Abstract
Many studies have linked ambient fine particulate matter (aerodynamic diameters less than 2.5 μm, PM2.5) air pollution to increased morbidity and mortality of cardiovascular diseases in the general population, but the biologic mechanisms of these associations are yet to be elucidated. In this study, we aimed to evaluate the relationship between daily variations in exposure to PM2.5 and inflammatory responses in mice during and for 2 months after the Beijing Olympic Games. Male C57BL/6 mice were exposed to Beijing PM2.5 or filtered air (FA) in 2008 during the 2 months of Beijing Olympic and Paralympic Games, and for 2 months after the end of the Games. During the Games, circulating monocyte chemoattractant protein 1 and interleukin 6 were increased significantly in the PM2.5 exposure group, when compared with the FA control group, although there were no significant inter-group differences in tumor necrosis factor α or interferon γ, or in macrophages, neutrophils or lymphocytes in the spleen or thymus between these 2 groups. However, macrophages were significantly increased in the lung and visceral fat with increasing PM2.5. After the Olympic Games, there were no significant PM2.5-associated differences for macrophages, neutrophils or lymphocytes in the thymus, but macrophages were significantly elevated in the lung, spleen, subcutaneous and visceral fat with increasing PM2.5, and the numbers of macrophages were even higher after than those during the Games. Moreover, the number of neutrophils was markedly higher in the spleen for the PM2.5-exposed- than the FA-group. These data suggest that short-term increases in exposure to ambient PM2.5 leads to increased systemic inflammatory responses, primarily macrophages and neutrophils in the lung, spleen, and visceral adipose tissue. Short-term air quality improvements were significantly associated with reduced overall inflammatory responses.
Keywords: air pollution, inflammation, PM2.5
Introduction
Many studies from large population cohorts have provided compelling associations between ambient fine particulate matter (PM2.5, particles less than 2.5 μm in aerodynamic diameter) air pollution exposure and increased cardiovascular morbidity and mortality (Miller et al., 2007; Pope et al., 2004), but the biologic mechanisms of these associations and underlying factors remain unclear. Although inflammation appears to play a critical role in this process as demonstrated by numerous investigations (Dandona et al., 2004; Hotamisligil, 2006; Shoelson et al., 2006), the systemic responses in inflammation are yet to be elucidated.
The ambient PM2.5 air pollution in Beijing during the 2008 Olympic and Paralympic Games (from August 8th to September 17th) decreased remarkably as a result of a series of air quality control measurements that were implemented by the Beijing municipal government, including banning the use of more than half of the motor vehicles in Beijing, moving or closing pollution-emitting factories in and around the city, and reducing heavy-duty truck traffic (Wang et al., 2010; Wang et al., 2009). The marked changes of air quality during and after the Olympic Games provided a unique opportunity to investigate the effects of ambient pollutant exposure and the level changes associated with systemic and tissue inflammation. A number of investigations have focused on the effect of air quality improvement on the heart rate variability, asthma and other diseases in human by using this advantage (Li et al., 2010; Liang et al., 2011; Wu et al., 2010, 2011), few studies performed such an investigation on animals. We therefore conducted this study to evaluate the relationship between the PM2.5 exposure and changes in systemic and tissue inflammation in mice exposed to PM2.5 air pollution during and after the Olympic Games. The objectives of this study were also to determine whether reduced PM2.5 air pollution with improved air quality in Beijing during the Olympic Games was associated with a persistent reduction in inflammation.
Materials and Methods
2.1. Animals and diets
Male C57BL/6 mice (8-week-old) were purchased from Animal Center of Peking University (Beijing, China). All groups had access to rodent diet and water ad libitum throughout the study duration. The mice were housed on a 12-hour light-dark cycle in a temperature-controlled room at 23°C. NIH guidelines for the care and use of laboratory animals were strictly followed, and all experiments were approved by the Animal Care and Use Committee at Peking University and the Ohio State University.
2.2. Air pollutant exposure
Air pollutant concentrations were measured continuously using monitoring equipment installed inside and outside the exposure facility. The mice were exposed (for 24 hours/day) to ambient PM2.5 or filtered air (FA) during (August 1st to September 30th, During) or after (October 4th to December 4th, After) the Beijing 2008 Olympic and Paralympics Games in exposure chambers in Beijing, China. A cyclone was used in the PM2.5-exposed group to remove the particles larger than 2.5 μm. The mice in the FA group were exposed to an identical protocol with the exception that a High Efficiency Particulate Air filter (HEPA) was positioned in the inlet valve to the exposure system to remove all of the particles from that air stream.
2.3. Measurement of blood inflammatory biomarkers
After the exposures, the mice were euthanized by overdose isoflurane inhalation, and blood was collected, spun, and plasma was stored at −80°C for the analysis of cytokines. Cytokine levels were determined by Cytometric Bead Array (BD Biosciences). Serum was incubated with beads specific for tumor necrosis factor (TNF) α, interferon γ (IFN-γ), monocyte chemoattractant protein 1 (MCP-1), interleukin 6 (IL-6), IL-10, and IL-12p70 according to the manufacturer’s instructions. The total amount of cytokines was then determined using a BD LSR II instrument and analyzed by the BD CBA software (BD, Biosciences).
