparative studies between mice and bats indicate that expression of these genes is altered during development (61) and thought to be involved in transforming the forelimb into a wing (62, 63). This process is multilayered. Hoxd13 expression is posteriorly shifted in the developing forelimb at later developmental stages in bats compared with mice, which reduces some wing skeletal elements (61). Although bone morphogenic proteins (BMPs) trigger apoptosis of interdigit membranes in mouse fore- and hindlimbs and the bat hindlimb, in the bat forelimb BMPs are inhibited by Gremlin so that interdigit membranes are maintained (64). This reduction in BMPs is accompanied by an increase in Fgf8 in the apical ectodermal ridge and is responsible for the extended proximal to distal growth of the limb in the bat (65). BMP2 triggers proliferation and differentiation of chondroctyes, which increases digit length in bats (63). Thus, the amount, timing, and position of expression of genes during early forelimb development can induce dramatic alterations in the structure of the forelimb. As noted earlier, these alterations in forelimb morphology and the use of the forelimb covary with the size and internal organization of the cortical field. Compared with mice, bats have a larger forelimb representation within S1, and the topographic features of the wing representation within S1 relate uniquely to its altered position while the bat is at rest (21, 66).
Although phenotypic diversity in cortical organization is generated by modifying these intrinsic and extrinsic genetic contingencies, these same contingencies also serve to constrain alterations to the phenotype. The complex relationship between morphogens, the transcription factors they regulate, and in turn the target genes that they regulate, has been well described by O’Leary and Sahara (55). Most of these relationships are contingencies in which the actions of one node in a genetic regulatory network alter the trajectory of another node, which can potentially alter genetic regulatory networks associated with a completely different feature of organization. Such integration limits the magnitude of viable changes that can be made via genetic mechanisms. Although small alterations at early stages of these contingencies (e.g., morphogen or transcriptional factor gradients) can have a large impact on the resultant cortical organization (e.g., change in cortical field size), alterations early in this cascade are also more likely to result in a nonviable phenotype. This is supported by the presence of certain cortical fields in some animals despite the lack of apparent functional use (9), the limited ways in which the cortical phenotype has changed, and the convergent evolution of similar features of organization despite very distant phylogenetic relationships. While we have given many examples of phenotypic diversity in the present review, we could provide an equally compelling argument that this diversity is fairly restricted if one considers all of the possible ways in which information could be processed and behavior generated.
Extrinsic factors also generate phenotypic variability within the cortex. For example, the activity from different sensory effectors during development, and throughout life, affects brain organization. Experiments from our laboratory in short-tailed opossums (Monodelphis domestica) in which both eyes were removed before cortical and subcortical connections were formed demonstrate that all of what would be visual cortex contained neurons that were responsive to somatosensory and/or auditory stimulation. Thus, sensory domain allocation was dramatically altered (67). In addition, architectonically defined V1 was significantly smaller, whereas S1 was significantly larger than in normal animals, and “V1” received altered projections from cortical and subcortical somatosensory and auditory structures (68). Similar results have been observed in anophthalmic mice (69) and blind mole rats (6). In mutant mice in which the cochlea is dysfunctional but the eighth nerve is still present, all of cortex that would normally process auditory inputs contains neurons that respond to visual and somatosensory stimulation, and the size of A1 is significantly reduced, whereas the size of V1 is significantly increased (70). Finally, as noted above, alterations in cortical field size and neuronal density are observed in the same species of rat reared in radically different environments (wild-caught vs. laboratory). Thus, loss of sensory receptor arrays, loss of sensory-driven activity, or reduced patterns of activity can alter cortical domain allocation, cortical field size, connectivity, and neuronal density.
Other studies specifically manipulate the sensory environment in which the animal is reared and examine the effects on neocortical areas. For example, when ferrets are exposed to early training on a single axis of visual motion, neurons in V1 become preferentially responsive to movement along that axis (71). In rats, early and prolonged exposure to a particular auditory tone results in increased cortical magnification for that frequency in A1 (72). These changes in the internal organization of a sensory field and neuron response properties are similar to the types of differences observed across species and can be induced early in development by altering the sensory environment in which the animal develops.
