In a recent issue of PNAS, Kiaris et al. (1) reported that JV-1-36, a growth hormone-releasing hormone (GHRH) receptor antagonist, dramatically suppresses the proliferation of human small lung cell carcinoma cell lines grown in athymic nude mice and in culture. The antitumorigenic action of JV-1-36 is consistent with previous reports from the same laboratory demonstrating the effectiveness of this and other GHRH antagonists in suppressing both in vivo and in vitro proliferation of a wide variety of transformed human cell lines, including those derived from colorectal, pancreatic, renal, glial, bone, prostate, breast, and ovarian cancers (summarized in Table 1 and reviewed in ref. 2).
Table 1.
Effects of the GHRH antagonists vasoactive intestinal peptide (VIP) and cAMP on human cancer cell lines grown as xenografts in nude mice (in vivo) or maintained in culture (in vitro)
| Cell type | GHRH antagonists
|
VIP
|
cAMP*
|
||
|---|---|---|---|---|---|
| Proliferation†, in vivo/ in vitro | Insulin-like growth factor (IGF)-I production, in vivo/in vitro | IGF-II production, in vivo/in vitro | Proliferation, in vivo/ in vitro | Proliferation, in vivo/ in vitro | |
| Lung (refs. 1, 2, 16, 17, and 21–24) | |||||
| H-69 (SCLC)‡§ | ↓/↓ | /− | ↓/↓ | ↓/↓ | |
| 510A (SCLC)‡ | /↓ | ||||
| H157 (NSCLC) | ↓/↓ | ↓/ | ↓/ | ||
| Colorectal (refs. 2, 25, and 26) | |||||
| HT-29¶ | ↓/↓ | −/ | ↓/↓ | /↓ | /↓ |
| Pancreas (refs. 2, 16, 17, and 27–29) | |||||
| SW-1990§ | ↓/↓ | −/− | ↓/↓ | /↓ | |
| Capan-2§¶ | /↓ | /↓ | /↑ | ||
| MiaPaCa-2§∥ | /↓ | /↓ | |||
| Kidney (refs. 2 and 30) | |||||
| Caki-1 | ↓/↓ | ↓/ | ↓/ | /− | |
| Glial (refs. 2, 31, and 32) | |||||
| U-87MG‡ | ↓/ | ↓/ | /↓ | ||
| Bone (refs. 2 and 6) | |||||
| SK-ES-1 | ↓/↓ | ↓/− | |||
| MNNG/HOS | ↓/↓ | ↓/− | |||
| Prostate (refs. 2, 15–17, 23, and 33–35) | |||||
| PC-3§ | ↓/↓ | ↓/ | ↓/↓ | /− | /↓ |
| DU-145§ | ↓/↓ | −/ | ↓/↓ | /↑ | /↑ |
| Breast (refs. 2, 14, 16, 17, 23, and 36) | |||||
| MDA-MB-468‡§ | ↓/↓ | ↓/ | ↓/↓ | ||
| ZR-75-1‡§¶ | /↓ | /↓ | |||
| Ovary (refs. 2, 14, 17, and 23) | |||||
| OV-1063 | /↓ | /↓ | |||
↓, Decreased; ↑, increased; −, unchanged; blank, not tested.
Treatment with nonselective protein kinase A (PKA) activators (dbcAMP, 8-Br-cAMP, forskolin, or phosphodiesterase inhibitors).
† Proliferation measured in vivo by tumor volume or weight and in vitro by changes in cell numbers, [3H]thymidine incorporation, or colorimetric assay.
‡Cell lines shown to express GHRH.
§Cell lines shown to respond to GHRH and VIP with a rise in cAMP.
¶Cell lines shown to express VIP1/pituitary adenylate cyclase-activating polypeptide (PACAP)2 receptors.
∥Cell lines shown not to express the VIP1/PACAP2 receptor.