2.4. Immunohistochemistry
Following the exposures, tissue samples of the spleen, thymus, lung, subcutaneous and epididymal adipose tissues were harvested. Immunohistochemical staining was performed as previously described (Xu et al., 2010). Briefly, deparaffinized sections (5 μm) were subjected to heat-induced antigen retrieval. The slides were dipped in 0.3% H2O2 for 10 minutes to quench the endogenous peroxidase and the sections were incubated in 1% BSA for 10 minutes, followed by overnight incubation with primary antibodies at 4°C. Then the slides were rinsed and incubated at room temperature for 2 hours with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies. The stain was developed using Fast 3,3′-diaminobenzidine tablet sets (D4293; Sigma), and the sections were counterstained with hematoxylin and examined by light microscopy. The primary antibodies were rat anti-mouse F4/80 (macrophage marker, AbD Serotec, Raleigh, NC), Armenian hamster anti-mouse T cell receptor (TCR)-β (Biolegend, San Diego, CA), and rat anti-mouse NIMP-R14 (neutrophil marker, Abcam, Cambridge, MA). All measurements were conducted in a double-blinded manner by two independent investigators. The slides were analyzed with a Nikon Eclipse FN1 microscope (Nikon, Tokyo, Japan) and the automatic computer-based analysis was performed using MetaMorph software. The pixel intensity of the positive threshold area was determined as a percentage of the total area for each image.
2.5. Real-time PCR
Epididymal adipose tissues from the mice were excised, minced, and RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Total RNA were then converted into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied biosystems, Foster City, CA). The quantification of gene expression was determined by real-time PCR. All reactions were performed under the same conditions: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. The primers for mouse IL-6, MCP-1, TNF-α, and β–actin are shown in Table 1, and β–actin was used as the housekeeping gene. All data are represented as relative mRNA levels to FA group during the Olympic Games.
Table 1.
Primers used for real-time PCR
| Primer | Forward oligonucleotides | Reverse oligonucleotides |
|---|---|---|
| IL-6 | 5′-ATCCAGTTGCCTTCTTGGGACTGA-3′ | 5′-TAAGCCTCCGACTTGTGAAGTGGT-3′ |
| MCP-1 | 5′-TCACCTGCTGCTACTCATTCACCA-3′ | 5′-TACAGCTTCTTTGGGACACCTGCT-3′ |
| TNF-α | 5′-CAACGGCATGGATCTCAAAGAC-3′ | 5′-AGATAGCAAATCGGCTGACGGT-3′ |
| β-actin | 5′-TGTGATGGTGGGAATGGGTCAGAA-3′ | 5′-TGTGGTGCCAGATCTTCTCCATGT-3′ |
2.6. Statistical analysis
Data are expressed as mean ± s.e. unless otherwise indicated. The experimental results were analyzed by several statistical methods (unpaired t tests, analysis of variance) and were performed using Graphpad Prism v5.0 (GraphPad Software, San Diego, CA). In all cases, a P value of < 0.05 was considered statistically significant. When multiple testing procedures were used (i.e, multiple t tests), the Bonferroni correction was applied.
Results
3.1. Exposure Characterization
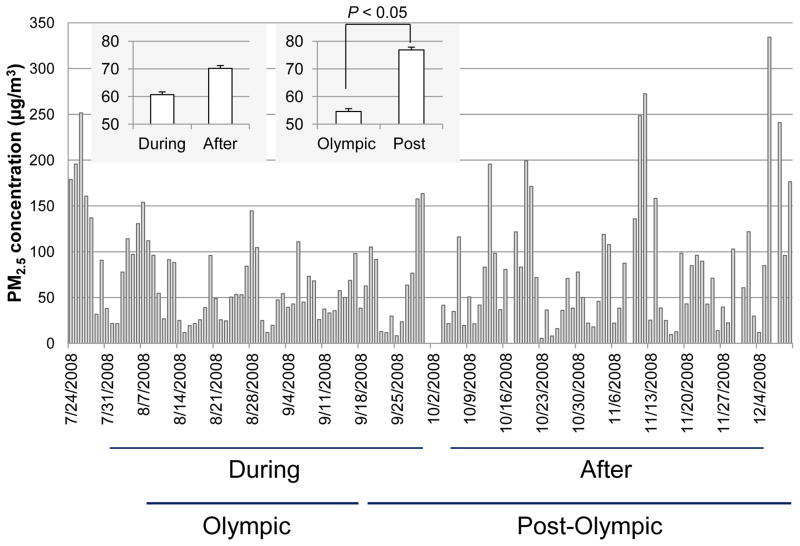
As shown in Figure 1, PM2.5 exposure levels are presented as daily averaged PM2.5 mass concentrations measured from the sampling filter of the exposure chamber. The mean of the individual PM2.5 mass concentrations over the period of the Olympic Games (from August 8th to September 17th, 54.62 ± 5.03 μg/m3) were significantly lower than those of the period after (from September 18th to December 10th, 76.88 ± 7.68 μg/m3, P < 0.05), but still well above the 24-hour U.S. National Ambient Air Quality Standard (NAAQS) with a PM2.5 concentration limit of 35 μg/m3, the 2005 WHO Air Quality Guideline (AQG) interim target-2 (50 μg/m3), and the WHO AQG goal of 25 μg/m3. We took these marked differences of air quality as a unique opportunity to investigate the effects of air quality improvement on inflammatory responses. The mice were exposed to PM2.5 for 2 months. The mean individual PM2.5 exposure levels over 2 months covering the period during the Olympic Games (from August 1st to September 30th, 60.67 ± 5.16 μg/m3) were modestly lower than after the Olympic Games (from October 4th to December 4th, 70.21 ± 7.72 μg/m3, respectively).