Thus, a high degree of phenotypic variability can be induced without invoking genetic mechanisms that control brain development. The cortex has evolved to match the sensory environment in which it develops and produce highly adaptive behavior for that context. Although we have focused this review on how sensory systems and cortical areas are modified, if one considers both social and cultural influences on the brain as complex patterns of sensory stimuli that groups of brains generate, then the same rules of construction and modification apply. However, as with genes, the environmental factors that generate phenotypic variability also serve to constrain the types of changes that can be made to the brain. For example, although photons can be differentially distributed in an aquatic, cave, or terrestrial environment, they have the same intrinsic properties, are uniformly defined as a discrete quantum of electromagnetic energy, are always in motion, and in a vacuum travel at the speed of light. These immutable characteristics of a stimulus that the nervous system must detect, transduce, and ultimately translate, constrain the evolution and construction of the effector organ that initially captures some portion of the spectrum of this energy, and also impacts how higher-level structures transmit specific information about its presence, magnitude, and dispersal within an environment.
Conclusions
We have discussed phenotypic variability across and within species and conclude that the ways in which animals and brains change are limited and predictable. Further, we show that a specific characteristic, such as the size of a cortical field, can be generated by different genetic mechanisms and/or activity-dependent mechanisms. Thus, similar features of organization that have independently arisen in different lineages may not have similar underpinnings. Examination of variability at multiple levels of organization indicates that although genes are not directly related to a specific behavior, they covary with aspects of body and brain organization, which in turn covary with the targets of selection (Fig. 7). For example, the wing of a bat is constructed in development through complex interactions between genes and morphogens. Slight variations in the amount, location, and timing of these factors can generate phenotypic diversity within a population. The presence of the highly derived wing with its array of specialized touch domes covaries with both the size of the forelimb representation and neural response properties in S1. Together such morphological and cortical specializations are critical for detecting and processing inputs that provide motor cortex with information necessary to produce fine muscle control during self-propelled flight. It is the resulting morphology and behavior, the efficiency with which a bat navigates, captures, and consumes insects using a wing of a given size, shape, tensor properties, and receptor distribution, which are the targets of selection.
Fig. 7.
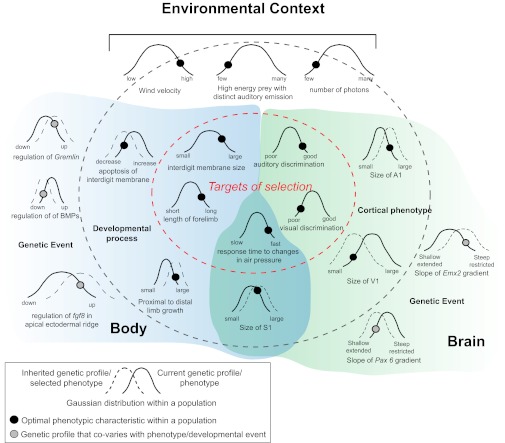
Schematic illustration demonstrating how covaration between the targets of selection, phenotypic organization, and genetic events could lead to inheritance of genes that generate a population of future individuals with a unique combination of phenotypic characteristics. Blue shading corresponds to factors associated with forelimb morphology, and green shading corresponds to factors associated with brain organization. These are not mutually exclusive but interact to some extent (overlapped shading). The Gaussian curves represent the range of naturally occurring variability in a specific characteristic, with narrower curves representing robust characteristics and wider curves representing stochastic characteristics. The black and gray circles represent the location of the optimal characteristic along the current distribution (solid curve). Selection pressures will eventually push the population to a new distribution, centered around the optimal characteristic (dashed curve). In this example our species is an echolocating bat, and our environmental context is illustrated at the top. Some of the targets of selection (Gaussian curves inside the red, dashed oval) would be characteristics of the forelimb that allow for flight, as well as behaviors such as fast response time and good auditory discrimination. Cortical phenotypic characteristics (located between the dark gray and red dashed lines) that underlie auditory and tactile discriminatory ability would include an increase in the size of S1 and A1, as well as an increase in the wing representation within S1. Underlying developmental processes associated with wing formation include a decrease in apoptosis in the interdigit membrane and the growth of the limb. At the far perimeter (far left and far right) of this illustration are the genetic events that covary with aspects of the body and brain phenotypes.
