Abstract
The discovery of functional magnetic resonance imaging (fMRI) has greatly impacted neuroscience. The blood oxygenation level-dependent (BOLD) signal, using deoxyhemoglobin as an endogenous paramagnetic contrast agent, exposes regions of interest in task-based and resting-state paradigms. However the BOLD contrast is at best a partial measure of neuronal activity, because the functional maps obtained by differencing or correlations ignore the total neuronal activity in the baseline state. Here we describe how studies of brain energy metabolism at Yale, especially with 13C magnetic resonance spectroscopy and related techniques, contributed to development of quantitative functional brain imaging with fMRI by providing a reliable measurement of baseline energy. This narrative takes us on a journey, from molecules to mind, with illuminating insights about neuronal-glial activities in relation to energy demand of synaptic activity. These results, along with key contributions from laboratories worldwide, comprise the energetic basis for quantitative interpretation of fMRI data.
Keywords: calibrated fMRI, GABA, glutamate, glutamine, field potentials, multi-unit activity, neuroimaging
Introduction
In 1992, while the first demonstrations of blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) (Bandettini et al., 1992; Blamire et al., 1992; Frahm et al., 1992; Kwong et al., 1992; Ogawa et al., 1992) caused great excitement in the functional brain imaging community, there were concerns about how well the BOLD signal reflected underlying changes in neuronal activity (Barinaga, 1997; Fitzpatrick and Rothman, 1999). This personal account by the authors describes how studies at Yale, in particular those utilizing 13C magnetic resonance spectroscopy (MRS) in conjunction with calibrated fMRI techniques and electrophysiology, helped reveal fundamental relationships between brain function and energy metabolism in the context of BOLD signal change, as well as the overall importance of baseline neuronal activity. These studies arose from basic work on brain energy metabolism using 13C MRS that had begun at Yale in the 1980’s under the direction of Professor Robert G. Shulman (Behar et al., 1986; Petroff et al., 1986; Rothman et al., 1985). Although we focus on work at Yale, we cover key contributions made at sites worldwide and apologize for any work we may have neglected due to space limitations.
Human brain mapping became possible about three decades ago with positron emission tomography (PET). Within a decade, however, PET functional measurements were replaced by fMRI. But the basic concepts for mapping brain function are similar (for a historical perspective see (Raichle, 2009)). PET measured changes in blood flow or volume (CBF, CBV) and/or changes in glucose or oxygen metabolism (CMRglc, CMRO2) induced by external stimuli, and fMRI uses the blood oxygenation level-dependent (BOLD) contrast, which is a consequence of a complex combination of changes in CBV, CBF, and CMRO2 (Ogawa et al., 1990). The main advantage of fMRI over PET is that BOLD signal can be mapped repeatedly in the same session because no external tracer is needed as the changing concentrations of oxyhemoglobin and deoxyhemoglobin (i.e., diamagnetic and paramagnetic respectively) during functional hyperemia provide the contrast.
However both contrasts revealed by PET and fMRI are qualitative because localization of brain regions ignores the total neuronal activity in the baseline state. But both methods have a key underlying assumption that changes in neuronal activity will be proportional to changes in neuronal energy consumption (Shulman and Rothman, 1998). This energy consumption may be measured directly via CMRO2 in PET and calibrated fMRI, as described later which is a method that extracts change in CMRO2 with CBF and CBV imaging by combining other magnetic resonance imaging (MRI) methods with BOLD contrast. However PET mostly used CBF to reflect the neuronal activity indirectly, whereas fMRI used BOLD contrast.
When the first PET and fMRI studies were performed in the human brain it was not known what the relationship between functional parameters familiar to neuroscientists such as neurotransmission or spiking activity and neuronal energy consumption. The work described in this article focuses on two main questions relevant to interpretation of neuroimaging data:
What is the relationship between changes quantitative measures of neuronal activity (such as neurotransmitter cycling, synaptic and spiking activity) and oxygen consumption?
What fraction of total neuronal activity does the contrast measured by BOLD represent?
Early PET and 1H MRS studies suggest non-oxidative energy contribution during brain function
It had long been established that glucose is the main energy substrate in the brain and its oxidation efficiently produces energy to support its function (Siesjo, 1978). However in the late 1980’s Fox et al published some PET data that revealed diminished glucose oxidation during sensory processing in awake human brain, where CMRglc and CBF changed significantly but CMRO2 did not (Fox and Raichle, 1986; Fox et al., 1988). These PET results, along with early theoretical calculations by Creutzfeld, supported a model of brain function in which neuronal signaling had minimal oxidative energy costs (Creutzfeldt, 1975). This conclusion was surprising based on the generally accepted view of disproportionately high energy costs of human brain function at rest, given its size relative to the body (Aiello and Wheeler, 1995; Sokoloff, 1991). An implication of this model is that the changes in activity mapped by fMRI were much larger than the brain activity in the resting-state, and therefore to a first approximation, the resting brain activity could be neglected. As we will describe below that MRS and other results, initially form Yale and later from elsewhere, showed that in fact the opposite is true. But the concept of negligible resting-state activity still has a strong influence on the interpretation of fMRI results.
Since the radioactivity in PET lacks specificity, the molecular basis of MRS signals provided an opportunity to test the Fox et al PET findings (Prichard and Shulman, 1986). Unlike 1H MRI, which measures water in all compartments, 1H MRS measures other endogenous molecules specific to intracellular and/or extracellular milieu. MRS is similar to MRI, except that it uses differences in resonance frequency between dissimilar chemical groups to measure the regional concentrations of endogenous molecules, including lactate, glucose, glutamate, GABA, glutamine, etc. However, as discussed in more detail below, 13C MRS in combination with infusion of 13C-labeled substrates like glucose, acetate, etc. the rates of 13C label incorporation into cell-specific pools – e.g., glutamate and GABA are predominantly neuronal, glutamine is predominantly glial – can be detected (for a recent review see (Rothman et al., 2011)).
In the early in 1990’s, the Yale group utilized 1H MRS to measure transient lactate increase and glucose decrease in the human brain during a similar visual stimulation paradigm as in Fox et al (Chen et al., 1993; Prichard et al., 1991). While these results suggested some level of non-oxidative glycolysis, the amount of lactate produced was quantitatively much smaller than predicted by the Fox et al PET studies. Moreover, subsequent PET, 1H MRS, and calibrated fMRI (see below) studies have shown there is several-fold larger increase in CMRO2 with functional activation than originally measured by Fox et al, especially in relation to the change in CBF which gives rise to the CMRO2-CBF coupling underlying the BOLD contrast (Hyder et al., 2000; Hyder et al., 1998) – an issue which is discussed below for calibrated fMRI. Therefore there was still a significant role for oxidative energy during brain function that could potentially be tracked by 13C MRS (see below). These early 1H MRS results from Yale were in good agreement with independent measurements from other laboratories using 1H MRS and PET, at that time (Frahm et al., 1996; Merboldt et al., 1992; Roland et al., 1987; Sappey-Marinier et al., 1992; Seitz and Roland, 1992; Vafaee et al., 1998; Vafaee et al., 1999) and more recent measurements (Mangia et al., 2007; Mintun et al., 2002).
13C MRS studies reveal high demand for oxidative energy during functional activation
Pioneering studies by Shulman and colleagues, originally at Bell Laboratories and later at Yale, showed that 13C MRS had the potential to provide detailed measurements of metabolic fluxes (for a historical perspective see (Alger and Shulman, 1984)). Following initial studies in the late 1970’s in cell suspensions and perfused organs, the 13C MRS method, and a related 1H MRS method called proton observe carbon edit (POCE), was applied to the rodent and human brain (Behar et al., 1986; Cerdan et al., 1990; Fitzpatrick et al., 1990; Gruetter et al., 1992; Petroff et al., 1986; Rothman et al., 1985; Rothman et al., 1992). A surprising initial finding from the in vivo studies was that there was rapid 13C labeling of glutamate and glutamine from glucose or acetate, which stood in contrast with results from brain slices (Badar-Goffer et al., 1990; Badar-Goffer et al., 1992). Because the brain slices were not activated, this feature (i.e., significant 13C labeling of glutamate and glutamine) provided a clue about the relation of energy metabolism and function discussed in the next sections. Based on the ability to detect in vivo turnover of 13C label from substrates (e.g., glucose or acetate) into various metabolites (e.g., glutamate, glutamine, lactate, or aspartate) in real time, several metabolic fluxes (e.g., tricarboxylic acid cycle, glutamine synthesis, neurotransmitter cycle, etc.) could be extracted with compartmental modeling of neuronal-glial trafficking of metabolites (Gruetter et al., 1996; Mason et al., 1995; Mason et al., 1992). The rate determined for glucose oxidation has been shown to be in good agreement with CMRglc measured by other methods in animal models (e.g., in rats with arterio-venous difference or 2-deoxyglucose autoradiographic methods (Hyder et al., 2000) or in primates with PET (Boumezbeur et al., 2005)) and in humans (Rothman et al., 2011)).