Figure 1.
Levels of PM2.5 concentration measured from filters during and after the Beijing 2008 Olympic and Paralympics Games. The mice were exposed to ambient PM2.5 or filtered air (FA) for 2 months during and after the Beijing 2008 Olympic and Paralympics Games in the exposure chambers, respectively. During, denotes the period from August 1st to September 30th; After, denotes the period from October 4th to December 4th. Olympic, denotes the period of the Olympic Games (from August 8th to September 17th). Post-Olympic, denotes period post-Olympic Games (from September 18th to December 10th).
3.2. Serum Cytokine Measurement
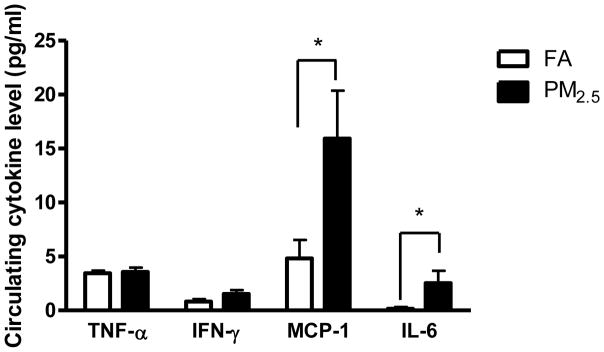
After 2 months of the exposure during the Olympic Games, the level of IL-10 or IL-12p70 in serum was low and non-detectable. As shown in Figure 2, although the levels of TNFα and IFN-γ were comparable between PM2.5- and FA-exposed groups, serum MCP-1 and IL-6 were both significantly increased in PM2.5-exposed mice when compared with the FA-exposed mice.
Figure 2.
Circulating cytokine levels after the Olympic Games. *, P < 0.05.
3.3. Effect of PM2.5 exposure on gene expression in visceral adipose tissue
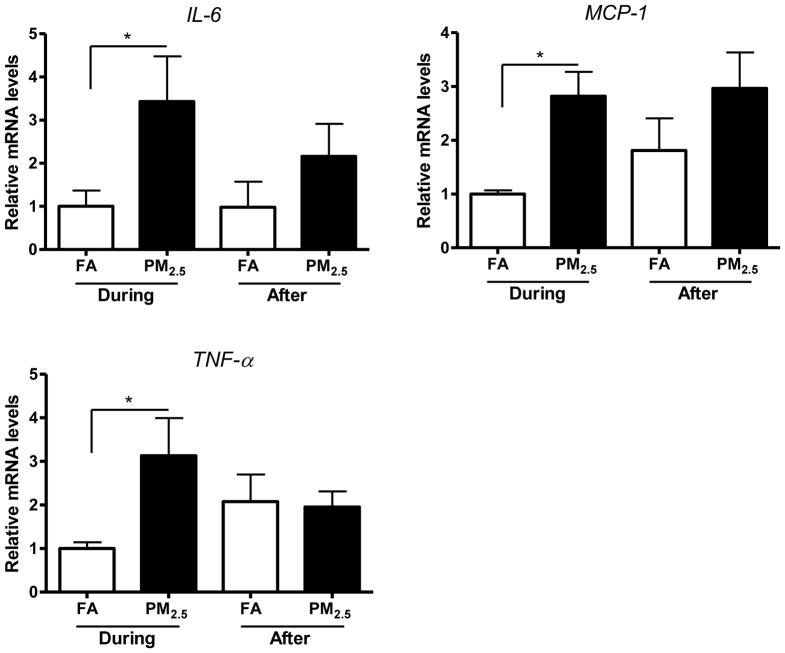
Adipose tissue macrophages (ATM), especially in visceral adipose tissue, are thought to represent key cellular mediators of adipose tissue inflammatory response and insulin resistance (IR) development. Previous studies have shown that exposure to concentrated PM2.5 (CAPs) may result in inflammation and IR systemically and locally in adipose tissues, especially visceral adipose tissue (Sun et al., 2005; Sun et al., 2009; Xu et al., 2011; Xu et al., 2010). We therefore measured inflammatory gene expression in epididymal (visceral) adipose tissue in these mice. As shown in Figure 3, gene expressions of IL-6, MCP-1, and TNF-α were all increased by PM2.5 exposure during the Olympic Games. Similarly, after the Olympic Games, the gene levels of MCP-1 and TNF-α were also increased in response to PM2.5 exposure.
