Abstract
Background:
Results of randomized controlled trials evaluating zinc for the treatment of the common cold are conflicting. We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of zinc for such use.
Methods:
We searched electronic databases and other sources for studies published through to Sept. 30, 2011. We included all randomized controlled trials comparing orally administered zinc with placebo or no treatment. Assessment for study inclusion, data extraction and risk-of-bias analyses were performed in duplicate. We conducted meta-analyses using a random-effects model.
Results:
We included 17 trials involving a total of 2121 participants. Compared with patients given placebo, those receiving zinc had a shorter duration of cold symptoms (mean difference −1.65 days, 95% confidence interval [CI] −2.50 to −0.81); however, heterogeneity was high (I2 = 95%). Zinc shortened the duration of cold symptoms in adults (mean difference −2.63, 95% CI −3.69 to −1.58), but no significant effect was seen among children (mean difference −0.26, 95% CI −0.78 to 0.25). Heterogeneity remained high in all subgroup analyses, including by age, dose of ionized zinc and zinc formulation. The occurrence of any adverse event (risk ratio [RR] 1.24, 95% CI 1.05 to 1.46), bad taste (RR 1.65, 95% CI 1.27 to 2.16) and nausea (RR 1.64, 95% CI 1.19 to 2.27) were more common in the zinc group than in the placebo group.
Interpretation:
The results of our meta-analysis showed that oral zinc formulations may shorten the duration of symptoms of the common cold. However, large high-quality trials are needed before definitive recommendations for clinical practice can be made. Adverse effects were common and should be the point of future study, because a good safety and tolerance profile is essential when treating this generally mild illness.
The common cold is a frequent respiratory infection experienced 2 to 4 times a year by adults and up to 8 to 10 times a year by children.1–3 Colds can be caused by several viruses, of which rhinoviruses are the most common.4 Despite their benign nature, colds can lead to substantial morbidity, absenteeism and lost productivity.5–7
Zinc, which can inhibit rhinovirus replication and has activity against other respiratory viruses such as respiratory syncytial virus,8 is a potential treatment for the common cold. The exact mechanism of zinc’s activity on viruses remains uncertain. Zinc may also reduce the severity of cold symptoms by acting as an astringent on the trigeminal nerve.9,10
A recent meta-analysis of randomized controlled trials concluded that zinc was effective at reducing the duration and severity of common cold symptoms.11 However, there was considerable heterogeneity reported for the primary outcome (I2 = 93%), and subgroup analyses to explore between-study variations were not performed. The efficacy of zinc therefore remains uncertain, because it is unknown whether the variability among studies was due to methodologic diversity (i.e., risk of bias and therefore uncertainty in zinc’s efficacy) or differences in study populations or interventions (i.e., zinc dose and formulation).
We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of zinc for the treatment of the common cold. We sought to improve upon previous systematic reviews11–17 by exploring the heterogeneity with subgroups identified a priori, identifying new trials by instituting a broader search and obtaining additional data from authors.
Methods
Eligibility criteria
We included studies if they were randomized controlled trials; involved patients of any age with the common cold; and compared oral zinc treatment started within three days of symptoms with placebo or no intervention. We excluded studies in which zinc was administered intranasally or that used zinc in a combined formulation with other minerals or vitamins. The primary outcome was the duration of cold symptoms. Secondary outcomes included the severity of cold symptoms, the presence of symptoms after three and seven days, and adverse events.
Literature search
We searched MEDLINE (1948 to Sept. 30, 2011), Embase (1980 to 2011 [week 40]), the Cochrane Central Register of Controlled Trials (until the third quarter of 2011), CINAHL (1982 to Sept. 30, 2011) and AMED (Allied and Complementary Medicine Database) for relevant studies. Details of the strategies used to search these databases are provided in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.111990/-/DC1). No restrictions were placed on year or language. We also searched conference proceedings from 2005 to 2011 through the Web of Science and Open-SIGLE databases, and clinical trial registries (ClinicalTrials.gov, Current Controlled Trials [controlled-trials.com] and the US National Institutes of Health database). Finally, we reviewed reference lists of key articles.
Data extraction
Two reviewers (J.J. and M.S.) independently screened the titles and abstracts of identified studies. All potentially relevant articles were then obtained and screened independently for eligibility. Disagreements were resolved by consensus or third-party adjudication. Study authors were contacted for information when required (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.111990/-/DC1).
Assessment of risk of bias
The Cochrane risk-of-bias tool was used to assess the risk of bias in included trials.18 The reviewers collected these data independently and in duplicate. The risk of bias for each outcome was assessed using the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation).19 The reviewers examined the included trials, and an overall quality of the summary statistic was determined by discussion after this independent assessment. Summary tables were prepared using the GRADE profiler (GRADEpro).20
Statistical analysis
Means and standard deviations were collected for the continuous outcomes. When these data were not reported and not available after contacting authors, we followed the methods outlined in the Cochrane handbook for obtaining standard deviations from confidence intervals and p values.18 When only the median and interquartile range (IQR) were reported, we used the median to reflect the mean,21 and we calculated the standard deviation by dividing the IQR by 1.35 standard deviations.18
Statistical analysis was conducted using Review Manager Software (RevMan, version 5.1).22 Data were pooled using a random-effects model. For continuous outcomes, we used mean differences to pool results when the measurement scale was the same (duration of cold symptoms) and standardized mean differences when the scale varied (symptom severity). Risk ratios were used for dichotomous outcomes. Heterogeneity was evaluated using the I2 statistic. When substantial heterogeneity was found (I2 ≥ 40%), subgroup analyses were performed.
