Abstract
We developed retrotransposon-based insertional polymorphism (RBIP) markers based on the long terminal repeat (LTR) sequences of copia-like retrotransposon Ppcrt4 and flanking genome sequences, which were derived from 454 sequencing data from Japanese pear (Pyrus pyrifolia) ‘Hosui’. Out of 40 sequences including both LTR and flanking genome regions, we developed 22 RBIP markers and used them for DNA profiling of 80 pear cultivars: 64 Japanese, 10 Chinese (Pyrus ussuriensis) and 6 European (Pyrus communis). Three RBIP markers were enough to differentiate ‘Hosui’ from the other Japanese pear cultivars. The 22 RBIP markers could also distinguish 61 of the 64 Japanese pear cultivars. European pears showed almost no amplification of the 22 RBIP markers, which might suggest that retrotransposons had transposed during Asian pear evolution or reflect the genetic relationship between Asian and European pears. Sixteen of the RBIP markers could be positioned on a genetic linkage map of ‘Hosui’. The RBIP loci were distributed in 10 linkage groups, and some loci were very closely located within the same linkage group. The information obtained will be applicable to developing cultivar-specific RBIP marker sets in plants.
Keywords: copia, DNA marker, LTR, Pyrus, retrotransposon
Introduction
The interest in plant breeders’ rights is increasing worldwide. The International Union for the Protection of New Varieties of Plant (UPOV; http://www.upov.int/index_en.html), an intergovernmental organization, was established in 1961, to provide and promote an effective system of plant variety protection with the aim of encouraging the development of new varieties of plants for the benefit of society. The BMT (Biochemical and Molecular Techniques and DNA-Profiling in Particular) working group of UPOV agreed to establish DNA profiling techniques for protecting plant breeders’ rights. In Japan, a plant variety protection system was established by the Ministry of Agriculture, Forestry and Fisheries under the Agricultural Seeds and Seedlings Law (PVP home page: http://www.hinsyu.maff.go.jp/en/en_top.html). Ten DNA profiling manuals for major crops, including Japanese pear (Pyrus pyrifolia Nakai), have already been released on the PVP home page.
Several DNA profiling techniques have been developed and used for plant variety protection; these include restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), cleaved amplified polymorphic sequence (CAPS), single-nucleotide polymorphism (SNP) and simple sequence repeat (SSR). In pears (Pyrus spp.), Teng et al. (2002) reported that 118 Asian pear cultivars could be differentiated by RAPD markers and Monte-Corvo et al. (2001) noted that ISSR (inter-simple sequence repeat) markers could be used to identify 24 European pears (P. communis L.). Polymorphism at the S-RNase locus, which control self-incompatibility, has been used for identification of Japanese pear cultivars (Ishimizu et al. 1999). The use of chloroplast DNA polymorphism was efficient for classification of pear cultivars (Iketani et al. 1998, Kimura et al. 2003a). A large number of SSR markers have been developed from genomic DNAs and ESTs in Japanese and European pears (Fernandez-Fernandez et al. 2006, Nishitani et al. 2009, Yamamoto et al. 2002). The use of these SSR markers has allowed DNA profiling, parentage analysis, and evaluation of genetic diversity in pear cultivars and species (Bao et al. 2007, Kimura et al. 2002, Sawamura et al. 2008). Because of their abundance in the genome and high degree of polymorphism, SSR markers have became the standard tool for DNA profiling. However, almost all SSRs developed in pear have di-nucleotide repeat motifs, and “stutter bands” can interfere with accurate profiling (Diwan and Cregan 1997). In addition, high mutation rates within the repeat sequences might be a problem for SSR markers (Henderson and Petes 1992, Weber and Wong 1993). Therefore, alternative DNA profiling techniques such as retrotransposon-based markers are being developed.
