Background: TGF-β1 positively and negatively regulates osteoblast differentiation.
Results: Repeated TGF-β1 negatively regulates osteoblast differentiation caused by inhibiting IGF-1 expression and Akt phosphorylation.
Conclusion: Prolonged TGF-β1 treatment inhibits osteoblast differentiation of mesenchymal stem cells via the suppression of the IGF-1 signaling pathway.
Significance: IGF-1 administration may recover the suppression of osteogenesis and promotion of bone resorption due to chronic inflammation by TGF-β1.
Keywords: Akt, Cell Culture, Cell Differentiation, Cell Signaling, Insulin-like Growth Factor (IGF), Molecular Cell Biology, Osteoblasts, Transforming growth factor-β (TGFbeta)
Abstract
TGF-β1 can regulate osteoblast differentiation not only positively but also negatively. However, the mechanisms of negative regulation are not well understood. We previously established the reproducible model for studying the suppression of osteoblast differentiation by repeated or high dose treatment with TGF-β1, although single low dose TGF-β1 strongly induced osteoblast differentiation. The mRNA expression and protein level of insulin-like growth factor-1 (IGF-1) were remarkably decreased by repeated TGF-β1 administration in human periodontal ligament cells, human mesenchymal stem cells, and murine preosteoblast MC3T3-E1 cells. Repeated TGF-β1 administration subsequently decreased alkaline phosphatase (ALP) activity and mRNA expression of osteoblast differentiation marker genes, such as RUNX2, ALP, and bone sialoprotein (BSP). Additionally, repeated administration significantly reduced the downstream signaling pathway of IGF-1, such as Akt phosphorylation in these cells. Surprisingly, exogenous and overexpressed IGF-1 recovered ALP activity and mRNA expression of osteoblast differentiation marker genes even with repeated TGF-β1 administration. These facts indicate that the key mechanism of inhibition of osteoblast differentiation induced by repeated TGF-β1 treatment is simply due to the down-regulation of IGF-1 expression. Inhibition of IGF-1 signaling using small interfering RNA (siRNA) against insulin receptor substrate-1 (IRS-1) suppressed mRNA expression of RUNX2, ALP, BSP, and IGF-1 even with single TGF-β1 administration. This study showed that persistence of TGF-β1 inhibited osteoblast differentiation via suppression of IGF-1 expression and subsequent down-regulation of the PI3K/Akt pathway. We think this fact could open the way to use IGF-1 as a treatment tool for bone regeneration in prolonged inflammatory disease.
Introduction
Insulin-like growth factor-1 (IGF-1) plays an important role in cell growth, differentiation, survival, and cell cycle progression. IGF-1 is secreted by mature osteoblasts and can be stored locally in the bone matrix until its release during bone resorption. IGFs stimulate in vitro and in vivo osteoblast proliferation and differentiation through specific membrane receptors (1–4). IGF-1 up-regulation may partially mediate increased expression of bone matrix proteins and bone anabolic effects in aged ovariectomized rats (5). Although IGF-1 does not direct undifferentiated stromal cells to differentiate into cells of an osteoblast lineage, it enhances the function of mature osteoblasts (6). IGF-1 also plays a role in the regulation of chondrocyte differentiation (7, 8) and promotes longitudinal bone growth by augmenting chondrocyte hypertrophy (8). Exogenous IGF-1 markedly improves chondrocyte matrix biosynthesis (9).
Transforming growth factor-β1 (TGF-β1) is crucial for connective tissue regeneration and bone remodeling, as demonstrated by several in vivo and in vitro studies. It affects osteoblast differentiation and bone formation (10–14) and increases mRNA levels of osteoblast differentiation markers and alkaline phosphatase (ALP)2 activity in murine bone marrow stromal cells (12). However, TGF-β1 also blocks osteogenesis by various mechanisms depending on its concentration, cell density, and differentiation stage of the cells (15–17) and blocks odontogenesis by down-regulating dentin sialophosphoprotein (18). The mitogen-activated protein kinase (MAPK) pathway negatively regulates the Smad pathway and osteoblast mineralization (19, 20). Some studies have reported that TGF-β1 has biphasic and concentration-dependent effects on osteoblast differentiation (15, 21). Although the TGF-β/Smad pathway could be the major inducer of osteogenesis, the dual effect of TGF-β signaling and the mechanism by which TGF-β1 influences osteogenesis remain unexplained. TGF-β is also an anti-inflammatory cytokine. However, it induces the development of Th17 cells, which produce the proinflammatory cytokine IL-17 (22). Many cytokines, including TGF-β1, IL-1, IL-6, and TNF-α, appear to be involved in degenerative diseases such as osteoarthritis, although the extent of involvement is unknown. Like other cytokines, TGF-β1 may inhibit osteoregeneration during inflammation.
We recently established an experimental model to study the inhibitory mechanism of TGF-β1 in osteoblast differentiation and found that a single low dose TGF-β1 administration significantly promoted osteoblast differentiation, but its repeated or high dose administration inhibited osteoblast differentiation in normal human periodontal ligament (HPDL) cells (23). DNA microarray analysis revealed that repeated TGF-β1 administration markedly reduced IGF-1 expression. Therefore, we studied the effects of TGF-β1 on mRNA expression and production of IGF-1 to elucidate its action mechanism in HPDL cells and MC3T3-E1 cells. We found that repeated TGF-β1 administration caused IGF-1 down-regulation and Akt phosphorylation, resulting in the inhibition of osteoblast differentiation. Moreover, the inhibition was reversed by treatment with endogenous IGF-1.
EXPERIMENTAL PROCEDURES
Cell Culture and Osteogenic Differentiation
Normal HPDL cells and human mesenchymal stem cells (hMSC) were purchased from Lonza (Basel, Switzerland) and cultured in BulletKit® stromal cell growth medium (Lonza) and BulletKit® mesenchymal stem cell growth medium (Lonza), respectively. HPDL cells and hMSC of passages 5–8 and 3–4, respectively, were seeded at a density of 1 × 105 cells/cm2 for each assay. MC3T3-E1 cells were purchased from RIKEN BioResource Center (Ibaraki, Japan). MC3T3-E1 cells were seeded at a density of 1.6 × 105 cells/cm2 for each assay. Osteoblast differentiation was induced by replacing with the osteoblast differentiation medium (OBM), comprising α-MEM (Invitrogen) supplemented with 50 μg/ml l-ascorbic acid (Wako Pure Chemical Industries Ltd., Osaka, Japan) and 10 mm β-glycerophosphate (Wako), with or without rhTGF-β1 (Wako), and this was added the following day. Under the single TGF-β1 administration condition, the medium was not changed until day 3 or 4, whereas under repeated TGF-β1 administration conditions, OBM containing fresh TGF-β1 was changed every 12 h. Control cells were treated identically except that they did not receive TGF-β1.
