The authors have used a reversible cross-linking–peptide fingerprinting approach to study the contacts between the nascent RNA and HCV RNA polymerase during RNA synthesis. Their results surprisingly suggest that the nascent RNA exits the polymerase in close proximity to the channel thought to be used by rNTPs to access the active site. Their results are thus more compatible with the template/primer duplex within the polymerase being in the opposite orientation to that suggested from crystal structures.
Keywords: hepatitis C virus, positive-strand RNA virus, NS5B RNA-dependent RNA polymerase, nascent RNA channel, RNA binding
Abstract
Understanding how the hepatitis C virus (HCV) RNA-dependent RNA polymerase (RdRp) interacts with nascent RNA would provide valuable insight into the virus's mechanism for RNA synthesis. Using a peptide mass fingerprinting method and affinity capture of peptides reversibly cross-linked to an alkyn-labeled nascent RNA, we identified a region below the Δ1 loop in the fingers domain of the HCV RdRp that contacts the nascent RNA. A modification protection assay was used to confirm the assignment. Several mutations within the putative nascent RNA binding region were generated and analyzed for RNA synthesis in vitro and in the HCV subgenomic replicon. All mutations tested within this region showed a decrease in primer-dependent RNA synthesis and decreased stabilization of the ternary complex. The results from this study advance our understanding of the structure and function of the HCV RdRp and the requirements for HCV RNA synthesis. In addition, a model of nascent RNA interaction is compared with results from structural studies.
INTRODUCTION
The causative agent of hepatitis C, the hepatitis C virus (HCV), has become a well-studied system for viral RNA replication. HCV is a single-stranded RNA (ssRNA) virus in the Flaviviridae family. The key enzyme for HCV RNA replication and transcription is NS5B, the RNA-dependent RNA polymerase (RdRp). Studies of the HCV RdRp have provided useful information about interactions with inhibitors and insights to the requirements for viral RNA-dependent RNA synthesis (Chinnaswamy et al. 2010a).
All polymerases can be described using the analogy of a right hand, with the fingers, thumb, and palm subdomains (Joyce and Steitz 1995). The fingers and thumb subdomains guide the template RNA, while the active-site motif resides in the palm (Ranjith-Kumar and Kao 2006). In vitro, the recombinant HCV RdRp is capable of initiating RNA synthesis de novo from purine nucleotides, which is the likely mechanism of RNA synthesis during infection (Ferrari et al. 1999; Bressanelli et al. 2002). GTP is the preferred initiation nucleotide in vitro and has recently been shown to stimulate the transition from initiation to elongation in trans (Harrus et al. 2010). Binding to the initiation nucleotide occurs through a region called the primer grip that lies adjacent to the divalent metal binding motif in the catalytic pocket of the HCV RdRp (Bressanelli et al. 2002; O'Farrell et al. 2003).
How the viral RdRp structure affects function has been the subject of considerable analysis (Appel et al. 2006; Powdrill et al. 2010; Rigat et al. 2010). The template channel has been characterized using proteomic approaches and mutational analysis (Kim et al. 2005; Deval et al. 2007). A complex of NS5B crystallized with a short RNA also provided valuable information on the interaction between the 5′ end of the single-stranded RNA template and the template channel (O'Farrell et al. 2003). A crystal structure of the HCV polymerase in a partially open form has also been determined (Biswal et al. 2005). It suggests that an extensive conformational change must occur for the HCV RdRp to accommodate the partial duplex formed between the template and nascent RNA (O'Farrell et al. 2003; Dutartre et al. 2005; Chinnaswamy et al. 2008). Single-molecule reconstructions of the monomeric form of the HCV RdRp as well as analysis of crystal structures of the HCV RdRp in complex with non-nucleoside inhibitors are consistent with large conformational changes being important for activities of the HCV RdRp (Chinnaswamy et al. 2010b; Caillet-Saguy et al. 2011). A key element involved in the conformational change is named the Δ1 loop, which extends from the fingers to contact the thumb subdomain largely through hydrophobic interactions (Chinnaswamy et al. 2008). Mutations or inhibitors that affect the Δ1 loop–thumb interaction reduced de novo–initiated RNA synthesis and were detrimental for HCV subgenomic replicon replication (Tomei et al. 2003; Chinnaswamy et al. 2010a).
An important feature of RNA synthesis by the HCV RdRp that has not been determined experimentally is the location where the nascent RNA emerges from the ternary complex. This project seeks to map the nascent RNA channel and characterize how the channel affects RNA-dependent RNA synthesis.
RESULTS
Identification of the nascent RNA channel of NS5B using mass spectrometry
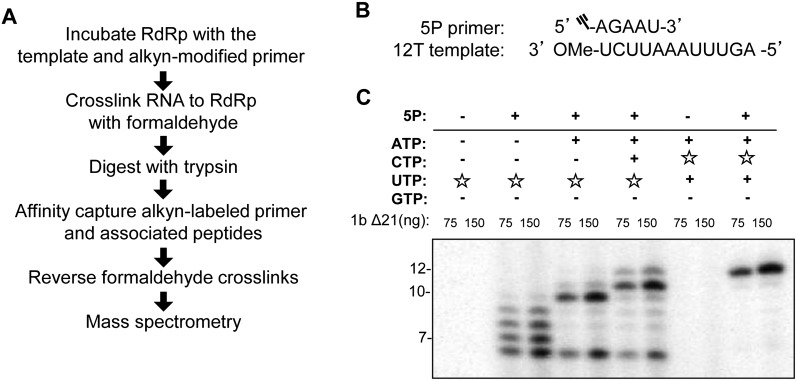
The goals of this study are to identify the nascent RNA exit site in the HCV RdRp and to examine its role in RNA-dependent RNA synthesis. This study used the NS5B from the 1b Con1 strain that was expressed without the C-terminal membrane–anchoring residues. The resulting protein is named 1bΔ21. Previously, a reversible cross-linking–peptide fingerprinting method (RCAP) was used to map the regions of the polymerase that contact RNA (Kim et al. 2005). A technical challenge in the identification of the nascent RNA exit channel was the specific capture of the nascent RNA in the presence of molar excess of the template RNA. Therefore, we modified the RCAP assay (Fig. 1A) to include an alkyn-modified 5-nt primer (5P) that can be extended in the nascent RNA channel to enable its specific capture using the highly efficient click chemistry (Speers and Cravatt 2004). Viral RdRps have been shown to use a 5-nt primer in place of GTP for de novo–initiated RNA synthesis (Ranjith-Kumar et al. 2002a). The template for the RNA synthesis reaction was a 12-nt RNA (12T) that has 5 nt at its 3′ terminus that can anneal with 5P as well as allow stepwise extension from 5P by the addition of specific NTP(s) (Fig. 1B).
FIGURE 1.
Conditions to examine nascent RNA synthesis using the RCAP assay. (A) Schematic of the reversible cross-linking affinity purification (RCAP) strategy used to identify nascent RNA contact sites on the HCV NS5B protein. (B) Sequences of the nascent RNA mimic (5P) and template (12T) used in this study. The alkyn modification is indicated by the Ξ symbol on the 5′ end of 5P. (C) Autoradiogram of a urea PAGE to illustrate walking NS5B down the template. (☆) Radiolabeled NTPs; (+) presence of cold NTPs. In the presence of only CTP or ATP, no RNA synthesis is observed. In the presence of UTP and of both ATP and UTP, synthesis of a 7-nt and 10-nt product is observed, respectively. In the presence of ATP, UTP, and CTP, full-length 12-nt product formation is detected. The product lengths were assigned by electrophoresis of chemically synthesized RNAs of known lengths in the same gel. These control RNAs were then visualized by staining with Toluidine Blue (data not shown).
To ensure that the HCV RdRp can perform stepwise synthesis with the 5P/12T RNA complex, one or more NTPs were added to the reactions, and the lengths of the products were monitored (Fig. 1C). The presence of only ATP resulted in no obvious product synthesis, due to the need to incorporate two uridylates after 5P. Similar results were observed in reactions with only CTP. In the presence of radiolabeled UTP, products extended from 5P of 6–9 nt were observed. While only the 6-nt to 7-nt products are expected based on the template sequence, the HCV RdRp has been reported to add nontemplated nucleotides to the 3′ terminus of the nascent RNA (Ranjith-Kumar et al. 2001). Interestingly, in the presence of both UTP and ATP, the majority of the products were of 10 and 11 nt. In the presence of the 5P primer, UTP, ATP, and CTP, the products were further extended to be of 11 and 12 nt (Fig. 1C). The 12-nt product represents a complete template-dependent RNA synthesis, while the 11-nt product terminated prematurely. RdRps have been documented to terminate prematurely if the template contains adenylates and uridylates at positions −2 to −4 from the 5′ terminus (Kim et al. 2000; Tayon et al. 2001). Synthesis of all of the products required the 5P/primer (Fig. 1C). These results have been independently confirmed in more than five independent experiments that manipulated the various NTP concentrations. While the pausing of the 1b RdRp elongation products was imprecise, these results demonstrate that intermediates in RNA synthesis could be produced in the absence of one or more of the NTPs.