Figure 3.
Inflammatory gene expressions of IL-6, MCP-1, and TNF-α in epididymal adipose tissue. *, P < 0.05.
3.4. Effect of PM2.5 exposure on inflammatory cell infiltration in lung
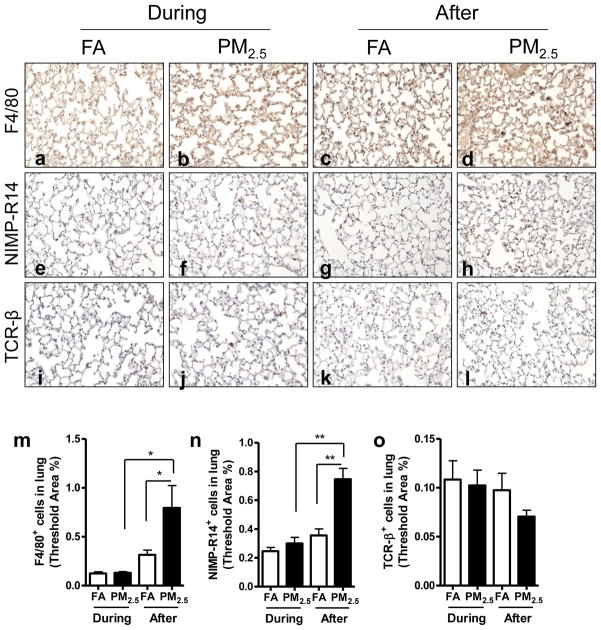
As shown in Figure 4, we did not find any significant difference in macrophage, neutrophil or lymphocyte infiltration in the lung by PM2.5 exposure during the Olympic Games time period. However, macrophages and neutrophils were significantly elevated in the lung by exposure to PM2.5 after the Olympic Games.
Figure 4.
Effect of PM2.5 exposure on inflammatory cell infiltration in lung. Representative images of immunohistochemistry for macrophages (F4/80+) (a–d), neutrophils (NIMP-R14+) (e–h), and T lymphocytes (TCR-β+) (i–l). Original magnification ×200. m–o, the analyses of the inflammatory cell infiltration in the lung. *, P < 0.05; **, P < 0.001.
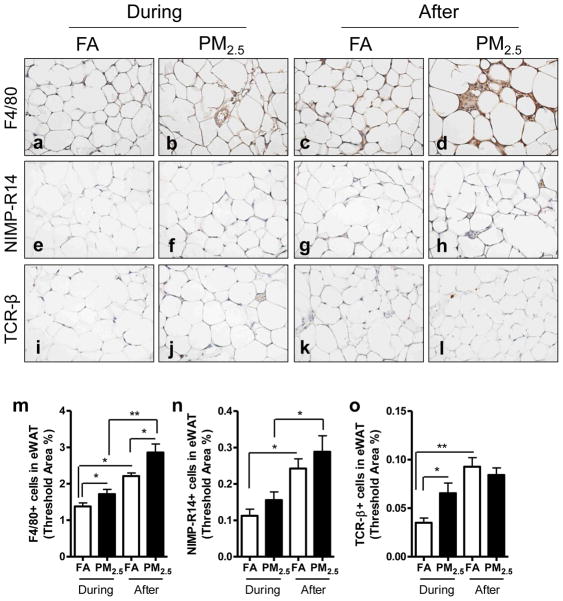
3.5. Effect of PM2.5 exposure on inflammatory cell infiltration in visceral adipose tissue
As shown in Figure 5, more macrophages (F4/80+) and lymphocytes (TCR-β+) were recruited in the visceral fat (epididymal fat) by PM2.5 exposure during the Olympic Games compared with FA-exposed control. Moreover, PM2.5 exposure after the Olympic Games resulted in a significant increase in macrophages and neutrophils (NIMP-R14+) recruited in the visceral fat depot. As unexpectedly, these cells were also significantly increased in the FA group after the Olympic Games.
Figure 5.
Effect of PM2.5 exposure on inflammatory cell infiltration in epididymal adipose tissue. Representative images of immunohistochemistry for macrophages (F4/80+) (a–d), neutrophils (NIMP-R14+) (e–h), and T lymphocytes (TCR-β+) (i–l). Original magnification ×400. m–o, the analyses of the inflammatory cell infiltration in epididymal adipose tissue. *, P < 0.05; **, P < 0.001.