To test the oxidative energy demand of functional brain activation, studies were performed in animal and human visual cortex. At Yale, POCE was used in an anesthetized rat model to demonstrate a large increase in neuronal glucose oxidation in the somatosensory cortex during forepaw activation (Hyder et al., 1996; Hyder et al., 1997). Because the earliest interpretations of BOLD signal increase were interpreted to reflect minimal rise in CMRO2 (Kim and Ugurbil, 1997; Ogawa et al., 1993), this Yale finding was especially controversial at the time because a large increase in CMRO2 in the presence of a positive BOLD signal was considered an artifact. This issue will be discussed more below in context of calibrated BOLD and electrical activity recordings, but POCE studies of the human brain conducted independently at Universities of Minnesota and Nottingham also showed a substantial increase in CMRO2 with visual stimulation in the primary visual cortex (Chen et al., 2001; Chhina et al., 2001). These POCE studies along with parallel studies using calibrated fMRI (see below) have led to the general acceptance that increases in brain activity are primarily fueled by ATP for oxidative glucose consumption, which in primary sensory cortices can be metabolically quite significant (Shulman et al., 2001b). However the mechanism and function of the enhanced non-oxidative metabolism remains an intense area of research, although several models have been proposed (Chih et al., 2001; Magistretti et al., 1999; Shulman et al., 2001a).
Relationship between oxidative energy and glutamatergic neurotransmission
While 13C MRS and POCE (and early calibrated fMRI, see below) studies provided insights into changes in oxidative energy metabolism with brain function, they did not deliver a quantitative relationship between a parameter measuring neuronal function such as glutamate neurotransmission or neuronal signaling as conventionally represented by electrical activity. A potential way to study this relationship was inferred from the rapid 13C labeling from glucose of glutamine in human and animal 13C MRS and POCE studies. In the cerebral cortex, glutamate released from nerve terminals is taken up by surrounding glial cells and returned to the nerve terminals as glutamine (for details of the process see (Rothman et al., 1999)). The glutamatergic neurotransmission pathway is referred to as the glutamate-glutamine cycle. If glutamine labeling from the glutamate-glutamine cycle could be distinguished from other labeling sources for glutamine (e.g., removal of brain ammonia in the glia by glutamine synthesis) this pathway of glutamatergic neurotransmission could be quantitated by 13C MRS.
At Yale, the first study that tried to disentangle the contribution of the glutamateglutamine cycle to glutamine synthesis was performed by measuring the increase in the rate of 13C labeling of glutamine (from glucose) as a function of ammonia concentration and then extrapolating to basal ammonia level. This study lead to an estimate of de novo glutamine synthesis to remove ammonia being no more than a 10–20% contribution even in the anesthetized rat cerebral cortex when metabolic rates are extremely slow compared to the awake state (Sibson et al., 1997). These early 13C MRS studies of the glutamate-glutamine cycle were supported by other studies at Yale and California Institution of Technology using alternate labeling strategies with 15N and 13C labeled precursors enabling separate and direct measurement of glutamine labeling from both de novo synthesis and the glutamate-glutamine cycle (Kanamori et al., 1993; Kanamori et al., 1995; Shen et al., 1998; Sibson et al., 2001). These heteronuclear MRS studies, in conjunction with advanced metabolic modeling (Gruetter et al., 2001; Gruetter et al., 1998; Mason and Rothman, 2004), have been consistent with the glutamate-glutamine cycle accounting for the majority of mass flow into the brain glutamine pool (for a recent review see (Rothman et al., 2011)).
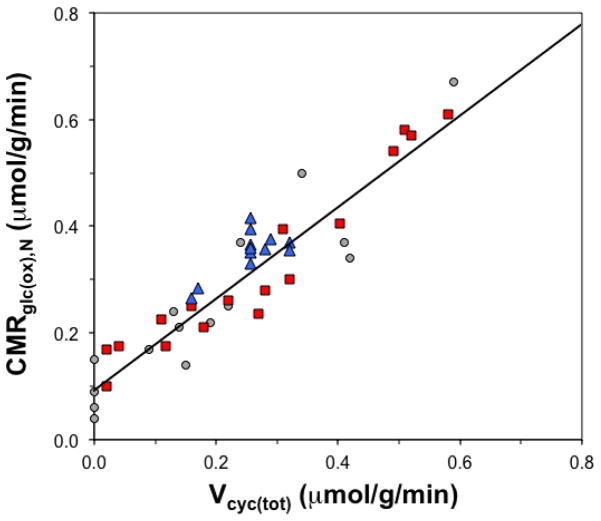
After the demonstration that 13C MRS could measure the glutamate-glutamine cycle, the Yale group performed a study in a rat model in which they measured the rate of the glutamate-glutamine cycle and neuronal glucose oxidation (both fluxes measured in the same 13C MRS experiment) at different brain electrical activities to address the question of the neuronal energy cost of function (Sibson et al., 1998). 13C MRS was used to measure the rates of neuronal glucose oxidation (CMRglc(ox),N) and neurotransmitter flux (Vcyc(tot)), localized in the rat somatosensory cortex over a wide range of activities: from isoelectric pentobarbital anesthesia, under which there is no neuronal signaling, to mildly anesthetized states with higher neuronal signaling. The study found a linear relationship between Vcyc(tot) vs. CMRglc(ox),N which is significant in several respects (Figure 1). Firstly it demonstrated a linear relationship between neuronal activity as measured by glutamatergic function and neuronal energy metabolism. Furthermore at the intercept where Vcyc(tot) falls to zero, CMRglc(ox),N is about 0.1 μmol/g/min. Hence in the awake brain approximately 80% of the neuronal energy demand is devoted to events associated with neuronal signaling, which contrasted expectations at the time that majority of resting brain energy not being related to signaling (see discussion above). 13C MRS experiments looking simultaneously at neuronal energy consumption and the rate of the glutamate glutamine cycle have subsequently been performed by several laboratories in both rats and humans (Bluml et al., 2002; Boumezbeur et al., 2010; Chen et al., 2001; Chhina et al., 2001; Choi et al., 2002; Chowdhury et al., 2007; de Graaf et al., 2004; Duarte et al., 2011; Gruetter et al., 2001; Gruetter et al., 1998; Henry et al., 2010; Jiang et al., 2011; Lebon et al., 2002; Mason et al., 1995; Mason et al., 2007; Oz et al., 2004; Pan et al., 2000; Patel et al., 2005a; Patel et al., 2005b; Serres et al., 2008; Shen et al., 1999; van Eijsden et al., 2010; Wang et al., 2010; Yang and Shen, 2005). The results of these studies have been highly consistent with the original findings of Sibson et al and furthermore the human results also agree with the findings in rodent brain (Figure 1).
Figure 1.
High neuronal energy demand and its relation to neurotransmitter cycling reveal a sizeable level of neuronal activity in the awake brain. Experimental estimates of relationship between total neurotransmitter cycling rate (Vcyc(tot)) and the cerebral metabolic rate of glucose oxidation in neurons (CMRglc(ox),N derived using 13C MRS localized to the rat somatosensory cortex (grey circles for Sibson et al (1998) and red squares are for all other studies since then) and human visual cortex (blue triangles). The results suggest that about 80% of the resting energy consumption in the awake rat brain is dedicated to events associated with neuronal activity. The trends, to a first-order, are similar between rat and human brain 13C MRS data.