In addition there are genetic regulatory networks in the neocortex that are responsible for providing the scaffold of organization that includes a constellation of cortical fields and their connectional relationships that all mammals share. These networks can vary to produce phenotypic change in cortical field size, relative location, and connectivity within individuals in a population. This in turn generates changes in sensory mediated behaviors, and as in the example above, it is behavior, not genes or features of cortical organization, that are the targets of selection (Fig. 7). Given this complex, multilayered relationship between genes, brains, bodies, the environment, and the targets of selection, the dialect of the current scientific culture, which proposes to study “the gene” for autism, language, memory, or any other class of complex behaviors, is inaccurate and certainly misleading.
Although variability is the cornerstone of evolution, it is difficult to find studies that specifically examine and quantify naturally occurring variability in any aspect of neural organization. As the title indicates, such variability is unwelcome in most studies. We strive to underscore common features or the sameness of our data and reduce the error bars on our histograms. For experimentation purposes, variability is in fact “the bane of our existence.” However, this same variability provides a deep insight into how evolution proceeds and the complex, sometimes tortuous path of phenotypic change. Although the evolution of future forms is not completely known, we can predict the types of changes that will occur and know with certainty that at all levels of organization, there will be variability.
Supplementary Material
Acknowledgments
We thank Dylan Cooke for his many helpful and insightful comments on the manuscript. This work was supported by Grants R01NS035103-13A1 and R21NS071225-02 from the National Institute of Neurological Disorders and Stroke (to L.A.K.).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VI: Brain and Behavior,” held January 19–21, 2012, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vi.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201891109/-/DCSupplemental.
References
- 1.Aristotle , Waterfield R, Bostock D. Physics. Oxford: Oxford Univ Press; 2008. p. 301. [Google Scholar]
- 2.Darwin C. On the Origin of Species by Means of Natural Selection. London: J. Murray; 1859. ix, 1, 502. [Google Scholar]
- 3.Dawkins R. Replicator selection and the extended phenotype. Z Tierpsychol. 1978;47:61–76. doi: 10.1111/j.1439-0310.1978.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 4.Krubitzer L. In search of a unifying theory of complex brain evolution. Ann N Y Acad Sci. 2009;1156:44–67. doi: 10.1111/j.1749-6632.2009.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krubitzer L. The magnificent compromise: Cortical field evolution in mammals. Neuron. 2007;56:201–208. doi: 10.1016/j.neuron.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HM, Herbin M, Nevo E. Visual system of a naturally microphthalmic mammal: The blind mole rat, Spalax ehrenbergi. J Comp Neurol. 1993;328:313–350. doi: 10.1002/cne.903280302. [DOI] [PubMed] [Google Scholar]
- 7.Nemec P, et al. The visual system in subterranean African mole-rats (Rodentia, Bathyergidae): Retina, subcortical visual nuclei and primary visual cortex. Brain Res Bull. 2008;75:356–364. doi: 10.1016/j.brainresbull.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 8.Heil P, Bronchti G, Wollberg Z, Scheich H. Invasion of visual cortex by the auditory system in the naturally blind mole rat. Neuroreport. 1991;2:735–738. doi: 10.1097/00001756-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Bronchti G, et al. Auditory activation of “visual” cortical areas in the blind mole rat (Spalax ehrenbergi) Eur J Neurosci. 2002;16:311–329. doi: 10.1046/j.1460-9568.2002.02063.x. [DOI] [PubMed] [Google Scholar]