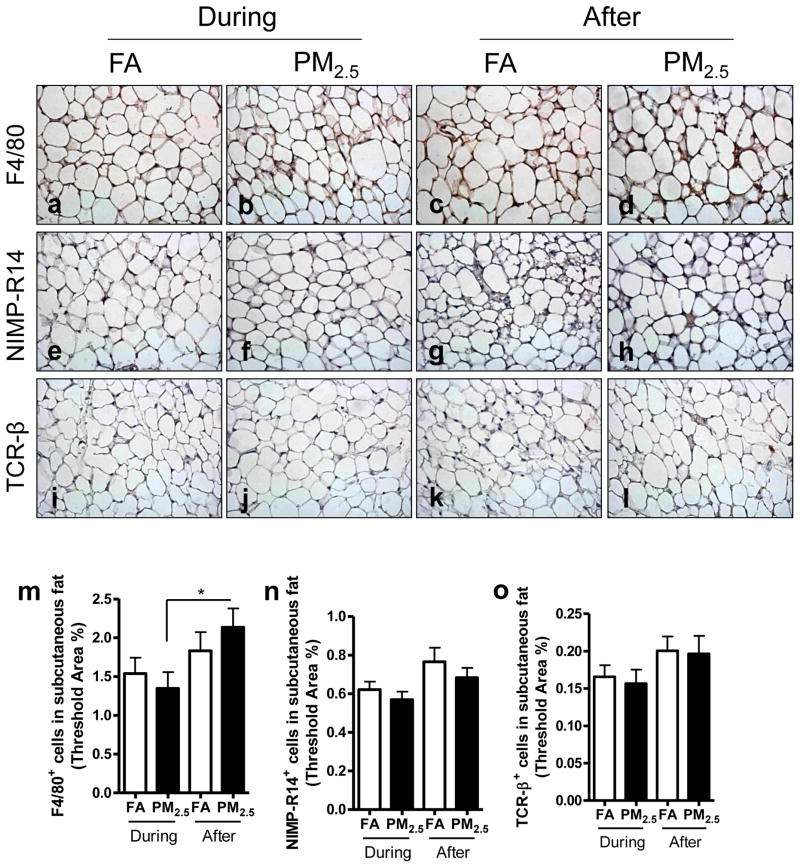
3.6. Effect of PM2.5 exposure on inflammatory cell infiltration in subcutaneous adipose tissue
As shown in Figure 6, more macrophages (F4/80+) were recruited in the subcutaneous adipose tissue by PM2.5 exposure after the Olympic Games compared with PM2.5-exposed group during the Olympic Games, although there were no significant differences among the groups for neutrophils and lymphocytes.
Figure 6.
Effect of PM2.5 exposure on inflammatory cell infiltration in subcutaneous adipose tissue. Representative images of immunohistochemistry for macrophages (F4/80+) (a–d), neutrophils (NIMP-R14+) (e–h), and T lymphocytes (TCR-β+) (i–l) in subcutaneous adipose tissue. Original magnification ×400. m–o, the analyses of the inflammatory cell infiltration in subcutaneous adipose tissue. *, P < 0.05.
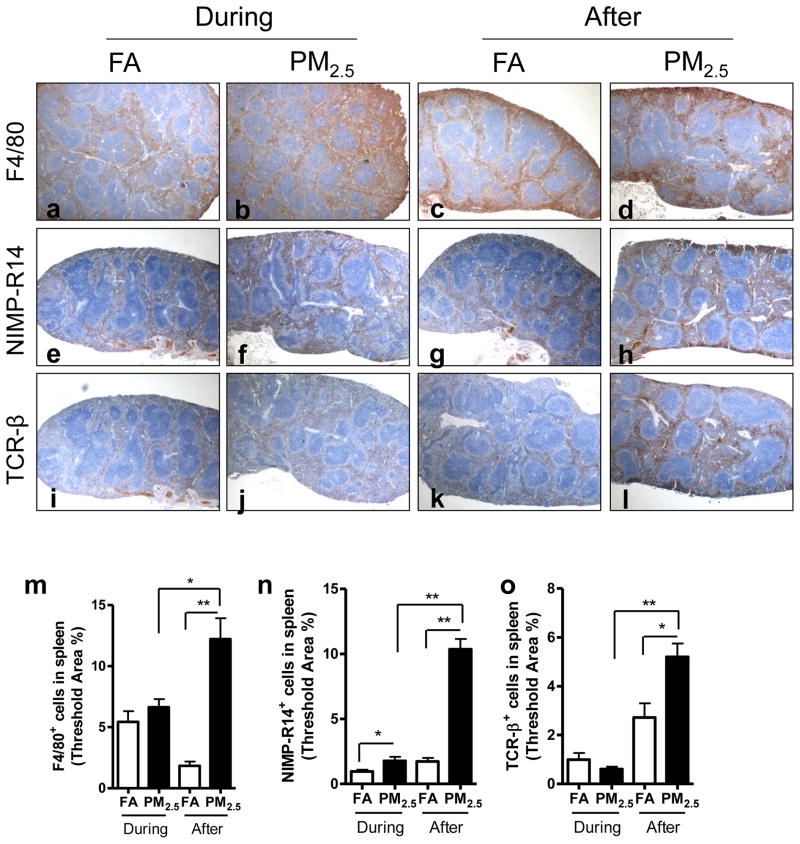
3.7. Effect of PM2.5 exposure on inflammatory cell infiltration in spleen
As shown in Figure 7, there was a significant increase in neutrophils recruited in the spleen by PM2.5 exposure during the Olympic Games compared with FA-exposed control. However, PM2.5 exposure after the Olympic Games led to a dramatic increase in macrophages, neutrophils, and lymphocytes in the spleen.