We performed subgroup analyses defined a priori to investigate the effects of age (< 18 years v. ≥ 18 years), experimentally induced versus naturally acquired colds, zinc formulation, daily dose of ionized zinc (≥ 75 mg v. < 75 mg), high versus low risk of bias, timing of treatment initiation (< 24 hours v. ≥ 24 hours) and funding source (industry v. non-industry). Interaction tests for subgroup differences were performed using the χ2 test and the I2 statistic. Subgroup credibility was assessed using the criteria described by Sun and colleagues23 (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.111990/-/DC1).
Sensitivity analysis
Given the potential differences between naturally acquired and experimentally induced colds, we performed a sensitivity analysis in which we excluded trials with induced colds.
Results
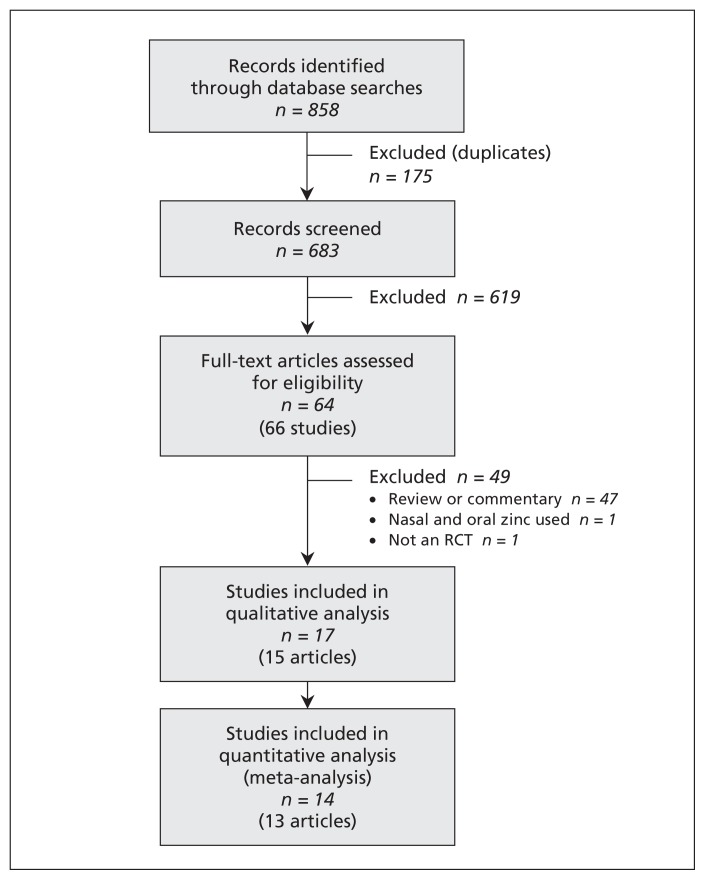
We screened the titles and abstracts of 683 unique records identified through our literature search. Of the 64 full-text articles assessed for eligibility, 17 studies were included in the systematic review and 14 in the meta-analysis (Figure 1). The kappa value for agreement between the reviewers was 0.96. Two trials presented in one paper included three zinc treatment arms and one placebo arm.24 In order to avoid a unit-of-analysis error,25 the three zinc arms were combined and compared with the one placebo arm.
Figure 1:
Selection of studies for the qualitative and quantitative analyses. RCT = randomized controlled trial.
Study characteristics
Seventeen trials involving 2121 patients ranging from 1 to 65 years of age were included (Table 1).24,26–39 Three trials included children less than 18 years old, 13 included adults, and 1 trial included both adults and children. Colds were either naturally acquired (13 trials) or experimentally induced (4). Treatment regimens included zinc gluconate lozenges (8 trials) or tablets (1), zinc acetate lozenges (4), zinc sulfate syrup (2), and either zinc gluconate or zinc acetate (2) compared with placebo. The duration of treatment was different in all trials (range 3–14 days or until symptom resolution).
Table 1:
Characteristics of randomized controlled trials included in the qualitative analysis
| Study | Country | n | Study period | Population | Type of cold | Zinc formulation, dosage | Duration of symptoms before treatment, h | Duration of treatment | Duration of follow-up | Definition of symptom resolution | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Nakib et al., 198726 | United Kingdom | 12 | NR | Adults, age 18–50 yr | I | ZG lozenge; 23 mg every 2 h while awake | < 24 | 6 d | NR | NR | Pharma (RBS) |
| Douglas et al., 198727 | Australia | 55 | Apr–Nov 1985 | Adults | N | ZA lozenge; 10 mg every 2 h while awake (6–8 daily) | < 48 | Until symptom resolution (minimum 3 d, maximum 6 d) | 14 d | No nasal, throat or cough symptoms | Pharma (Fauldings Ltd.) |
| Eby et al., 198428 | United States | 146 | Fall 1981 | Children and adults | N | ZG tablet; loading dose 46 mg, then 23 mg every 2 h while awake | < 72 | Until symptom resolution | 7 d | Absence of all cold symptoms* | Pharma (Truett laboratories) |
| Farr et al. (A), 198729 | United States | 32 | NR | Adults | I | ZG lozenge (citric acid); loading dose 46 mg, then 23 mg every 2 h while awake | < 36 | 5 d | NR | NR | Pharma (Bristol Myers) |
| Farr et al. (B), 198729 | United States | 45 | NR | Adults | I | ZG lozenge (citric acid); 23 mg every 2 h while awake | < 24 | 7 d | NR | NR | Pharma (Bristol Myers) |
| Godfrey et al., 199230 | United States | 87 | NR | Adults | N | ZG lozenge (glycine); 23.7 mg every 2 h while awake (maximum 8) | < 48 | Until symptom resolution | NR | Absence of all cold symptoms* | Pharma (Rorer), Godfrey Science and Design Inc. |
| Kurugöl et al., 200631 | Turkey | 200 | Oct 2004–May 2005 | Children, age 2–10 yr | N | ZS syrup; 15 mg twice daily | < 24 | Until symptom resolution (maximum 10 d) | 7 mo | Symptom score ≤ 1† on 2 consecutive days | Pharma (Berko Ilaç) |