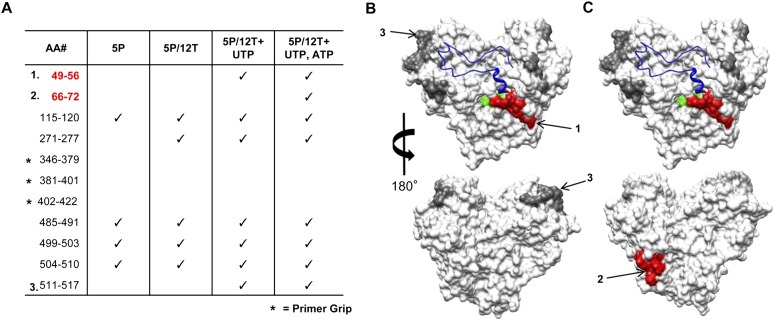
The conditions used to form the ternary complex in an NTP-dependent manner were used for the RCAP assay. Reactions performed with 1bΔ21 incubated with only 5P or with the 5P/12T complex but no NTPs, were processed in parallel to serve as controls for the identification of regions of the 1bΔ21 that can contact RNA (but not specifically contact the nascent RNA exit site). Several peptides associated with 5P or 5P/12T were identified (Fig. 2A). Upon the addition of UTP, two peptides in addition to those identified with 5P or 5P/12T were reproducibly observed. The first contained residues 49–56 that mapped to the base of the Δ1 loop, while the second mapped to residues 511–517 (Fig. 2A,B). When both UTP and ATP were present in the reaction, peptide consisting of residues 66–72 was observed. Since this peptide was located next to amino acids 49–56 in the NS5B structure (Fig. 2B), we hypothesize that residues 49–72 contact the nascent RNA.
FIGURE 2.
Identification of peptides associated with the nascent RNA by the RCAP assay. (A) Peptides identified in the RCAP assay performed with nascent RNAs synthesized in the presence of different nucleotides. Peptides were resolved by a Bruker Autoflex III MALDI-TOF mass spectrometer set in reflectron mode. The reactions were performed as described in Materials and Methods. Peak assignments to peptides within NS5B were based on theoretical digests that match calculated masses within 0.5 Da. (Red) Peptides within a region only identified during RNA synthesis; (*) peptides from the primer grip region that were not identified in any of the experimental setups. (B,C) Molecular model of the peptides within NS5B found to contact RNA (PDB: 1QUV). (Gray) Peptides found to contact 5P or 5P/12T; (red) peptides present only with the ternary complex generated in the presence of UTP or UTP and ATP; (green) the active site of NS5B; (blue in the ribbon structure) the Δ1 loop.
When viewed from the front face of the RdRp, residues 49–72 are located on the right side of the central cavity of the HCV RdRp, while the primer grip is located to the left side of the cavity (Fig. 2B). This result suggests that the nascent RNA needs to turn from the left side of the polymerase to the right in its path out of the central cavity. To confirm that the region encompassed by peptide 49–72 does contact the nascent RNA using an independent method, we incorporated aminoallyl-UTP in place of UTP in the RNA synthesis reaction that will add a free amine for cross-linking. Aminoallyl-uridylate can be used by the HCV RdRp in the RNA synthesis assay (Supplemental Fig. 1). Within the nascent RNA, the primary amine from aminoallyl-uridylate increases its coupling efficiency to the nascent RNA channel. However, an additional radius of cross-linking should be expected due to the allyl moiety. Cross-linking of the ∼10-nt nascent RNA should enrich for peptides from residues 49–72 from NS5B as well as from residues 21–43 that correspond to the Δ1 loop that lies above peptide 49–72 (Fig. 3; Supplemental Fig. 2A). These results are consistent with those from the RCAP assay.
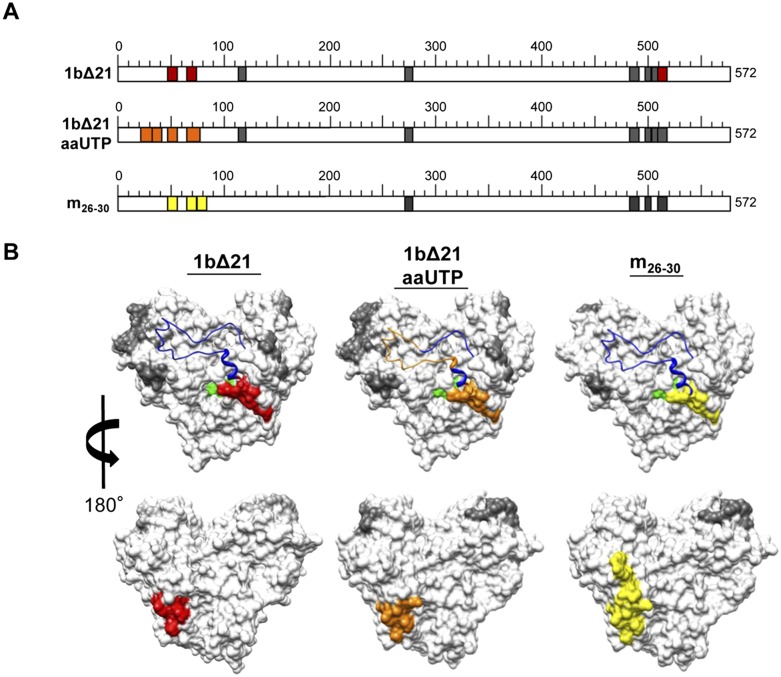
FIGURE 3.
Mapping of the residues in the HCV RdRp that contact the nascent RNA. (A) Schematic representation of peptides identified in the RCAP assay variations. Colored regions represent peptides identified during RNA synthesis: (red) regions identified by the RCAP assay of 1bΔ21; (orange) regions identified during RNA synthesis using a nucleotide analog, aminoallyl-UTP (aaUTP); (yellow) regions identified within m26-30, an RdRp mutant defective in de novo initiation. Details regarding the peptide assignments can be found in Supplemental Figure 2. The reactions were performed as described in Materials and Methods. Peak assignments to peptides within NS5B were based on theoretical digests that match calculated masses within 0.5 Da. (B) Molecular model of the peptides within NS5B found to contact RNA (PDB: 1QUV). (Gray) Peptides found to contact 5P or 5P/12T; ones present only with the ternary complex generated in the presence of UTP (red), UTP (orange), or ATP (yellow); (green) the active site of NS5B; (blue in the ribbon structure) the Δ1 loop.
As an additional test to the nascent RNA exiting from the right side of the RdRp, a de novo initiation-defective RdRp mutant, m26-30, which retained the ability to elongate RNA in the presence of a primer–template duplex, was characterized (Chinnaswamy et al. 2008, 2010b). M26-30 was used to form a ternary complex and subjected to the RCAP assay. Peptides that contain residues 51–56 and 66–74 were observed in the complex with 5P/12T. The same two peptides along with one that contains residues 73–81 were observed in the ternary complex that contained 5P/12T and both ATP and UTP (Fig. 3; Supplemental Fig. 2B). Residues 73–81 lie on the back face of the RdRp and contain the same region that contacted the nascent RNA generated by 1bΔ21 (Fig. 3B). In summary, two versions of the 1b HCV RdRp that are capable of elongative RNA synthesis identified that the nascent RNA exits to the right of the central cavity.
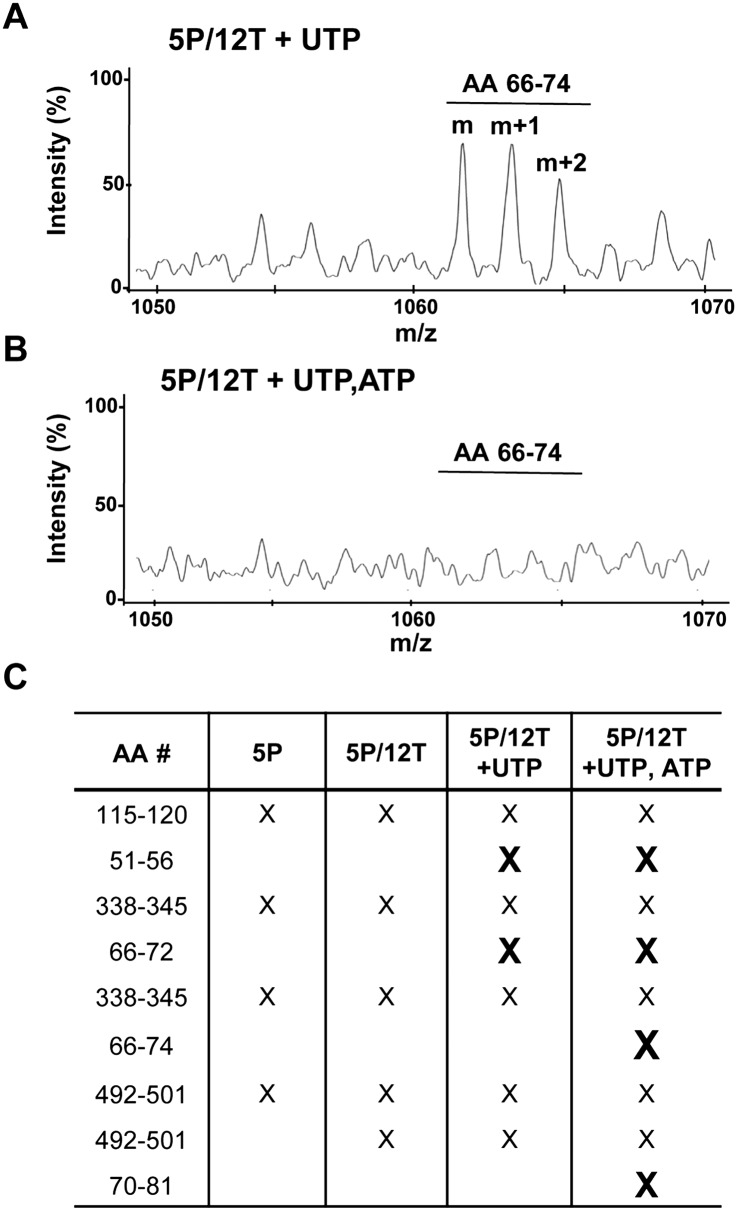
To further examine 1bΔ21 contact with the nascent RNA, we used positive-ion-mode mass spectrometry in an attempt to identify peptides that are not in contact with RNA (Steen and Jensen 2002). The negative charges of the RNA should bias against the RNA-contacting peptides once they are covalently linked with formaldehyde. To ensure that only peptides derived from NS5B that are in contact with the RNA were visualized, RNA–protein conjugates were purified by cyclo-addition before digestion with trypsin. Peptides derived from the polymerase in the presence of UTP reproducibly contained a peptide that corresponds to residues 66–74 (Fig. 4A). However, peptides released from tryptic digests of the ternary complex that formed in the presence of UTP and ATP did not contain single or multiply charged ions for residues 66–74 (Fig. 4B). These results suggest that this region of NS5B is in contact with the nascent RNA. Additionally, peptides of residues 51–56 and 66–72 were absent in the ternary complex formed in the presence of UTP. Reactions performed with both ATP and UTP resulted in the lack of peptides corresponding to amino acids 66–74 and 70–81 (Fig. 4C). These results are also in good agreement with those from the RCAP assay (Figs. 2, 3) in identifying residues that contact the nascent RNA.