Figure 7.
Effect of PM2.5 exposure on inflammatory cell infiltration in spleen. Representative images of immunohistochemistry for macrophages (F4/80+) (a–d), neutrophils (NIMP-R14+) (e–h), and T lymphocytes (TCR-β+) (i–l) in spleen. Original magnification ×40. m–o, the analyses of the inflammatory cell infiltration in spleen. *, P < 0.05; **, P < 0.001.
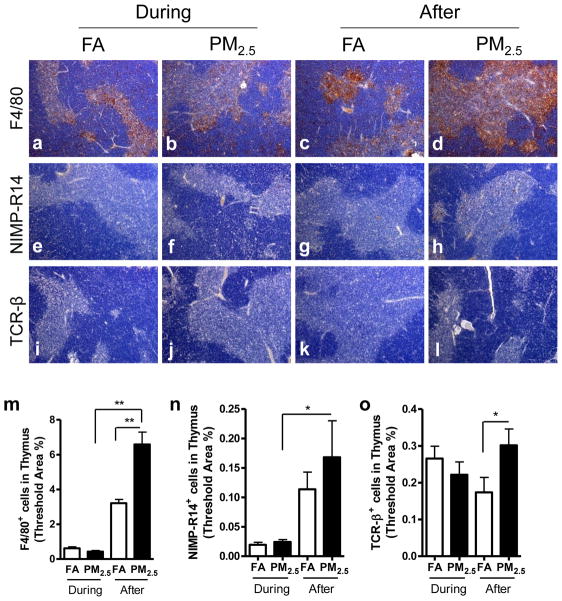
3.8. Effect of PM2.5 exposure on inflammatory cell infiltration in thymus
As shown in Figure 8, we did not find any significant difference in macrophages, neutrophils, or lymphocytes in the thymus by PM2.5 exposure during the Olympic Games compared with FA-exposed control. However, PM2.5 exposure after the Olympic Games resulted in significantly more macrophages, neutrophils, and lymphocytes recruited in the thymus than FA-exposed group or PM2.5-exposed group during the Olympic Games.
Figure 8.
Effect of PM2.5 exposure on inflammatory cell infiltration in thymus. Representative images of immunohistochemistry for macrophages (F4/80+) (a–d), neutrophils (NIMP-R14+) (e–h), and T lymphocytes (TCR-β+) (i–l) in thymus. Original magnification ×100. m–o, the analyses of the inflammatory cell infiltration in thymus. *, P < 0.05; **, P < 0.001.
Discussion
In this study, in view of the significant air quality improvement in Beijing during the 2008 Olympic Games, we assessed real-time, “real world” exposure to PM2.5 during and after the Olympic Games associated with statistically significant differences in exposure levels, and evaluated the relationship between PM2.5 and systemic and tissue inflammation with three major inflammatory cell lineages: neutrophil, monocyte/macrophage, and lymphocyte. The main findings of this investigation were that increased PM2.5 exposure was associated with increases of the systemic chemokine levels of MCP-1 and IL-6, and with induced infiltration of macrophages and neutrophils in the adipose tissue, lung, spleen, and thymus. T lymphocytes were also observed in these tissues other than lung in relation to PM2.5 exposure. In addition, air quality improvement during the Olympic Games was significantly associated with reduced overall inflammatory responses in the mice, especially in the lung, visceral adipose tissue, spleen, and thymus.
The influence of ambient air PM2.5 on systemic response in inflammation has been unclear. A number of studies have shown that exposure to PM2.5 is associated with a systemic pro-inflammatory response (e.g, increased TNF-α and IL-6 levels) in humans and animals (Calderon-Garciduenas et al., 2008; Mutlu et al., 2007; Thompson et al., 2010; Xu et al., 2010). Inflammation and oxidative stress pathways play critical roles in the process of IR, adiposity, and the development of cardiovascular risk in response to PM2.5, as demonstrated by numerous investigations (Dandona et al., 2004; Hotamisligil, 2006; Houstis et al., 2006; Shoelson et al., 2006; Xu et al., 2010). In addition, oxidative stress leads to an increase in the release of cytokines, especially MCP-1 (also known as CCL2), which plays an important role in the recruitment of monocytic cells to the site of inflammation. Our results demonstrate that exposure to PM2.5 can increase the MCP-1 and IL-6 levels, indicating that both cytokines are involved the inflammatory response to PM2.5.