- 10.Doron N, Wollberg Z. Cross-modal neuroplasticity in the blind mole rat Spalax ehrenbergi: A WGA-HRP tracing study. Neuroreport. 1994;5:2697–2701. doi: 10.1097/00001756-199412000-00072. [DOI] [PubMed] [Google Scholar]
- 11.Deschênes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: Reciprocity versus parity. Brain Res Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 12.Catania KC, Leitch DB, Gauthier D. A star in the brainstem reveals the first step of cortical magnification. PLoS One. 2011;6:e22406. doi: 10.1371/journal.pone.0022406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catania KC. The sense of touch in the star-nosed mole: From mechanoreceptors to the brain. Philos Trans R Soc Lond B Biol Sci. 2011;366:3016–3025. doi: 10.1098/rstb.2011.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis OJ. Joint remodelling and the evolution of the human hand. J Anat. 1977;123:157–201. [PMC free article] [PubMed] [Google Scholar]
- 15.Marzke MW, Marzke RF. Evolution of the human hand: Approaches to acquiring, analysing and interpreting the anatomical evidence. J Anat. 2000;197:121–140. doi: 10.1046/j.1469-7580.2000.19710121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper LN, Berta A, Dawson SD, Reidenberg JS. Evolution of hyperphalangy and digit reduction in the cetacean manus. Anat Rec (Hoboken) 2007;290:654–672. doi: 10.1002/ar.20532. [DOI] [PubMed] [Google Scholar]
- 17.Reidenberg JS. Anatomical adaptations of aquatic mammals. Anat Rec (Hoboken) 2007;290:507–513. doi: 10.1002/ar.20541. [DOI] [PubMed] [Google Scholar]
- 18.Dudzinski KM, Gregg JD, Ribic CA, Kuczaj SA. A comparison of pectoral fin contact between two different wild dolphin populations. Behav Processes. 2009;80:182–190. doi: 10.1016/j.beproc.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Zook JM. Somatosensory adaptations of flying mammals. In: Kaas J, Krubitzer L, editors. Evolution of Nervous systems. Vol 3. San Diego, CA: Academic Press; 2007. [Google Scholar]
- 20.Sterbing-D’Angelo S, et al. Bat wing sensors support flight control. Proc Natl Acad Sci USA. 2011;108:11291–11296. doi: 10.1073/pnas.1018740108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calford MB, Graydon ML, Huerta MF, Kaas JH, Pettigrew JD. A variant of the mammalian somatotopic map in a bat. Nature. 1985;313:477–479. doi: 10.1038/313477a0. [DOI] [PubMed] [Google Scholar]
- 22.Krubitzer L, Huffman KJ, Disbrow E, Recanzone G. Organization of area 3a in macaque monkeys: Contributions to the cortical phenotype. J Comp Neurol. 2004;471:97–111. doi: 10.1002/cne.20025. [DOI] [PubMed] [Google Scholar]
- 23.Nelson RJ, Sur M, Felleman DJ, Kaas JH. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol. 1980;192:611–643. doi: 10.1002/cne.901920402. [DOI] [PubMed] [Google Scholar]
- 24.Seelke AM, et al. Topographic maps within Brodmann’s area 5 of macaque monkeys. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz S, Crespo P, Romo R. Representation of moving tactile stimuli in the somatic sensory cortex of awake monkeys. J Neurophysiol. 1995;73:525–537. doi: 10.1152/jn.1995.73.2.525. [DOI] [PubMed] [Google Scholar]
- 26.Sur M, Wall JT, Kaas JH. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b of somatosensory cortex in monkeys. J Neurophysiol. 1984;51:724–744. doi: 10.1152/jn.1984.51.4.724. [DOI] [PubMed] [Google Scholar]
- 27.Qi HX, Kaas JH. Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J Comp Neurol. 2004;477:172–187. doi: 10.1002/cne.20247. [DOI] [PubMed] [Google Scholar]
- 28.Gibson KR. Cognition, brain size, and the extraction of embedded food resources. In: Else JG, Lee PC, editors. Primate Ontogeny, Cognition, and Social Behavior. Cambridge, UK: Cambridge Univ Press; 1986. pp. 93–105. [Google Scholar]
