Kokoza et al. (1) recently reported the genetic engineering of Aedes aegypti mosquitoes to express abundantly an anti-microbial immune effector molecule in the hemolymph of a vector. In the realm of vector-borne diseases, this is a very significant event with provocative implications. Vector-borne diseases, such as malaria, leishmania, trypanosomiasis, dengue, yellow fever, West Nile encephalitis, etc., are resurgent through out much of their former distributions and/or are emerging in new geographic areas. The morbidity and mortality associated with these diseases are overwhelming: billions of people are now at risk for infection, each year hundreds of millions of infections occur, resulting in millions of deaths, and the morbidity associated with vector-borne pathogen infections is inestimable [see Nakajima, H. (1996) The World Health Report (World Health Organization) at http://www.who.int/whr/1996/whr-e.htm]. In addition, these diseases impose major economic and social burdens on populations least able to afford them. The situation is bleak, and it is especially disconcerting that many of these diseases were previously controlled to some extent.
These vector-borne diseases of humans and domestic animals are proving to be almost intractable to control by conventional means. The reasons for this are multifactorial and include such problems as (i) the lack of effective vaccines for diseases such as malaria and dengue, (ii) the demise of public health programs for vector control, (iii) the decline in vector-borne disease specialists, (iv) the development of pesticide resistance in vector populations, and (v) the development of drug resistance in parasites. The situation is exacerbated for some of these diseases by socioeconomic issues including dramatic population growth, dramatically increased urbanization, lack of planning and sanitation in the face of unprecedented urbanization, deforestation, rapid movement of pathogens and vectors by jet travel, etc. (2).
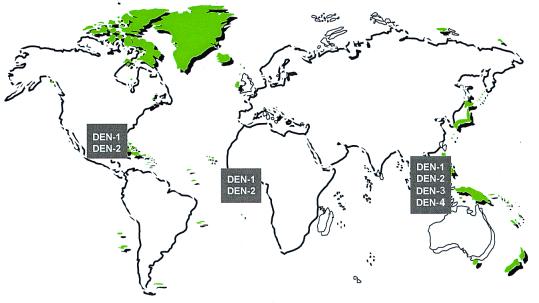
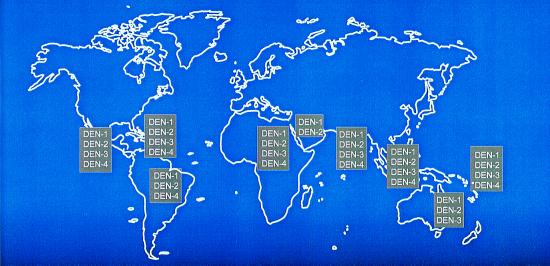
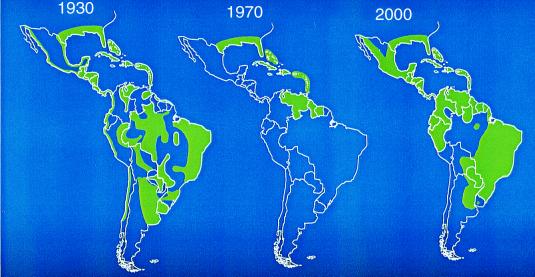
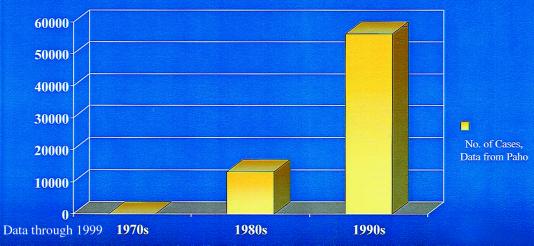
The situation is illustrated by the recent emergence of dengue/dengue hemorrhagic fever as a major public health problem in the Americas (3). Before the 1980s the severe form of dengue, hemorrhagic fever, was very rare in the Western Hemisphere, and typically only one or two of the four dengue virus serotypes circulated in the Americas at one time (Fig. 1 Upper). Aedes aegypti was controlled to a large extent in the Americas in a remarkably effective campaign to prevent yellow fever emerging from its sylvatic foci. With the demise of these programs, Aedes aegypti has returned with a vengeance (Fig. 2). During the same period, multiple serotypes of dengue (some of which are more virulent than those viruses that had previously caused human infections in the Americas) began to circulate, and molecular epidemiological investigations revealed that these were being introduced from different parts of the world (Fig. 1 Lower). This hyperendemicity with multiple serotypes and genotypes of dengue viruses circulating and Aedes aegypti resurgent and hyperabundant throughout much of its previous distribution have contributed to the emergence of dengue/dengue hemorrhagic fever as a major public health problem in the Americas (ref. 3; Fig. 3). Indeed, dengue emergencies are now declared each year in many countries in Central and South America.