A third highly significant finding of the Sibson et al study is that the slope between glutamate release/recycling and neuronal glucose oxidation is close to 1:1, or approximately one molecule of glucose is consumed for every glutamate released. The high value of this slope indicated that the glutamate-glutamine cycle is a major metabolic flux in the brain – on the order of the tri-carboxylic acid cycle and glucose consumption (which has considerable importance for models of glutamate excitotoxicity in disease). Furthermore the close to stoichiometric 1:1 ratio implies a molecular mechanism for the coupling of the two fluxes. The early 13C MRS results of Sibson et al were consolidated into a model of neuronal-glial interactions proposing a novel role for lactate, which conventionally had been thought to be an end product of glycolysis (Magistretti et al., 1999). The fundamental hypothesis was that glial glycolytic ATP was produced rapidly enough to clear glutamate from the extracellular space to prepare the nerve terminal for the next synaptic event. The recycling of one mole of glutamate neurotransmitter between glia and neurons was associated with oxidation of one mole of glucose molecule in neurons. However only by glia took up the glucose whereas all the lactate generated by glycolysis in the glia was transferred to neurons for subsequent oxidation.
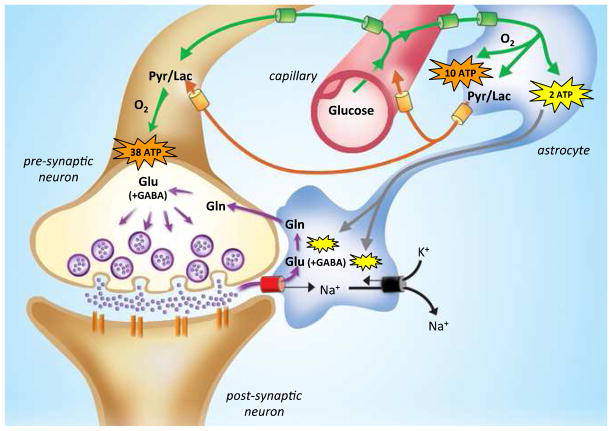
While this model was consistent with the 1:1 observed relationship between ΔCMRglc(ox),N and ΔVcyc(tot) measured by 13C MRS, it could not specify the energetics of glia and GABAergic neurons because quantitative values for these pathways were not available back then. Later we revised this model to include more up-to-date measurements (Hyder et al., 2006). The revised model showed that glia could produce around 10% of total oxidative ATP and GABAergic neurons could potentially generate about 20% of total oxidative ATP in neurons. Neurons could produce around 90% of total oxidative ATP and could be required to take up directly as much as 25% of the total glucose oxidized. However glial lactate would still make a major contribution to neuronal oxidation, but about 30% less than predicted before. The relationship observed between ΔCMRglc(ox),N and ΔVcyc(tot) could still be determined by glial glycolytic ATP as before. Quantitative aspects of this model (Figure 2) are currently being tested, but so far the Yale group has shown that interruption of glutamate release/recycling affects both BOLD contrast and oxidative demand (Kida et al., 2001; Kida et al., 2006).
Figure 2.
A revised coupling between synaptic activity and glucose metabolism. Glucose uptake (green) is now ubiquitous. Astrocytic lactate flow (orange) to neurons is now less, with a small amount being effluxed to blood. ATP is produced oxidatively (orange) in neurons and astrocytes. ATP produced by glycolysis (yellow) fuels ion pumping and glutamine synthesis in astrocytes. Abbreviations: Glu, glutamate; Gln, glutamine; Pyr, pyruvate; Lac, lactate in astrocytes. See Hyder et al (2006) for details. Modified from Hyder et al (2006), Journal of Cerebral Blood Flow and Metabolism, NPG.
It should be pointed, however, that other models have been proposed to explain the relationship based upon other molecular mechanisms, including redox potential balancing requirements and the need of glycolytic ATP to pump glutamate into vesicles (Hertz et al., 2007; Mangia et al., 2009; Simpson et al., 2007). The hopeful discovery of the molecular/cellular mechanism(s) underlying the relationship between ΔCMRglc(ox),N and ΔVcyc(tot) will provide important insight into why the brain requires glucose as its major fuel and may be of importance in understanding the finding of altered brain glucose metabolism in a variety of diseases. Thus the most up-to-date 13C MRS data from the rat and human brain suggest that about 20% of neuronal energy demand in the awake brain is dedicated for non-signaling or housekeeping needs, whereas the remaining 80% fraction of the energy supports the signaling needs within cortical networks. This large 80% of the baseline energy, which is necessary for consciousness (Shulman et al., 2009), as discussed below remains largely unexplored in brain studies.
Implications of the 13C MRS findings for the interpretation of fMRI
As described above, due to the belief of minimal resting brain activity at the time the most accepted model for interpreting fMRI was based on the changes seen being proportional to the total neuronal activity engaged in the task, an assumption that was inherent in the early versions of statistical parametric mapping which remains the most popular method for analyzing functional imaging data (Frackowiak et al., 2004). However by the mid 1990’s there were findings of negative changes in the BOLD signal (as well as negative CBF changes reported from PET studies), which represented a paradox from the viewpoint of there being little resting neuronal activity compared with activated state. In 1998, Shulman and Rothman proposed an explanation for the negative BOLD signal by applying the relationship discovered by the 13C MRS results between neuronal glucose oxidation and neuronal activity (as assessed by the glutamate-glutamine cycle) to estimate the total resting brain activity versus the change in activity measured in the increments reported by PET and early quantitative fMRI studies (Shulman and Rothman, 1998). They concluded, contrary to common belief at the time, that the task-induced changes measured by BOLD signal were substantially lower than the neuronal activity in the same regions when no task was present. From this perspective the negative BOLD was no longer paradoxical – it simply meant the brain region exhibiting the change during the task had less neuronal activity than in the resting state. They also presented examples of how the large resting activity could change the interpretation of an fMRI study. For example, no change in BOLD signal during a task did not mean the region was not involved in its performance – its neuronal activity devoted to the task could be the same as that used in its resting state (see the later section regarding studies that examined the question of how much neuronal activity is needed for function). Soon after the publication of the initial 13C MRS studies looking at the energy costs of neuronal activity and resting state activity, Raichle and colleagues at Washington University incorporated these findings along with other results from PET and fMRI to develop a model explaining the function of resting state activity in terms of a default state of function, which has extensively influenced the analysis and interpretation of fMRI studies of the resting-state paradigm (Gusnard and Raichle, 2001).
Calibrating the BOLD contrast to reflect oxidative demand during task paradigms
From the earliest descriptions of BOLD contrast (Boxerman et al., 1995; Kennan et al., 1994; Ogawa et al., 1993), it was clear that CMRO2 could be extracted, provided that CBF and CBV were measured with the baseline BOLD signal. As fMRI and 13C MRS developments continued to flourish in the 1990’s, several groups focused on novel MRI methods for CBF and CBV imaging in vivo which proved to be seminal for the development of calibrated fMRI studies (Detre et al., 1992; Kennan et al., 1998; Kim, 1995; Mandeville et al., 1998; Williams et al., 1992). Because magnetic labeling of arterial blood proved to be a sensitive enough perfusion tracer in brain tissue, the CBF method did not require exogenous contrast agents and thus could be imaged reliably in both humans and animals. The CBV method, however, was based on intravascular-borne exogenous paramagnetic contrast agents that drowned out the diamagnetic BOLD effect during functional hyperemia. Because this contrast agent-based CBV method was limited to animals, early human “calibrated fMRI” studies combined BOLD and CBF measurements to estimate CMRO2 changes during functional activation (Davis et al., 1998; Hoge et al., 1999; Kastrup et al., 1999; Kim et al., 1999; Kim and Ugurbil, 1997),
| eq. [1] |
where the subscript “o” represents the baseline state values for each parameter, S represents the BOLD signal, M represents the baseline BOLD signal which is the product of the transverse relaxation rate (with either spin-echo or gradient-echo) and the echo time, and the parameters α and β are assumed to be 0.4 and 1.5, respectively, for field strengths of 1.5T.