Retrotransposons are the predominant class of mobile genetic elements in plants. They transpose via reverse-transcribed RNA intermediates and integrate into new locations within the host genome by means of a “copy-and-paste” mechanism. This mechanism can cause the copy number of the retrotransposons to increase and create stable insertion mutations in the host genome (Flavell et al. 1994). The characteristics of retrotransposons, such as ubiquitous distribution, abundant copy number, high heterogeneity and insertional polymorphism both within and between plant species, provide an excellent basis for the development of molecular markers (Flavell et al. 1992, Hirochika and Hirochika 1993). In Japanese pear, eight novel Ty1-copia-like retrotransposons were identified and designated Ppcrt1–Ppcrt8 (Kim et al. 2011). Phylogenetic analysis grouped these elements with the copia-like retrotransposons RIRE1 in rice (Noma et al. 1997) and BARE-1 (Manninen and Schulman 1993) in barley. Two Ty1-copia retrotransposons (CTcrm1 and CTcrm2) were identified in apple, which is closely related to pear (both belonging to subfamily Spiraeoideae in the family Rosaceae), and bud sports of ‘Fuji’ apple could be distinguished from the original cultivar with sequence-specific amplified polymorphism (SSAP) markers (Zhao et al. 2010).
Several molecular marker systems based on the available retrotransposon sequence information have been developed for plants, including retrotransposon-based insertional polymorphism (RBIP, Kalendar et al. 2011), inter-retrotransposon amplified polymorphism (IRAP, Kalendar et al. 1999), retrotransposon microsatellite amplified polymorphism (REMAP, Kalendar et al. 1999) and SSAP (Waugh et al. 1997). These retrotransposon-based DNA markers have been utilized for DNA profiling of varieties (both within and between species) and for evolutional and genetic diversity studies. Among the classes of retrotransposon-based markers, RBIP markers can detect polymorphism caused by the integration of an element at a particular locus and supply an accurate DNA profile. Active Ty1-copia retrotransposons were isolated and used for differentiation of cultivars in sweet potato (Ipomoea batatas) (Tahara et al. 2004, Yamashita and Tahara 2006). Yamashita et al. (2008) identified Adzuki bean (Vigna angularis) cultivars by retrotransposon-based DNA markers. When retrotransposon insertion is found in only one cultivar or occurs after the cross-breeding process, the insertion tag should be applicable as a cultivar-specific DNA marker. Furthermore, suitable combinations of retrotransposon insertions will be also useful for cultivar-specific DNA markers.
In this study, long terminal repeat (LTR) sequences of copia-like retrotransposon Ppcrt4 from Japanese pear were used to screen data obtained from 454 sequencing, and RBIP markers were established on the basis of the junctions between LTR retrotransposons and the genome sequence. These new RBIP markers were applied to DNA profiling of Japanese, Chinese and European pear cultivars. Transposition of retrotransposons was examined by placing these markers onto a genetic linkage map.
Materials and Methods
Plant materials and DNA extraction
The Japanese pear (Pyrus pyrifolia Nakai) cultivar ‘Hosui’ (syn. ‘Housui’) was used for genome sequencing analysis and marker development. A total of 80 pear cultivars were used for PCR analysis with retrotransposon-based DNA markers: 64 Japanese pear, 10 Chinese pear (Pyrus ussuriensis Maxim.) and 6 European pear (Pyrus communis L.). All plant materials were maintained and collected at the National Institute of Fruit Tree Science (NIFTS, Ibaraki, Japan). Genomic DNA was isolated from young leaves by using a Genomic-tip 20/G (Qiagen, Germany) as described by Yamamoto et al. (2006).
454 sequencing and data analysis
The total genomic DNA of ‘Hosui’ was sheared by nebulization, used to 454 library preparation and shotgun sequenced with a Roche/454 Genome Sequencer (GS)-FLX Titanium platform (Roche Diagnostics, USA). LTR nucleotide sequences were identified by BLASTN sequence-similarity searches based on the 5′- and 3′-LTR regions of the copia-like retrotransposon Ppcrt4 from Japanese pear (AB550654; Kim et al. 2011) and CTcrm2 from apple (Zhao et al. 2010).