Assay of ALP Activity and Mineralization
Three days after stimulation, cells were washed two times with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 5 min at room temperature, and washed three times with water. For staining, an ALP substrate solution (Roche Diagnostics) was added to the fixed cells for 60 min at room temperature. After staining, cells were washed three times with distilled water, and images were scored. ALP activity was measured as follows. The cells were washed twice with PBS and lysed with lysis buffer (10 mm Tris-HCl (pH 7.5), 150 mm NaCl, complete protease inhibitor mixture, and 1% Nonidet P-40). ALP activity was assayed using p-nitrophenyl phosphate as a substrate, and the protein content was measured with a DC protein assay kit (Bio-Rad) according to the manufacturer's instructions. ALP activity was then expressed as micromoles of p-nitrophenol/min/mg of protein.
To detect calcium deposits in mineralized tissue, cells were fixed by the same method and then stained with Alizarin Red S solution (pH 6.38) (Wako) for 5 min at room temperature. After washing five times with PBS, images captured with phase-contrast microscope.
Quantitative Real Time-PCR (qRT-PCR)
qRT-PCR was used to examine the expression of osteoblast differentiation markers. Following incubation for 48 and 72 h in the differentiation medium, total RNA was extracted using QIAzol reagent (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions. cDNA was synthesized using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). qRT-PCR analysis was performed using the Premix Ex TaqTM reagent (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. Target genes included RUNX2, ALP, BSP, and insulin-like growth factor 1 (IGF1), and osteocalcin (OC). 18 S rRNA was used as an internal control. All primers and probes are presented in Table 1 and were designed using Probefinder Version 2.45. Relative expression of genes of interest was estimated using ΔΔCt method.
TABLE 1.
Primers used for quantitative real time PCR
| Gene symbol | GenBankTM accession no. | Forward primer sequence | Reverse primer sequence |
|---|---|---|---|
| RUNX2 | NM_001024630.2 | gtgcctaggcgcatttca | gctcttcttactgagagtggaagg |
| ALP | NM_000478.3 | caaccctggggaggagac | gcattggtgttgtacgtcttg |
| BSP | NM_004967.3 | caatctgtgccactcactgc | tcattttggtgattgcttcct |
| IGF-1 | NM_000618.3 | tgtggagacaggggctttta | atccacgatgcctgtctga |
| OC | NM_199173.4 | tgagagccctcacactcctc | acctttgctggactctgcac |
| Alp | NM_007431.2 | cggatcctgaccaaaaacc | tcatgatgtccgtggtcaat |
| Igf-1 | NM_001111274.1 | tcggcctcatagtacccact | acgacatgatgtgtatctttattgc |
| Oc | NM_007541.2 | agactccggcgctacctt | ctcgtcacaagcagggttaag |
| 18 S rRNA | M11188.1 | cggacaggattgacagattg | cgctccaccaactaagaacg |
Protein Extraction and Immunoblotting
Cells were lysed with lysis buffer (10 mm Tris-HCl (pH 7.5), 150 mm NaCl, complete protease inhibitor mixture, 1 mm sodium orthovanadate, and 1% Nonidet P-40), and the protein content was measured using a DC protein assay kit (Bio-Rad). Equivalent protein concentrations were resolved by electrophoresis on NuPAGE 4–12% BisTris gels (Invitrogen) and transferred to a PVDF membrane. The membrane was probed with anti-phosphorylated Akt (1:2000; Cell Signaling Technology Inc., Danvers, MA) and anti-IGF1 (1:2500; Abcam, Cambridge, UK) antibodies, followed by HRP-conjugated goat anti-rabbit IgG. Bound antibodies were visualized using a chemiluminescent substrate (ECL plus; GE Healthcare) and ImageQuant LAS 4000 mini (GE Healthcare).
Small Interfering RNA (siRNA) Transfection
HPDL cells were transfected with siRNA for insulin receptor substrate 1 (IRS-1) (Ambion, siRNA ID s7520) using LipofectamineTM RNAiMAX (Invitrogen). An siRNA consisting of a scrambled sequence of similar length was transfected as a control. siRNA was diluted in 100 μl of Opti-MEM I medium without serum in each well of a new tissue culture plate. Subsequently, RNAiMAX was added to each well, followed by addition of an adequate number of cells in 500 μl of growth medium without antibiotics such that they were 30–50% confluent 24 h after plating. After 24 h, the medium was replaced with α-MEM containing 5% FBS, and cells were cultured for 48 h until confluent. To determine whether IRS-1 siRNA specifically silenced IRS-1 expression, we evaluated its protein expression in transfected HPDL cells by Western blotting.
Plasmid Construction and Transfection
A human IGF-1 expression plasmid was generated by inserting full-length IGF-1 cDNA into the expression vector pEF1/V5-His at the BamHI and EcoRI sites. MC3T3-E1 cells were plated in 12-well plates at 70–80% confluence (1 × 105 cells). Cells were transfected with plasmid mixtures containing 1.6 μg/well of pEF1/IGF1 or pEF1/mock using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. After 6 h, the medium was replaced with α-MEM containing 5% FBS, and cells were cultured for 72 h until confluent.
Statistical Analysis
All data are expressed as mean ± S.E. When analysis of variance indicated differences among the groups, multiple comparisons between each experimental group were performed using the Bonferroni test. Statistical significance was defined as p < 0.05.