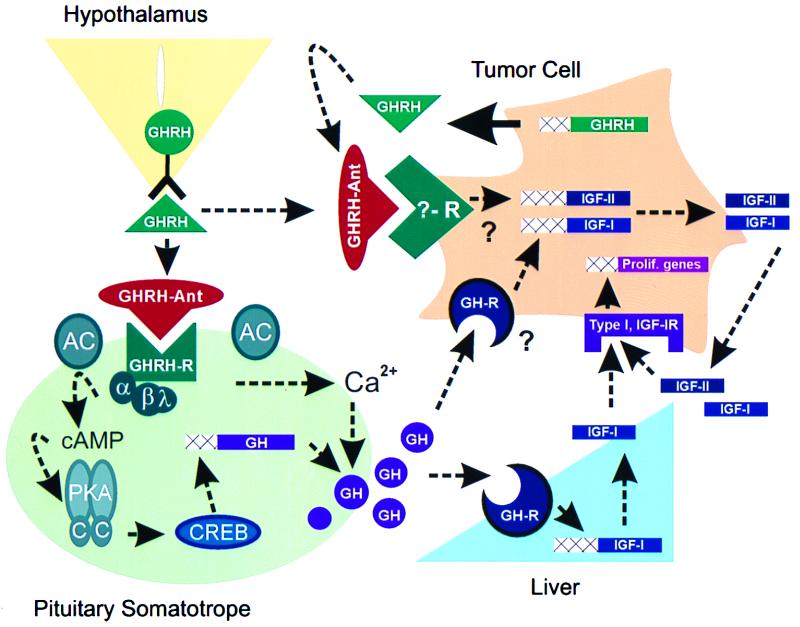
GHRH-antagonists bind to GHRH receptors located on pituitary somatotropes, thereby blocking the hypothalamic GHRH-mediated activation of the intracellular cAMP signal transduction pathway; a requirement for optimum GH synthesis and release (summarized in Fig. 1 and reviewed in ref. 3). A decline in circulating GH levels leads to the reduction in IGF-I production from the liver, the primary contributor to circulating IGF-I concentrations (Fig. 1; ref. 4). The suppressive effects of GHRH antagonists on the GH/IGF-I axis have been demonstrated in normal rats, in transgenic mice expressing the human GHRH transgene, and in nude mice bearing human tumor xenografts (2).
Figure 1.
Potential mechanisms mediating the antitumorigenic actions of GHRH antagonists (GHRH-Ant). GHRH antagonists bind to GHRH receptors (GHRH-R), located on pituitary somatotropes, and block GH synthesis and release. The GHRH receptor is a seven-transmembrane, G-protein-coupled receptor and is a member of the receptor superfamily that includes the VIP and PACAP receptors. Binding of GHRH to its receptor activates the α-subunit (Gs) of the closely associated G-protein complex, thus stimulating membrane bound adenylyl cyclase (AC) and increasing intracellular cAMP concentrations. cAMP binds to and activates the regulatory subunits of PKA, which in turn release catalytic subunits (C) that translocate to the nucleus and phosphorylate the cAMP response element binding protein, CREB. CREB, via direct and indirect mechanisms, stimulates GH gene transcription (3). In addition, GHRH-mediated cAMP-dependent and cAMP-independent pathways cause an influx of extracellular Ca2+, leading to the release of GH secretory vesicles and resulting in a rapid increase in circulating GH concentrations (3). GH stimulates liver IGF-I gene transcription (37) and could directly stimulate tumor IGF-I production. GH-induced increases in IGF-I could activate type I IGF-I receptors located on tumor cells, thereby mediating the transcription of genes important for cell proliferation (5). It is also possible that GHRH antagonists directly bind to and block a yet to be identified receptor that mediates the stimulatory effects of locally produced GHRH on IGF-II production. Locally produced IGF-II can in turn activate cell proliferation by binding to type I IGF-I receptors (5, 12). Dashed arrows indicate pathways suppressed after application of GHRH antagonists. Theoretical pathways are denoted by question marks.
The use of GHRH antagonists to suppress the GH/IGF-I axis as a potential anticancer therapy evolved from a plethora of reports demonstrating that most normal and transformed tumor cell lines express receptors for IGF-I and proliferate in response to supplemental IGF-I treatment (for review, see ref. 5). In addition, GH directly stimulates IGF-I production in cell lines derived from osteosarcomas (6). Therefore, it could be reasoned that reducing liver or tumor production of IGF-I by inhibiting pituitary GH production would slow tumor growth. In support of this hypothesis, Pollak and coworkers (7, 8) found that the metastatic behavior of murine osteosarcoma and fibrosarcoma cell lines in vivo was decreased by hypophysectomy and restored by GH replacement. In addition, somatostatin, which also suppresses the GH/IGF-I axis, can decrease tumor growth in nude mice bearing a human pancreatic cell line that does not express somatostatin receptors (9). Finally, a positive correlation between serum IGF-I concentrations and malignancies has been reported in patients with prostate (10) and breast (11) cancers. Taken together, these observations indicate that a component of the antitumorigenic effects of GHRH antagonists likely involves the inhibition of the pituitary GH/IGF-I axis.