| Kurugöl et al., 200732 | Turkey | 120 | Dec 2004–Mar 2005 | Children | N | ZS syrup; 15 mg twice daily | < 48 | 10 d | Until symptom resolution or 7 d | Resolution of all or all but one mild symptom* | Pharma (Berko Ilaç) |
| Macknin et al., 199833 | United States | 249 | Oct 1996–Mar 1997 | Children, grades 1–12 | N | ZG lozenge (glycine); 10 mg five times daily (grades 1–6) or six times daily (grades 7–12) | < 24 | Until symptom resolution | Until symptom resolution | Absence of nine symptoms* (excluding fever) | Pharma (Quigley) |
| Mossad et al., 199634 | United States | 100 | Oct 1994–Nov 1994 | Adults | N | ZG lozenge (glycine); 13.3 mg every 2 h | < 24 | Until symptom resolution | 18 d | Resolution of all or all but one mild symptom* | Pharma (Quigley), Cleveland Clinic Foundation |
| Petrus et al., 199835 | United States | 102 | July 1997–Aug 1997 | Adults | N | ZA lozenge; 9 mg every 1.5 h on day 1, then every 2 h while awake | NR | Until symptom resolution (maximum 14 d) | Until symptom resolution | Resolution of 11 cold symptoms (10 listed* plus malaise) | Pharma (Weider Nutrition) |
| Prasad et al., 200036 | United States | 50 | Jan 1998–Dec 1998 | Adults | N | ZA lozenge; 12.8 mg every 2–3 h while awake | < 24 | Until symptom resolution | 1 d after symptom resolution | Resolution of all or all but one mild symptom* | George and Patsy Eby Research Foundation |
| Prasad et al., 200837 | United States | 50 | Jan 1999–Jan 2003 | Adults | N | ZA lozenge; 13.3 mg every 2–3 h while awake | < 24 | Until symptom resolution | 1 d after symptom resolution | Resolution of all or all but one mild symptom* | George and Patsy Eby Research Foundation, NIH |
| Smith et al., 198938 | United States | 174 | Jan 1986–May 1986 | Adults | N | ZG lozenge; loading dose 46 mg, then 23 mg every 2 h while awake | NR | 7 d or until symptom resolution | 7 d | Resolution of 11 symptoms (10 listed* plus chilliness) | Pharma (McNeil Consumer Products) |
| Turner et al. (A), 200024 | United States | 273 | NR | Adults, age 18–65 yr | I | ZG or ZA lozenge; ZG 13.3 mg, or ZA 11.5 mg or 5 mg; every 2–3 h (total 6/d) | < 48 | Until symptom resolution (minimum 3 d, maximum 14 d) | 14 d | 2 consecutive symptom scores ≤ 1‡ | Pharma (Warner Lambert Consumer) |
| Turner et al. (B), 200024 | United States | 281 | NR | Adults, age 18–65 yr | N | ZG or ZA lozenge; ZG 13.3 mg, or ZA 11.5 mg or 5 mg; every 2–3 h (total 6/d) | < 36 | Until symptom resolution (minimum 3 d, maximum 14 d) | 14 d | 2 consecutive symptom scores ≤ 1‡ | Pharma (Warner Lambert Consumer) |
| Weismann et al., 199039 | Denmark | 145 | Feb 1987–Feb 1988 | Adults, age 18–65 yr | N | ZG lozenge; 4.5 mg every 1–1.5 h while awake (10 daily) | < 24 | Until symptom resolution (maximum 10 d) | 10 d | Absence of 9 symptoms (10 listed* plus indisposition and excluding sneezing and scratchy throat) | Pharma (Kirsten B Staehr) |
Note: NR = not reported, N = natural, I = induced, ZG = zinc gluconate, ZA = zinc acetate, ZS = zinc sulfate, SR = symptom resolution.
Headache, fever, muscle pain, sneezing, nasal drainage, nasal obstruction, sore throat, scratchy throat, cough and hoarseness.
Symptom score: 0 = none, 1 = mild, 2 = moderate, 3 = severe for 10 cold symptoms.*
Symptom score: 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe for 7 cold symptoms (sneezing, rhinorrhea, nasal obstruction, sore throat, cough, headache and hoarseness).
The findings of our risk-of-bias assessment are presented in Table 2 (and Appendix 4, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.111990/-/DC1). Most of the trials did not provide adequate information on allocation concealment. All of the trials reported blinding of patients and health care professionals with placebos identical in appearance or with no identifying features. The risk of bias is summarized for each outcome in the GRADE Evidence Profile (Appendix 5, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.111990/-/DC1) and in Table 3.