RESULTS
Repeated TGF-β1 Administration Inhibits Osteoblast Differentiation in HPDL Cells, MC3T3-E1 Cells, and hMSC
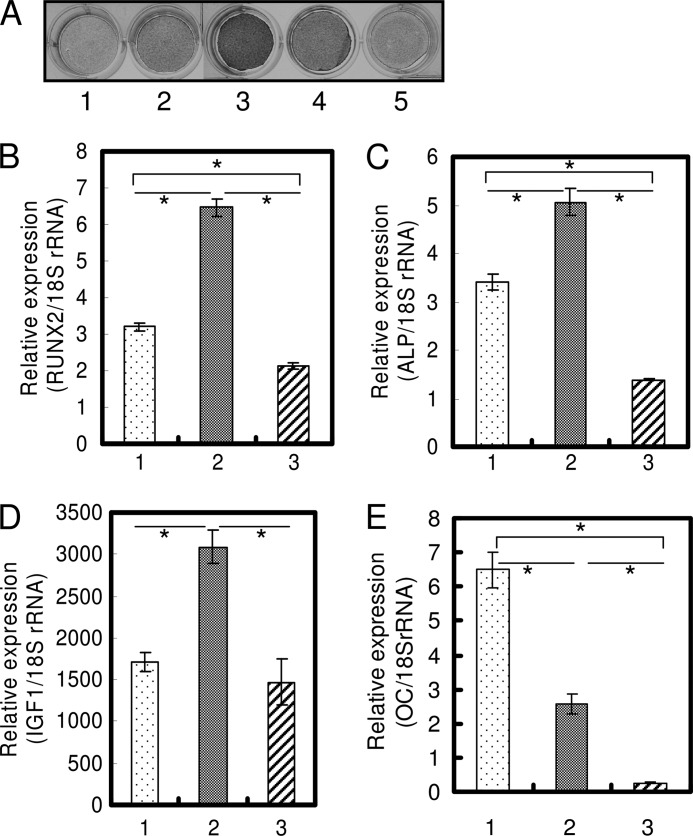
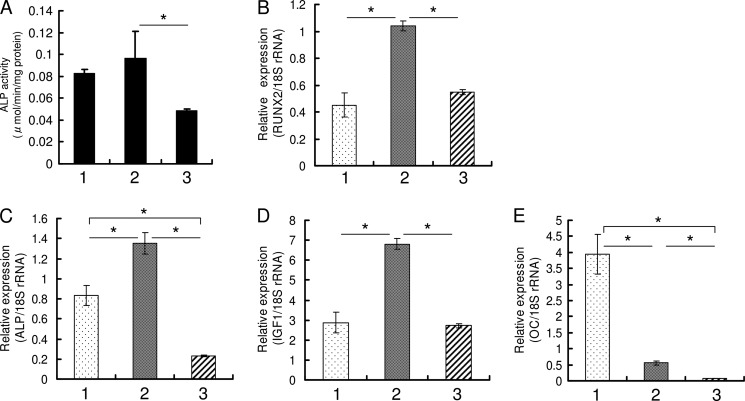
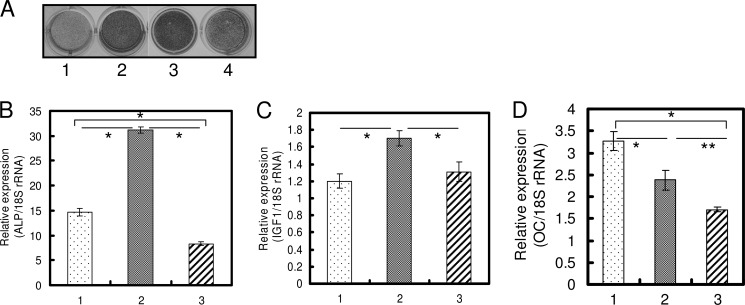
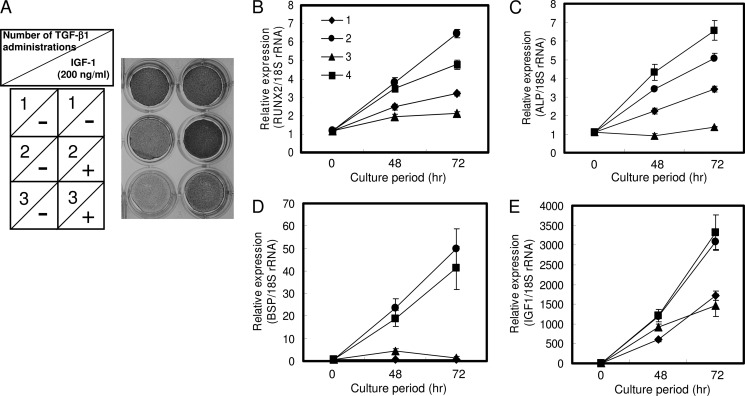
We found that the same dose of TGF-β1 could positively as well as negatively regulate osteoblast differentiation, and we reported that single treatment with 1 ng/ml TGF-β1 induced osteoblast differentiation (23). The direction of regulation was determined by the number of administrations. In HPDL cells and hMSC, 1 ng/ml TGF-β1 was suitable to induce osteoblast differentiation, and in MC3T3-E1 cells 0.1 ng/ml TGF-β1 was suitable in our preliminary experiments (data not shown). We investigated the differences between a single and repeated TGF-β1 administration by comprehensive analysis using DNA microarray and found that IGF-1 expression decreased ∼10-fold in cells administered repeated doses of TGF-β1 compared with cells administered a single dose of TGF-β1 (data not shown). In HPDL cells and hMSC, as the number of administrations of 1.0 ng/ml TGF-β1 increased, ALP activity and mRNA expression of osteoblast differentiation-related genes (RUNX2 and ALP) were significantly decreased (Figs. 1, A–C, and 2, A–C). The decrease in IGF-1 mRNA expression revealed by DNA microarray was confirmed by qRT-PCR (Figs. 1D and 2D). Repeated TGF-β1 administration caused the decrease of ALP activity and IGF-1 mRNA expression regardless of serum condition (supplemental Fig. S1). After 2 weeks of osteogenic culture in the presence of serum, HPDL cells also showed increased mineralization by a single administration of 1.0 ng/ml TGF-β1 and showed decreased mineralization by repeated administration of 1.0 ng/ml TGF-β1 (supplemental Fig. S2). OC mRNA expression level was decreased by TGF-β1 treatment (Figs. 1E and 2E). Sowa et al. (32) also reported that TGF-β1 treatment reduced the Oc mRNA level in MC3T3-E1 cells. In MC3T3-E1 cell line, which is a well characterized murine preosteoblast cell line for studying osteoblast differentiation, ALP activity and mRNA expression of ALP and IGF-1 were significantly decreased by repeated administration of 0.1 ng/ml TGF-β1 (Fig. 3). Oc mRNA level was decreased by TGF-β1 treatment in MC3T3-E1 cells.
FIGURE 1.