- 29.Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- 30.Bortoff GA, Strick PL. Corticospinal terminations in two new-world primates: Further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J Neurosci. 1993;13:5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padberg J, et al. Parallel evolution of cortical areas involved in skilled hand use. J Neurosci. 2007;27:10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riddle DR, Purves D. Individual variation and lateral asymmetry of the rat primary somatosensory cortex. J Neurosci. 1995;15:4184–4195. doi: 10.1523/JNEUROSCI.15-06-04184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlen SJ, Krubitzer L. Phenotypic diversity is the cornerstone of evolution: Variation in cortical field size within short-tailed opossums. J Comp Neurol. 2006;499:990–999. doi: 10.1002/cne.21156. [DOI] [PubMed] [Google Scholar]
- 34.Campi KL, Krubitzer L. Comparative studies of diurnal and nocturnal rodents: Differences in lifestyle result in alterations in cortical field size and number. J Comp Neurol. 2010;518:4491–4512. doi: 10.1002/cne.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Essen DC, Newsome WT, Maunsell JH, Bixby JL. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: Asymmetries, areal boundaries, and patchy connections. J Comp Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- 36.Dougherty RF, et al. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis. 2003;3:586–598. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- 37.Karlen SJ, Krubitzer L. The functional and anatomical organization of marsupial neocortex: Evidence for parallel evolution across mammals. Prog Neurobiol. 2007;82:122–141. doi: 10.1016/j.pneurobio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albus K, Beckmann R. Second and third visual areas of the cat: Interindividual variability in retinotopic arrangement and cortical location. J Physiol. 1980;299:247–276. doi: 10.1113/jphysiol.1980.sp013123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merzenich MM, et al. Variability in hand surface representations in areas 3b and 1 in adult owl and squirrel monkeys. J Comp Neurol. 1987;258:281–296. doi: 10.1002/cne.902580208. [DOI] [PubMed] [Google Scholar]
- 40.Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: Do New World monkeys have an area 2? Cereb Cortex. 2005;15:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- 41.Tennant KA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neafsey EJ, et al. The organization of the rat motor cortex: A microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 43.Cooke DF, Padberg J, Zahner T, Krubitzer L. The functional organization and cortical connections of motor cortex in squirrels. Cereb Cortex. 2011 doi: 10.1371/journal.pone.0032322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- 45.Jain N, Catania KC, Kaas JH. A histologically visible representation of the fingers and palm in primate area 3b and its immutability following long-term deafferentations. Cereb Cortex. 1998;8:227–236. doi: 10.1093/cercor/8.3.227. [DOI] [PubMed] [Google Scholar]
- 46.Jain N, Qi HX, Catania KC, Kaas JH. Anatomic correlates of the face and oral cavity representations in the somatosensory cortical area 3b of monkeys. J Comp Neurol. 2001;429:455–468. doi: 10.1002/1096-9861(20010115)429:3<455::aid-cne7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 47.Adams DL, Horton JC. Capricious expression of cortical columns in the primate brain. Nat Neurosci. 2003;6:113–114. doi: 10.1038/nn1004. [DOI] [PubMed] [Google Scholar]
- 48.Krubitzer L, Campi KL, Cooke DF. All rodents are not the same: A modern synthesis of cortical organization. Brain Behav Evol. 2011;78:51–93. doi: 10.1159/000327320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campi KL, Collins CE, Todd WD, Kaas J, Krubitzer L. Comparison of area 17 cellular composition in laboratory and wild-caught rats including diurnal and nocturnal species. Brain Behav Evol. 2011;77:116–130. doi: 10.1159/000324862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smale L, Heideman PD, French JA. Behavioral neuroendocrinology in nontraditional species of mammals: Things the ‘knockout’ mouse CAN’T tell us. Horm Behav. 2005;48:474–483. doi: 10.1016/j.yhbeh.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinman MQ, Knight JA, Trainor BC. Effects of photoperiod and food restriction on the reproductive physiology of female California mice. Gen Comp Endocrinol. 2012;176:391–399. doi: 10.1016/j.ygcen.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heideman PD. Top-down approaches to the study of natural variation in complex physiological pathways using the white-footed mouse (Peromyscus leucopus) as a model. ILAR J. 2004;45:4–13. doi: 10.1093/ilar.45.1.4. [DOI] [PubMed] [Google Scholar]