Table 2:
Risk-of-bias review of included studies
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding (performance bias and detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias |
|---|---|---|---|---|---|---|
| Al-Nakib et al.26 | Unclear | Unclear | Low | Low | High | High |
| Douglas et al.27 | Unclear | Unclear | Low | High | Unclear | High |
| Eby et al.28 | Unclear | Unclear | Low | High | Low | High |
| Farr et al. (A)29 | Low | Unclear | Low | Unclear | High | Unclear |
| Farr et al. (B)29 | Low | Unclear | Low | Unclear | Unclear | Unclear |
| Godfrey et al.30 | Low | Low | Low | High | Unclear | Unclear |
| Kurugöl et al.31 | Low | Unclear | Low | Low | Low | Low |
| Kurugöl et al.32 | Low | Low | Low | Low | Low | Low |
| Macknin et al.33 | Low | Low | Low | Low | Low | Low |
| Mossad et al.34 | Low | Low | Low | Low | Low | Unclear |
| Petrus et al.34 | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Prasad et al.36 | Low | Low | Low | Low | Low | Low |
| Prasad et al.37 | Low | Low | Low | Low | Low | Low |
| Smith et al.38 | Unclear | Unclear | Low | High | High | Unclear |
| Turner et al. (A)24 | Unclear | Unclear | Low | High | Low | Unclear |
| Turner et al. (B)24 | Unclear | Unclear | Low | High | Low | Unclear |
| Weismann et al.39 | Unclear | Unclear | Low | High | High | Unclear |
Table 3:
Summary of findings for oral zinc therapy for the common cold in children and adults with the common cold in outpatient or ambulatory settings
| Outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | GRADE quality of evidence† | |
|---|---|---|---|---|---|
| Assumed risk for control group | Corresponding risk with zinc | ||||
| Duration of symptoms | 1.65 days lower (2.5 to 0.81 days lower) | 934 (8) | Moderatea,b,c | ||
| Severity of symptoms | 0.27 SDs lower (0.58 lower to 0.05 higher) | 412 (4) | Lowd,e | ||
| No. of symptomatic patients after 3 d of treatment | 858 per 1000 | 789 per 1000 (712 to 875 per 1000) | RR 0.92 (0.83 to 1.02) | 1252 (8) | Lowf,g |
| No. of symptomatic patients after 7 d of treatment | 471 per 1000 | 297 per 1000 (207 to 424 per 1000) | RR 0.63 (0.44 to 0.9) | 1325 (9) | Lowh,i |
| Adverse events leading to stopping treatment | 0 per 1000 | 0 per 1000 (0 to 0 per 1000) | RR 11 (0.62 to 193.8) | 230 (2) | Lowj,k |
| Adverse event | |||||
| Any | 385 per 1000 | 477 per 1000 (404 to 562 per 1000) | RR 1.24 (1.05 to 1.46) | 1487 (9) | Moderatel,m |
| Bad taste | 204 per 1000 | 337 per 1000 (259 to 441 per 1000) | RR 1.65 (1.27 to 2.16) | 961 (8) | Moderaten,o |
| Nausea | 102 per 1000 | 167 per 1000 (121 to 232 per 1000) | RR 1.64 (1.19 to 2.27) | 973 (9) | Moderatep,q |
| Abdominal pain | 94 per 1000 | 112 per 1000 (78 to 162 per 1000) | RR 1.19 (0.83 to 1.72) | 876 (7) | Moderater |
| Diarrhea | 29 per 1000 | 55 per 1000 (28 to 108 per 1000) | RR 1.88 (0.95 to 3.72) | 831 (7) | Moderates |
| Constipation | 23 per 1000 | 33 per 1000 (15 to 72 per 1000) | RR 1.42 (0.64 to 3.12) | 876 (7) | Moderatet |
Note: CI = confidence interval, GRADE = Grading of Recommendations Assessment, Development and Evaluation, RR = risk ratio, SD = standard deviation.
The basis for the assumed risk (e.g., the median risk for the control group across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence: high quality: further research is very unlikely to change our confidence in the estimate of effect; moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low quality: we are very uncertain about the estimate.
No serious design limitations: Blinding was adequate in all trials. Two trials had unclear allocation concealment.31,35 One trial did not describe random sequence generation,35 and two trials had unclear selective reporting bias.30,35 One trial had incomplete outcome data.30 Other bias was unclear in three trials.30,34,35 Sensitivity analysis excluding these trials did not change the results, so the evidence was not downgraded.
Serious inconsistency: Very high statistical heterogeneity (I2 = 95%). Age, ionic zinc dose and zinc formulation partially accounted for between-study variation.
No serious imprecision: Cumulative sample size was appropriate. The optimal information size to detect a one-day difference in duration of symptoms (α = 0.05, 90% power) assuming a mean of 7 days (SD 3 days) was 190 participants per arm. The 95% CI (2.50 to 0.81) crossed the minimally important difference of one day. However, the CI was narrow and did not include “no treatment effect.”
Serious inconsistency: Substantial heterogeneity (I2 = 55%).
Serious imprecision: Total sample size 412. The optimal information size to detect a 1-point difference in score for severity of symptoms (α = 0.05, 80% power) assuming a mean score of 3 (SD 4) was 252 participants per arm.
Serious design limitations: Five trials had significant design limitations (all had incomplete outcome data, unclear allocation concealment and did not report the method of randomization).28,38,24[A,B],39 The remaining three trials had no significant limitations.
Serious Inconsistency: High statistical heterogeneity (I2 = 80%) not explained by subgroup analyses.
Serious design limitations: Six trials had significant design limitations.28,30,38,24[A,B],39 All had incomplete outcome data. Allocation concealment and method of randomization were unclear in all but one of the six.30 Other bias was present in one trial.28
Serious inconsistency: High statistical heterogeneity (I2 = 78%).
Serious design limitations: One trial had serious design limitations.39 This trial had incomplete outcome data and was high risk for selective reporting. It also had unclear allocation concealment and did not report the method of randomization.
Serious imprecision: Only two trials reported on this outcome, and one of these trials had no outcomes to report in either group;39 this resulted in a large 95% CI and small sample size.
No serious limitations: Five trials had serious design problems.28,30,24[A,B],39 All five had incomplete outcome data and unclear allocation concealment, and all but one trial30 did not report the randomization method. Sensitivity analysis excluding these trials did not change the results, so the evidence was not downgraded.
Serious imprecision: Estimated range of adverse events from 19 more to 177 more per 1000 versus placebo.
No serious design limitations: Two trials had serious design limitations.28,39 Sensitivity analysis excluding these trials did not change the results, so the evidence was not downgraded.
Serious imprecision: Estimated range of “bad taste” events from 55 to 237 more per 1000 versus placebo.