FIGURE 4.
Analysis of the peptides from the RdRp ternary complex that are not cross-linked to the RNA. (A) MALDI-TOF mass spectra of a peptide detected in the presence of 5P/12T and UTP. The spectrum was generated using positive-mode ionization. (B) MALDI-TOF mass spectra of the same m/z region as in A in the presence of 5P/12T and both UTP and ATP. The peptide corresponding to amino acids 66–72 is no longer detectable. (C) Table of peptides that are lost during RNA synthesis. (X) A peptide no longer detected in the mass spectra under the experimental setup indicated. Peptides no longer detected during RNA synthesis are in bold.
Mapping of the nascent RNA exit site using an amidination interference assay
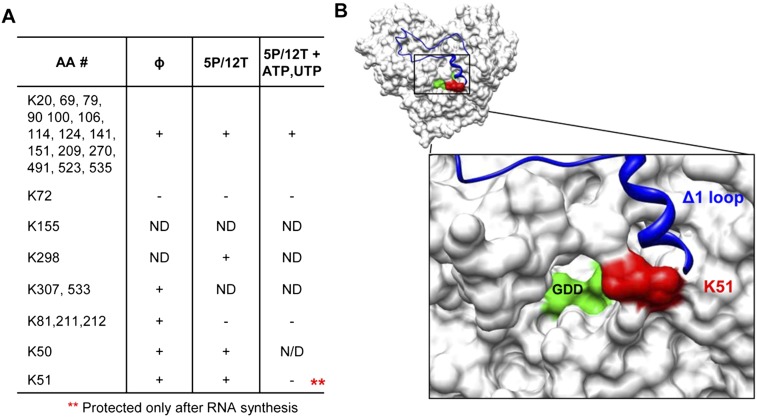
A fourth method was used to map the nascent RNA-contacting residues in 1bΔ21 independently. S-Methyl thioacetimidate (SMTA) can covalently modify surface-exposed lysine side chains and the N-terminal amine without major perturbations in the protein structure (Janecki et al. 2005). A comparison of the sites of SMTA modification from peptides derived from an apoprotein to those generated by the protein–ligand complex will identify regions of the protein that are changed upon ligand binding. SMTA-modified lysine will be marked in two ways: (1) by a 41-Da mass change and (2) by the loss of trypsin cleavage.
We assessed whether nascent RNA synthesis would result in the increased protection of specific lysines. In the absence of RNA, all except two of the surface-exposed lysines were observed to be amidinated in multiple experiments (Fig. 5A). In the presence of 5P/12T, amidination at lysines 81, 211, and 212 were decreased to background. In the presence of 5P/12T, UTP, and ATP, only one additional lysine, K51, was protected from amidination. Notably, K51 lies within the peptides identified in the RCAP assay, as well as the peptide missing in the positive-ion-mode experiment (Figs. 2, 4). The assignments of the differentially amidinated peptide were confirmed by MS/MS fragmentation spectra (Supplemental Fig. 3). Altogether, four independent approaches identified the right side of the central cavity of the HCV RdRp to contact the nascent RNA. The region contains α-helices B and C in residues 49–72 and lies underneath the Δ1 loop in the fingers domain of NS5B (Bressanelli et al. 1999).
FIGURE 5.
Mapping interactions in the RdRp ternary complex using an aminidation interference assay. (A) Lysines modified in the presence of S-methyl thioacetimidate (SMTA) as a function of RNA synthesis condition. Briefly, 0.3 μM 1bΔ21 was added to a 50-μL reaction containing 100 mM HEPES, 4 mM MgCl2, 12.5 mM dithiothreitol, 0.5% Triton X-100 (v/v), and the RNAs and NTPs indicated. An equal volume of 200 mM SMTA dissolved in the same buffer was added for 1 h to allow modification of all surface-exposed lysines. After exchanging the buffer with 100 mM NH4HCO3, trypsin was added and it was incubated overnight at 37°C. The peptides were then analyzed using a LTQ linear ion trap mass spectrometer (Thermo Scientific). (ND) No data were obtained for that residue. (B) Molecular models of NS5B that highlight K51, which is differentially protected during RNA synthesis. (Red) K51; (blue) the Δ1 loop; (green) the GDD active-site motif.
Mutations in the nascent RNA channel can affect HCV replication
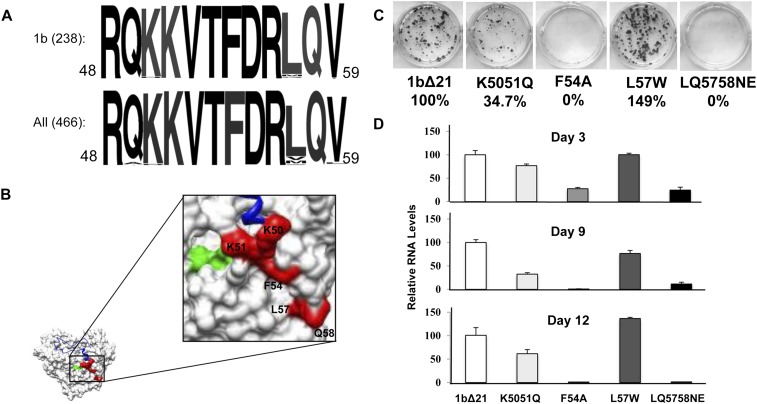
Residues 49–71 contain several charged residues as well as a phenylalanine that could interact with the nascent RNA. To assess whether this sequence was conserved in the HCV genotypes, the sequences corresponding to residues 49–59 in the HCV genome database were analyzed. Ninety-eight percent of genotype 1b HCV strains and 91% of all genotypes in the HCV database had the exact sequence. The most variable residue was L57 (Fig. 6A). The high degree of conservation suggests that this region is important for NS5B function.
FIGURE 6.
Nascent RNA channel mutants and their effect on replicon replication. (A) Sequence alignment of the region of NS5B found to contact nascent RNA. Alignments are of the 1b genotype and for all genotypes deposited in the HCV database. (B) Location of mutated residues on the structure of NS5B. (Red) Mutated residues; (green) the GDD active site; (blue in the ribbon structure) the Δ1 loop. (C) Colony formation assay. One microgram of replicon RNA containing either wild type (WT) NS5B or mutant NS5B was electroporated into Huh7.5 cells and plated to assay for the presence of G418-resistant colonies as described in Materials and Methods. G418 was present in the medium at 0.5 mg/mL G418 (Invitrogen) and was stained by crystal violet after 3 wk. (D) Transient replication of replicon RNAs. Total RNA was extracted from replicon cells using TRIzol reagent (Invitrogen) at the days of post-transfection indicated, and viral RNAs were quantified by real-time RT-PCR. The percent change of each mutant was done in triplicate and compared with wild-type RNA levels.
Several mutations within the putative nascent RNA binding region of NS5B were generated and analyzed for defects in RNA synthesis in vitro and in the context of the replicon. The mutations represent our best guess concerning features within the putative nascent RNA channel that could affect interactions with RNA while retaining NS5B structure. The mutants were K5051Q (both lysines substituted with glutamines), F54A, L57W, and LQ5758NE (L57 substituted with asparagine and Q58 substituted with a glutamate) and were initially tested in the 1b/Con1 subgenomic replicon to assess for effects on HCV replication (Fig. 6B,C). Mutants K5051Q, F54A, and LQ5758NE showed significant reductions in the HCV replication-dependent colony formation assay (Fig. 6C). The same three mutants reduced HCV RNA as determined by qRT-PCR (Fig. 6D). Mutant L57W, the least conserved residue in the putative nascent RNA channel (Fig. 6A), did not exhibit a defect in the HCV subgenomic replicon assay.