Figure 1.
Global distribution of dengue virus serotypes in 1970 (Upper) and 2000 (Lower). Figure kindly provided by D. Gubler, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO.
Figure 2.
Aedes aegypti distribution in the Americas. Figure kindly provided by D. Gubler.
Figure 3.
Emergence of dengue hemorrhagic fever in the Americas. Figure kindly provided by D. Gubler.
The situation with other vector-borne diseases (especially malaria) is equally bleak, and novel and efficacious control strategies are sorely needed for this epidemiological group of pathogens. New information concerning the molecular biology of vectors and the determinants of productive pathogen–vector interactions is necessary to develop new strategies for control of vector-borne diseases. One particularly provocative approach is to molecularly perturb the ability of vectors to transmit pathogens (4–6). Theoretically, vectors could be genetically immunized to express pathogen-specific molecules that would impair the competence of the vector. Competent vectors are those that are susceptible to pathogen infection, replication, and dissemination to epidemiologically significant target organs (e.g., salivary glands) and that efficiently transmit the pathogen. Incompetent vectors are those that are resistant or refractory to infection and that inefficiently transmit the pathogen. Should mechanisms become available to drive effector molecules that condition vector incompetence into mosquito populations, the cycle of pathogen transmission could be interrupted and controlled.
To accomplish this, (i) transgenesis systems must be developed for efficient transformation of targeted vector species, (ii) effector molecules must be identified that will induce the anti-pathogen phenotype in the vector, and (iii) mechanisms must be developed to drive the effector system into the vector population (4–6). These certainly are not trivial undertakings, and the list is purposefully simplified. However, there has been significant progress recently in development of new technologies and tools for vector manipulation and investigation.
Landmark studies have now demonstrated the transformation of important vector mosquitoes by using transposable element-based systems (7–10). Aedes aegypti (the urban vector of yellow fever and dengue viruses) and Anopheles stephensi (an important vector of malaria) have been transformed by using the transposable elements mariner, Hermes (7–9), and minos (10), respectively, and others such as piggyBac (11) also offer great promise in this regard. Although these systems are not as robust as the P element system, which revolutionized genetic investigations in Drosophila, it is now likely that more and more laboratories will be able to apply the power of transgenesis in the investigation of the molecular biology of vectors.
Concomitantly, modern molecular biological and genomic investigations are revealing a plethora of new vector genes, which potentially condition important vector phenotypes. The publication of the Drosophila genome sequence provides an unparalleled opportunity for vector biologists to identify genes that could be exploited to control vector-borne diseases (12). Field and laboratory investigations are now being linked in the emerging field of population genomics (13), which effects a marriage of field oriented research, population and molecular genetics, and functional genomics to identify important vector genes that condition pathogen maintenance and transmission. In this regard, new virus transduction systems are additional tools that allow rapid biological characterization of vector genes in vivo, thereby permitting focus of transgenesis efforts on those with demonstrated efficacy. For example, recombinant Sindbis viruses expressing antisense sequences to LaCrosse and dengue viruses were used to rapidly define virus sequences that conferred resistance to infection with and transmission of the respective human pathogens by their mosquito vectors (14, 15). The virus systems can also be used to silence expression of endogenous genes for rapid characterization in vivo in vectors (16).