No serious design limitations: Three trials had significant design concerns.28,29[B],38 All had incomplete outcome data and unclear allocation concealment. The method of randomization was not reported in two trials.28,38 The remaining six trials had low risk of bias. Sensitivity analysis excluding the trials with high risk of bias did not change the results, so the evidence was not downgraded.
Serious imprecision: Estimated range of nausea events from 19 more to 130 more per 1000 versus placebo.
Serious imprecision: Low number of events (102) and 95% CI crosses no treatment effect (1.0) and the threshold for appreciable harm (1.25).
Serious imprecision: Low number of events (36) and 95% CI crosses no treatment effect (1.0) and the threshold for appreciable harm (1.25).
Serious imprecision: Low number of events (28) and 95% CI crosses no treatment effect (1.0) and threshold for appreciable harm (1.25).
Duration of symptoms
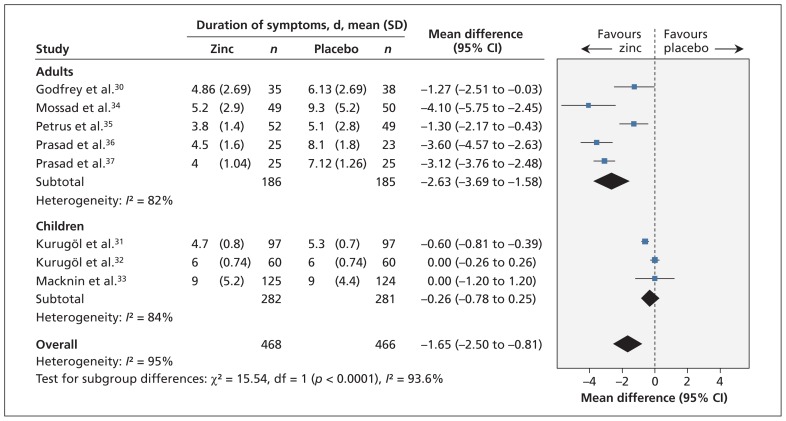
Thirteen trials reported on the duration of cold symptoms. The remaining four trials either measured but did not report the outcome26,29 or reported the proportion of symptomatic patients on each day.38,39 Eight trials (n = 934) could be pooled in the meta-analysis (Figure 2). All trials involved patients with naturally acquired colds. Treatment with zinc compared with placebo significantly reduced the duration of cold symptoms (mean difference −1.65 days, 95% CI −2.50 to −0.81). Because of significant heterogeneity (I2 = 95%), however, the quality of the evidence for this finding was considered moderate (Table 3, Appendix 5).
Figure 2:
Meta-analysis of the duration of cold symptoms, by age, in randomized controlled trials of oral zinc therapy for the common cold. A value less than zero indicates a benefit from zinc. CI = condifence interval, df = degrees of freedom.
Subgroup analyses showed a statistically significant interaction between trials involving adults compared with those involving children (interaction p < 0.001) (Figure 2) (Appendix 6, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.111990/-/DC1). Zinc reduced the duration of cold symptoms in adults (mean difference −2.63, 95% CI −3.69 to −1.58) but not in children (mean difference −0.26, 95% CI −0.78 to 0.25). Heterogeneity was slightly reduced in this subgrouping (adults: I2 = 82%; children: I2 = 84%).
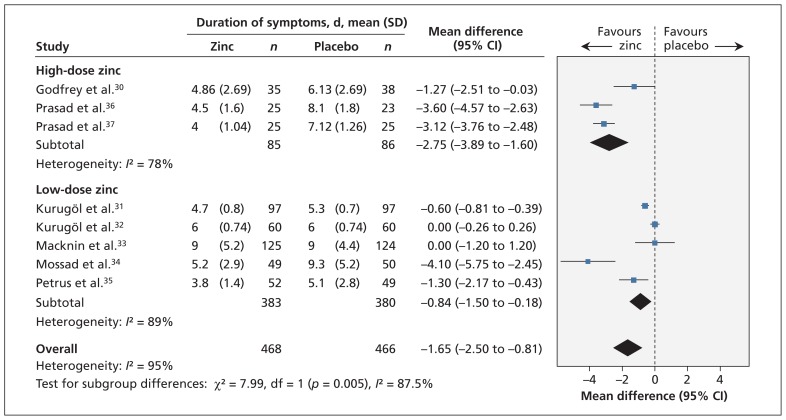
The reduction in the duration of cold symptoms was greater with high doses of ionic zinc (mean difference −2.75, 95% CI −3.89 to −1.60) than with lower doses (mean difference −0.84, 95% CI −1.50 to −0.18) (Figure 3) (Appendix 6). There was a significant interaction effect (p = 0.005), and heterogeneity was reduced (high dose: I2 = 78%; low dose: I2 = 89%).
Figure 3:
Meta-analysis of the duration of cold symptoms, by dose of ionic zinc, in randomized controlled trials of oral zinc therapy for the common cold. A value less than zero indicates a benefit from zinc. CI = confidence interval, df = degrees of freedom.
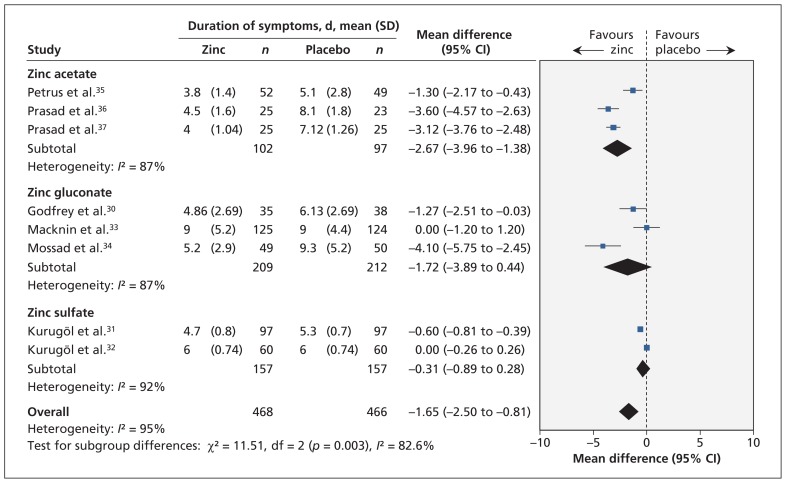
The effect of zinc on the duration of symptoms also varied by zinc formulation (interaction p = 0.003) (Appendix 6). The duration of symptoms was significantly reduced in trials using zinc acetate (mean difference −2.67, 95% CI −3.96 to −1.38) but was not different in trials using zinc gluconate or zinc sulfate (Figure 4). This subgroup effect may reflect the fact that zinc acetate was used only in adults and zinc sulfate only in children (Appendix 3).