Effects of mutation on properties of recombinant HCV RdRps
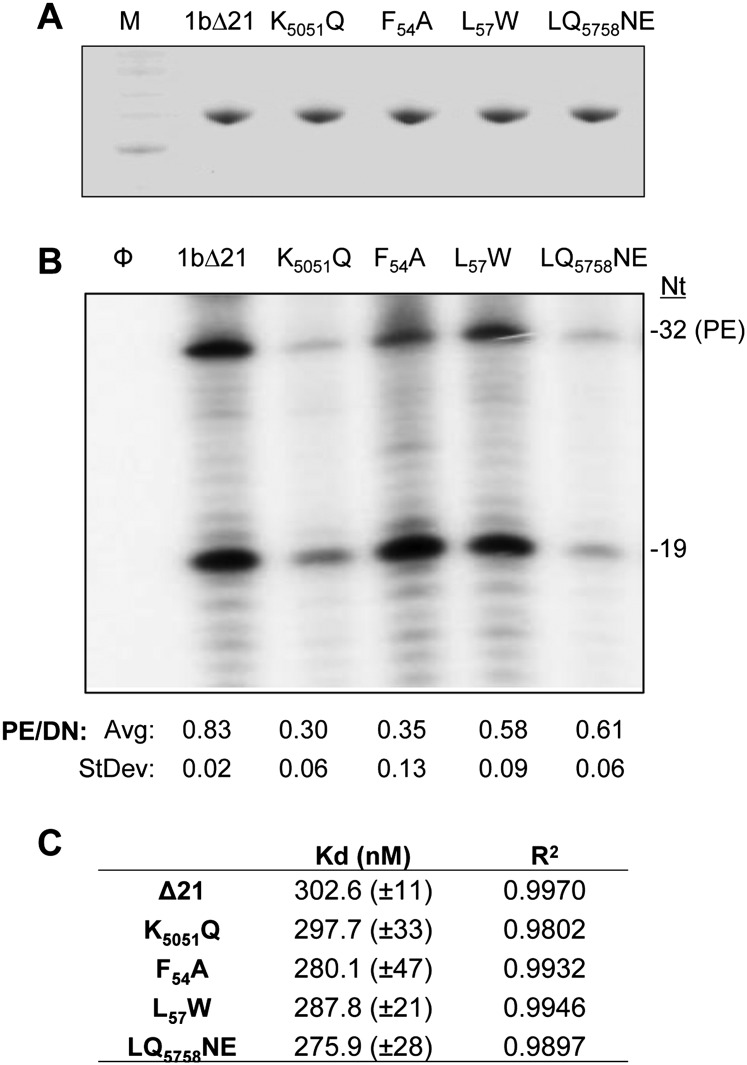
Results from the subgenomic replicon suggest that the changes in residues 49–59 could affect RNA-dependent RNA synthesis by the recombinant NS5B. Therefore, all four mutations were individually engineered into the recombinant protein 1bΔ21. All of the proteins were soluble (Fig. 7A). To determine whether the mutant proteins were affected for interaction with the template RNA, the anisotropy of a version of 12T that contained a 5′ fluorescein was measured (Fig. 7B; Supplemental Fig. 4). 1bΔ21 had an affinity of 300 nM with a standard error of 11 nM, and all mutant proteins had comparable affinity for the labeled 12T (Fig. 7B). These results suggest that these mutations did not significantly affect their ability to bind template RNA.
FIGURE 7.
Biochemical characterization of the nascent RNA channel mutants. (A) Recombinant protein purification. The purified proteins were separated by SDS-PAGE (4%–12% polyacrylamide) and visualized by Coomassie Blue stain. (B) RNA synthesis using LE19 as the template RNA sequence. LE19 is capable of directing primer-dependent (32-nt product) and primer-independent (de novo) RNA synthesis (Ranjith-Kumar et al. 2001). Larger species are products of template-switch RNA synthesis. Quantification of de novo and primer extension RNA synthesis by 1bΔ21 NS5B and mutant proteins are below the gel. Averages and standard errors were from five independent experiments. (C) Affinities of RdRp and mutants to 12T as determined by fluorescence anisotropy experiments. 12T, at a final concentration of 0.2 μM, was resuspended in a buffer containing 50 mM HEPES (pH 7.5), 5 mM MgCl2, 0.002% Tween 20, and 50 mM NaCl; RdRp or the nascent RNA channel mutant was titrated in. Sixty anisotropy values were taken after the RdRp was allowed to equilibrate for 1 min. The data were fitted into the Hill equation using KaleidaGraph software.
To examine whether the mutations affected RNA-dependent RNA synthesis, LE19 was used as a template. LE19 can report on the ability of the polymerase to extend from a primed template and to initiate de novo on the same template sequence (Ranjith-Kumar et al. 2002b). The mutant polymerases had different total levels of RNA synthesis, with K5051Q and LQ5758NE being less active for RNA synthesis than 1bΔ21, while F54A and L57W retained highly active RNA synthesis when both the de novo–initiated and the primer extension products were quantified (Fig. 7B). RNA synthesis by K5051Q, L57W, and LQ5758 approximately corresponded to RNA replication by the subgenomic HCV replicons. However, mutant F54A was defective in the replicons but retained robust activity as the recombinant protein. We do not know the reason for this discrepancy, but it is known that other viral proteins and/or the environment within the cell will affect RNA synthesis by NS5B (Kang et al. 2009; Ranjith-Kumar et al. 2011).
Characterization of the RdRp ternary complex
All four of the mutant polymerases, including F54A, produced a lower ratio of the primer-extended products from LE19 than the de novo–initiated product (p < 0.05) (Fig. 7C). The reduction in primer extension could indicate that the mutant RdRps have some defect in elongative RNA synthesis.
To examine this possibility, we first used the RCAP assay to separate the peptides generated from ternary complexes generated from the wild-type (WT) and K5051Q RdRps along with 5P/12T in the presence of UTP and ATP (generating nascent RNAs of 10 nt or longer) (Fig. 1). The peptides bound or unbound to the alkyn-labeled nascent RNA were analyzed according to the protocols diagrammed in Supplemental Figure 5A. With K5051Q, the loss of the lysines will prevent formaldehyde cross-linking to the RNA (Metz et al. 2004); however, if it retains binding to the nascent RNA, it should not be present in the unbound fraction. For the 1bΔ21 ternary complex, peptide 49–56 was present in the fractions associated with the alkyn-labeled nascent RNA and absent in the unbound fraction (Supplemental Fig. 5B). With the ternary complex of mutant K5051Q, the glutamine substitutions altered the mass of peptide 49–56 by only 0.072 Da, but the peptide was absent in the RNA-bound fraction and present in the unbound fraction (Supplemental Fig. 5C). This demonstrates that this region is directly involved in interacting with the nascent RNA, and a mutation would likely have an effect on its biochemical activity.
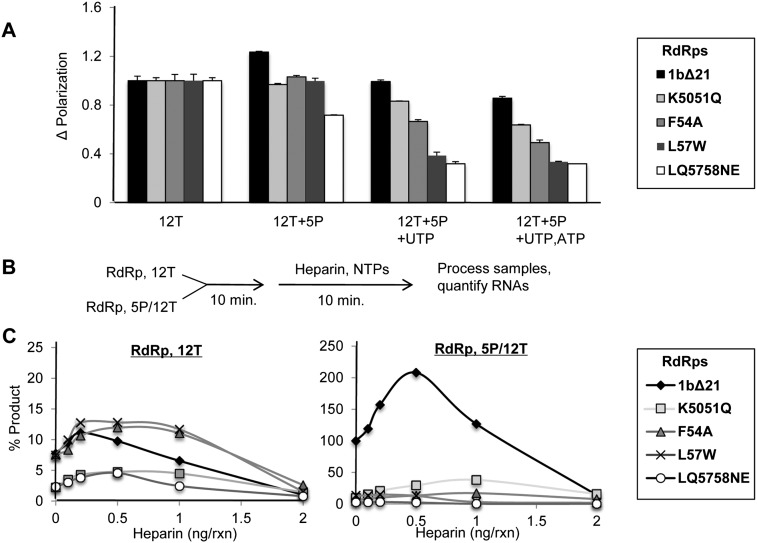
A defect in the contact with the nascent RNA channel should result in the RdRps exhibiting increased tendencies to release from their ternary complex. To test this directly, the polarization of 1bΔ21 and the mutant RdRps was determined with complexes formed with the fluorescein-labeled 12T and/or 5P and NTPs. The ability to form a ternary complex with 1bΔ21 resulted in a slight enhancement in fluorescence polarization. However, all four mutants either exhibited a reduction in polarization or remained unchanged (Fig. 8A). With the addition of UTP, or UTP and ATP, a reduction in the relative change in polarization was observed with the four mutant RdRps when compared with 1bΔ21. This suggests that the mutants form less stable ternary complexes when compared with 1bΔ21.
FIGURE 8.
RNA synthesis by the putative nascent RNA channel mutants in the presence of a nascent RNA mimic. (A) Change in fluorescence polarization while walking RdRps down 12T. Plotted is the change in fluorescence polarization under the conditions listed. All values are normalized to RdRp+12T. (B) Outline of the heparin titration experiment. RdRps were preincubated with either the 12T template alone or in the presence of the 5P primer. Heparin along with nucleotides were then added, and RNA synthesis was quantified by radiolabel incorporation. (C) The effect of heparin on RNA synthesis in the presence and absence of the 5P nascent RNA mimic used in the RCAP assay. Plotted is the relative abundance of RNA synthesized in the presence of different concentrations of heparin as indicated. In the presence of the 12T template alone, 1bΔ21 and the nascent RNA channel mutants showed discernable amounts of RNA synthesis (left panel). However, in the presence of 12T/5P, RNA synthesis by 1bΔ21 is significantly enhanced (right panel). This enhancement is not seen among any of the nascent RNA channel mutants.