The study by Kokoza et al. (1) builds upon these marvelous advances in the field of vector biology by exploiting the new transgenesis capabilities to transform mosquitoes with a potentially biologically relevant effector molecule under the regulation of an inducible promoter system. The authors engineered Aedes aegypti mosquitoes to express defensin, a potent anti-microbial gene, in the hemolymph of transgenic mosquitoes. In brief, the investigators used a Hermes-based system to transform the mosquitoes. The construct contained a marker cinnabar gene to rescue eye color in transformed white-eyed Aedes aegypti (7) and a promoter sequence of the vitellogenin gene (Vg; ref. 17) to regulate expression of the Aedes aegypti defensin A gene, which encodes the pre-pro-defensin (18). This construct and a helper plasmid containing the Hermes transposase were microinjected into the mosquito eggs, transformants were selected by red eye color, and a transgenic line of Aedes aegypti was established. In the transgenic mosquitoes, high levels of biologically active defensin accumulate after a blood meal. This is a very important issue—the Vg promoter is activated only in the fat body of mosquitoes after a blood meal. Thus, the putative anti-pathogen defensin molecule is expressed only during critical stages of vector–pathogen interactions, and it accumulates abundantly in the hemolymph. Many vector-borne pathogens must traffic through the hemolymph to infect the salivary glands and then be transmitted. Thus, the pathogen and effector molecule should be temporally and spatially colocalized. In addition, the “inducible” strategy minimizes the “genetic load” of the transgene on vectors, thereby minimizing effects on the fitness of transgenic organisms (6).
Kokoza et al. (1) have provided the proof of concept that anti-pathogen effector molecules can be engineered into vectors and be expressed stably in a biologically relevant fashion. A plethora of potentially anti-pathogenic molecules (e.g., alternative anti-microbials, Mx protein, single-chain antibodies, antisense sequences) are being investigated as potent effector molecules for vector immunization against a variety of pathogens. Similarly, a variety of mechanisms, such as transposable elements (19), Wolbachia and other symbionts (20–22), and densoviruses (23), are being investigated for their potential to deliver or drive the effector molecules into vector populations. Such agents continuously traffic through vector populations and thus could provide mechanisms to disseminate immunizing effector molecules in vectors. If a sufficient proportion of vectors became incompetent, the cycle of transmission of the pathogen could theoretically be interrupted. Obviously, numerous and important scientific, ethical, safety, regulatory, and biopolitical issues will have to be addressed before such a strategy could be used to attempt to control a vector-borne disease. The benefits and risks associated with such an approach will have to be critically assessed (24). However, the inexorable increase in the public health importance of these pathogens, the failure of conventional approaches to control them, and the lack of alternatives in the face of pesticide resistance in vectors and drug resistance in pathogens, mandates examination of all possible approaches for control. Importantly, investigations into molecular genetic approaches for control of these diseases is providing invaluable spin-off information concerning vector molecular biology and vector–pathogen interactions, which may also lead to novel and efficacious control strategies for these diseases.
Considering the tenacity of vector-borne pathogen cycles and the evolutionary potential of the pathogens and their arthropod vectors, successful control strategies for these diseases will undoubtedly require integrated approaches. Such an approach would include the development of new vaccines and drug therapies, invigoration of classical vector control and disease prevention and surveillance strategies, and the application of molecular approaches to manipulate and monitor vector populations. The advances reported by Kokoza et al. (1) are a beginning.
Acknowledgments
All figures were kindly provided by Dr. Duane Gubler. This review was supported in part by Grants AI 46753 and AI 45430 from the National Institutes of Health.
Footnotes
See companion article on page 9144 in issue 16 of volume 97.