Lung is an entry and front target organ for inhalants, rendering it as one of the most vulnerable organs resulting from inhalation exposure. Epidemiologic and animal studies have shown that airborne toxicant exposures may induce functional and/or histological lung changes, apoptosis and inflammation (Martin et al.; Riva et al.; Tamagawa et al., 2008). Since the lung inflammatory response is characterized by increased numbers of neutrophils, macrophages and T lymphocytes in pulmonary diseases (Keatings and Barnes, 1997; Saetta et al., 1993), whether these cells increase in response to PM2.5 exposure was investigated in this study. Although no significant difference of these inflammatory cells in lung was observed in response to the reduction in PM2.5 concentrations during the Olympic Games, our data showed that higher PM2.5 exposures in the 2 months of more normal Beijing exposures that followed the Olympic Games resulted in recruitment of macrophages and neutrophils, indicating that short-term air quality improvements were helpful in decreasing transient pulmonary inflammation.
Adipose tissue is not only a triglyceride-storage organ, but also an inflammation “reservoir”. Recent studies have shown the role of white adipose tissue as a producer of certain inflammatory substances called adipokines, including leptin, plasminogen activator inhibitor-1 (PAI-1), TNF-α, resistin, IL-6 (Fernandez-Sanchez et al., 2011; Fonseca-Alaniz et al., 2007). Data have shown that the irregular production of TNF-α participates in the pathogenesis of the obesity-associated metabolic syndrome (Dahlman et al., 2005; Ouchi et al.; Weisberg et al., 2006). IL-6 exerts many effects, ranging from defense to inflammation and tissue damage (Fonseca-Alaniz et al., 2007). Monocyte chemoattractant proteins (MCPs) and their receptors play pivotal roles in the development of inflammatory responses and are crucial for the recruitment of immune cells to sites of inflammation. In view of the inflammatory macrophage phenotype in adipose tissues, we showed increased expressions of IL-6, MCP-1, and TNF-α in response to PM2.5 exposure both during and after the Olympic Games, supported by a exposure led to a significant increase in the expression of prior study which showed PM2.5 proinflammatory genes (M1, or “classically” activated) (Sun et al., 2009; Xu et al., 2010).
By taking advantage of a highly sophisticated system of chemokines and chemokine receptors, leukocytes such as neutrophils, macrophages, and T-lymphocytes are targeted to the precise location of inflammation in response to inflammation reaction. Recruitment of adipose tissue macrophages (ATMs) are thought to represent key cellular mediators of adipose tissue inflammatory response, IR, and obesity development (Sun et al., 2009; Surmi and Hasty; Xu et al., 2010). In this study, PM2.5 exposure after the Olympic Games induced infiltration of macrophages both in the visceral adipose (epididymal fat) and in the subcutaneous adipose tissue, even though the elicited adipose inflammatory response was lower during the Olympic Games in comparison with that sustained after the Olympic Games. Consistent with it, our earlier studies have demonstrated ATMs play a pivotal role in the PM2.5-mediated inflammation in mice fed on both normal diet and high-fat diet (Sun et al., 2009; Xu et al., 2010). Since CCR2 deficiency decreased macrophage content of adipose tissue (Weisberg et al., 2006), the enhanced MCP1 expression may explain recruitment of these inflammatory cells in epididymal fat. To our knowledge, this study is the first to show neutrophils and T-lymphocytes are recruited to adipose tissue in response to PM2.5 exposure. However, visceral adipose tissue was shown to be more vulnerable than subcutaneous adipose tissue in the present study, the mechanism of which remains further investigation.
Although spleen and thymus are two important immune organs, there were few studies that show the inflammatory alterations, in response to airborne exposure, in these two organs. Similar to the result of adipose tissues, this study demonstrated that PM2.5 lead to an increase in macrophages, neutrophils and T lymphocytes both in spleen and thymus during 2 months of exposure after the Olympic Games, even though there was no significant difference in these organs during the Olympic Games in comparison with that sustained after the Olympic Games. These findings suggest that PM2.5 exposure at a relatively higher level leads to a multisystem inflammatory reaction with inflammatory cell infiltration in several organs, while air quality control and improvement could reduce these chronic adverse inflammatory responses.
While our study produced important new insights, there were several limitations. First, a more comprehensive mechanistic investigation and intervention was not possible due to the limitations of the “real world” exposures, and the distance of the exposure location to the Olympic Games Stadium. Second, although oxidative stress has been shown to play a pivotal role in pulmonary inflammation induced by airborne exposure (Martin et al.; Rhoden et al., 2008; Riva et al.), it was not measured in other organs. Third, the serum cytokine levels were measured only in the period after the Olympic Games. Fourth, although there was a control group (FA) for each time period that controlled for the co-presence of pollutant gases, the potential for the effects of differences between the during and after periods in seasonal, temperature, bioactive (pollens, mold, et al) agents, and the possible impacts of the pre-exposure environment of the animals could not be studied in this project due to technical limitations. In addition, the morphological changes of these organs induced by PM2.5 air pollution and related inflammatory gene profiles need to be further investigated.
In summary, our data suggest that ambient PM2.5 air pollution induces inflammatory responses in multiple organs and systemic inflammation as well, and that a modest air quality improvement substantially reduced the overall inflammatory response. These findings suggest an important public health impact on the health of human populations. Further understanding of the factors regulating the activation and accumulation of inflammatory cells in these organs may provide novel prevention and therapeutic strategies for better control and treatment of obesity and IR.