To further investigate the stability of the ternary complex with the four putative nascent RNA channel mutants, the polyanionic heparin was used to inhibit additional rounds of RNA synthesis from 12T (Fig. 8B). Heparin acts as a template mimic to trap free polymerases after they release from the template (Walter et al. 1967). All of the RdRps preincubated with only 12T were highly sensitive to heparin (Fig. 8C, left panel), indicating that the binary complex is highly dynamic. Preincubation with 5P/12T resulted in 1bΔ21 being significantly more resistant to heparin when compared with the four mutant polymerases (Fig. 8C, right panel). In fact, lower concentrations of heparin actually increased RNA synthesis from the 5P/12T complex from 1bΔ21, possibly due to the molecular crowding effect of the long heparin polymers. Similar results were seen in early studies on the effects of heparin on polymerase activity (Walter et al. 1967; Novello and Stirpe 1969). Taken together, these data show that mutations in the putative nascent RNA channel affected the stability of the ternary complex.
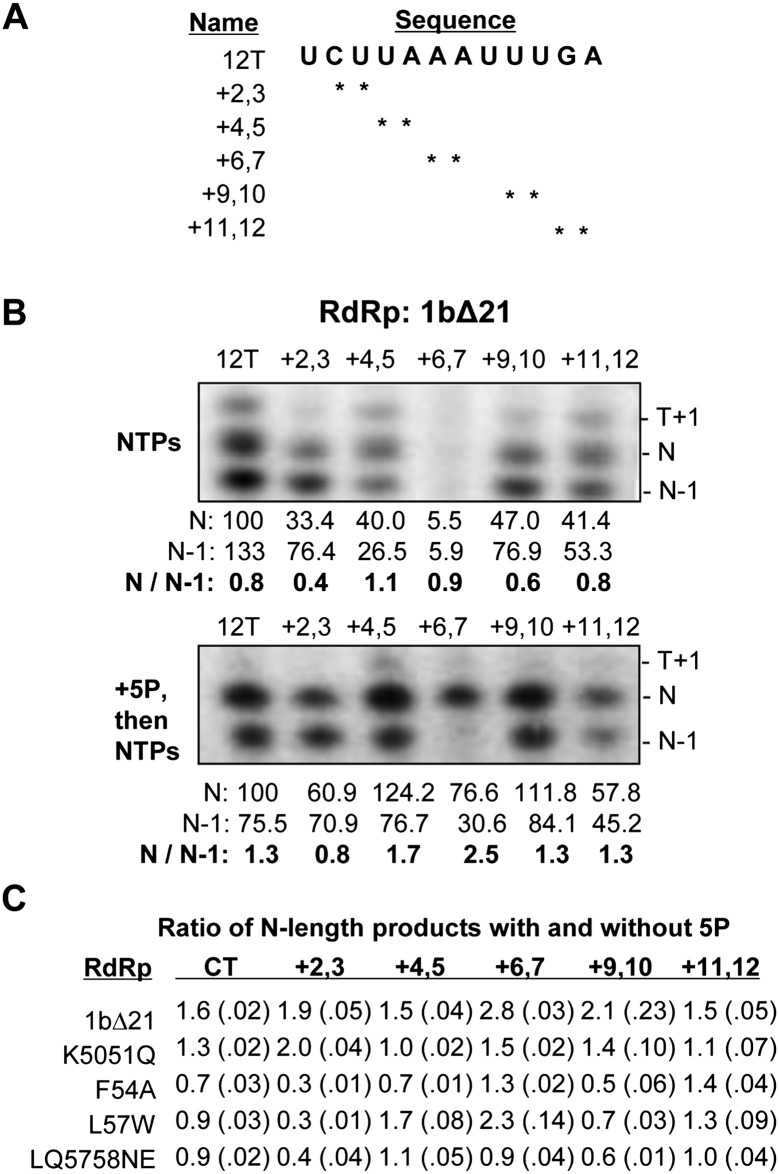
Finally, we reasoned that a reduction in the interactions between the mutant RdRps and the nascent RNA will be exacerbated when the template RNA contained modifications that could further destabilize the ternary complex. Modifications at specific 2′-hydroxyls were found to affect the length of the RNA products synthesized by the replicases of two plant RNA viruses (brome mosaic virus and cowpea chlorotic mottle virus) and the recombinant RdRp from the flavivirus bovine viral diarrhea virus (Kim et al. 2000; Tayon et al. 2001). Therefore, a series of templates derived from 12T with pairs of consecutive 2′-deoxyribose modifications was synthesized and tested for RNA synthesis under standard conditions (Fig. 9A).
FIGURE 9.
Effects of 2′-deoxyribose modification on RNA-dependent RNA synthesis by 1bΔ21 and the putative nascent RNA mutants. (A) Sequence of RNAs used to investigate N-length RNA synthesis. (*) The absence of a 2′-hydroxyl at the positions indicated. (B) Autoradiogram of RNA synthesis by wild-type 1bΔ21 NS5B in the presence and absence of 5P on the templates indicated. (T+1) The band corresponding to terminal nucleotide addition; (N) the full-length RNA product; (N−1) a prematurely terminated product. Below the gels are quantifications of RNA synthesis by 1bΔ21 NS5B normalized to 12T's N-length product. Ratios of N/(N−1) are taken to represent the relative amount of N-length synthesis. (C) Average of the ratios of products formed with and without 5P. The products are equal to the length of the template (N), 1 nt longer (N+1) or shorter (N−1), as indicated. The range for one standard error is shown in parentheses. These values were calculated from five independent experiments that had each sample assayed in triplicate per experiment.
RNA synthesis by 1bΔ21 from the set of modified templates was initially determined after a 2-min incubation. All of the deoxyribose (2′H)-containing templates had discernible effects on RNA synthesis by 1bΔ21 when compared with the unmodified control, 12T. Based on the sizes of the products relative to molecular weight markers and the availability of NTPs, the three predominant products observed had a terminal nucleotide added to the template (T+1) of 13 nt, a de novo–initiated 12-nt product (N), and a prematurely terminated product of 11 nt (N−1) (Fig. 9B, upper image). Notably, 2′H substitutions at the positions +6 and +7 in the template had the most dramatic defect, as determined by the amount of N-length product. In the presence of the nascent RNA mimic 5P, the N-length product increased in abundance relative to the N−1 product (Fig. 9B, bottom gel image). Notably, even abundant N-length product was observed with 2′H substitutions at the +6,+7 positions (Fig. 9B). This is likely due to an increase in stabilization of the ternary complex in the presence of the nascent RNA mimic before RNA synthesis.
The ratio of the products made by the four mutant polymerases in the presence of 5P and in its absence was determined in five independent experiments, and the results are summarized in Figure 9C. All four mutant polymerases exhibited a significantly reduced ratio of N to N−1 product compared with 1bΔ21, indicating that 5P had less of a stabilizing effect on RNA synthesis by the mutants when compared with 1bΔ21 (Fig. 9C). The mutants also exhibited reproducible differences in the products made from the different templates. In the presence of 5P, mutants F54A, L57W, and LQ5758NE had decreased products with the templates containing +2,+3 and +9,+10 substitutions, while mutant K5051Q exhibited larger defects in templates containing deoxyribose modifications from positions +4,+5 to +10,+11, but was unaffected by 2′H at the +2,+3 positions (Fig. 9C). These results confirm that the mutations in the putative nascent RNA channel had defects in product synthesis. Furthermore, the four recombinant proteins had distinguishable differences in RNA synthesis with the four mutant polymerases.
DISCUSSION
In this study, we mapped a region of the HCV RdRp that contacts nascent RNA using a combination of mass spectrometry–based approaches. Four mutations were made in the putative nascent RNA exit channel, and all four mutations exhibited defects in elongative RNA synthesis in biochemical assays, while three were also debilitated for HCV subgenomic RNA synthesis in Huh7.5 cells.
The nascent RNA channel in NS5B was identified using multiple independent approaches. The RCAP assay with an alkyn-labeled primer captured peptides derived from the region immediately below the Δ1 loop of the HCV NS5B protein. The same region was identified using a modified nucleotide, as well as a de novo initiation-defective RdRp mutant. The corresponding peptide from the K5051Q mutant did not bind the nascent RNA. An amidination interference assay showed that K51 was protected from modification only during RNA synthesis. Previous studies have identified that this part of the RdRp contacts RNA, but those studies were done in the absence of RNA synthesis and using longer templates (Kim et al. 2005; Deval et al. 2007).