Repeated TGF-β1 administration inhibits expression of osteoblast differentiation markers in HPDL cells. A, HPDL cells were cultured in α-MEM (1), OBM (2), OBM with a single administration of 1 ng/ml TGF-β1 (3), OBM with a double administration of 1 ng/ml TGF-β1 (4), and OBM with a triple administration of 1 ng/ml TGF-β1 (5) for 72 h. ALP activity was visualized by ALP activity staining of cells. A single TGF-β1 administration markedly induced ALP activity. However, as the number of administrations increased, ALP activity gradually decreased. B–E, confluent cells were treated for 72 h with OBM (1), OBM with a single administration of 1 ng/ml TGF-β1 (2), and OBM with a double administration of 1 ng/ml TGF-β1 (3). Repeated TGF-β1 administration inhibited the expression of RUNX2 (B), ALP (C), IGF-1 (D), and OC (E). Expression of these genes was analyzed by qRT-PCR, and their mRNA levels were normalized to that of 18 S rRNA and measured in triplicate. Figures shown represent at least three independent experiments. Values represent mean ± S.E. (n = 4). Bonferroni correction for multiple comparisons was applied. *, p < 0.001.
FIGURE 2.
Repeated TGF-β1 administration inhibits expression of osteoblast differentiation markers in hMSC. A, cells were cultured in OBM (1), OBM with a single administration of 1 ng/ml TGF-β1 (2), OBM with a triple administration of 1 ng/ml TGF-β1 (3) for 4 days after which ALP activity was measured. B–E, confluent cells were treated for 96 h with OBM (1), OBM with a single administration of 1 ng/ml TGF-β1 (2), and OBM with a triple administration of 1 ng/ml TGF-β1 (3). Repeated TGF-β1 administration inhibited the expression of RUNX2 (B), ALP (C), IGF-1 (D), and OC (E). Expression of these genes was analyzed by qRT-PCR, and their mRNA levels were normalized to that of 18 S rRNA and measured in triplicate. Values represent mean ± S.E. (n = 4). Bonferroni correction for multiple comparisons was applied. *, p < 0.001.
FIGURE 3.
Repeated TGF-β1 administration inhibits expression of osteoblast differentiation markers in MC3T3-E1 cells. A, MC3T3-E1 cells were cultured in α-MEM (1), OBM (2), OBM with a single administration of 0.1 ng/ml TGF-β1 (3), and OBM with repeated administration of 0.1 ng/ml TGF-β1 (4) for 72 h. ALP activity was visualized by ALP activity staining of cells. A single TGF-β1 administration induced ALP activity. However, repeated TGF-β1 administration suppressed ALP activity. B, confluent cells were treated for 72 h with OBM (1), OBM with a single administration of 0.1 ng/ml TGF-β1 (2), and OBM with repeated administration of 0.1 ng/ml TGF-β1 (3). Repeated TGF-β1 administration inhibited the expression of Alp (B), Igf-1 (C), and Oc (D). Expression of these genes was analyzed by qRT-PCR, and their mRNA levels were normalized to that of 18 S rRNA and measured in triplicate. Figures shown represent at least three independent experiments. Values represent mean ± S.E. (n = 4). Bonferroni correction for multiple comparisons was applied. *, p < 0.001; **, p < 0.01.
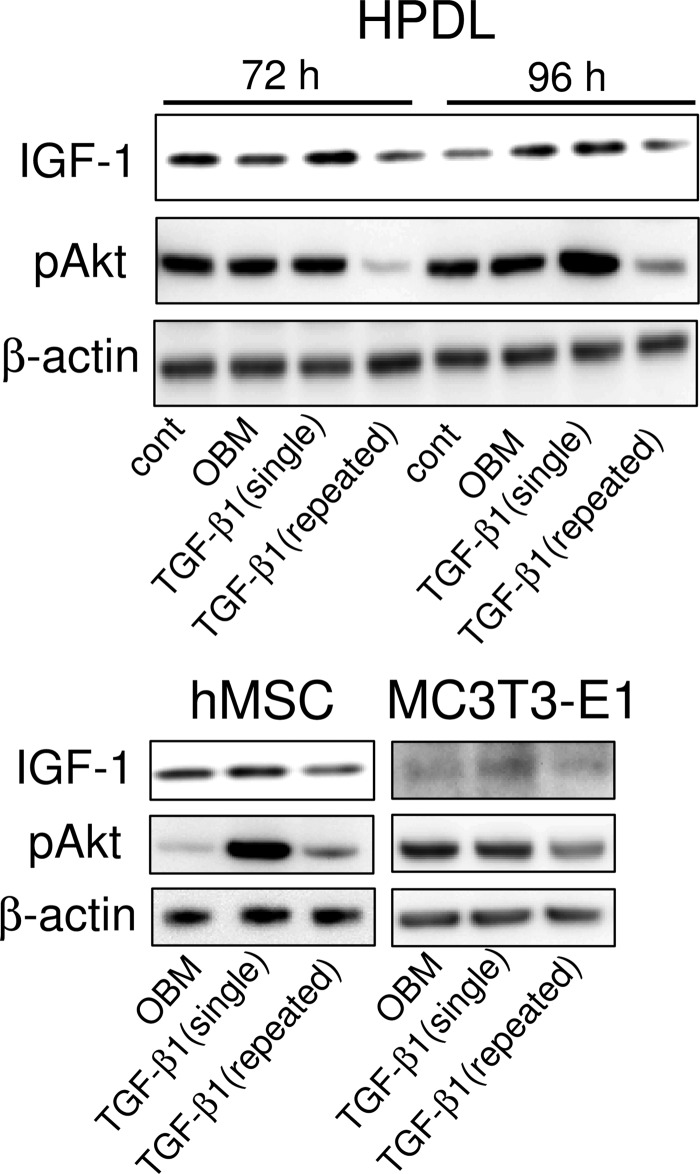
Repeated TGF-β1 Treatment Inhibits IGF-1 Expression and Akt Phosphorylation
We conducted Western blot analysis to examine the levels of IGF-1 protein and phosphorylated Akt (pAkt), a downstream effector of PI3K. In HPDL cells, repeated TGF-β1 administration significantly decreased IGF-1 protein at 72 and 96 h (Fig. 4). Akt phosphorylation was also remarkably reduced after repeated TGF-β1 administration at 72 and 96 h. Similarly, IGF-1 and pAkt protein level was decreased by repeated TGF-β1 treatment in both hMSC and MC3T3-E1 cells. However, both IGF-1 protein and phosphorylated Akt increased after a single TGF-β1 administration compared with the untreated control.
FIGURE 4.
Repeated TGF-β1 administration inhibits IGF-1/Akt signaling. Confluent cells were cultured in OBM and treated with a single or repeated TGF-β1 administration for 72 or 96 h without serum starvation culture. Top panel, HPDL cells were cultured for 72 or 96 h. cont, control. Bottom left panel, hMSC were cultured for 96 h. Bottom right panel, MC3T3-E1 cells were cultured for 72 h. The levels of IGF-1 protein and phosphorylated Akt were determined by Western blot analysis. Blots are representative of three experiments.