However, reduced circulating GH/IGF-I cannot entirely account for the antitumorigenic actions of the GHRH antagonists in that these agents are also effective inhibitors of tumor IGF-II production (Table 1), where regulation of IGF-II synthesis is considered independent of GH actions (12). These findings suggest that the GHRH antagonists might also have a direct effect on tumor physiology. Indeed, GHRH antagonists effectively inhibit the proliferation of a variety of human cancer cell lines in vitro, and this inhibition is associated with a decline in tumor IGF-II production (Table 1). Because many tumors show elevated levels of IGF-II and because IGF-II is a mitogen acting through the type I IGF-I receptor (12), these observations suggest that GHRH antagonists may inhibit tumor cell proliferation by directly inhibiting tumor production of IGF-II.
A direct action of the GHRH antagonists on tumor growth implies that GHRH or a GHRH-like compound produced by the tumor acts as a paracrine/autocrine growth factor. Ectopic GHRH production, that occurring outside the accepted “normal” framework of hypothalamic GHRH, was first reported in carcinoids and pancreatic tumors (13), and it was from such tumors that GHRH was purified and later found to be identical to hypothalamic GHRH. Since that time, a variety of tumors that are GHRH-immunopositive has been described, including hypothalamic-pituitary gangliocytomas; bronchial, intestinal, and liver carcinoids; pancreatic islet cell tumors; sympathoadrenergic tumors; adrenal tumors (pheochromocytomas); pituitary adenomas; and thyroid medullary carcinomas. In addition, sensitive reverse transcription–PCR methods have detected GHRH expression in many normal tissues (3). Consistent with these observations is the fact that, of the human cancer cell lines examined that are responsive to GHRH antagonists, all express the GHRH gene (see ‡ in Table 1), and the resultant peptide is both immunologically and biologically active (14). Therefore, the antiproliferative effects of GHRH antagonists might be mediated by blocking the actions of locally produced GHRH.
The question arises: how does locally produced tumor GHRH transduce a mitogenic signal? A logical candidate is the GHRH receptor, because this receptor is essential for the proliferation of the pituitary somatotrope population (3). However, in preliminary studies, use of reverse transcription–PCR to detect GHRH receptor mRNA in GHRH antagonist responsive tumor cell lines has proven unsuccessful (2, 14, 15). Interestingly, the GHRH receptor is not detected in many normal tissues (testis, ovary, and placenta) shown to produce GHRH and respond to the ligand by a rise in cAMP (3). Likewise, Csernus et al. (16, 17) have recently shown that GHRH can elicit a rise in intracellular cAMP in many human cancer cell lines. Given the structural similarities of GHRH, VIP, and PACAP and the fact that GHRH can bind to the VIP1/PACAP2 receptor at high concentrations and elicit a cAMP response (18, 19), coupled with the observation that VIP1/PACAP2 receptors are expressed in some tumor cell lines (see ¶ in Table 1), it is possible that GHRH modulates tumor-cell proliferation through VIP1/PACAP2 receptors. A heterologous action of GHRH is supported by the recent findings that VIP and PACAP, as well as GHRH, increase cAMP in some human cancer cell lines that respond to the inhibitory effects of GHRH antagonists (see § in Table 1). In addition, VIP antagonist can block a rise in cAMP initiated by GHRH, and the reciprocal is also true (i.e., GHRH-antagonist can block the VIP-mediated cAMP rise; refs. 16 and 17).