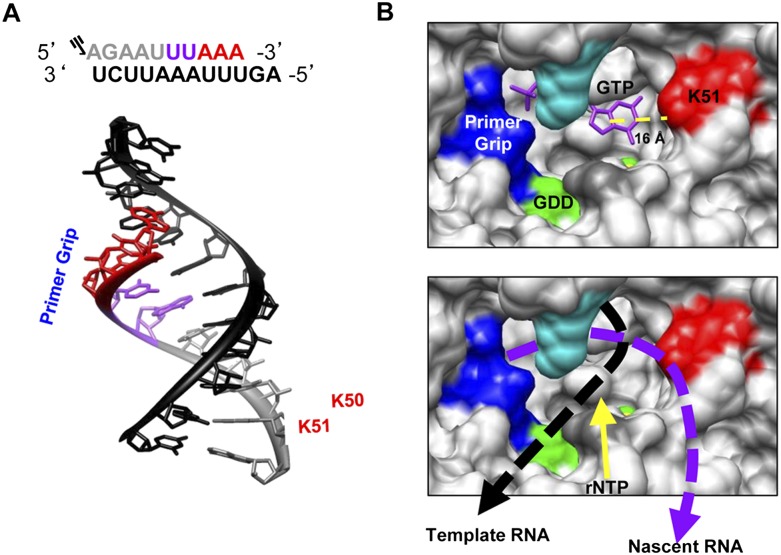
Structural comparison of the HCV RdRp to other related viral RdRps such as HIV-1 have identified the primer-grip motif that is thought to interact with the nascent primer strand at the juncture between the palm and the fingers domain (Bressanelli et al. 1999; Liu et al. 2008, 2011; Leung et al. 2011). Notably, peptides from the primer grip were not identified by any of the methods used in this work. However, the primer grip may contact the RdRp primarily through electrostatic interactions (Tao et al. 2002) and, hence, was not as conducive to cross-linking that required the bases in the primer–template duplex. Should the primer-grip interaction constrain the 5′-terminal portion of the RdRp–nascent RNA interaction, our mapping result on the portion of the RdRp that contacts the nascent RNA contacts would require that the RNA extend toward the base of the Δ1 loop. The distance of initiation GTP to K51, as determined from the structure of Harrus et al. (2010) (PDB: 2X13) in the RdRp, is ∼16 Å, which is sufficient to accommodate an RNA duplex of ∼6 nt assuming that the rise between base pairs in an A-form RNA duplex is ∼2.6 Å. In fact, an A-form RNA helix of ∼6 bp has sufficient pitch to contact the primer-grip region and contact residue K51. A model for the path of the nascent RNA–template duplex is shown in Figure 10.
FIGURE 10.
A model for the paths of the nascent and template RNAs as they exit the HCV RdRp. (A) Schematic and structure of 5P and 12T relative to the primer grip and residues K50 and K51. The structure shown is an A-form RNA helix, trimmed to match the size of 12T and 5P (PDB: 1RNA). (Gray) 5P; (black) 12T; (purple and red residues) the walking of the polymerase. (B, upper panel) Regions and structural features within NS5B to contact the nascent RNA. The location of the initiation GTP was used to denote the position of the first nucleotide that could form the nascent RNA. (Yellow sphere) The Mg2+ ion that coordinates the GTP to the GDD active site (green). (Red) Residue K51 that forms the beginning of the nascent RNA channel. The distance between C5 of the guanine to the terminal ɛ-amino atom of K51 is shown below the yellow dashed line. (B, lower panel) The proposed paths of the nascent and template RNA. The models used the coordinates in the polymerase–GTP complex of Harrus et al. (2010) (PDB: 2XI3). The figures were drawn with the Chimera program (UCSF, San Francisco) (Pettersen et al. 2004), and labels and features within the polymerase were added using Microsoft PowerPoint.
K51 has been previously hypothesized to be part of the rNTP channel (Schuwirth et al. 2005). While our mapping of the nascent RNA contact site seems initially to be incompatible with the traffic of rNTPs to the active site, it is also possible that the rNTPs may enter the active site in closer proximity to the ternary complex than previously thought. RdRps have a slower rate of RNA synthesis when compared with other template-dependent polymerases (Tomei et al. 2000; Adelman et al. 2002), possibly due to more constrained diffusion of rNTPs to the active site.
Only with a fully extended nascent RNA of ∼10 nt was the RCAP assay able to purify peptides on the opposite face of the RdRp. These results suggest that the nascent RNA, in the absence of the other replicase subunits, could wrap around the RdRp as it grows in length. Whether this interaction will take place within the membrane-embedded replicase complex remains to be determined. Previous studies have shown that NS5B transitions from a closed, de novo initiation-competent conformation to an open conformation upon its transition to elongation, after synthesis of ∼7–8 nt (Chinnaswamy et al. 2008; Chinnaswamy et al. 2010b). Because of this, it cannot be ruled out that this transition leads to a conformation that would allow this region to come into contact with the nascent RNA without the need to wrap around to the other side of the polymerase. Even as such, this would imply that the nascent RNA exits the polymerase beneath the Δ1 loop at the right side of the central cavity.
Substitutions K5051Q, F54A, and L5758NE exhibited defects in the HCV subgenomic RNA replication as well as contact with the nascent RNA in biochemical assays. L57W exhibited a defect in nascent RNA binding as determined by the RNA synthesis assays in vitro, but it was not apparently detrimental to subgenomic replicon replication (Figs. 6B, 7B). We do not have a suitable explanation for this discrepancy, but can only speculate that lack of cellular factors or the presence of other nonstructural proteins could have exacerbated the defect of the L57W mutation in vitro.
The in vitro RNA synthesis reactions with the 2′-deoxy substituted templates also provide information concerning the HCV RdRp elongative complex. Two results are particularly intriguing. First, 2′-deoxy modifications at +6 and +7 had the most dramatic effect on RNA synthesis in the absence of a preformed nascent RNA. Since this length corresponds to the bite size of the RdRp (Kao et al. 2000; Deval et al. 2007), it may be that contacts at these residues are especially critical for translocation of the RdRp to downstream sequences in the template. Additionally, the stability of the ternary complex is reduced for all nascent RNA channel mutants tested, as shown by a greater change in polarization while walking the RdRp down 12T (Fig. 8A), and by an increased sensitivity to heparin (Fig. 8B,C). This shows that RdRp contacts with the nascent RNA enhance the stability of the ternary complex, and every mutant tested has defective contacts with the nascent RNA. Finally, we note that the ratios of the products formed without and with 5P differed with the four polymerases. For example, mutant L57W produced high levels of products in the presence of 5P with the +6,+7 template in a manner similar to 1bΔ21, while mutants K5051Q, F54A, and LQ7558NE all had higher levels of product synthesis in the presence of 5P with other members of the template series. These results suggest that changes in the template channel may affect how the RdRp contacts the template RNA.
Interaction between the T7 RNA polymerase and nascent RNA has been documented to regulate several properties of RNA synthesis, including processive elongation and nucleotide misincorporation (Macdonald et al. 1993; Lyakhov et al. 1998; Temiakov et al. 2000; Toulokhonov et al. 2001; Durniak et al. 2008). We expect that our proposed location of the nascent RNA channel in the HCV RdRp should affect HCV genome replication and could be useful for studies of polymerase error and RNA recombination in cells. Finally, our biochemical results make several predictions concerning the HCV RdRp ternary complex that should fuel additional experimentation on an important aspect of viral RNA-dependent RNA synthesis.
MATERIALS AND METHODS
Protein expression and purification
For protein expression, wild-type and mutant HCV NS5B protein from the 1b Con1 strain without the C-terminal membrane-anchoring residues (Δ21) was expressed with a C-terminal His6 tag in Escherichia coli. Recombinant protein was purified through a Talon cobalt affinity column (Invitrogen) as previously described by Chinnaswamy et al. (2008). However, a nickel-NTA agarose column rather than a MonoS ion exchange resin was used to further purify 1bΔ21 and its derivatives (Invitrogen). The purified proteins were dialyzed and stored in 50 mM Tris (pH 7.5), 400 mM NaCl, 5 mM β-mercaptoethanol, and 10% (v/v) glycerol at −80°C. Protein concentrations were determined by measuring the absorbance at 280 nm, confirmed by SDS-PAGE stained with Coomassie Blue, and comparing the band intensity to bovine serum albumin of known quantity.
Oligonucleotides, site-directed mutagenesis, and DNA sequencing
Oligonucleotides, including those with chemical modifications, were synthesized by Integrated DNA Technologies. Mutations in the plasmid expressing 1bΔ21 were introduced using the QuikChange site-directed mutagenesis kit (Agilent Technologies). The complete open reading frame was sequenced to confirm the presence of directed mutation and absence of unintended mutations.
Reversible cross-linking–peptide fingerprinting analysis (RCAP)
NHS-agarose resin (Sigma-Aldrich) was coupled to azide-PEG4-amine (Click Chemistry Tools, Inc.) to affinity-purify alkyn-labeled RNAs. The coupling reaction was performed in 0.1 M sodium tetraborate (pH 8.5) and quenched by the addition of glycine to 1 M final concentration. A typical RCAP assay consists of a 200-μL reaction containing 100 mM HEPES, 4 mM MgCl2, 12.5 mM dithiothreitol, 0.5% Triton X-100 (v/v), and 0.3 μM 1bΔ21 that had been preincubated for 15 min in the presence of the RNAs and NTPs, where appropriate. Formaldehyde was then added to a final concentration of 0.1% (v/v) for 10 min to cross-link the RNA–protein complexes followed by the addition of glycine to a final concentration of 0.2 M to quench the cross-linking reaction. After 5 min, sequencing-grade trypsin (Promega) was added at 1:50 (w/w) in 100 mM NH4HCO3 (pH 7.8), and the reaction was incubated overnight at 37°C. Cyclo-addition of the alkyn-labeled RNA to the azide-containing resin was induced by the addition of 1 mM CuSO4 and 1 mM TCEP for 1 h. Unbound materials were washed away with three 1-mL washes of a buffer containing 20 mM HEPES (pH 7.5), 1 M NaCl, 1 mM EDTA, and 1 mM dithiothreitol. RNA–peptide conjugates were then reversed by incubating the samples for 1 h at 70°C in a buffer containing 50 mM Tris (pH 7.5), 200 mM NaCl, and 0.1% trifluoroacetic acid. The samples were centrifuged at 3000g for 5 min, and the supernatants containing the peptides were passed through a Ziptip column (Millipore). The bound peptides were eluted in 2.5 μL of 70% acetonitrile and 0.1% trifluoroacetic acid and analyzed by MALDI-TOF (Bruker Autoflex III; Agilent Technologies) in positive-ion mode. Assigned peaks all corresponded to within 0.5 Da of the theoretical masses of peptides from 1bΔ21.