RBIP primer design and multiplex PCR analysis
Primer sets were designed to amplify specific junction regions between LTR retrotransposon sequences and pear genome sequences (Fig. 1A). One primer in each set was designated within the 5′- or 3′-LTR of the retrotransposon; the other was designed within the pear genomic DNA region by using Primer3 software (Rozen and Skaletsky 2000) and labeled with a fluorescent chemical (FAM, VIC, or NED). PCR amplification was performed in a 5-μL reaction mixture containing 2.5 μL of Multiplex PCR Master Mix including HotStar Taq Polymerase (Qiagen), 5 pmol each of forward and reverse primer and 5 ng of genomic DNA. The multiplex PCR amplification was performed with three to six primer combinations in a single reaction. The PCR profile consisted of initial denaturation for 15 min at 95°C followed by 35 cycles of 60 s at 94°C, 90 s at 55°C, 90 s at 72°C and a final extension of 10 min at 72°C. The amplified PCR products were separated and detected in a PRISM 3100 DNA sequencer (Applied Biosystems, USA). The sizes of the amplified bands were scored against internal standard DNA (400HD-ROX, Applied Biosystems) by GeneScan software (Applied Biosystems). The single PCR amplicons for all markers and cultivars were also evaluated by agarose gel electrophoresis.
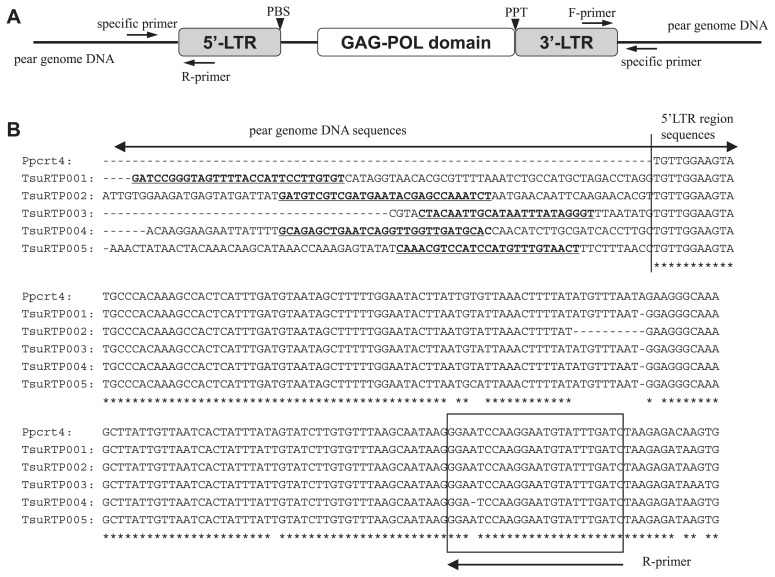
Fig. 1.
(A) Structure of the Ppcrt copia-like retrotransposon of Japanese pear. (B) Nucleotide sequences of five RBIP markers and the Ppcrt4 retrotransposon. LTR, long terminal repeat; PBS, primer binding site; GAG, group-specific antigen; POL, RNA-dependent DNA polymerase; PPT, polypurine tract; F-primer, forward primer; R-primer, reverse primer. Forward primers in B are indicated by italics and underlined, and reverse primers are boxed.
Because retrotransposon-based markers show dominant inheritance, it is sometimes difficult to interpret “no amplification”. Therefore, chloroplast DNA markers such as atpB–rbcL (intergenic spacer between ATPase B subunit and ribulose 1,5-bisphosphate carboxylase/oxygenase large subunit genes) and/or trnL–trnF (intergenic spacer between tRNA-Leu and tRNA-Phe genes) (Yamamoto et al. 2006) were included in the multiplex PCR amplification with one-fifth to one-tenth of primer concentration of RBIP markers. The PCR products were electrophoresed in 2%–3% agarose gel, stained with ethidium bromide and visualized under UV light. The target sizes of trnL–trnF and atpB–rbcL in ‘Hosui’ are 477 bp and ca. 800 bp, respectively. Successful amplification of these chloroplast markers was used as an indicator of a successful reaction.
MinimalMarker software (Fujii et al. 2007) was used to identify minimal marker subsets to distinguish ‘Hosui’ from the other cultivars and to find identical genotypes generated from the 22 RBIP markers for the 64 Japanese pear cultivars.