The aforementioned studies clearly demonstrate that agonists and antagonists of GHRH and VIP receptors can modulate intracellular cAMP levels in short-term experiments; however, the question remains: does ligand activation of the cAMP-dependent signal transduction pathway induce proliferation of human cancer cell lines? By focusing on the cell lines previously shown to respond to GHRH antagonists, it is clear that the effect of elevating intracellular cAMP concentrations on cell growth is cell-line specific (Table 1). Two cell lines (Capan-2 and DU-145) have been shown to proliferate in response to VIP and ligand-independent increases in intracellular cAMP. These findings are consistent with the hypothesis that GHRH antagonists suppress tumor growth by blocking a VIP receptor-mediated increase in cAMP. However, in the majority of cell lines tested, VIP and cAMP do not affect (Caki-1) or actually inhibit (H-69, HT-29, SW-1990, U-87MG, and PC-3) proliferation. One possible explanation given for the variable effects of cAMP on cell proliferation is the relative expression and activity level of the PKA regulatory subunits (RI and RII; ref. 20). Cells that express a higher ratio of RI:RII proliferate in response to increases in intracellular cAMP, whereas the proliferation of cells with lower RI:RII ratios is inhibited by cAMP. Of particular relevance to this discussion is the response of the small lung cell carcinoma-derived cell line, H-69, to GHRH, VIP, and cAMP mediators (Table 1). Kiaris et al. (1) have reported that H-69 cells synthesize GHRH and proliferate in response to exogenous GHRH treatment. In contrast, Maruno et al. (21) have demonstrated previously that the proliferation of H-69 cells is inhibited by VIP and forskolin, a direct activator of adenylyl cyclase activity. The fact that the growth of some human cancer cell lines can be inhibited by GHRH antagonists as well as VIP or ligand-independent increases in cAMP presents the intriguing possibility that locally produced GHRH stimulates (and the GHRH antagonists inhibit) human tumor cell proliferation by binding to a unique receptor that mediates cell proliferation via a cAMP-independent pathway (Fig. 1). A VIP receptor-independent action of GHRH antagonists is also supported by recent work showing that in vitro growth of both VIP1/PACAP2 receptor negative (MiaCaPa-2) and positive (LNCaP) cell lines can be inhibited by GHRH-selective antagonists but not VIP-selective antagonists (Z. Rekasi and A. V. Schally, personal communication).
In summary, GHRH antagonists suppress the in vivo and in vitro growth of human cell lines from diverse tissue origins, making them interesting candidates for anticancer therapy. The mechanisms that mediate the antitumorigenic actions of GHRH antagonists might include suppression of the GH/IGF-I axis, in addition to direct inhibition of tumor growth that may be related to suppression of tumor IGF-II production. The direct effects of the GHRH antagonists on tumor growth could be mediated by blocking the paracrine/autocrine actions of locally produced GHRH. These preliminary studies not only have direct clinical relevance but also fuel more basic questions: what is the physiological, as well as pathophysiological, role of extrahypothalamic GHRH, and are some of the effects of extrahypothalamic GHRH mediated through unique, yet to be identified receptors?
Footnotes
See companion article on page 14894 in issue 26 of volume 96.
References
- 1.Kiaris H, Schally A V, Varga J L, Groot K, Armatis P. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schally A V, Varga J L. Trends Endocrinol Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 3.Frohman L A, Kineman R D. In: Handbook of Physiology: Hormonal Control of Growth. Kostyo J L, Goodman H M, editors. New York: Oxford Univ. Press; 1999. pp. 189–221. [Google Scholar]
- 4.Sjogren K, Liu J L, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson O, Jansson J, et al. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeRoith D, Lakesley V A, Werner H. In: Handbook of Physiology: Hormonal Control of Growth. Kostyo J L, Goodman H M, editors. New York: Oxford Univ. Press; 1999. pp. 633–662. [Google Scholar]
- 6.Pinski J, Schally A V, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. J Natl Cancer Inst. 1995;87:1787–1794. doi: 10.1093/jnci/87.23.1787. [DOI] [PubMed] [Google Scholar]
- 7.Sekyi-Otu A, Bell R, Andrulis I, Pollak M. J Natl Cancer Inst. 1994;86:628–632. doi: 10.1093/jnci/86.8.628. [DOI] [PubMed] [Google Scholar]