Figure 4:
Meta-analysis of the duration of cold symptoms, by zinc formulation, in randomized controlled trials of oral zinc therapy for the common cold. A value less than zero indicates a benefit from zinc. CI = confidence interval, df = degrees of freedom.
No subgroup effect was found for differences in risk of bias or symptom duration before intervention (Appendix 6). All of the trials were funded by industry; therefore, this subgroup analysis was not possible.
To address concerns over potentially inadequate blinding to the taste of the placebo, we conducted a post hoc sensitivity analysis in which we excluded trials that reported significant differences in bad taste between zinc and placebo arms33,34 or did not report a bad taste outcome.30,35 After excluding these trials, the duration of cold symptoms still favoured zinc, with a mean difference of −1.74 days (95% CI −2.90 to −0.58). On subgroup analysis, there was no significant difference between trials with or without likelihood of blinding broken by bad taste (interaction p = 0.85) (Appendix 6).
Severity of symptoms
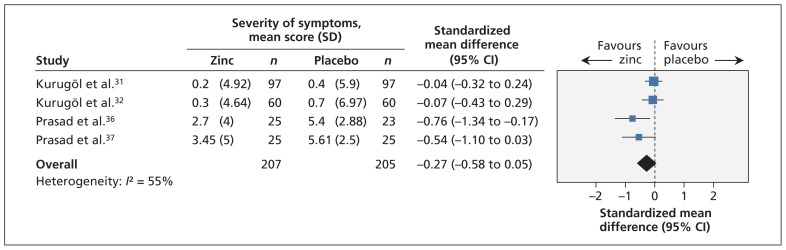
Sixteen trials reported on the severity of symptoms, but only four trials reported the data in such a way that they could be combined in the meta-analysis.31,32,36,37 When data from these trials were combined, we found no significant difference in severity of symptoms between the zinc group and the placebo group (standardized mean difference −0.27, 95% CI −0.58 to 0.05) (Figure 5). The quality of evidence was low given the substantial heterogeneity (I2 = 55%) and imprecision in the summary estimate.
Figure 5:
Meta-analysis of the severity of symptoms in randomized controlled trials of oral zinc therapy for the common cold. A value less than zero indicates a benefit from zinc. CI = confidence interval.
Subgroup analyses showed that there was a significant difference in the mean severity score between children and adults (interaction p = 0.01), with children having a nonsignificant difference between the zinc and placebo groups (standardized mean difference −0.05, 95% CI −0.27 to 0.17) and adults having a significant difference favouring zinc (standardized mean difference −0.64, 95% CI −1.05 to −0.24) (Appendix 6). However, the same trials were included in the subgroup analysis by zinc formulation (all children received zinc sulfate) and in the subgroup analysis by dose of ionic zinc (all children received a low dose). Therefore, although a subgroup difference may exist, it is not clear whether age, zinc formulation or dose of ionic zinc contributed to this difference (Appendix 3).
Presence of symptoms at three and seven days
Eight trials involving 1252 patients reported the proportion of patients who were symptomatic after three days; no difference between the zinc and placebo groups was found (risk ratio [RR] 0.92, 95% CI 0.83 to 1.02). Nine trials involving 1325 patients reported the proportion of patients who were symptomatic after seven days; a significant reduction in the number was reported in the zinc arm compared with placebo group (RR 0.63, 95% CI 0.44 to 0.90). Both outcomes were associated with high heterogeneity, and the quality of evidence was considered low given this inconsistency and risk of bias in the trials. No significant subgroup effects were found (Appendix 6).
Adverse events
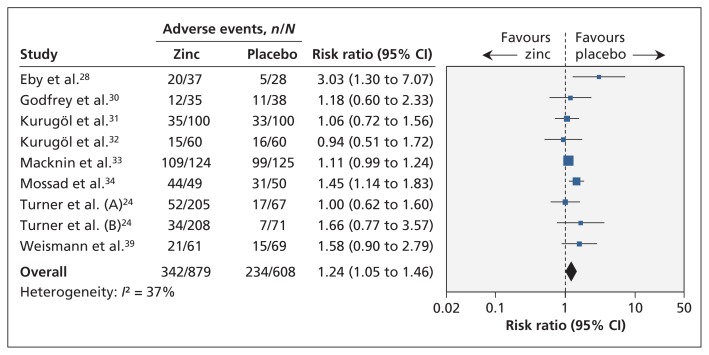
Nine trials involving 1487 patients found that the proportion of patients with any adverse events was higher in the zinc group than in the placebo group (RR 1.24, 95% CI 1.05 to 1.46) (Figure 6). Heterogeneity was moderate (I2 = 37%).
Figure 6:
Meta-analysis of adverse events in randomized controlled trials of oral zinc therapy for the common cold. A risk ratio less than zero indicates a benefit from zinc. CI = confidence interval.