Identification of RBIP markers on the genetic linkage map
A previously constructed genetic linkage map of ‘Hosui’ (Nishitani et al. 2009, Terakami et al. 2009) was used to identify the linkage group and position of each of the RBIP markers, using 63 F1 plantlets obtained from the interspecific cross between the European pear ‘Bartlett’ and the Japanese pear ‘Hosui’. The genetic linkage map of ‘Hosui’ was constructed by JoinMap v. 3.0 software (Van Ooijen and Voorrips 2001), and a double pseudo-testcross strategy was used to create the map. An LOD score of 4.0 was used to define linkage groups, and map distances were calculated according to Kosambi’s mapping function. The map was drawn using MapChart 2.2 software (Voorrips 2002).
Results
Generation of 454 sequencing data of ‘Hosui’ and identification of retrotransposon insertions
Using a Roche/454 Genome Sequencer (GS)-FLX Titanium, we generated 2,654,964 sequences from genomic DNA of ‘Hosui’, with an average read length of 374 bp, yielding a total sequenced length of 995 Mb. This is equivalent to twice the haploid pear genome (1C = 500 Mb). Among these sequences, 11,312 showed homology to the LTR domain region of Ppcrt4 (Japanese pear copia-like retrotransposon) and 170 showed homology to the LTR domain region of sequences CTcmr2 (apple copia-like retrotransposon), with E-values below 10E-5. Out of the 11,312 Ppcrt4-homologous sequences, 40 sequences of at least 300 bp in length were chosen as showing high similarity (>97%) to the LTR regions of Ppcrt4. Seventeen of the 40 sequences included the 5′-LTR region of the retrotransposon and pear genome DNA; the remaining 23 sequences included the 3′-LTR region of the retrotransposon and pear genome DNA. None of the CTcmr2-homologous sequences showed high similarity (>97%) to the LTR regions of CTcmr2; therefore, they were not considered further.
Because more than 150 bp of nucleotide sequence from the start position of the 5′-LTR of Ppcrt4 were highly conserved in 17 sequences (Fig. 1B), we concluded that these 17 sequences were derived from the Ppcrt4-type copia-like retrotransposon. The regions upstream from the start position of the 5′-LTR showed no homology among the 17 sequences, indicating that they were flanking pear genome regions of inserted retrotransposons. Similarly, more than 150 bp upstream from the end position of the 3′-LTR of Ppcrt4 were highly conserved in 23 of the sequences, whereas the downstream regions showed no homology among the sequences and were identified as pear genome regions.
Evaluation of RBIP markers
We designed 22 RBIP primer sets to amplify specific junction regions between LTR sequences and pear genome sequences with an expected size from 197 to 275 bp: 17 sets for 5′-LTR regions and 5 for 3′-LTR regions (Table 1). All primers showed single clear amplified bands in ‘Hosui’ with the expected size. Polymorphism among cultivars detected by markers generated from 5′-LTR and 3′-LTR regions of the same retrotransposon insertion should be identical or very similar, so the remaining 18 sequences of 3′-LTR regions were not used for primer design.