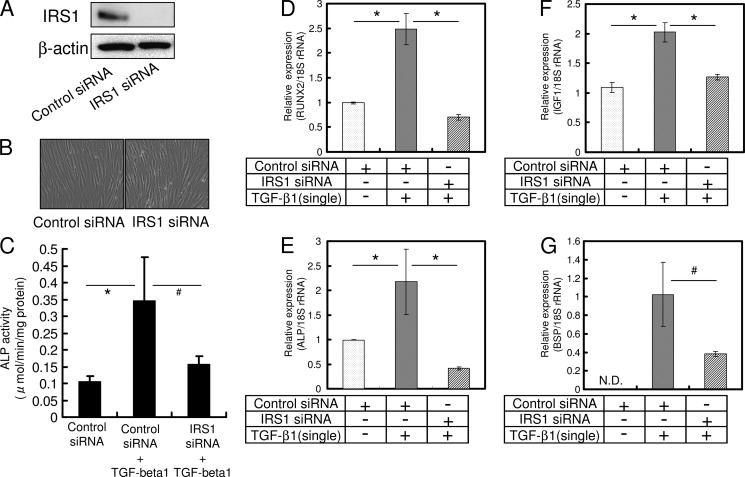
Inhibition of IRS-1 Inhibits Osteoblast Differentiation Initiated by a Single TGF-β1 Treatment in HPDL Cells
We examined whether the inhibition of IGF-1 signaling could suppress a promotion of osteoblast differentiation by a single administration of TGF-β1 (1 ng/ml). siRNA transfection did not affect proliferation or cell density (Fig. 5B). ALP activity was suppressed by single TGF-β1 treatment in cells with knockdown of IRS-1, whereas single TGF-β1 treatment stimulated ALP activity in control siRNA-transfected cells (Fig. 5C). Analysis by qRT-PCR showed that knockdown of ISR-1 by siRNA resulted in a marked reduction of mRNA expression of RUNX2, ALP, IGF-1, and BSP in cells administered a single dose of TGF-β1 (Fig. 5, D–G).
FIGURE 5.
Inhibition of IGF-1 signaling by a specific siRNA suppresses TGF-β1-induced expression of osteoblast differentiation marker genes. HPDL cells were transfected with IRS-1 siRNA or control siRNA (A) and cultured in α-MEM for 3 days until confluent (B). Confluent cells were treated for 72 h in the presence or absence of 1 ng/ml TGF-β1. Transfection with IRS-1 siRNA reduced the expression of RUNX2 (D), ALP (E), IGF-1 (F), and BSP (G). Expression of these genes was analyzed by qRT-PCR, and their mRNA levels were normalized to that of 18 S rRNA and measured in triplicate. Figures shown represent at least three independent experiments. Values represent mean ± S.E. (n = 4). Bonferroni correction for multiple comparisons was applied. #, p < 0.05; *, p < 0.01.
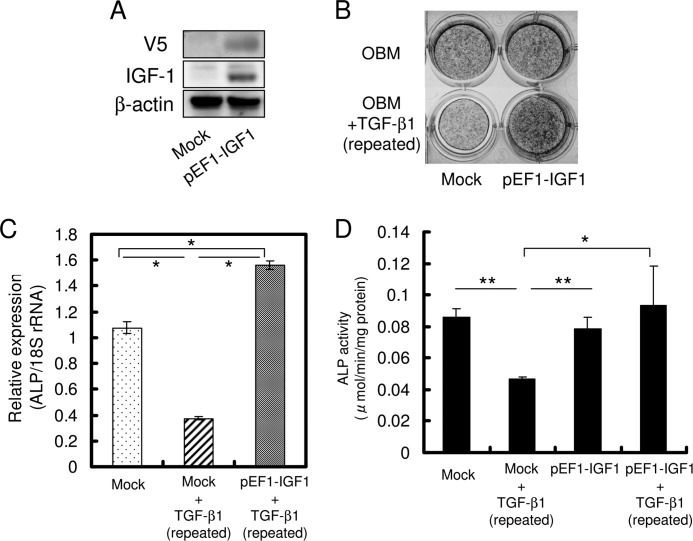
Exogenous IGF-1 Recovers the Suppression of Osteoblast Differentiation by Repeated TGF-β1 Administration in HPDL Cells
IGF-1 stimulates osteoblast differentiation and bone formation. Furthermore, IGF-1 is essential for TGF-β1-induced osteoblast differentiation, as indicated by our results. However, the effect of IGF-1 on the inhibition of osteoblast differentiation by repeated TGF-β1 administration is not well known. We therefore evaluated the effect of exogenous IGF-1 on HPDL cells. As shown in Fig. 6A, ALP activity was remarkably diminished with increased TGF-β1 administrations. Interestingly, the addition of 200 ng/ml IGF-1 fully recovered the suppression of ALP activity by repeated TGF-β1 administration. Exogenous IGF-1 also significantly increased mRNA expression of RUNX2, ALP, and BSP (Fig. 6, B–D). We found that IGF-1 expression markedly increased on treatment with exogenous IGF-1 (Fig. 6E).
FIGURE 6.
Exogenous IGF-1 restores inhibition of osteoblast differentiation by repeated TGF-β1 administration in HPDL cells. A, HPDL cells were cultured in OBM with a single administration of 1 ng/ml TGF-β1, OBM with a double administration of 1 ng/ml TGF-β1, and OBM with a triple administration of 1 ng/ml TGF-β1 for 72 h. Exogenous IGF-1 (200 ng/ml) was added concomitantly with double or triple TGF-β1 administration. ALP activity was visualized by ALP activity staining of cells. It gradually decreased as the number of TGF-β1 administrations increased. However, exogenous IGF-1 completely restored the down-regulation of ALP activity by repeated TGF-β1 administration. B, confluent cells were treated for 72 h with OBM (1), OBM with a single administration of 1 ng/ml TGF-β1 (2), OBM with a double administration of 1 ng/ml TGF-β1 (3), and OBM with a double administration of 1 ng/ml TGF-β1 along with 200 ng/ml IGF-1 (4). Addition of IGF-1 fully recovered the expression of RUNX2 (B), ALP (C), BSP (D), and IGF-1 (E). Expression of these genes was analyzed by qRT-PCR, and their mRNA levels were normalized to that of 18 S rRNA and measured in triplicate. Figures shown represent at least three independent experiments. Values represent mean ± S.E. (n = 4).