Because 13C MRS methods can measure CMRO2 changes directly, an opportunity presented itself at Yale to test the validity of eq. [1] by conducting multi-modal measurements of CMRO2, CBF, CBV, and BOLD signal (with both spin-echo or gradient-echo) under various levels of brain activity in rats at 7.0T (Hyder et al., 2001; Kida et al., 1999; Kida et al., 2000). Some important observations from these studies have relevance for calibrated fMRI studies, especially for those conducted at fields higher than 1.5T. First, it was observed that the parameter β, which designates the CMRO2-CBF coupling in relation to the BOLD effect, is closer to 1 at 7.0T (or higher). This β value is within expectations from stimulations of the BOLD effect extrapolated to fields beyond 1.5T (Boxerman et al., 1995; Kennan et al., 1994; Ogawa et al., 1993). Second, the value of the parameter α which describes the CBV-CBF coupling is probably less than 0.4 and was estimated by PET in primate brain during CO2 challenges (Grubb et al., 1974).
Most early human calibrated fMRI studies assumed a specific value of α because CBV could not be independently measured. Animal studies at Yale suggested that the α value could range from 0.1 to 0.2 (Hyder et al., 2001) because CBV and CBF dynamics were not fixed during the time course of the functional activation (Mandeville et al., 1999). These suggestions are now independently supported by subsequent findings from studies in rat, cat, and human brain where CBV and CBF have been measured in the same session (Chen and Pike, 2009; Jin and Kim, 2008; Kida et al., 2007). However it should be noted that these CBV measurements reflect the entire blood volume in the vascular branching, whereas the BOLD effect is sensitive to mainly the venous compartment. Recently some novel CBV methods developed at University of Pittsburgh, which allows separation of the arterial and venous compartments, suggest that the contribution of CBV changes incorporated into calibrated fMRI may be too large because it is the arterial compartment that contributes most during functional hyperemia (Kim and Kim, 2005, 2011). These results have serious consequences for calibrated fMRI because of the potential to underestimate changes in CMRO2 by exaggerated contributions from CBV in eq. [1].
Another intrinsic problem in calibrated fMRI is the assessment of an independent measure (or assumption) about the baseline BOLD signal (i.e., the so-called M parameter), which is affected by a variety of experimental settings including the static magnetic field shim, which can vary from study to study. By comparing ΔCMRO2 predicted from calibrated fMRI and ΔCMRO2 measured from POCE, the Yale group showed good accuracy of calibrated fMRI in animals with independent CBF and CBV imaging and direct M measurement under optimal shim conditions (Hyder et al., 2001; Kida et al., 2000). An important component of this early work was that M can be directly measured, from both spin-echo and gradient-echo calibrated fMRI studies, a point that has been raised recently in the literature (Blockley et al., 2012; Gauthier et al., 2011).
The use of calibrated fMRI has advanced considerably in many different laboratories (Bulte et al., 2012; Chen and Pike, 2010; Chiarelli et al., 2007a; Chiarelli et al., 2007b; Leontiev and Buxton, 2007; Leontiev et al., 2007; Lin et al., 2009; Liu et al., 2004; Restom et al., 2007; Restom et al., 2008; Shen et al., 2008; Stefanovic et al., 2005; Uludag et al., 2004). However the neuronal basis of the CMRO2 changes derived from calibrated fMRI remained unresolved until recently. The Yale group compared neuronal activity recordings of local field potential (LFP) and/or multi-unit activity (MUA) with calibrated fMRI results, in an animal model, to show tight neurovascular and neurometabolic couplings in block-design (Maandag et al., 2007; Smith et al., 2002) and event-related paradigms (Herman et al., 2009; Sanganahalli et al., 2009) of normal brain and various types of epileptic seizure models (Englot et al., 2008; Mishra et al., 2011; Schridde et al., 2008). Although relationships between neuronal activity and stimulus features can range from linear to nonlinear, associations between hyperemic components (i.e., BOLD, CBF, CBV) and neuronal activity (i.e., LFP, MUA) are linear. These results showed that CMRO2 changes are correlated with LFP and MUA in cerebral cortex.
Overall, the calibrated fMRI studies at Yale quantitatively explained the presence of large CMRO2 changes observed during sensory stimulation in anesthetized rats with a positive BOLD signal (Hyder et al., 1996; Hyder et al., 1997) with concomitant large changes in CBF (Hyder et al., 2000; Silva et al., 1999). Notably, both in human and animal studies, the measured ΔCBF/CBF and calculated ΔCMRO2/CMRO2 from calibrated fMRI studies (i.e., the CMRO2-CBF coupling) show a linear trend suggesting rapid oxygen equilibration between blood and tissue pools within the physiological range (Hyder et al., 1998). In support of this hypothesis, independent studies from Universities of Pennsylvania and Pittsburgh show that tissue pO2 dynamics are just as fast as the CBF dynamics during functional activation (Ances et al., 2001; Vazquez et al., 2008). Together, these results propose that calibrated fMRI at high magnetic fields can provide high spatiotemporal mapping of CMRO2 changes (Hyder et al., 2010).
What fraction of neuronal ensemble’s activity is needed for brain function?
Although BOLD signal had been shown to correlate with LFP and MUA dynamics (Logothetis et al., 2001), no specific mechanism for this linkage had been identified yet. The Yale group sought an energetic basis for this question because the model on the basis of the Sibson et al 13C MRS studies (Figure 2) proposed a testable hypothesis that neurotransmitter flux, and consequently oxidative demand, should be proportional to spiking frequency of glutamatergic neurons (Smith et al., 2002). In an anesthetized rat model, Smith et al derived change in oxidative energy (ΔCMRO2/CMRO2) from calibrated fMRI during sensory stimulation and compared it to changes in spiking frequency of a neuronal ensemble (Δν/ν) within activated voxels identified by BOLD contrast. Smith et al examined the relationships from two anesthetized levels and found good agreement between ΔCMRO2/CMRO2 and Δν/ν, thereby relating the energetic basis of neuronal spiking frequency and neurotransmitter flux.
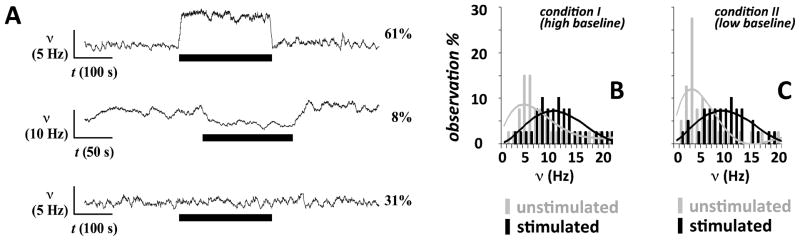
A surprising finding by Smith et al was that not all neurons in the recorded ensemble responded the same way to the forepaw stimulation (Figure 3). The histograms of neuronal firing rates showed different distributions in the resting and stimulated states. For example, in the deeply anesthetized level (or a low baseline state) about 60% of the population showed an increase in firing, about 10% of the population showed a decrease in firing, and about 30% of the population showed no change in firing. But these fractional responses of the population were different from the lightly anesthetized level (or a high baseline state), where the fraction that decreased their firing remained at 10% (see (Smith et al., 2002) and (Hyder et al., 2002). for details). These fMRI and electrophysiology results from Yale showed that the fraction of neuronal ensemble’s activity that underlies BOLD contrast is highly dependent on the baseline state. Similar observations had been made with electrophysiological measurements under a variety of conditions (McCasland and Woolsey, 1988; Scannell and Young, 1999). Overall these studies suggest that collaboration among a large number of neurons – some of which are firing faster and others slower but all requiring energy – is crucial for neuronal signaling.
Figure 3.
Neuronal ensemble recordings. (A) Experimental variations across electrophysiological measurements (shown for condition II, i.e., high baseline state, only) from the contralateral forepaw region. Significant increases and decreases in spiking frequency (ν) within were observed in ~60% and ~10% of the recordings, respectively (top and middle), whereas in ~30% of the recordings the stimulation did not induce any significant changes in n (bottom). The vertical and horizontal bars represent the scales for ν and time, respectively. The thick black horizontal bar represents the stimulus duration. (B and C) The comparison in a small neuronal ensemble (72 neurons) between basal activity achieved with two dosages of α-chloralose shows significantly different spiking frequencies at rest (P < 0.01), whereas upon stimulation the spiking frequencies became similar (P > 0.25). See Smith et al (2002) for details. Modified from Smith et al (2002), Proceedings of National Academy of Sciences, USA.