Table 1.
Characteristics of 22 retrotransposon-based insertional polymorphism markers
| Marker name | Primer sequence (5′-3′) | Target size (bp) | Position of LTR | Sequence ID DDBJ accession nos. |
|---|---|---|---|---|
| TsuRTP001 | F: GATCCGGGTAGTTTTACCATTCCTTGTGT R1*: GATCAAATACATTCCTTGGATTCC |
236 | 5′-LTR | FZII0R302IGWK7com AB665188 |
| TsuRTP002 | F: GATGTCGTCGATGAATACGAGCCAAATCT R1*: GATCAAATACATTCCTTGGATTCC |
207 | 5′-LTR | FZII0R302J38JUcom AB665189 |
| TsuRTP003 | F: CTACAATTGCATAATTTATAGGGT R1*: GATCAAATACATTCCTTGGATTCC |
197 | 5′-LTR | FZII0R301DIMYLcom AB665190 |
| TsuRTP004 | F: GCAGAGCTGAATCAGGTTGGTTGATGCAC R1*: GATCAAATACATTCCTTGGATTCC |
215 | 5′-LTR | FZII0R301EE5FE AB665191 |
| TsuRTP005 | F: CAAACGTCCATCCATGTTTGTAACT R1*: GATCAAATACATTCCTTGGATTCC |
200 | 5′-LTR | FZII0R302HLAXN AB665192 |
| TsuRTP006 | F1**: CTTGAAGGCCCTCCACTTGGGTGAATC R: TTCGCTCATTGTGCCGATGACTCCATC |
355 | 3′-LTR | FZII0R302HWJVH AB665193 |
| TsuRTP007 | F1**: CTTGAAGGCCCTCCACTTGGGTGAATC R: TGGTTCTTAAACGGAGCTTTG |
331 | 3′-LTR | FZII0R302IQHOUcom AB665194 |
| TsuRTP008 | F1**: CTTGAAGGCCCTCCACTTGGGTGAATC R: AGGCTTAAGGCCTTCGAGCCGTTTCC |
354 | 3′-LTR | FZII0R302JG2OA AB665195 |
| TsuRTP009 | F1**: CTTGAAGGCCCTCCACTTGGGTGAATC R: TCACAAACTCCGTTAAGCTTTTCCTG |
341 | 3′-LTR | FZII0R302FYYLT AB665196 |
| TsuRTP010 | F1**: CTTGAAGGCCCTCCACTTGGGTGAATC R: TAAAGAGTGCCGTAGCAAGGA |
293 | 3′-LTR | FZII0R301A1VUKcom AB665197 |
| TsuRTP011 | F: TCCGAGTCACAACTTTGATTCTGTAGGT R1*: GATCAAATACATTCCTTGGATTCC |
236 | 5′-LTR | FZII0R302HK1DMcom AB665198 |
| TsuRTP012 | F: GACGCCTTGATTCCAAATCCGCACTTCGT R1*: GATCAAATACATTCCTTGGATTCC |
238 | 5′-LTR | FZII0R302IVOE6 AB665199 |
| TsuRTP013 | F: CACTCAATTGTGGGCTCTTCATTCTGAG R1*: GATCAAATACATTCCTTGGATTCC |
275 | 5′-LTR | FZII0R302H05XFcom AB665200 |
| TsuRTP014 | F: TGCCCGTCATAGTTTCCTCATCCCTCA R1*: GATCAAATACATTCCTTGGATTCC |
259 | 5′-LTR | FZII0R301EVKBVcom AB665201 |
| TsuRTP015 | F: ACTCAAAATAAGTCCACAAGACTCCA R1*: GATCAAATACATTCCTTGGATTCC |
235 | 5′-LTR | FZII0R302JLE1L AB665202 |
| TsuRTP016 | F: TGTTGTGGATTGAGTGCTCGATTAGCA R1*: GATCAAATACATTCCTTGGATTCC |
232 | 5′-LTR | FZII0R302I5E7C AB665203 |
| TsuRTP017 | F: CTCCAAGATTGACTTGAGGTCGGGTTACC R1*: GATCAAATACATTCCTTGGATTCC |
240 | 5′-LTR | FZII0R302IB05Mcom AB665204 |
| TsuRTP018 | F: GAGATGTAATCCTAAAAGGGTATGGATTC R1*: GATCAAATACATTCCTTGGATTCC |
234 | 5′-LTR | FZII0R301B7ENDcom AB665205 |
| TsuRTP019 | F: CAGGGAATTAAGCCCTTGAATTAAACCAC R1*: GATCAAATACATTCCTTGGATTCC |
247 | 5′-LTR | FZII0R301EEA32 AB665206 |
| TsuRTP020 | F: TCGTGTCAGCTTCTCACATGCTGCACATC R1*: GATCAAATACATTCCTTGGATTCC |
243 | 5′-LTR | FZII0R301EOB61 AB665207 |
| TsuRTP021 | F: TCCGGATCAAGTGATGGAGATTGCA R1*: GATCAAATACATTCCTTGGATTCC |
260 | 5′-LTR | FZII0R302HIJ4L AB665208 |
| TsuRTP022 | F: GCCAACTTCCATTAACTAGGCCCCAA R1*: GATCAAATACATTCCTTGGATTCC |
265 | 5′-LTR | FZII0R301B5G6X AB665209 |
R1 primer was designed based on nucleotide sequences of 5′-LTR region.
F1 primer was designed based on nucleotide sequences of 3′-LTR region.