References
- 1.Kokoza V, Ahmed A, Cho W-L, Jasinskiene N, James A A, Raikhel A. Proc Natl Acad Sci USA. 2000;97:9144–9149. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratz N G. Annu Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Gubler D. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins F H, James A A. Sci Med. 1996;3:52–61. [Google Scholar]
- 5.Beaty B J, Carlson J O. Curr Opin Infect Dis. 1997;10:372–376. [Google Scholar]
- 6.O'Brochta D A, Atkinson P W. Sci Am. 1998;279(6):90–95. doi: 10.1038/scientificamerican1298-90. [DOI] [PubMed] [Google Scholar]
- 7.Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A, Collins F H. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates C J, Jasinskiene H, Miyashiro L, James A A. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catteruccia F, Nolan T, Loukeris T G, Blass C, Savakis C, Kafatos F C, Crisanti A. Nature (London) 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- 10.Coates C J, Jasinskiene N, Pott G B, James A A. Gene. 1999;21:317–325. doi: 10.1016/s0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 11.Grossman G L, Rafferty C S, Fraser M J, Benedict M Q. Insect Biochem Mol Biol. 2000;30:909–914. doi: 10.1016/s0965-1748(00)00092-8. [DOI] [PubMed] [Google Scholar]
- 12.Adams M D, Celniker S E, Holt R A, Evans C A, Gocayne J D, Amanatides P G, Scherer S E, Li P W, Hoskins R A, Galle R F, et al. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 13.Black, W. C., Baer, C. F., Antolin, M. F. & DuTeau, N. M. (2000) Annu. Rev. Entomol., in press. [DOI] [PubMed]
- 14.Powers A, Kamrud K, Olson K, Higgs S, Carlson J, Beaty B. Proc Natl Acad Sci USA. 1996;93:4187–4191. doi: 10.1073/pnas.93.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson K, Higgs S, Powers A, Davis B, Carlson J, Blair C, Beaty B J. Science. 1996;272:884–886. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- 16.Johnson B, Olson K, Allen-Miura T, Carlson J O, Coates C J, Jasinskiene N, James A A, Beaty B J, Higgs S. Proc Natl Acad Sci USA. 1999;96:13399–13403. doi: 10.1073/pnas.96.23.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romans P, Tu Z, Ke Z, Hagedorn H H. Insect Biochem Mol Biol. 1995;25:936–958. doi: 10.1016/0965-1748(95)00037-v. [DOI] [PubMed] [Google Scholar]
- 18.Lowenberger C A, Smartt C T, Bulet P, Ferdig M T, Severson D W, Hoffmann J A, Christensen B M. Insect Mol Biol. 1999;8:107–118. doi: 10.1046/j.1365-2583.1999.810107.x. [DOI] [PubMed] [Google Scholar]
- 19.Kidwell M G, Ribeiro J M C. Parasitol Today. 1992;8:325–329. doi: 10.1016/0169-4758(92)90065-a. [DOI] [PubMed] [Google Scholar]
- 20.Durvasula R V, Gumbs A, Pahackal A, Kruglov O, Aksoy S, Merrifield R B, Richards F F, Beard C B. Proc Natl Acad Sci USA. 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis C F, Sinkins S P. Parasitology. 1998;116:111–115. doi: 10.1017/s0031182000084997. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Q, Ruel T D, Zhou W, Moloo S K, Majiwa P, O'Neill S L, Aksoy S. Med Vet Entomol. 2000;14:44–50. doi: 10.1046/j.1365-2915.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 23.Afanasiev B N, Ward T W, Beaty B J, Carlson J O. Virology. 1999;257:62–72. doi: 10.1006/viro.1999.9621. [DOI] [PubMed] [Google Scholar]
- 24.Coates C J. Nature (London) 2000;405:900–901. doi: 10.1038/35016192. [DOI] [PubMed] [Google Scholar]