Another interesting feature of the Smith et al studies was that the same forepaw stimulation was applied from two anesthetized levels (i.e., low and high baseline states). Although the histograms representing the two rest conditions showed different distributions, the histograms for the two stimulated conditions were nearly identical (Figure 3). In other words, the final values of CMRO2 and ν (and thus BOLD signal) reached upon stimulation were approximately the same from both baselines (Hyder et al., 2002). If only increments were required to support function then magnitudes should be independent of the baseline. But if particular magnitudes of activity were required, then incremental sizes should inversely correlate with baseline, being larger from a lower baseline (Shulman et al., 1999). In support of this hypothesis, the results of Smith et al showed that particular magnitudes of activity support neural function. These results confirmed that disregard of baseline activity in fMRI experiments by differencing (or correlations) removes a large and necessary component of the total neuronal activity in the resting brain.
The Smith et al findings are supported by other results, which have been measured by a variety of techniques (e.g., fMRI, optical imaging, electrophysiology), applied to different sensory systems (e.g., somatosensory cortex, visual cortex, olfactory bulb), across a wide range of species (e.g., rat, cat, monkey, human), and under quite diverse behavioral states (e.g., awake, sleep, and anesthetized. Measurements show that the magnitude of the evoked response upon stimulation is, to a first-order, inversely related to the level of baseline activity (Chen et al., 2005; Issa and Wang, 2008, 2009; Li et al., 2011; Masamoto et al., 2007; Pasley et al., 2007; Portas et al., 2000; Smith et al., 2002; Uludag et al., 2004; Zhu et al., 2009).
More recent studies at Yale by Maandag et al showed that the spatial extent of activation spreading is also inversely conditional on the level of baseline activity (Maandag et al., 2007). Since the separation of the two baseline states (e.g., as reflected by ΔCMRO2) in the Smith et al and Maandag et al studies differed significantly, these early results about the relevance of baseline for fMRI studies suggest that careful consideration about an absolute measure of resting state is necessary. These results from Maandag et al also find support from other studies in the literature (Antognini et al., 1997; Disbrow et al., 1999; Disbrow et al., 2000; Dueck et al., 2005; Erchova et al., 2002; Heinke et al., 2004; Imas et al., 2006; Sperling et al., 2002). Most of these studies, which used either an anesthetic or a sedative to alter the baseline state, suggest that evoked activity is more localized under levels of deep anesthesia or higher sedation (i.e., low baseline state), whereas the response patterns expand beyond the primary area under levels of light anesthesia or lower sedation (i.e., high baseline state).
Thus contrary to the traditional approach of just varying the stimulus input, the brain’s input can also be treated as an independent variable, because perception measured at the cortical level depends on both exogenous and endogenous inputs (Ebner and Armstrong-James, 1990). While recent experimental evidence is changing the opinion that spontaneous neuronal activity is simply “noise” (for recent reviews see (Hyder and Rothman, 2011; Northoff et al., 2010; Ringach, 2009; Shulman et al., 2007)), it should be noted that classical studies had raised the importance of spontaneous activity for understanding brain function (Adrian, 1941). Future studies need considerations about properties of the stimulus (e.g., amplitude, contrast, etc.) in relation to baseline to study interactions of exogenous and endogenous inputs (Davis et al., 2007; Silva et al., 2011). Since anesthetics and sedatives are the primary means to experimentally alter the baseline states, better mechanistic understanding is needed (Alkire et al., 2008; Franks, 2008). An alternate means of varying resting brain activity without exogenous chemicals, as recently featured in studies from Universities of California in San Diego and Berkley (Pasley et al., 2007; Uludag et al., 2004), are needed for translation to humans.
Understanding healthy aging with multi-modal studies of 13C MRS and calibrated fMRI
As applications of fMRI to disease states rise (Matthews et al., 2006), there is a growing need to assess effects of healthy human aging because alterations in brain morphology, cellular density, or metabolism could significantly influence the BOLD contrast (d’Esposito et al., 2003). Alterations in mitochondrial function have been implicated in age-related neurodegenerative diseases through reactive oxygen species hypothesis and have been suggested to have a role in the loss of brain function with healthy aging (Reddy, 2007). Healthy human aging is associated with a decline in cognitive, memory, and sensory processes (Hedden and Gabrieli, 2004). But is there a quantitative neuroimaging correlate of these cognitive findings?
Recent task-based fMRI studies in healthy human aging show that the BOLD response is greater in younger subjects, but the CMRO2-CBF couplings established from calibrated fMRI in aging and younger subjects are nearly identical (Ances et al., 2009). Moreover, the resting-state fMRI studies show very little difference between the networks revealed in the healthy young and aging brains (Koch et al., 2010). PET studies have noted 10–20% reductions in overall CMRglc and CMRO2 with normal aging (Kalpouzos et al., 2009; Martin et al., 1991), but these may not all be related to mitochondrial changes. Furthermore, it is unclear whether these metabolic decreases are due to changes at the cellular level or are secondary to brain shrinkage (Ibanez et al., 2004). Recent 13C MRS studies from the Yale group shows that, compared with young subjects, neuronal mitochondrial metabolism and neurotransmitter cycle flux (assessed with 13C-labelled glucose) was approximately 30% lower in elderly subjects, but glial mitochondrial metabolism (assessed with 13C-labelled acetate) was approximately 30% higher in elderly subjects (Boumezbeur et al., 2010). Taken together, these multi-modal studies suggest that healthy aging is associated with reduced neuronal mitochondrial metabolism and altered glial mitochondrial metabolism, which may in part be responsible for declines in brain function.
Concluding remarks
Functional brain imaging methods like PET and fMRI that highlight areas of activity in vivid hues have revolutionized neuroscience, but has been limited by these methods not directly capturing changes in neuronal activity or reflecting a measure of total neuronal activity. Studies using multi-modal MRI, MRS, and electrical recordings have contributed to quantitating the energetic changes underlying the BOLD signal and directly relating them to changes in neurotransmission and electrical activity. They also have shown that the baseline neuronal activity is much larger than the changes in activity induced by tasks, which in turn has contributed to the recent interest in understanding its functional significance. The response to task has been shown to be dependent on this baseline activity, which strongly argues for its functional relevance, and thus every attempt should be made to image the resting brain activity and metabolism quantitatively and new schemes should be sought for its inclusion in data analysis. The underlying theme in these early Yale studies, and those that continue today in several other laboratories around the world, is that functional brain imaging studies can go beyond just mapping indirect correlates of function and instead directly study total brain activity as well as the neurometabolic and neurovascular components that support it in health and disease.