Amidination protection assay
Differential modification of surface-exposed lysines was analyzed as described previously (Janecki et al. 2005). Briefly, 1bΔ21 (0.3 μM) was added to a 50-μL reaction containing 100 mM HEPES, 4 mM MgCl2, 12.5 mM dithiothreitol, 0.5% Triton X-100 (v/v), and preincubated for 15 min in the presence of the RNAs and NTPs indicated. An equal volume of 200 mM S-methyl thioacetimidate (SMTA) dissolved in the same buffer was added for 1 h. After exchanging the buffer with 100 mM NH4HCO3, trypsin was added at a ratio of 1:50 (w/w) of the polymerase and incubated overnight at 37°C. The samples were stored at 4°C before analysis using an LTQ linear ion trap mass spectrometer (Thermo Scientific). The tryptic digests were injected onto the C18 column (Waters NonaAcquity UPLC column: 100 μm × 100 mm, 1.7 μm, BEH130 C18) and eluted with a linear gradient of 1%–45% acetonitrile (in water with 0.1% FA) developed over 90 min at room temperature. The flow rate was 500 nL/min, and effluent was electro-sprayed into the LTQ mass spectrometer. MS/MS spectra were obtained by collision-induced dissociation.
A database search was performed using both Sequest and X!Tandem algorithms against the HCV protein sequence database from NCBI. Molecular modeling used the crystal structure of the 1b NS5B (Protein Data Bank code 1QUV) and the Chimera program (Pettersen et al. 2004).
HCV subgenomic replicon assay
EcoRI–SpeI restriction fragments containing a portion of the NS5A and the intact NS5B coding sequence of the 1b/Con1 replicon pFKI389/NS3-3′/WT (Lohmann et al. 1999) were subcloned into pCR-Blunt II TOPO (Invitrogen) for site-directed mutagenesis. The restriction fragment was used to replace the comparable sequence in pFKI389/NS3-3′/WT to generate the mutant replicons. ScaI-linearized plasmids purified by a QIAGEN column were used for in vitro transcription using the AmpliScribe T7-Flash Transcription Kit (Epicentre Technologies).
Huh7.5 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units of penicillin–streptomycin/mL, and 0.1 mM nonessential amino acids. Trypsin-liberated Huh7.5 cells were washed twice with ice-cold Cytomix (120 mM KCl; 0.15 mM CaCl2, 10 mM K2HPO4/KH2PO4, 25 mM HEPES, 2 mM EGTA, 5 mM MgCl2, at pH 7.6) and suspended at 1 × 107 cells/mL in ice-cold Cytomix for electroporation. Two hundred microliters of the cell suspension, 5 μg of HCV replicon RNA, and 5 μg of carrier RNA (total RNA extracted from naive Huh7.5 cells) were pulsed at 270 V, 96 mF in a electroporation cuvette with a 2-mm gap (Bio-Rad Gene-pulser). The cells were allowed to recover for 10 min at room temperature before dilution in complete medium.
The colony formation assay used 1/10 of the electroporated cells seeded in the first well of a 12-well plate, followed by a twofold serial dilution of the cells into growth medium added to the remaining wells. G418 (0.5 mg/mL; Invitrogen) was added to culture media 24 h after electroporation. The selective medium was replaced every 3 d for 3 wk. Replicon-harboring colonies were stained with crystal violet, to allow enumeration of colony formation per microgram of HCV replicon RNA.
Real-time RT-PCR used total RNA extracted from Huh7.5 cells electroporated with the replicon RNAs as described above. First-strand cDNA synthesis used 1 μg of total RNA extracted using the TRIzol reagent (Invitrogen) along with M-MulV (New England Biolabs) and 4 μM a randomized 9-nt primer mix. RT-PCR used the Bio-Rad IQTM SYBR Green kit (Bio-Rad), and the primers used were HCV 5′-UTRsense (5′-AGCCATGGCGTTAGTATGAGTGTC-3′) and 5′-UTRanti (5′-ACAAGGCCTTTCGCGACCCAAC-3′). The message for GAPDH was detected as an internal control using the sense and antisense oligonucleotides (5′-GAGTCAACGGATTTGGTCGT-3′ and 5′-TGGGATTTCCATTGATGACA-3′, respectively). All reaction mixtures were heated for 10 min to 95°C, followed by 40 cycles of PCR for 15 sec at 95°C, 20 sec at 55°C, and 30 sec at 72°C. The percent change of each mutant was compared with wild type as previously described (Livak and Schmittgen 2001).
Fluorescence spectroscopy
Fluorescence measurements were made using a PerkinElmer luminescence spectrometer LS50B luminescence spectrometer and a cuvette with an optical path length of 0.4 cm. Equilibrium anisotropy or polarization measurements were taken of a fluorescein-labeled 12T (0.2 μM final concentration) in binding buffer (50 mM HEPES at pH 7.5, 5 mM MgCl2, 0.002% Tween 20, and 50 mM NaCl). The integration time of 1 sec and a slit width of 5 nm were used throughout the measurements. Titrations for anisotropy measurements were performed by successive addition of 1bΔ21 with constant stirring. Polarization measurements were taken with 1bΔ21 at a constant concentration of 300 nM. All values collected were averaged from 60 measurements after each sample had equilibrated for 1 min.
Binding data were analyzed by nonlinear least-squares fitting using KaleidaGraph software (Synergy Software). The equilibrium dissociation constants (Kd) were fitted into the Hill equation (ΔA = Bmaxxn/[xn + Kdn]) as a binding model. ΔA is the value of anisotropy change by the ligand binding, Bmax is the value of maximum anisotropy change, x is the total concentration of the input ligand, and n is the Hill coefficient.
RNA synthesis assay
Standard RdRp assays consisted of 2.5 pmol of template RNA, and 0.08 μM NS5B in a 20-μL reaction containing 20 mM sodium glutamate (pH 8.2), 4 mM MgCl2, 1 mM MnCl2, 12.5 mM dithiothreitol, 0.5% Triton X-100 (v/v), and 50 mM the NTPs indicated. Where present, radiolabeled NTPs were used at a final concentration of 250 nM. Aminoallyl-UTP was from Thermo Scientific. Heparin inhibition assays (Sigma-Aldrich) used a template RNA concentration of 60 pmol per reaction, an amount determined empirically to be twice the half-maximal concentration needed to saturate RNA synthesis. The reactions were incubated at 30°C for the amount of time indicated, and terminated by the addition of formamide-containing loading buffer. RNA products were loaded directly onto a 20% polyacrylamide gel containing 7.5 M urea and electrophoresed at 400 V for 4 h. RNA synthesis was visualized and quantitated by the amount of [α-32P]CTP (MP Biomedicals, LLC) incorporated in RdRp products using a PhosphorImager (Typhoon 9210; Amersham Biosciences). All data were from a minimum of three independent experiments.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank C.T. Ranjith-Kumar and Hui Cai for helpful discussions. This work was supported by grant 1RO1AI073335 from the NIAID to C.K.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.031914.111.