IGF-1 Overexpression Restores Osteoblast Differentiation in MC3T3-E1 Cells
To determine whether IGF-1 was essential for osteoblast differentiation, MC3T3-E1 cells were transfected with the pEF1-IGF1 plasmid. Repeated administration of 0.1 ng/ml TGF-β1 in mock-transfected cells resulted in a decrease in ALP activity and mRNA expression (Fig. 7, B–D). However, ALP activity was not decreased by repeated administration of 0.1 ng/ml TGF-β1 in pEF1-IGF1-transfected cells. These pEF1-IGF1-transfected cells with repeated administration of TGF-β1 had higher ALP activity than cells that were mock-transfected without treatment. These results indicate that IGF-1 enhances osteoblast differentiation only after it is induced by TGF-β1.
FIGURE 7.
Overexpression of IGF-1 restores inhibition of osteoblast differentiation by repeated TGF-β1 administration in MC3T3-E1 cells. A, MC3T3-E1 cells were transfected with pEF1-V5-IGF1 vector or pEF1-mock vector. Transfected cells were cultured in α-MEM for 3 days until confluent, and then cells were treated with or without repeated administration of 0.1 ng/ml TGF-β1 for 72 h. Transfection with the pEF1-V5-IGF1 vector completely recovered the inhibition of ALP activity staining (B), ALP mRNA expression (C), and ALP activity (D) by repeated TGF-β1 administration. Expression of ALP was analyzed by qRT-PCR. The mRNA level of Alp was normalized to that of 18 S rRNA and measured in triplicate. Figures shown represent at least three independent experiments. Values represent mean ± S.E. (n = 4). Bonferroni correction for multiple comparisons was applied. *, p < 0.001; **, p < 0.01.
DISCUSSION
Growth factors such as TGF-β, bone morphogenetic protein, fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) are proteins secreted by cells that act on target cells to promote bone regeneration. TGF-β1 is abundantly expressed in the periosteum, osteocytes (24), and during fracture healing in vivo (25, 26). TGF-β1 also exerts antiproliferative effects and functions as a tumor suppressor during early stages of tumorigenesis and as a tumor promoter aiding in metastatic progression at later stages (27–29). Similarly, TGF-β signaling also regulates osteoblast differentiation both positively and negatively. Recombinant human TGF-β1 induces in vivo endochondral bone formation in extraskeletal sites of adult baboons (14, 30). TGF-β1-loaded microgranules have a high bone regenerative capacity, ALP activity, and osteocalcin content in rabbit calvarial defects (11). Smad3 null mice are osteopenic, with less cortical and cancellous bones compared with wild-type littermates (31). Overexpression of Smad3 enhances ALP activity, mineralization, and production of bone matrix proteins in MC3T3-E1 cells (32). Despite evidence supporting its positive effect on osteoblast differentiation, TGF-β1 has been shown to inhibit osteocalcin promoter activity and osteocalcin expression in rat primary osteoblasts (19). TGF-β1 suppresses mRNA expression of osteoblast differentiation genes, such as Alp, Bsp, and Oc, in MC3T3-E1 cells (33). Although these inhibitory effects on osteoblast differentiation have been described, the mechanism of suppression remains unknown. We had previously established a reproducible model for studying the suppression of osteoblast differentiation by TGF-β1, showing that TGF-β1 negatively regulates osteoblast differentiation by prolonged and repeated TGF-β1 treatment (23).
In this study, we found that repeated TGF-β1 administration remarkably decreased IGF-1 expression in HPDL, hMSC, and MC3T3-E1 cells. IGF-1 is an important regulator of bone homeostasis and is central to the achievement of normal longitudinal bone growth and bone mass. Therefore, the inhibition of autocrine IGF-1 expression may decrease the effects of IGF-1 on these cells and cause inhibition of osteoblast differentiation. The IGF-1 gene knock-out mice revealed that the fetal mice demonstrated short limb dwarfism delays in mineralization and increased chondrocyte apoptosis (34). Long term recombinant human IGF-1 therapy for short children with severe IGF-1 deficiency caused an increase in height velocity from 2.8 cm/year on average at base line to 8.0 cm/year during the 1st year of treatment (1). The combined delivery of BMP-7 and IGF-1 genes synergistically enhanced the differentiation of HPDL cells while suppressing their proliferation (4). Once activated, IGF-1 can interact with its receptor, IGF-1R, to induce signaling. Following stimulation by a ligand, the IRSs are rapidly phosphorylated on multiple tyrosine residues (35). These phosphorylated substrates bind to proteins containing Src homology 2 domains and later activate PI3K/Akt signaling to regulate cell differentiation, growth, and metabolism (36). We previously found that the PI3K inhibitor LY294002 completely abrogated TGF-β1-induced osteoblast differentiation of HPDL cells (data not shown). Repeated TGF-β1 administration markedly inhibited IGF-1 expression and subsequently Akt phosphorylation and then decreased osteoblast differentiation marker expression. Thus, these results suggest that the inhibition of osteoblast differentiation caused by repeated TGF-β1 administration is associated with the down-regulation of IGF-1 expression and Akt inactivation.
To examine whether the IGF-1 signaling cascade plays an important role in TGF-β1-induced osteoblast differentiation, siRNAs for IRS-1 were used. The inhibition of IGF-1 signaling resulted in down-regulation of mRNA expression of osteoblast differentiation marker gene, although a single TGF-β1 administration induced osteoblast differentiation of HPDL cells (Fig. 5). Our results revealed that TGF-β1-induced osteoblast differentiation requires IGF-1 expression. However, repeated TGF-β1 treatment down-regulates IGF-1 expression that appears to be the key mechanism for the negative effects on osteoblast differentiation caused by TGF-β1.