Acknowledgments
Thanks to colleagues at Yale University for insightful comments. Supported by National Institutes of Health Grants (R01 MH-067528 to FH, P30 NS-052519 to FH, R01 AG-034953 to DLR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian ED. Afferent discharges to the cerebral cortex from peripheral sense organs. J Physiol. 1941;100:159–191. doi: 10.1113/jphysiol.1941.sp003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello LC, Wheeler P. The Expensive-Tissue Hypothesis - the Brain and the Digestive-System in Human and Primate Evolution. Current Anthropology. 1995;36:199–221. [Google Scholar]
- Alger JR, Shulman RG. Metabolic applications of high-resolution 13C nuclear magnetic resonance spectroscopy. Br Med Bull. 1984;40:160–164. doi: 10.1093/oxfordjournals.bmb.a071963. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Buerk DG, Greenberg JH, Detre JA. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci Lett. 2001;306:106–110. doi: 10.1016/s0304-3940(01)01868-7. [DOI] [PubMed] [Google Scholar]
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antognini JF, Buonocore MH, Disbrow EA, Carstens E. Isoflurane anesthesia blunts cerebral responses to noxious and innocuous stimuli: a fMRI study. Life Sci. 1997;61:PL 349–354. doi: 10.1016/s0024-3205(97)00960-0. [DOI] [PubMed] [Google Scholar]
- Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by 13C-n. m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990;266:133–139. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar-Goffer RS, Ben-Yoseph O, Bachelard HS, Morris PG. Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. Biochem J. 1992;282 (Pt 1):225–230. doi: 10.1042/bj2820225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Barinaga M. What makes brain neurons run? Science. 1997;276:196–198. doi: 10.1126/science.276.5310.196. [DOI] [PubMed] [Google Scholar]
- Behar KL, Petroff OA, Prichard JW, Alger JR, Shulman RG. Detection of metabolites in rabbit brain by 13C NMR spectroscopy following administration of [1-13C]glucose. Magn Reson Med. 1986;3:911–920. doi: 10.1002/mrm.1910030611. [DOI] [PubMed] [Google Scholar]
- Blamire AM, Ogawa S, Ugurbil K, Rothman D, McCarthy G, Ellermann JM, Hyder F, Rattner Z, Shulman RG. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blockley NP, Griffeth VE, Buxton RB. A general analysis of calibrated BOLD methodology for measuring CMRO(2) responses: Comparison of a new approach with existing methods. Neuroimage. 2012;60:279–289. doi: 10.1016/j.neuroimage.2011.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15:1–5. doi: 10.1002/nbm.725. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Besret L, Valette J, Gregoire MC, Delzescaux T, Maroy R, Vaufrey F, Gervais P, Hantraye P, Bloch G, Lebon V. Glycolysis versus TCA cycle in the primate brain as measured by combining 18F-FDG PET and 13C-NMR. J Cereb Blood Flow Metab. 2005;25:1418–1423. doi: 10.1038/sj.jcbfm.9600145. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Bulte DP, Kelly M, Germuska M, Xie J, Chappell MA, Okell TW, Bright MG, Jezzard P. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage. 2012;60:582–591. doi: 10.1016/j.neuroimage.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem. 1990;265:12916–12926. [PubMed] [Google Scholar]
- Chen JJ, Pike GB. BOLD-specific cerebral blood volume and blood flow changes during neuronal activation in humans. NMR Biomed. 2009;22:1054–1062. doi: 10.1002/nbm.1411. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. J Cereb Blood Flow Metab. 2010;30:1094–1099. doi: 10.1038/jcbfm.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Friedman RM, Roe AW. Optical imaging of SI topography in anesthetized and awake squirrel monkeys. J Neurosci. 2005;25:7648–7659. doi: 10.1523/JNEUROSCI.1990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Novotny EJ, Zhu XH, Rothman DL, Shulman RG. Localized 1H NMR measurement of glucose consumption in the human brain during visual stimulation. Proc Natl Acad Sci U S A. 1993;90:9896–9900. doi: 10.1073/pnas.90.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu XH, Gruetter R, Seaquist ER, Adriany G, Ugurbil K. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using (1)H-[(13)C] MRS and fMRI. Magn Reson Med. 2001;45:349–355. doi: 10.1002/1522-2594(200103)45:3<349::aid-mrm1045>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Chhina N, Kuestermann E, Halliday J, Simpson LJ, Macdonald IA, Bachelard HS, Morris PG. Measurement of human tricarboxylic acid cycle rates during visual activation by (13)C magnetic resonance spectroscopy. J Neurosci Res. 2001;66:737–746. doi: 10.1002/jnr.10053. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Gallichan D, Piechnik SK, Wise R, Jezzard P. Flow-metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn Reson Med. 2007a;57:538–547. doi: 10.1002/mrm.21171. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Wise R, Gallichan D, Jezzard P. A calibration method for quantitative BOLD fMRI based on hyperoxia. Neuroimage. 2007b;37:808–820. doi: 10.1016/j.neuroimage.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Chih CP, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- Chowdhury GM, Patel AB, Mason GF, Rothman DL, Behar KL. Glutamatergic and GABAergic neurotransmitter cycling and energy metabolism in rat cerebral cortex during postnatal development. J Cereb Blood Flow Metab. 2007;27:1895–1907. doi: 10.1038/sj.jcbfm.9600490. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O. Neurophysiological correlates of different functional states of the brain. In: Ingvar D, Lassen N, editors. Brain Work. The Coupling of Function, Metabolism and Blood Flow in the Brain. Alfred Benzon Symposium VIII. Academic Press; New York: 1975. pp. 21–46. [Google Scholar]
- d’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, Owen AM, Menon DK. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci U S A. 2007;104:16032–16037. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf RA, Mason GF, Patel AB, Rothman DL, Behar KL. Regional glucose metabolism and glutamatergic neurotransmission in rat brain in vivo. Proc Natl Acad Sci U S A. 2004;101:12700–12705. doi: 10.1073/pnas.0405065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Roberts TP, Slutsky D, Krubitzer L. The use of fMRI for determining the topographic organization of cortical fields in human and nonhuman primates. Brain Res. 1999;829:167–173. doi: 10.1016/s0006-8993(99)01297-4. [DOI] [PubMed] [Google Scholar]
- Disbrow EA, Slutsky DA, Roberts TP, Krubitzer LA. Functional MRI at 1.5 tesla: a comparison of the blood oxygenation level-dependent signal and electrophysiology. Proc Natl Acad Sci U S A. 2000;97:9718–9723. doi: 10.1073/pnas.170205497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JM, Lanz B, Gruetter R. Compartmentalized Cerebral Metabolism of [1,6-C]Glucose Determined by in vivoC NMR Spectroscopy at 14.1 T. Front Neuroenergetics. 2011;3:3. doi: 10.3389/fnene.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck MH, Petzke F, Gerbershagen HJ, Paul M, Hesselmann V, Girnus R, Krug B, Sorger B, Goebel R, Lehrke R, Sturm V, Boerner U. Propofol attenuates responses of the auditory cortex to acoustic stimulation in a dose-dependent manner: a FMRI study. Acta Anaesthesiol Scand. 2005;49:784–791. doi: 10.1111/j.1399-6576.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- Ebner FF, Armstrong-James MA. Intracortical processes regulating the integration of sensory information. Prog Brain Res. 1990;86:129–141. doi: 10.1016/s0079-6123(08)63172-6. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erchova IA, Lebedev MA, Diamond ME. Somatosensory cortical neuronal population activity across states of anaesthesia. Eur J Neurosci. 2002;15:744–752. doi: 10.1046/j.0953-816x.2002.01898.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SM, Hetherington HP, Behar KL, Shulman RG. The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy. J Cereb Blood Flow Metab. 1990;10:170–179. doi: 10.1038/jcbfm.1990.32. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SM, Rothman D. New approaches to functional neuroenergetics. J Cogn Neurosci. 1999;11:467–471. doi: 10.1162/089892999563454. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Ashburner JT, Penny WD, Zeki S, Friston KJ, Frith CD, Dolan RJ, Price CJ. Human Brain Function. 2. Elsevier Academic Press; San Diego, California: 2004. [Google Scholar]
- Frahm J, Bruhn H, Merboldt KD, Hanicke W. Dynamic MR imaging of human brain oxygenation during rest and photic stimulation. J Magn Reson Imaging. 1992;2:501–505. doi: 10.1002/jmri.1880020505. [DOI] [PubMed] [Google Scholar]