- 8.Pollak M, Sem A W, Richard M, Tetenes E, Bell R. J Natl Cancer Inst. 1992;84:966–971. doi: 10.1093/jnci/84.12.966. [DOI] [PubMed] [Google Scholar]
- 9.Weckbecker G, Raulf F, Bodmer D, Bruns C. Yale J Biol Med. 1997;70:549–554. [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J M, Stampfer M J, Giovannucci E, Gann P H, Ma J, Wilkinson P, Hennekens C H, Pollak M. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson S E, Willett W C, Colditz G A, Hunter D J, MIchaud D S, Deroo B, Rosner B, Speizer F E, Pollak M. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 12.Richman R A. In: Handbook of Physiology: Hormonal Control of Growth. Kostyo J L, Goodman H M, editors. New York: Oxford Univ. Press; 1999. pp. 701–736. [Google Scholar]
- 13.Szabo M, Frohman L A. In: Hormones in Normal and Abnormal Human Tissues. Fotherby K, Pal S B, editors. New York: de Gruyter; 1981. pp. 415–535. [Google Scholar]
- 14.Kahan Z, Arencibia J M, Csernus V J, Groot K, Kineman R D, Robinson W R, Schally A V. J Clin Endocrinol Metab. 1998;84:582–589. doi: 10.1210/jcem.84.2.5487. [DOI] [PubMed] [Google Scholar]
- 15.Lamharzi N, Schally A V, Koppán M, Groot K. Proc Natl Acad Sci USA. 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csernus V, Schally A V, Groot K. Peptides. 1999;20:843–850. doi: 10.1016/s0196-9781(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 17.Csernus V, Schally A V, Groot K. J Endocrinol. 1999;163:269–280. doi: 10.1677/joe.0.1630269. [DOI] [PubMed] [Google Scholar]
- 18.Campbell R M, Scanes C G. Growth Regul. 1992;2:175–191. [PubMed] [Google Scholar]
- 19.Robberecht P, Waelbroeck M. Ann NY Acad Sci. 1998;865:157–163. doi: 10.1111/j.1749-6632.1998.tb11174.x. [DOI] [PubMed] [Google Scholar]
- 20.Cho-Chung Y S, Pepe S, Clair T, Budillon A, Nesterova M. Crit Rev Oncol Hematol. 1995;21:33–61. doi: 10.1016/1040-8428(94)00166-9. [DOI] [PubMed] [Google Scholar]
- 21.Maruno K, Absood A, Said S I. Proc Natl Acad Sci USA. 1998;95:14373–14378. doi: 10.1073/pnas.95.24.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinski J, Schally A V, Jungwirth A, Groot K, Halmos G, Armantis P, Zarandi M, Vadillo-Buenfil M. Int J Oncol. 1996;9:1099–1105. doi: 10.3892/ijo.9.6.1099. [DOI] [PubMed] [Google Scholar]
- 23.Csernus V J, Schally A V, Kiaris H, Armatis P. Proc Natl Acad Sci USA. 1999;96:3098–3103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan M Z, Freshney R I, McNicol A M, Murray A M. Ann Oncol. 1993;4:499–507. doi: 10.1093/oxfordjournals.annonc.a058562. [DOI] [PubMed] [Google Scholar]
- 25.Sreedharan S P, Robichon A, Peterson K E, Goetzl E J. Proc Natl Acad Sci USA. 1991;88:4986–4990. doi: 10.1073/pnas.88.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamet L, Murat J C, Remaury A, Remesy C, Valet P, Paris H, Denis-Pouxviel C. J Cell Physiol. 1992;150:501–509. doi: 10.1002/jcp.1041500310. [DOI] [PubMed] [Google Scholar]
- 27.Szepeshazi, K., Schally, A. V., Groot, K., Armatis, P., Hebert, F. & Halmos, G. (1999) Eur. J. Cancer, in press. [DOI] [PubMed]
- 28.Jiang S, Kopras E, McMichael M, Bell R H, Jr, Ulrich C D, II. Cancer Res. 1997;57:1475–1480. [PubMed] [Google Scholar]
- 29.Sack T L, Gum J R, Kim Y S. Int J Pancreatol. 1988;3:171–184. doi: 10.1007/BF02798929. [DOI] [PubMed] [Google Scholar]
- 30.Jungwirth A, Schally A V, Pinski J, Groot K, Armatis P, Halmos G. Proc Natl Acad Sci USA. 1997;94:5810–5813. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiaris, H., Schally, A. V. & Varga, J. L. (1999) Neoplasia, in press. [DOI] [PMC free article] [PubMed]
- 32.Chen T C, Hinton D R, Zidovetzki R, Hofman F M. Lab Invest. 1998;78:165–174. [PubMed] [Google Scholar]
- 33.Jungwirth A, Schally A V, Pinski J, Halmos G, Groot K, Armatis P, Vadillo-Buenfil M. Br J Cancer. 1997;75:1585–1592. doi: 10.1038/bjc.1997.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jongsma J, Oomen M H, Noordzij M A, Romijn C, van Der Kwast T H, Schroder F H, van Steenbrugge G J. Prostate. 2000;42:34–44. doi: 10.1002/(sici)1097-0045(20000101)42:1<34::aid-pros5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Bang Y J, Kim S J, Danielpour D, O'Reilly M A, Kim K Y, Myers C E, Trepel J B. Proc Natl Acad Sci USA. 1992;89:3556–3560. doi: 10.1073/pnas.89.8.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madsen B, Georg B, Vissing H, Fahrenkrug J. Cancer Res. 1998;58:4845–4850. [PubMed] [Google Scholar]
- 37.Lund P K. In: Handbook of Physiology: Hormonal Control of Growth. Kostyo J L, Goodman H M, editors. New York: Oxford Univ. Press; 1999. pp. 537–571. [Google Scholar]