Interestingly, exclusive exogenous IGF-1 markedly restored the down-regulated ALP staining and osteoblast differentiation marker gene expression caused by repeated TGF-β1 administration (Fig. 6). IGF-1 has been found to be very potent and is localized at healing fracture sites in rats and humans. Locally applied IGF-1 and TGF-β1 from IGF-1/TGF-β1-coated polylactide membranes distinctly increases bone area in healing fractures in a sheep model of delayed callus formation (2). According to our results, TGF-β1 is inadequate for stimulation of osteoblast differentiation. Topical IGF-1 application using gelatin hydrogels is well tolerated and may be efficacious for hearing recovery in patients with sudden sensorineural hearing loss that is resistant to systemic glucocorticoids (37). Thus, topical IGF-1 treatment may be utilized as a new therapeutic measure by inducing osteoblastic differentiation. Next, we investigated whether overexpression of IGF-1 can promote osteoblast differentiation. Osteoblast differentiation was inhibited in HPDL, MC3T3-E1 cells, and hMSC by repeated TGF-β1 administration (Figs. 1–3). IGF-1 overexpression successfully recovered the suppression of osteoblast differentiation by repeated TGF-β1 administration. ALP expression in pEF1-IGF1-transfected cells repeatedly administered TGF-β1 was higher compared with pEF1-IGF1-transfected cells that were not administered TGF-β1 (Fig. 7, B–D). We also observed that a single TGF-β1 administration is sufficient to up-regulate ALP expression (data not shown). IGF-1 enhances the function of mature osteoblasts, although IGF-1 does not direct the differentiation of undifferentiated stromal cells toward the osteoblast lineage (38). Our observations support the fact that IGF-1 acts as an enhancer only after osteoblast differentiation is initiated by TGF-β1. Therefore, IGF-1 secreted from cells could induce osteoblast differentiation through paracrine or autocrine mechanisms. IGF-1 signaling is essential for osteoblast differentiation induced by TGF-β1 administration.
TGF-β1 influences a broad range of cellular activities, including anti-inflammatory processes, growth, differentiation, and extracellular matrix synthesis. TGF-β1 also induces IL-6 release in human bronchial epithelial cells and prostate cancer cells (39, 40). Our experimental conditions involving repeated TGF-β1 administration may correspond to the suppression of osteogenesis and promotion of bone resorption due to chronic inflammation, because chronic inflammation could induce prolonged TGF-β1 expression.
In conclusion, our results reveal that TGF-β1 inhibits osteoblast differentiation via suppression of IGF-1 expression and subsequent down-regulation of the PI3K/Akt pathway. Our results suggest that exclusive IGF-1 administration recovers the suppression of osteogenesis and promotion of bone resorption due to chronic inflammation, such as during periodontal disease and rheumatoid arthritis. Thus, local application of IGF-1 may be useful as a treatment tool for bone regeneration in prolonged inflammatory disease.
Supplementary Material
This work was supported by Oral Health Science Center Grant hrc8 from Tokyo Dental College and by a Project for Private Universities, matching fund subsidy from Ministry of Education, Culture, Sports, Science and Technology of Japan, 2010-2012.

This article contains supplemental Figs. S1 and S2.
- ALP
- alkaline phosphatase
- HPDL
- human periodontal ligament
- hMSC
- human mesenchymal stem cell
- BSP
- bone sialoprotein
- OBM
- osteoblast differentiation medium
- α-MEM
- α-minimal essential medium
- qRT
- quantitative real time
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- OC
- osteocalcin.
REFERENCES
- 1. Chernausek S. D., Backeljauw P. F., Frane J., Kuntze J., Underwood L. E., and GH Insensitivity Syndrome Collaborative Group (2007) Long term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-I deficiency due to growth hormone insensitivity. J. Clin. Endocrinol. Metab. 92, 902–910 [DOI] [PubMed] [Google Scholar]
- 2. Bernstein A., Mayr H. O., Hube R. (2010) Can bone healing in distraction osteogenesis be accelerated by local application of IGF-1 and TGF-β1? J. Biomed. Mater. Res. B Appl. Biomater. 92, 215–225 [DOI] [PubMed] [Google Scholar]
- 3. Nakayama Y., Nakajima Y., Kato N., Takai H., Kim D. S., Arai M., Mezawa M., Araki S., Sodek J., Ogata Y. (2006) Insulin-like growth factor-I increases bone sialoprotein (BSP) expression through fibroblast growth factor-2 response element and homeodomain protein-binding site in the proximal promoter of the BSP gene. J. Cell Physiol. 208, 326–335 [DOI] [PubMed] [Google Scholar]
- 4. Yang L., Zhang Y., Dong R., Peng L., Liu X., Wang Y., Cheng X. (2010) Effects of adenoviral-mediated coexpression of bone morphogenetic protein-7 and insulin-like growth factor-1 on human periodontal ligament cells. J. Periodontal Res. 45, 532–540 [DOI] [PubMed] [Google Scholar]
- 5. Power R. A., Iwaniec U. T., Wronski T. J. (2002) Changes in gene expression associated with the bone anabolic effects of basic fibroblast growth factor in aged ovariectomized rats. Bone 31, 143–148 [DOI] [PubMed] [Google Scholar]
- 6. Walsh S., Jefferiss C. M., Stewart K., Beresford J. N. (2003) IGF-I does not affect the proliferation or early osteogenic differentiation of human marrow stromal cells. Bone 33, 80–89 [DOI] [PubMed] [Google Scholar]
- 7. Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (IGF-1) and type 1 IGF receptor (IGF1r). Cell 75, 59–72 [PubMed] [Google Scholar]
- 8. Wang J., Zhou J., Bondy C. A. (1999) IGF1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 13, 1985–1990 [DOI] [PubMed] [Google Scholar]
- 9. Davies L. C., Blain E. J., Gilbert S. J., Caterson B., Duance V. C. (2008) The potential of IGF-1 and TGFβ1 for promoting “adult” articular cartilage repair. An in vitro study. Tissue Eng. Part A 14, 1251–1261 [DOI] [PubMed] [Google Scholar]
- 10. Janssens K. (2005) Transforming Growth Factor-1 to the bone. Endocr. Rev. 26, 743–774 [DOI] [PubMed] [Google Scholar]
- 11. Lee J. Y., Kim K. H., Shin S. Y., Rhyu I. C., Lee Y. M., Park Y. J., Chung C. P., Lee S. J. (2006) Enhanced bone formation by transforming growth factor-β1-releasing collagen/chitosan microgranules. J. Biomed. Mater. Res. A 76, 530–539 [DOI] [PubMed] [Google Scholar]
- 12. Zhao L., Jiang S., Hantash B. M. (2010) Transforming growth factor β1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng. Part A 16, 725–733 [DOI] [PubMed] [Google Scholar]