Patients treated with zinc more frequently experienced bad taste (eight trials, RR 1.65, 95% CI 1.27 to 2.16) and nausea (nine trials, RR 1.64, 95% CI 1.19 to 2.27). We found no difference between groups in the occurrence of abdominal pain (seven trials, RR 1.19, 95% CI 0.83 to 1.72), constipation (seven trials, RR 1.42, 95% CI 0.64 to 3.12) or diarrhea (seven trials, RR 1.88, 95% CI 0.95 to 3.72) (Appendix 7, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.111990/-/DC1).
Sensitivity analysis
When we excluded the two trials in which colds were experimentally induced,24,29 we found no significant change in the number of symptomatic participants at three days and seven days, or in the incidence of any adverse events and nausea.
Interpretation
We found that orally administered zinc shortened the duration of cold symptoms. These findings, however, are tempered by significant heterogeneity and quality of evidence. Although there was low-quality evidence that participants receiving zinc were less likely than controls to be symptomatic at one week, there was no difference between groups in symptom severity or presence of symptoms at three days. Our findings question the importance of zinc and suggest that any benefit may be outweighed by adverse events, which were more common among participants given zinc than among controls.
Our demonstration of a reduced duration of cold symptoms (mean difference −1.65 days, 95% CI −2.50 to −0.81) is consistent with the results of the most recent systematic review.11 However, the effect of zinc differed in three subgroup analyses (by age, zinc formulation and ionic zinc dose).
Zinc reduced the duration of cold symptoms in adults; however, the effect was greatly attenuated and not statistically significant among children. Possible explanations include age-related differences in the host inflammatory responses,40 different viruses involved41 with varying abilities of zinc to inhibit these viruses, and consequences of third-party reporting of symptoms in children. Other possible factors include the use of lower doses of ionic zinc in the pediatric studies, as well as the use of syrup formulation (v. lozenge) and less frequent administration (resulting in less local exposure).
With respect to the dose of ionic zinc and the zinc formulation, greater reductions in the duration of symptoms occurred with higher doses than with lower doses, and zinc acetate reduced the duration of symptoms whereas the other formulations showed no effect. These findings suggest a possible dose-dependent effect associated with ionic zinc and is consistent with results of a previous report showing an association between the amount of ionic zinc and the magnitude of clinical response.14 However, these characteristics only partially explain between-study differences.
Our review has several other key differences from the Cochrane review.11 First, we used a different approach to estimating means and standard deviations in trials that reported only medians.32,33 In the Cochrane review, the authors calculated the means and standard deviations by assuming that the 95% CIs presented around the medians also reflected 95% CIs around the means.11 However, this approach resulted in one trial estimate showing a significant difference between the zinc and placebo groups,32 a finding inconsistent with the authors’ conclusion of no difference. In contrast, our approach enabled inclusion of effect estimates in the meta-analysis that were qualitatively consistent with the trial conclusions.
Finally, we included additional trials, obtained additional data from study authors and corrected data that had been incorrectly extracted from one trial.35 For the primary outcome, we were able to obtain data from eight studies, as compared with six studies in the previous review. We also included two additional trials that had previously been excluded because they were not considered to be randomized trials.24 However, the methods described appeared appropriate for inclusion, and we confirmed the methodology with the author. These two trials may have influenced the outcome, because they showed no effect.24
Limitations
The limitations of our review predominantly relate to the large heterogeneity that remained unexplained despite exploration of several subgroups a priori and the quality of reported summary data. Assumptions were made to calculate the means and standard deviations of several trial estimates, and all studies were industry funded. Although the trials reported double blinding, ineffective blinding related to taste of the placebo may have contributed to bias. Finally, the majority of trials were conducted in developed countries.
Conclusion
We found moderate quality of evidence to suggest that orally administered zinc reduces the duration of symptoms of the common cold. However, the evidence of benefit was limited to adults, and even in this patient group, uncertainty remained about its clinical benefit. Although oral zinc treatment may attenuate the symptoms of the common cold, large high-quality trials enrolling adults and children are needed. Future trials should be designed to maximize the tolerable doses of bioavailable zinc with a balanced consideration toward potential dose-related adverse effects. Until further evidence becomes available, there is only a weak rationale for physicians to recommend zinc for the treatment of the common cold. The questionable benefits must be balanced against the potential adverse effects.
Supplementary Material
Acknowledgements
The authors thank Elizabeth Uleryk for providing invaluable feedback on the literature search. They also thank Drs. Ronald B. Turner, Sherif B. Mossad and Michael Macknin for responding with additional study data. Drs. Science and Johnstone receive salary support from the Canadian Institutes for Health Research. Dr. Loeb holds the Michael DeGroote Institute for Infectious Disease Research Chair.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Michelle Science, Jennie Johnstone and Daniel Roth conceived the study. Michelle Science, Jennie Johnstone and Mark Loeb designed the study. Michelle Science and Jennie Johnstone reviewed the titles and abstracts, selected studies for full-text review, reviewed the papers for inclusion, and extracted data. Michelle Science analyzed the data and drafted the manuscript. All authors interpreted the data, reviewed the draft and approved the final version for publication.
Funding: There was no dedicated funding to support this study.