REFERENCES
- Adelman K, La Porta A, Santangelo TJ, Lis JT, Roberts JW, Wang MD 2002. Single molecule analysis of RNA polymerase elongation reveals uniform kinetic behavior. Proc Natl Acad Sci 99: 13538–13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel N, Schaller T, Penin F, Bartenschlager R 2006. From structure to function: New insights into hepatitis C virus RNA replication. J Biol Chem 281: 9833–9836 [DOI] [PubMed] [Google Scholar]
- Biswal BK, Cherney MM, Wang M, Chan L, Yannopoulos CG, Bilimoria D, Nicolas O, Bedard J, James MN 2005. Crystal structures of the RNA-dependent RNA polymerase genotype 2a of hepatitis C virus reveal two conformations and suggest mechanisms of inhibition by non-nucleoside inhibitors. J Biol Chem 280: 18202–18210 [DOI] [PubMed] [Google Scholar]
- Bressanelli S, Tomei L, Roussel A, Incitti I, Vitale RL, Mathieu M, De Francesco R, Rey FA 1999. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc Natl Acad Sci 96: 13034–13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressanelli S, Tomei L, Rey FA, De Francesco R 2002. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J Virol 76: 3482–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Saguy C, Simister PC, Bressanelli S 2011. An objective assessment of conformational variability in complexes of hepatitis C virus polymerase with non-nucleoside inhibitors. J Mol Biol 414: 370–384 [DOI] [PubMed] [Google Scholar]
- Chinnaswamy S, Yarbrough I, Palaninathan S, Kumar CT, Vijayaraghavan V, Demeler B, Lemon SM, Sacchettini JC, Kao CC 2008. A locking mechanism regulates RNA synthesis and host protein interaction by the hepatitis C virus polymerase. J Biol Chem 283: 20535–20546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaswamy S, Cai H, Kao C 2010a. An update on small molecule inhibitors of the HCV NS5B polymerase: Effects on RNA synthesis in vitro and in cultured cells, and potential resistance in viral quasispecies. J Virus Adapt Treat 2: 73–89 [Google Scholar]
- Chinnaswamy S, Murali A, Li P, Fujisaki K, Kao CC 2010b. Regulation of de novo-initiated RNA synthesis in hepatitis C virus RNA-dependent RNA polymerase by intermolecular interactions. J Virol 84: 5923–5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval J, D'Abramo CM, Zhao Z, McCormick S, Coutsinos D, Hess S, Kvaratskhelia M, Gotte M 2007. High resolution footprinting of the hepatitis C virus polymerase NS5B in complex with RNA. J Biol Chem 282: 16907–16916 [DOI] [PubMed] [Google Scholar]
- Durniak KJ, Bailey S, Steitz TA 2008. The structure of a transcribing T7 RNA polymerase in transition from initiation to elongation. Science 322: 553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutartre H, Boretto J, Guillemot JC, Canard B 2005. A relaxed discrimination of 2′-O-methyl-GTP relative to GTP between de novo and elongative RNA synthesis by the hepatitis C RNA-dependent RNA polymerase NS5B. J Biol Chem 280: 6359–6368 [DOI] [PubMed] [Google Scholar]
- Ferrari C, Urbani S, Penna A, Cavalli A, Valli A, Lamonaca V, Bertoni R, Boni C, Barbieri K, Uggeri J, et al. 1999. Immunopathogenesis of hepatitis C virus infection. J Hepatol (Suppl 1) 31: 31–38 [DOI] [PubMed] [Google Scholar]
- Harrus D, Ahmed-El-Sayed N, Simister PC, Miller S, Triconnet M, Hagedorn CH, Mahias K, Rey FA, Astier-Gin T, Bressanelli S 2010. Further insights into the roles of GTP and the C terminus of the hepatitis C virus polymerase in the initiation of RNA synthesis. J Biol Chem 285: 32906–32918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecki DJ, Beardsley RL, Reilly JP 2005. Probing protein tertiary structure with amidination. Anal Chem 77: 7274–7281 [DOI] [PubMed] [Google Scholar]
- Joyce CM, Steitz TA 1995. Polymerase structures and function: Variations on a theme? J Bacteriol 177: 6321–6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Choi JK, Kim SJ, Kim JH, Ahn DG, Oh JW 2009. Regulation of hepatitis C virus replication by the core protein through its interaction with viral RNA polymerase. Biochem Biophys Res Commun 386: 55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CC, Yang X, Kline A, Wang QM, Barket D, Heinz BA 2000. Template requirements for RNA synthesis by a recombinant hepatitis C virus RNA-dependent RNA polymerase. J Virol 74: 11121–11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Zhong W, Hong Z, Kao CC 2000. Template nucleotide moieties required for de novo initiation of RNA synthesis by a recombinant viral RNA-dependent RNA polymerase. J Virol 74: 10312–10322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Russell WK, Ranjith-Kumar CT, Thomson M, Russell DH, Kao CC 2005. Functional analysis of RNA binding by the hepatitis C virus RNA-dependent RNA polymerase. J Biol Chem 280: 38011–38019 [DOI] [PubMed] [Google Scholar]
- Leung AK, Nagai K, Li J 2011. Structure of the spliceosomal U4 snRNP core domain and its implication for snRNP biogenesis. Nature 473: 536–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, Davies DR 2008. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320: 379–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ghalei H, Luhrmann R, Wahl MC 2011. Structural basis for the dual U4 and U4atac snRNA-binding specificity of spliceosomal protein hPrp31. RNA 17: 1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285: 110–113 [DOI] [PubMed] [Google Scholar]
- Lyakhov DL, He B, Zhang X, Studier FW, Dunn JJ, McAllister WT 1998. Pausing and termination by bacteriophage T7 RNA polymerase. J Mol Biol 280: 201–213 [DOI] [PubMed] [Google Scholar]
- Macdonald LE, Zhou Y, McAllister WT 1993. Termination and slippage by bacteriophage T7 RNA polymerase. J Mol Biol 232: 1030–1047 [DOI] [PubMed] [Google Scholar]
- Metz B, Kersten GF, Hoogerhout P, Brugghe HF, Timmermans HA, de Jong A, Meiring H, ten Hove J, Hennink WE, Crommelin DJ, et al. 2004. Identification of formaldehyde-induced modifications in proteins: Reactions with model peptides. J Biol Chem 279: 6235–6243 [DOI] [PubMed] [Google Scholar]
- Novello F, Stirpe F 1969. Experimental conditions affecting ribonucleic acid polymerase in isolated rat liver nuclei. Effect of nucleoside triphosphate concentration, temperature, ammonium sulphate and heparin. Biochem J 112: 721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell D, Trowbridge R, Rowlands D, Jager J 2003. Substrate complexes of hepatitis C virus RNA polymerase (HC-J4): Structural evidence for nucleotide import and de-novo initiation. J Mol Biol 326: 1025–1035 [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Powdrill MH, Bernatchez JA, Gotte M 2010. Inhibitors of the hepatitis C virus RNA-dependent RNA polymerase NS5B. Viruses 2: 2169–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Kao CC 2006. Biochemical activities of the HCV NS5B RNA-dependent RNA polymerase. In Hepatitis C viruses: Genomes and molecular biology (ed. SL Tan), pp. 293–310. Taylor & Francis, Norfolk, UK [Google Scholar]
- Ranjith-Kumar CT, Gajewski J, Gutshall L, Maley D, Sarisky RT, Kao CC 2001. Terminal nucleotidyl transferase activity of recombinant Flaviviridae RNA-dependent RNA polymerases: Implication for viral RNA synthesis. J Virol 75: 8615–8623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Gutshall L, Kim MJ, Sarisky RT, Kao CC 2002a. Requirements for de novo initiation of RNA synthesis by recombinant flaviviral RNA-dependent RNA polymerases. J Virol 76: 12526–12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Kim YC, Gutshall L, Silverman C, Khandekar S, Sarisky RT, Kao CC 2002b. Mechanism of de novo initiation by the hepatitis C virus RNA-dependent RNA polymerase: Role of divalent metals. J Virol 76: 12513–12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Wen Y, Baxter N, Bhardwaj K, Cheng Kao C 2011. A cell-based assay for RNA synthesis by the HCV polymerase reveals new insights on mechanism of polymerase inhibitors and modulation by NS5A. PLoS ONE 6: e22575 doi: 10.1371/journal.pone.0022575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigat K, Wang Y, Hudyma TW, Ding M, Zheng X, Gentles RG, Beno BR, Gao M, Roberts SB 2010. Ligand-induced changes in hepatitis C virus NS5B polymerase structure. Antiviral Res 88: 197–206 [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH 2005. Structures of the bacterial ribosome at 3.5 Å resolution. Science 310: 827–834 [DOI] [PubMed] [Google Scholar]
- Speers AE, Cravatt BF 2004. Profiling enzyme activities in vivo using click chemistry methods. Chem Biol 11: 535–546 [DOI] [PubMed] [Google Scholar]
- Steen H, Jensen ON 2002. Analysis of protein–nucleic acid interactions by photochemical cross-linking and mass spectrometry. Mass Spectrom Rev 21: 163–182 [DOI] [PubMed] [Google Scholar]
- Tao Y, Farsetta DL, Nibert ML, Harrison SC 2002. RNA synthesis in a cage—structural studies of reovirus polymerase λ3. Cell 111: 733–745 [DOI] [PubMed] [Google Scholar]
- Tayon R Jr, Kim MJ, Kao CC 2001. Completion of RNA synthesis by viral RNA replicases. Nucleic Acids Res 29: 3576–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temiakov D, Mentesana PE, Ma K, Mustaev A, Borukhov S, McAllister WT 2000. The specificity loop of T7 RNA polymerase interacts first with the promoter and then with the elongating transcript, suggesting a mechanism for promoter clearance. Proc Natl Acad Sci 97: 14109–14114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomei L, Vitale RL, Incitti I, Serafini S, Altamura S, Vitelli A, De Francesco R 2000. Biochemical characterization of a hepatitis C virus RNA-dependent RNA polymerase mutant lacking the C-terminal hydrophobic sequence. J Gen Virol 81: 759–767 [DOI] [PubMed] [Google Scholar]
- Tomei L, Altamura S, Bartholomew L, Biroccio A, Ceccacci A, Pacini L, Narjes F, Gennari N, Bisbocci M, Incitti I, et al. 2003. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J Virol 77: 13225–13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulokhonov I, Artsimovitch I, Landick R 2001. Allosteric control of RNA polymerase by a site that contacts nascent RNA hairpins. Science 292: 730–733 [DOI] [PubMed] [Google Scholar]
- Walter G, Zillig W, Palm P, Fuchs E 1967. Initiation of DNA-dependent RNA synthesis and the effect of heparin on RNA polymerase. Eur J Biochem 3: 194–201 [DOI] [PubMed] [Google Scholar]