- 13. Lee K. S., Hong S. H., Bae S. C. (2002) Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-β and bone morphogenetic protein. Oncogene 21, 7156–7163 [DOI] [PubMed] [Google Scholar]
- 14. Ripamonti U., Ferretti C., Teare J., Blann L. (2009) Transforming growth factor-β isoforms and the induction of bone formation. J. Craniofac. Surg. 20, 1544–1555 [DOI] [PubMed] [Google Scholar]
- 15. Zhang H., Ahmad M., Gronowicz G. (2003) Effects of transforming growth factor-β1 (TGF-β1) on in vitro mineralization of human osteoblasts on implant materials. Biomaterials 24, 2013–2020 [DOI] [PubMed] [Google Scholar]
- 16. Alliston T., Choy L., Ducy P., Karsenty G., Derynck R. (2001) TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 20, 2254–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaji H., Naito J., Sowa H., Sugimoto T., Chihara K. (2006) Smad3 differently affects osteoblast differentiation depending upon its differentiation stage. Horm. Metab. Res. 38, 740–745 [DOI] [PubMed] [Google Scholar]
- 18. He W. X., Niu Z. Y., Zhao S. L., Jin W. L., Gao J., Smith A. J. (2004) TGF-β-activated Smad signaling leads to a Smad3-mediated down-regulation of DSPP in an odontoblast cell line. Arch. Oral Biol. 49, 911–918 [DOI] [PubMed] [Google Scholar]
- 19. Kwok S., Partridge N. C., Srinivasan N., Nair S. V., Selvamurugan N. (2009) Mitogen-activated protein kinase-dependent inhibition of osteocalcin gene expression by transforming growth factor-β1. J. Cell. Biochem. 106, 161–169 [DOI] [PubMed] [Google Scholar]
- 20. Sowa H., Kaji H., Yamaguchi T., Sugimoto T., Chihara K. (2002) Activations of ERK1/2 and JNK by transforming growth factor β negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J. Biol. Chem. 277, 36024–36031 [DOI] [PubMed] [Google Scholar]
- 21. Centrella M., Ji C., Casinghino S., McCarthy T. L. (1996) Rapid flux in transforming growth factor-β receptors on bone cells. J. Biol. Chem. 271, 18616–18622 [DOI] [PubMed] [Google Scholar]
- 22. Weaver C. T., Harrington L. E., Mangan P. R., Gavrieli M., Murphy K. M. (2006) Th17. An effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 [DOI] [PubMed] [Google Scholar]
- 23. Ochiai H., Yamamoto Y., Yokoyama A., Yamashita H., Matsuzaka K., Abe S., Azuma T. (2010) Dual nature of TGF-β1 in osteoblastic differentiation of human periodontal ligament cells. J. Hard Tissue Biol. 19, 187–194 [Google Scholar]
- 24. Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. (1991) Immunohistochemical localization of TGFβ1, TGFβ, and TGFβ3 in the mouse embryo. Expression patterns suggest multiple roles during embryonic development. J. Cell Biol. 115, 1091–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zimmermann G., Henle P., Küsswetter M., Moghaddam A., Wentzensen A., Richter W., Weiss S. (2005) TGF-β1 as a marker of delayed fracture healing. Bone 36, 779–785 [DOI] [PubMed] [Google Scholar]
- 26. Cho T. J., Gerstenfeld L. C., Einhorn T. A. (2002) Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J. Bone Miner. Res. 17, 513–520 [DOI] [PubMed] [Google Scholar]
- 27. Bakin A. V., Tomlinson A. K., Bhowmick N. A., Moses H. L., Arteaga C. L. (2000) Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 [DOI] [PubMed] [Google Scholar]
- 28. Akhurst R. J., Derynck R. (2001) TGF-β signaling in cancer, a double-edged sword. Trends Cell Biol. 11, S44–S51 [DOI] [PubMed] [Google Scholar]
- 29. Bierie B., Moses H. L. (2006) Tumor microenvironment. TGFβ, the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6, 506–520 [DOI] [PubMed] [Google Scholar]
- 30. Ripamonti U., Duneas N., Van Den Heever B., Bosch C., Crooks J. (1997) Recombinant transforming growth factor-β1 induces endochondral bone in the baboon and synergizes with recombinant osteogenic protein-1 (bone morphogenetic protein-7) to initiate rapid bone formation. J. Bone Miner. Res. 12, 1584–1595 [DOI] [PubMed] [Google Scholar]
- 31. Borton A. J., Frederick J. P., Datto M. B., Wang X. F., Weinstein R. S. (2001) The loss of Smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J. Bone Miner. Res. 16, 1754–1764 [DOI] [PubMed] [Google Scholar]
- 32. Sowa H., Kaji H., Yamaguchi T., Sugimoto T., Chihara K. (2002) Smad3 promotes alkaline phosphatase activity and mineralization of osteoblastic MC3T3-E1 cells. J. Bone Miner. Res. 17, 1190–1199 [DOI] [PubMed] [Google Scholar]
- 33. Miyazono A., Yamada A., Morimura N., Takami M., Suzuki D., Kobayashi M., Tezuka K., Yamamoto M., Kamijo R. (2007) TGF-β suppresses POEM expression through ERK1/2 and JNK in osteoblasts. FEBS Lett. 581, 5321–5326 [DOI] [PubMed] [Google Scholar]
- 34. Yakar S., Courtland H. W., Clemmons D. (2010) IGF-1 and bone. New discoveries from mouse models. J. Bone Miner. Res. 25, 2543–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kadowaki T., Tobe K., Honda-Yamamoto R., Tamemoto H., Kaburagi Y., Momomura K., Ueki K., Takahashi Y., Yamauchi T., Akanuma Y., Yazaki Y. (1996) Signal transduction mechanism of insulin and insulin-Like growth factor-1. Endocr. J. 43, S33–S41 [DOI] [PubMed] [Google Scholar]
- 36. Cusi K., Maezono K., Osman A., Pendergrass M., Patti M. E., Pratipanawatr T., DeFronzo R. A., Kahn C. R., Mandarino L. J. (2000) Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105, 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakagawa T., Sakamoto T., Hiraumi H., Kikkawa Y. S., Yamamoto N., Hamaguchi K., Ono K., Yamamoto M., Tabata Y., Teramukai S., Tanaka S., Tada H., Onodera R., Yonezawa A., Inui K., Ito J. (2010) Topical insulin-like growth factor 1 treatment using gelatin hydrogels for glucocorticoid-resistant sudden sensorineural hearing loss. A prospective clinical trial. BMC Med. 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giustina A., Mazziotti G., Canalis E. (2008) Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 29, 535–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park J. I., Lee M. G., Cho K., Park B. J., Chae K. S., Byun D. S., Ryu B. K., Park Y. K., Chi S. G. (2003) Transforming growth factor-β1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-κB, JNK, and Ras signaling pathways. Oncogene 22, 4314–4332 [DOI] [PubMed] [Google Scholar]
- 40. Ge Q., Moir L. M., Black J. L., Oliver B. G., Burgess J. K. (2010) TGFβ1 induces IL-6 and inhibits IL-8 release in human bronchial epithelial cells: the role of Smad2/3. J. Cell. Physiol. 225, 846–854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.