- 54.Macneil LT, Walhout AJ. Gene regulatory networks and the role of robustness and stochasticity in the control of gene expression. Genome Res. 2011;21:645–657. doi: 10.1101/gr.097378.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bishop KM, Rubenstein JL, O’Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamasaki T, Leingärtner A, Ringstedt T, O’Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Leingärtner A, et al. Cortical area size dictates performance at modality-specific behaviors. Proc Natl Acad Sci USA. 2007;104:4153–4158. doi: 10.1073/pnas.0611723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirth F, Reichert H. Basic nervous system types: One or many? In: Streidter G, Rubenstein JL, editors. The Evolution of Nervous Systems. Vol 1. Oxford: Academic Press; 2007. pp. 55–72. [Google Scholar]
- 60.Tallafuss A, Bally-Cuif L. Formation of the head-trunk boundary in the animal body plan: An evolutionary perspective. Gene. 2002;287:23–32. doi: 10.1016/s0378-1119(01)00829-0. [DOI] [PubMed] [Google Scholar]
- 61.Chen CH, Cretekos CJ, Rasweiler JJ, 4th, Behringer RR. Hoxd13 expression in the developing limbs of the short-tailed fruit bat, Carollia perspicillata. Evol Dev. 2005;7:130–141. doi: 10.1111/j.1525-142X.2005.05015.x. [DOI] [PubMed] [Google Scholar]
- 62.Cretekos CJ, Rasweiler JJ, Behringer RR. Comparative studies on limb morphogenesis in mice and bats: A functional genetic approach towards a molecular understanding of diversity in organ formation. Reprod Fertil Dev. 2001;13:691–695. doi: 10.1071/rd01115. [DOI] [PubMed] [Google Scholar]
- 63.Sears KE, Behringer RR, Rasweiler JJ, 4th, Niswander LA. Development of bat flight: morphologic and molecular evolution of bat wing digits. Proc Natl Acad Sci USA. 2006;103:6581–6586. doi: 10.1073/pnas.0509716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weatherbee SD, Behringer RR, Rasweiler JJ, 4th, Niswander LA. Interdigital webbing retention in bat wings illustrates genetic changes underlying amniote limb diversification. Proc Natl Acad Sci USA. 2006;103:15103–15107. doi: 10.1073/pnas.0604934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cretekos CJ, Deng JM, Green ED, Rasweiler JJ, Behringer RR. NISC Comparative Sequencing Program Isolation, genomic structure and developmental expression of Fgf8 in the short-tailed fruit bat, Carollia perspicillata. Int J Dev Biol. 2007;51:333–338. doi: 10.1387/ijdb.062257cc. [DOI] [PubMed] [Google Scholar]
- 66.Wise LZ, Pettigrew JD, Calford MB. Somatosensory cortical representation in the Australian ghost bat, Macroderma gigas. J Comp Neurol. 1986;248:257–262. doi: 10.1002/cne.902480208. [DOI] [PubMed] [Google Scholar]
- 67.Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proc Natl Acad Sci USA. 2002;99:11429–11434. doi: 10.1073/pnas.162342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karlen SJ, Kahn DM, Krubitzer L. Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience. 2006;142:843–858. doi: 10.1016/j.neuroscience.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 69.Chabot N, et al. Subcortical auditory input to the primary visual cortex in anophthalmic mice. Neurosci Lett. 2008;433:129–134. doi: 10.1016/j.neulet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Hunt DL, et al. Aberrant retinal projections in congenitally deaf mice: How are phenotypic characteristics specified in development and evolution? Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1051–1066. doi: 10.1002/ar.a.20251. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9:676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- 72.Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.