References
- 1.Schappert SM. National Ambulatory Medical Care Survey: 1989 summary. Vital Health Stat 13 1992;(110):1–80 [PubMed] [Google Scholar]
- 2.Gwaltney JM, Jr, Hendley JO, Simon G, et al. Rhinovirus infections in an industrial population. I. The occurrence of illness. N Engl J Med 1966;275:1261–8 [DOI] [PubMed] [Google Scholar]
- 3.Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA 1974;227:164–9 [PubMed] [Google Scholar]
- 4.Turner RB. The treatment of rhinovirus infections: progress and potential. Antiviral Res 2001;49:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellgren J, Cervin A, Nordling S, et al. Allergic rhinitis and the common cold — high cost to society. Allergy 2010;65:776–83 [DOI] [PubMed] [Google Scholar]
- 6.Nichol KL, D’Heilly S, Ehlinger E. Burden of upper respiratory illnesses among college and university students: 2002–2003 and 2003–2004 cohorts. Vaccine 2006;24:6724–5 [DOI] [PubMed] [Google Scholar]
- 7.Bramley TJ, Lerner D, Sames M. Productivity losses related to the common cold. J Occup Environ Med 2002;44:822–9 [DOI] [PubMed] [Google Scholar]
- 8.Suara RO, Crowe JE., Jr Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother 2004;48: 783–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novick SG, Godfrey JC, Godfrey NJ, et al. How does zinc modify the common cold? Clinical observations and implications regarding mechanisms of action. Med Hypotheses 1996;46:295–302 [DOI] [PubMed] [Google Scholar]
- 10.Godfrey JC, Conant Sloane B, Smith DS, et al. Zinc gluconate and the common cold: a controlled clinical study. J Int Med Res 1992;20:234–46 [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Das RR. Zinc for the common cold [review]. Cochrane Database Syst Rev 2011;(2):CD001364. [DOI] [PubMed] [Google Scholar]
- 12.Caruso TJ, Prober CG, Gwaltney JM., Jr Treatment of naturally acquired common colds with zinc: a structured review. Clin Infect Dis 2007;45:569–74 [DOI] [PubMed] [Google Scholar]
- 13.Hemilä H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir Med J 2011;5:51–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eby GA., III Zinc lozenges as cure for the common cold — a review and hypothesis. Med Hypotheses 2010;74:482–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson JL, Lesho E, Peterson C. Zinc and the common cold: a meta-analysis revisited. J Nutr May 2000;130(Suppl 5S):1512S–5S [DOI] [PubMed] [Google Scholar]
- 16.Jackson JL, Peterson C, Lesho E. A meta-analysis of zinc salts lozenges and the common cold. Arch Intern Med 1997;157: 2373–6 [PubMed] [Google Scholar]
- 17.Marshall I. Zinc for the common cold [review]. Cochrane Database Syst Rev 2000;(2):CD001364. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester (UK); Hoboken (NJ): Wiley-Blackwell; 2008 [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GRADEpro [computer program]; version 3.2 for Windows. Brozek Jan, Oxman Andrew, Schunemann Holger; 2008 [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manager R. (RevMan) [computer program]; version 5.1. Cophenhagen (Denmark): Nordic Cochrane Centre, Cochrane Collaboration; 2011 [Google Scholar]
- 23.Sun X, Briel M, Walter SD, et al. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340:c117. [DOI] [PubMed] [Google Scholar]
- 24.Turner RB, Cetnarowski WE. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clin Infect Dis 2000;31:1202–8 [DOI] [PubMed] [Google Scholar]
- 25.Whiting-O’Keefe QE, Henke C, Simborg DW. Choosing the correct unit of analysis in Medical Care experiments. Med Care 1984;22:1101–14 [DOI] [PubMed] [Google Scholar]
- 26.Al-Nakib W, Higgins PG, Barrow I, et al. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J Antimicrob Chemother 1987;20:893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas RM, Miles HB, Moore BW, et al. Failure of effervescent zinc acetate lozenges to alter the course of upper respiratory tract infections in Australian adults. Antimicrob Agents Chemother 1987;31:1263–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eby GA, Davis DR, Halcomb WW. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother 1984;25:20–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farr BM, Conner EM, Betts RF, et al. Two randomized controlled trials of zinc gluconate lozenge therapy of experimentally induced rhinovirus colds. Antimicrob Agents Chemother 1987; 31:1183–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey JC, Conant Sloane B, Smith DS, et al. Zinc gluconate and the common cold: a controlled clinical study. J Int Med Res 1992;20:234–46 [DOI] [PubMed] [Google Scholar]
- 31.Kurugöl Z, Akilli M, Bayram N, et al. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr 2006;95:1175–81 [DOI] [PubMed] [Google Scholar]
- 32.Kurugöl Z, Bayram N, Atik T. Effect of zinc sulfate on common cold in children: randomized, double blind study. Pediatr Int 2007;49:842–7 [DOI] [PubMed] [Google Scholar]
- 33.Macknin ML, Piedmonte M, Calendine C, et al. Zinc gluconate lozenges for treating the common cold in children: a randomized controlled trial. JAMA 1998;279:1962–7 [DOI] [PubMed] [Google Scholar]
- 34.Mossad SB, Macknin ML, Medendorp SV, et al. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann Intern Med 1996;125:81–8 [DOI] [PubMed] [Google Scholar]
- 35.Petrus EJ, Lawson KA, Bucci LR, et al. Randomized, double-masked, placebo-controlled clinical study of the effectiveness of zinc acetate lozenges on common cold symptoms in allergy-tested subjects. Curr Ther Res Clin Exp 1998;59:595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad AS, Fitzgerald JT, Bao B, et al. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2000;133:245–52 [DOI] [PubMed] [Google Scholar]
- 37.Prasad AS, Beck FWJ, Bao B, et al. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J Infect Dis 2008;197:795–802 [DOI] [PubMed] [Google Scholar]
- 38.Smith DS, Helzner EC, Nuttall CE, Jr, et al. Failure of zinc gluconate in treatment of acute upper respiratory tract infections. Antimicrob Agents Chemother 1989;33:646–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weismann K, Jakobsen JP, Weismann JE, et al. Zinc gluconate lozenges for common cold. A double-blind clinical trial. Dan Med Bull 1990;37:279–81 [PubMed] [Google Scholar]
- 40.Hendley JO. The host response, not the virus, causes the symptoms of the common cold. Clin Infect Dis 1998;26:847–8 [DOI] [PubMed] [Google Scholar]
- 41.Mäkelä MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998;36: 539–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.