- Frahm J, Kruger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med. 1996;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Gauthier CJ, Madjar C, Tancredi FB, Stefanovic B, Hoge RD. Elimination of visually evoked BOLD responses during carbogen inhalation: implications for calibrated MRI. Neuroimage. 2011;54:1001–1011. doi: 10.1016/j.neuroimage.2010.09.059. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Shulman RG. 1H NMR studies of glucose transport in the human brain. J Cereb Blood Flow Metab. 1996;16:427–438. doi: 10.1097/00004647-199605000-00009. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Rothman DL, Novotny EJ, Shulman RG. Localized 13C NMR spectroscopy of myo-inositol in the human brain in vivo. Magn Reson Med. 1992;25:204–210. doi: 10.1002/mrm.1910250121. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281:E100–112. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem. 1998;70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Heinke W, Fiebach CJ, Schwarzbauer C, Meyer M, Olthoff D, Alter K. Sequential effects of propofol on functional brain activation induced by auditory language processing: an event-related functional magnetic resonance imaging study. Br J Anaesth. 2004;92:641–650. doi: 10.1093/bja/aeh133. [DOI] [PubMed] [Google Scholar]
- Henry PG, Criego AB, Kumar A, Seaquist ER. Measurement of cerebral oxidative glucose consumption in patients with type 1 diabetes mellitus and hypoglycemia unawareness using (13)C nuclear magnetic resonance spectroscopy. Metabolism. 2010;59:100–106. doi: 10.1016/j.metabol.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P, Sanganahalli BG, Blumenfeld H, Hyder F. Cerebral oxygen demand for short-lived and steady-state events. J Neurochem. 2009;109(Suppl 1):73–79. doi: 10.1111/j.1471-4159.2009.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, Shulman RG. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci U S A. 1996;93:7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL. Evidence for the importance of measuring total brain activity in neuroimaging. Proc Natl Acad Sci U S A. 2011;108:5475–5476. doi: 10.1073/pnas.1102026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Mason GF, Rangarajan A, Behar KL, Shulman RG. Oxidative glucose metabolism in rat brain during single forepaw stimulation: a spatially localized 1H[13C] nuclear magnetic resonance study. J Cereb Blood Flow Metab. 1997;17:1040–1047. doi: 10.1097/00004647-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci U S A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Sanganahalli BG, Herman P, Coman D, Maandag NJ, Behar KL, Blumenfeld H, Rothman DL. Neurovascular and Neurometabolic Couplings in Dynamic Calibrated fMRI: Transient Oxidative Neuroenergetics for Block-Design and Event-Related Paradigms. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol. 1998;85:554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Pietrini P, Furey ML, Alexander GE, Millet P, Bokde AL, Teichberg D, Schapiro MB, Horwitz B, Rapoport SI. Resting state brain glucose metabolism is not reduced in normotensive healthy men during aging, after correction for brain atrophy. Brain Res Bull. 2004;63:147–154. doi: 10.1016/j.brainresbull.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Imas OA, Ropella KM, Wood JD, Hudetz AG. Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett. 2006;402:216–221. doi: 10.1016/j.neulet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Issa EB, Wang X. Sensory responses during sleep in primate primary and secondary auditory cortex. J Neurosci. 2008;28:14467–14480. doi: 10.1523/JNEUROSCI.3086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa EB, Wang X. Altered neural responses to sounds in primate primary auditory cortex during slow-wave sleep. J Neurosci. 2009;31:2965–2973. doi: 10.1523/JNEUROSCI.4920-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mason GF, Rothman DL, de Graaf RA, Behar KL. Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. J Cereb Blood Flow Metab. 2011;31:2313–2323. doi: 10.1038/jcbfm.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008;43:1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kanamori K, Parivar F, Ross BD. A 15N NMR study of in vivo cerebral glutamine synthesis in hyperammonemic rats. NMR Biomed. 1993;6:21–26. doi: 10.1002/nbm.1940060104. [DOI] [PubMed] [Google Scholar]
- Kanamori K, Ross BD, Kuo EL. Dependence of in vivo glutamine synthetase activity on ammonia concentration in rat brain studied by 1H - 15N heteronuclear multiple-quantum coherence-transfer NMR. Biochem J. 1995;311 (Pt 2):681–688. doi: 10.1042/bj3110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Glover GH, Moseley ME. Assessment of cerebral oxidative metabolism with breath holding and fMRI. Magn Reson Med. 1999;42:608–611. doi: 10.1002/(sici)1522-2594(199909)42:3<608::aid-mrm26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Scanley BE, Innis RB, Gore JC. Physiological basis for BOLD MR signal changes due to neuronal stimulation: separation of blood volume and magnetic susceptibility effects. Magn Reson Med. 1998;40:840–846. doi: 10.1002/mrm.1910400609. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med. 1994;31:9–21. doi: 10.1002/mrm.1910310103. [DOI] [PubMed] [Google Scholar]
- Kida I, Hyder F, Behar KL. Inhibition of voltage-dependent sodium channels suppresses the functional magnetic resonance imaging response to forepaw somatosensory activation in the rodent. J Cereb Blood Flow Metab. 2001;21:585–591. doi: 10.1097/00004647-200105000-00013. [DOI] [PubMed] [Google Scholar]
- Kida I, Hyder F, Kennan RP, Behar KL. Toward absolute quantitation of bold functional MRI. Adv Exp Med Biol. 1999;471:681–689. doi: 10.1007/978-1-4615-4717-4_78. [DOI] [PubMed] [Google Scholar]
- Kida I, Kennan RP, Rothman DL, Behar KL, Hyder F. High-resolution CMR(O2) mapping in rat cortex: a multiparametric approach to calibration of BOLD image contrast at 7 Tesla. J Cereb Blood Flow Metab. 2000;20:847–860. doi: 10.1097/00004647-200005000-00012. [DOI] [PubMed] [Google Scholar]
- Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: implications for dynamic functional magnetic resonance imaging calibration. J Cereb Blood Flow Metab. 2007;27:690–696. doi: 10.1038/sj.jcbfm.9600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida I, Smith AJ, Blumenfeld H, Behar KL, Hyder F. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. Neuroimage. 2006;29:216–224. doi: 10.1016/j.neuroimage.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ugurbil K. Comparison of blood oxygenation and cerebral blood flow effects in fMRI: estimation of relative oxygen consumption change. Magn Reson Med. 1997;38:59–65. doi: 10.1002/mrm.1910380110. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SG. Quantification of cerebral arterial blood volume and cerebral blood flow using MRI with modulation of tissue and vessel (MOTIVE) signals. Magn Reson Med. 2005;54:333–342. doi: 10.1002/mrm.20550. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SG. Temporal dynamics and spatial specificity of arterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J Cereb Blood Flow Metab. 2011;31:1211–1222. doi: 10.1038/jcbfm.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage. 2010;51:280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiev O, Buxton RB. Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated-BOLD fMRI. Neuroimage. 2007;35:175–184. doi: 10.1016/j.neuroimage.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO2 coupling measured with calibrated BOLD fMRI: sources of bias. Neuroimage. 2007;36:1110–1122. doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Gong L, Xu F. Brain-state-independent neural representation of peripheral stimulation in rat olfactory bulb. Proc Natl Acad Sci U S A. 2011;108:5087–5092. doi: 10.1073/pnas.1013814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Fox PT, Yang Y, Lu H, Tan LH, Gao JH. Time-dependent correlation of cerebral blood flow with oxygen metabolism in activated human visual cortex as measured by fMRI. Neuroimage. 2009;44:16–22. doi: 10.1016/j.neuroimage.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci U S A. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Mangia S, Giove F, Tkac I, Logothetis NK, Henry PG, Olman CA, Maraviglia B, Di Salle F, Ugurbil K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab. 2009;29:441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J Cereb Blood Flow Metab. 1995;15:12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petersen KF, de Graaf RA, Shulman GI, Rothman DL. Measurements of the anaplerotic rate in the human cerebral cortex using 13C magnetic resonance spectroscopy and [1-13C] and [2-13C] glucose. J Neurochem. 2007;100:73–86. doi: 10.1111/j.1471-4159.2006.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Rothman DL. Basic principles of metabolic modeling of NMR 13C isotopic turnover to determine rates of brain metabolism in vivo. Metab Eng. 2004;6:75–84. doi: 10.1016/j.ymben.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Mason GF, Rothman DL, Behar KL, Shulman RG. NMR determination of the TCA cycle rate and alphaketoglutarate/glutamate exchange rate in rat brain. J Cereb Blood Flow Metab. 1992;12:434–447. doi: 10.1038/jcbfm.1992.61. [DOI] [PubMed] [Google Scholar]
- Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7:732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- McCasland JS, Woolsey TA. High-resolution 2-deoxyglucose mapping of functional cortical columns in mouse barrel cortex. J Comp Neurol. 1988;278:555–569. doi: 10.1002/cne.902780407. [DOI] [PubMed] [Google Scholar]
- Merboldt KD, Bruhn H, Hanicke W, Michaelis T, Frahm J. Decrease of glucose in the human visual cortex during photic stimulation. Magn Reson Med. 1992;25:187–194. doi: 10.1002/mrm.1910250119. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Vlassenko AG, Shulman GL, Snyder AZ. Time-related increase of oxygen utilization in continuously activated human visual cortex. Neuroimage. 2002;16:531–537. doi: 10.1006/nimg.2002.1114. [DOI] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, Enev M, Sanganahalli BG, Hyder F, Blumenfeld H. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci. 2011;31:15053–15064. doi: 10.1523/JNEUROSCI.0101-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Duncan NW, Hayes DJ. The brain and its resting state activity--experimental and methodological implications. Prog Neurobiol. 2010;92:593–600. doi: 10.1016/j.pneurobio.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Stein DT, Telang F, Lee JH, Shen J, Brown P, Cline G, Mason GF, Shulman GI, Rothman DL, Hetherington HP. Spectroscopic imaging of glutamate C4 turnover in human brain. Magn Reson Med. 2000;44:673–679. doi: 10.1002/1522-2594(200011)44:5<673::aid-mrm3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Chowdhury GM, de Graaf RA, Rothman DL, Shulman RG, Behar KL. Cerebral pyruvate carboxylase flux is unaltered during bicuculline-seizures. J Neurosci Res. 2005a;79:128–138. doi: 10.1002/jnr.20311. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005b;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OA, Prichard JW, Ogino T, Avison M, Alger JR, Shulman RG. Combined 1H and 31P nuclear magnetic resonance spectroscopic studies of bicuculline-induced seizures in vivo. Ann Neurol. 1986;20:185–193. doi: 10.1002/ana.410200203. [DOI] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000;28:991–999. doi: 10.1016/s0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard JW, Shulman RG. NMR spectroscopy of brain metabolism in vivo. Annu Rev Neurosci. 1986;9:61–85. doi: 10.1146/annurev.ne.09.030186.000425. [DOI] [PubMed] [Google Scholar]
- Raichle ME. A brief history of human brain mapping. Trends Neurosci. 2009;32:118–126. doi: 10.1016/j.tins.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37:430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restom K, Perthen JE, Liu TT. Calibrated fMRI in the medial temporal lobe during a memory-encoding task. Neuroimage. 2008;40:1495–1502. doi: 10.1016/j.neuroimage.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach DL. Spontaneous and driven cortical activity: implications for computation. Curr Opin Neurobiol. 2009;19:439–444. doi: 10.1016/j.conb.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Eriksson L, Stone-Elander S, Widen L. Does mental activity change the oxidative metabolism of the brain? J Neurosci. 1987;7:2373–2389. [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hetherington HP, den Hollander JA, Bendall MR, Petroff OA, Shulman RG. 1H-Observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci U S A. 1985;82:1633–1637. doi: 10.1073/pnas.82.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Novotny EJ, Shulman GI, Howseman AM, Petroff OA, Mason G, Nixon T, Hanstock CC, Prichard JW, Shulman RG. 1H-[13C] NMR measurements of [4-13C]glutamate turnover in human brain. Proc Natl Acad Sci U S A. 1992;89:9603–9606. doi: 10.1073/pnas.89.20.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Sibson NR, Hyder F, Shen J, Behar KL, Shulman RG. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Philos Trans R Soc Lond B Biol Sci. 1999;354:1165–1177. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanganahalli BG, Herman P, Blumenfeld H, Hyder F. Oxidative neuroenergetics in event-related paradigms. J Neurosci. 2009;29:1707–1718. doi: 10.1523/JNEUROSCI.5549-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992;12:584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Young MP. Neuronal population activity and functional imaging. Proc Biol Sci. 1999;266:875–881. doi: 10.1098/rspb.1999.0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex. 2008;18:1814–1827. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Roland PE. Vibratory stimulation increases and decreases the regional cerebral blood flow and oxidative metabolism: a positron emission tomography (PET) study. Acta Neurol Scand. 1992;86:60–67. doi: 10.1111/j.1600-0404.1992.tb08055.x. [DOI] [PubMed] [Google Scholar]
- Serres S, Raffard G, Franconi JM, Merle M. Close coupling between astrocytic and neuronal metabolisms to fulfill anaplerotic and energy needs in the rat brain. J Cereb Blood Flow Metab. 2008;28:712–724. doi: 10.1038/sj.jcbfm.9600568. [DOI] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Sibson NR, Cline G, Behar KL, Rothman DL, Shulman RG. 15N-NMR spectroscopy studies of ammonia transport and glutamine synthesis in the hyperammonemic rat brain. Dev Neurosci. 1998;20:434–443. doi: 10.1159/000017341. [DOI] [PubMed] [Google Scholar]
- Shen Q, Ren H, Duong TQ. CBF, BOLD, CBV, and CMRO2 fMRI signal temporal dynamics at 500-msec resolution. J Magn Reson Imaging. 2008;27:599–606. doi: 10.1002/jmri.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci U S A. 2001a;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001b;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Baseline brain energy supports the state of consciousness. Proc Natl Acad Sci U S A. 2009;106:11096–11101. doi: 10.1073/pnas.0903941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci U S A. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci U S A. 1999;96:3245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. Neuroimage. 2007;36:277–281. doi: 10.1016/j.neuroimage.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci U S A. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during [2-13C]glucose infusion. J Neurochem. 2001;76:975–989. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Brain Energy Metabolism. Wiley and Sons, Ltd; New York, USA: 1978. [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Silva AC, Liu JV, Hirano Y, Leoni RF, Merkle H, Mackel JB, Zhang XF, Nascimento GC, Stefanovic B. Longitudinal functional magnetic resonance imaging in animal models. Methods Mol Biol. 2011;711:281–302. doi: 10.1007/978-1-61737-992-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Relationship between functional activity and energy metabolism in the nervous system: whether, where and why? In: Lassen NA, Ingvar DH, Raichle ME, Friberg L, editors. Brain work and mental activity. Munksgaard: Copenhagen; 1991. pp. 52–64. [Google Scholar]
- Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Cocchiarella A, Firth P, Rosen B, Lake S, Lange N, Routledge C, Albert M. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci U S A. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Kobayashi E, Bagshaw AP, Hawco C, Dubeau F, Gotman J, Pike GB. Hemodynamic and metabolic responses to activation, deactivation and epileptic discharges. Neuroimage. 2005;28:205–215. doi: 10.1016/j.neuroimage.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Uludag K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage. 2004;23:148–155. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Marrett S, Meyer E, Evans AC, Gjedde A. Increased oxygen consumption in human visual cortex: response to visual stimulation. Acta Neurol Scand. 1998;98:85–89. doi: 10.1111/j.1600-0404.1998.tb01724.x. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Meyer E, Marrett S, Paus T, Evans AC, Gjedde A. Frequency-dependent changes in cerebral metabolic rate of oxygen during activation of human visual cortex. J Cereb Blood Flow Metab. 1999;19:272–277. doi: 10.1097/00004647-199903000-00005. [DOI] [PubMed] [Google Scholar]
- van Eijsden P, Behar KL, Mason GF, Braun KP, de Graaf RA. In vivo neurochemical profiling of rat brain by 1H-[13C] NMR spectroscopy: cerebral energetics and glutamatergic/GABAergic neurotransmission. J Neurochem. 2010;112:24–33. doi: 10.1111/j.1471-4159.2009.06428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez AL, Masamoto K, Kim SG. Dynamics of oxygen delivery and consumption during evoked neural stimulation using a compartment model and CBF and tissue PO2 measurements. Neuroimage. 2008;42:49–59. doi: 10.1016/j.neuroimage.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang L, Jiang Y, Ma X, Chowdhury GM, Mason GF. Regional metabolite levels and turnover in the awake rat brain under the influence of nicotine. J Neurochem. 2010;113:1447–1458. doi: 10.1111/j.1471-4159.2010.06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shen J. In vivo evidence for reduced cortical glutamate-glutamine cycling in rats treated with the antidepressant/antipanic drug phenelzine. Neuroscience. 2005;135:927–937. doi: 10.1016/j.neuroscience.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]