Highlights.
During the Games, circulating MCP-1 and IL-6 were increased in the PM2.5 group.
Macrophages were increased in the lung and visceral fat with increasing PM2.5.
After the Games, macrophages were elevated in lung, spleen, and adipose by PM2.5.
After the Games, neutrophils were infiltrated in the spleen by PM2.5 exposure.
Acknowledgments
This work was supported in part by National Institute of Health grant ES016588 to Dr. Sun, Ministry of Science and Technology of China grant 2006BAI19B06 and National Science Foundation of China grant 30571534 to Dr. Guo. The authors would like to thank Hong Liu at Beijing University for her technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Calderon-Garciduenas L, Villarreal-Calderon R, Valencia-Salazar G, Henriquez-Roldan C, Gutierrez-Castrellon P, Torres-Jardon R, Osnaya-Brizuela N, Romero L, Torres-Jardon R, Solt A, Reed W. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AS, Wahlen K, Andersson J, Nordstrom EA, Blomqvist L, Sjogren A, Forsgren M, Attersand A, Arner P. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab. 2005;90:5834–5840. doi: 10.1210/jc.2005-0369. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, Morales-Gonzalez JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J) 2007;83:S192–203. doi: 10.2223/JPED.1709. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. Am J Respir Crit Care Med. 1997;155:449–453. doi: 10.1164/ajrccm.155.2.9032177. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang W, Kan H, Xu X, Chen B. Air quality and outpatient visits for asthma in adults during the 2008 Summer Olympic Games in Beijing. Sci Total Environ. 2010;408:1226–1227. doi: 10.1016/j.scitotenv.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Liang XY, Lan L, Chen WN, Zhang AP, Lu CY, Lu YW, Dai JP. Disease distribution and medical resources during the Beijing 2008 Olympic and Paralympic Games. Chin Med J (Engl) 2011;124:1031–1036. [PubMed] [Google Scholar]
- Martin S, Fernandez-Alanis E, Delfosse V, Evelson P, Yakisich JS, Saldiva PH, Tasat DR. Low doses of urban air particles from Buenos Aires promote oxidative stress and apoptosis in mice lungs. Inhal Toxicol. 22:1064–1071. doi: 10.3109/08958378.2010.523030. [DOI] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Rhoden CR, Ghelfi E, Gonzalez-Flecha B. Pulmonary inflammation by ambient air particles is mediated by superoxide anion. Inhal Toxicol. 2008;20:11–15. doi: 10.1080/08958370701758379. [DOI] [PubMed] [Google Scholar]
- Riva DR, Magalhaes CB, Lopes AA, Lancas T, Mauad T, Malm O, Valenca SS, Saldiva PH, Faffe DS, Zin WA. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol. 23:257–267. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Maestrelli P, Ferraresso A, Drigo R, Potena A, Ciaccia A, Fabbri LM. Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis. 1993;147:301–306. doi: 10.1164/ajrccm/147.2.301. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. Jama. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, Zhang X, Xing L, Li Y, Laher I, Sin DD, Man SF, van Eeden SF. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol. 2008;295:L79–85. doi: 10.1152/ajplung.00048.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Zanobetti A, Silverman F, Schwartz J, Coull B, Urch B, Speck M, Brook JR, Manno M, Gold DR. Baseline repeated measures from controlled human exposure studies: associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect. 2010;118:120–124. doi: 10.1289/ehp.0900550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhao M, Xing J, Wu Y, Zhou Y, Lei Y, He K, Fu L, Hao J. Quantifying the air pollutants emission reduction during the 2008 Olympic games in Beijing. Environ Sci Technol. 2010;44:2490–2496. doi: 10.1021/es9028167. [DOI] [PubMed] [Google Scholar]
- Wang X, Westerdahl D, Chen LC, Wu Y, Hao J, Pan X, Guo X, Zhang KM. Evaluating the air quality impacts of the 2008 Beijing Olympic Games: on-road emission factors and black carbon profiles. Atmospheric Environment. 2009;43:4535–4543. [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Deng F, Niu J, Huang Q, Liu Y, Guo X. Association of heart rate variability in taxi drivers with marked changes in particulate air pollution in Beijing in 2008. Environ Health Perspect. 2010;118:87–91. doi: 10.1289/ehp.0900818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Deng F, Niu J, Huang Q, Liu Y, Guo X. The relationship between traffic-related air pollutants and cardiac autonomic function in a panel of healthy adults: a further analysis with existing data. Inhal Toxicol. 2011;23:289–303. doi: 10.3109/08958378.2011.568976. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Long-term Exposure to Ambient Fine Particulate Pollution Induces Insulin Resistance and Mitochondrial Alteration in Adipose Tissue. Toxicol Sci. 2011;124:88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Effect of Early Particulate Air Pollution Exposure on Obesity in Mice: Role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30:2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]