Polymorphisms between pear cultivars
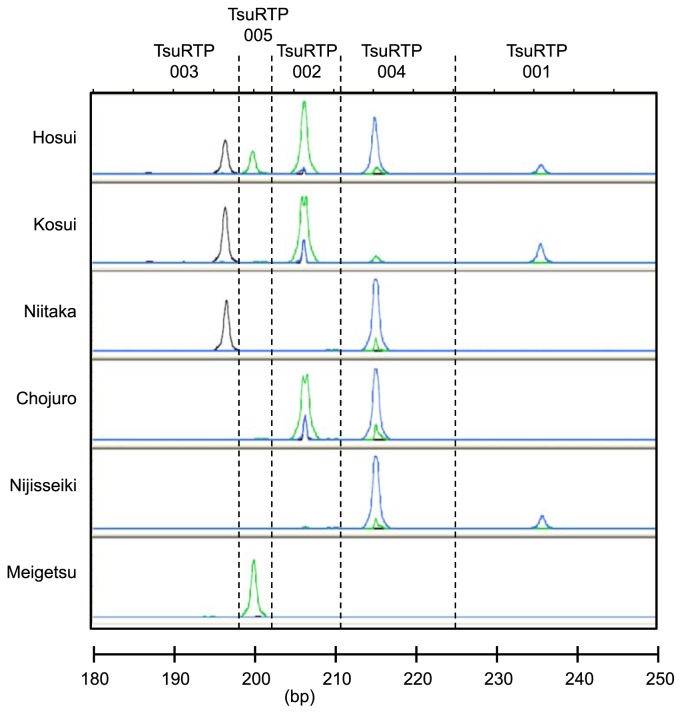
We analyzed the 80 pear cultivars with the 22 RBIP markers (Table 2). An example of the data output is shown in Fig. 2. Two markers, TsuRTP007 and TsuRTP014, generated amplified fragments of the size found in ‘Hosui’ in all 64 Japanese pear cultivars tested. Marker TsuRTP006 amplified fragments within all of the Japanese cultivars except ‘Amanogawa’, ‘Kimizukawase’ and ‘Shinsetsu’. TsuRTP022 amplified fragments in all except ‘Ichiharawase’, ‘Imamuraaki’ and ‘Niitaka’ and TsuRTP013 amplified fragments in all except ‘Echigonishiki’, ‘Nansui, ‘Natsuhikari’, ‘Shugyoku’ and ‘Shuurei’. On the other hand, TsuRTP010 showed amplification in only nine cultivars (‘Hosui’, ‘Akizuki’, ‘Ichiharawase’, ‘Ishiiwase’, ‘Okusankichi’, ‘Kumoi’, ‘Nikkori’, ‘Shinchu’ and ‘Yanaga’), TsuRTP005 showed amplification in 15 cultivars and TsuRTP002 showed amplification in 24 out of the 64 Japanese pear cultivars tested.
Table 2.
Amplified fragments of the 80 Japanese, Chinese and European pear cultivars revealed by 22 RBIP markers
| Cultivar name | Species | Marker name (TsuRTP) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||||
| 001 | 002 | 003 | 004 | 005 | 006 | 007 | 008 | 009 | 010 | 011 | 012 | 013 | 014 | 015 | 016 | 017 | 018 | 019 | 020 | 021 | 022 | ||
| Hosui | P. pyrifolia | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Aikansui | P. pyrifolia | − | + | + | − | − | + | + | + | − | − | + | + | + | + | + | + | + | + | − | + | − | + |
| Akiakari | P. pyrifolia | + | − | + | + | − | + | + | + | − | − | + | + | + | + | + | + | + | + | − | + | + | + |
| Akibae | P. pyrifolia | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | − | + |
| Akizuki | P. pyrifolia | + | − | + | + | − | + | + | + | − | + | − | − | + | + | + | + | + | − | + | + | + | + |
| Amanogawa | P. pyrifolia | − | − | + | − | − | − | + | + | + | − | − | + | + | + | − | − | − | + | + | − | − | + |
| Akemizu | P. pyrifolia | − | + | − | − | + | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | + | + |
| Asahi | P. pyrifolia | + | + | + | + | − | + | + | + | − | − | − | + | + | + | + | + | + | − | + | + | − | + |
| Atago | P. pyrifolia | − | − | + | + | − | + | + | + | − | − | + | + | + | + | + | − | + | + | + | + | + | + |
| Chikusui | P. pyrifolia | + | + | + | + | + | + | + | + | − | − | + | + | + | + | + | + | + | + | + | + | − | + |
| Choju | P. pyrifolia | − | + | + | − | − | + | + | + | − | − | + | + | + | + | + | + | + | + | − | + | − | + |
| Chojuro | P. pyrifolia | − | + | − | + | − | + | + | + | − | − | + | − | + | + | + | − | + | + | + | + | + | + |
| Doitsu | P. pyrifolia | − | + | − | + | − | + | + | + | − | − | + | + | + | + | + | − | + | + | + | + | − | + |
| Echigonishiki | P. pyrifolia | − | − | − | − | − | + | + | + | + | − | + | − | − | + | − | − | + | + | − | − | − | + |
| Gion | P. pyrifolia | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | − | + | + | − | + | − | + |
| Hakkou | P. pyrifolia | − | − | + | − | + | + | + | + | − | − | + | + | + | + | + | + | + | + | + | + | − | + |
| Hatsuaki | P. pyrifolia | − | − | + | − | − | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | − | + |
| Heiwa | P. pyrifolia | + | − | − | − | − | + | + | + | − | − | − | + | + | + | + | − | + | + | + | + | − | + |
| Hokushin | P. pyrifolia | + | − | − | + | − | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | − | + |
| Hokukan | P. pyrifolia | + | + | − | + | − | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | − | + |
| Hogetsu | P. pyrifolia | − | − | + | + | − | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | + |
| Ichiharawase | P. pyrifolia | − | − | − | − | − | + | + | + | − | + | − | + | + | + | + | − | − | + | + | + | − | − |
| Inagi | P. pyrifolia | + | − | + | + | − | + | + | + | − | − | − | + | + | + | + | + | + | − | + | + | − | + |
| Imamuraaki | P. pyrifolia | − | − | + | + | + | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | + | − |
| Ishiiwase | P. pyrifolia | − | − | + | + | + | + | + | − | − | + | + | + | + | + | − | + | + | + | − | − | + | + |
| Oushu | P. pyrifolia | + | − | + | + | − | + | + | + | + | − | − | + | + | + | + | − | − | − | + | + | + | + |
| Okusankichi | P. pyrifolia | − | − | + | + | − | + | + | − | + | + | + | + | + | + | + | − | + | + | − | + | − | + |
| Kikusui | P. pyrifolia | + | + | + | − | − | + | + | − | − | − | − | + | + | + | + | + | + | − | + | + | − | + |
| Kimizukawase | P. pyrifolia | − | − | − | − | − | − | + | + | − | − | + | + | + | + | + | − | − | + | + | + | + | + |
| Kisui | P. pyrifolia | + | + | + | − | + | + | + | + | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Kosui | P. pyrifolia | + | + | + | − | − | + | + | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Kumoi | P. pyrifolia | − | − | − | − | − | + | + | + | − | + | + | + | + | + | + | − | + | + | + | + | − | + |
| Kunitomi | P. pyrifolia | + | + | − | + | − | + | + | + | + | − | + | − | + | + | + | − | + | + | + | + | − | + |
| Meigetsu | P. pyrifolia | − | − | − | − | + | + | + | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Nangetsu | P. pyrifolia | + | − | + | − | − | + | + | + | − | − | − | + | + | + | + | − | + | − | + | + | + | + |
| Nansui | P. pyrifolia | + | − | + | + | − | + | + | + | − | − | − | + | − | + | − | − | + | − | + | − | + | + |
| Natsushizuku | P. pyrifolia | − | + | + | + | + | + | + | + | − | − | + | + | + | + | + | + | + | + | + | + | − | + |
| Niitaka | P. pyrifolia | − | − | + | + | − | + | + | + | − | − | + | − | + | + | + | − | − | + | + | + | − | − |
| Nijisseiki | P. pyrifolia | + | − | − | + | − | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | − | + |
| Nikkori | P. pyrifolia | + | − | + | − | − | + | + | + | − | + | − | + | + | + | + | + | + | − | + | + | + | + |
| Natsuhikari | P. pyrifolia | − | + | + | − | − | + | + | + | − | − | + | + | − | + | − | + | + | + | + | − | − | + |
| Sagami | P. pyrifolia | − | + | + | − | − | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | − | + |
| Seigyoku | P. pyrifolia | − | − | − | − | − | + | + | + | + | − | + | + | + | + | + | − | + | + | − | + | + | + |
| Seiryu | P. pyrifolia | + | − | − | + | − | + | + | + | + | − | − | − | + | + | + | + | − | + | + | + | + | + |
| Shinchu | P. pyrifolia | − | + | − | + | + | + | + | + | − | + | + | − | + | + | + | − | + | + | − | + | + | + |
| Shugyoku | P. pyrifolia | + | − | + | − | − | + | + | − | − | − | + | + | − | + | − | + | + | + | + | − | − | + |
| Shuurei | P. pyrifolia | − | − | + | − | + | + | + | − | − | − | + | + | − | + | − | + | + | + | + | − | + | + |
| Shinkou | P. pyrifolia | + | − | + | − | − | + | + | + | + | − | − | + | + | + | + | − | + | − | + | + | − | + |
| Shinsui | P. pyrifolia | + | − | + | − | − | + | + | + | − | − | + | + | + | + | − | + | + | + | + | − | + | + |
| Shinsei | P. pyrifolia | + | − | + | − | − | + | + | + | + | − | − | + | + | + | + | − | + | − | + | + | − | + |
| Shinseiki | P. pyrifolia | − | − | − | + | − | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | − | + |
| Shinsetsu | P. pyrifolia | − | − | + | + | − | − | + | + | + | − | − | + | + | + | + | − | + | + | + | + | + | + |
| Suisei | P. pyrifolia | + | + | − | − | + | + | + | + | − | − | + | + | + | + | + | − | + | + | + | + | − | + |
| Taihaku | P. pyrifolia | + | + | + | − | − | + | + | + | + | − | − | + | + | + | + | + | + | − | + | + | − | + |
| Tama | P. pyrifolia | − | + | + | − | − | + | + | − | − | − | + | − | + | + | + | + | + | + | + | + | − | + |
| Tanzawa | P. pyrifolia | + | + | − | + | − | + | + | + | + | − | − | − | + | + | + | − | + | + | + | + | − | + |
| Tayadama | P. pyrifolia | − | − | − | − | − | + | + | + | − | − | + | + | + | + | + | − | + | + | − | + | + | + |
| Touno | P. pyrifolia | + | − | − | + | − | + | + | + | − | − | − | + | + | + | + | − | + | + | + | + | − | + |
| Wakahikari | P. pyrifolia | + | + | + | + | + | + | + | + | + | − | − | + | + | + | + | + | + | − | + | + | + | + |
| Wasekozo | P. pyrifolia | − | − | + | − | − | + | + | + | − | − | + | + | + | + | − | + | + | + | + | − | + | + |
| Yachiyo | P. pyrifolia | − | − | + | + | − | + | + | + | − | − | − | − | + | + | − | + | + | + | + | − | − | + |
| Yanaga | P. pyrifolia | − | − | − | + | − | + | + | + | + | + | + | + | + | + | + | − | + | + | − | + | − | + |
| Yasato | P. pyrifolia | + | − | + | − | + | + | + | + | − | − | + | + | + | + | + | + | + | + | + | + | − | + |
| Yoshikaori | P. pyrifolia | − | − | − | + | − | + | + | + | − | − | + | + | + | + | + | − | + | + | + | + | + | + |
| Balixiang | P. ussuriensis | − | − | − | − | − | − | + | − | + | − | − | − | − | + | + | − | + | − | + | − | − | − |
| Beijinbaili | P. ussuriensis | − | − | − | − | − | − | + | − | + | + | − | − | + | + | + | − | − | − | − | − | + | − |
| Enli | P. ussuriensis | + | − | − | − | − | + | + | − | − | − | − | − | + | + | + | − | − | − | − | − | + | + |
| Hongli | P. ussuriensis | − | − | − | − | − | − | + | − | − | − | + | − | + | + | + | − | − | − | + | − | − | − |
| Huangli | P. ussuriensis | − | − | + | − | − | − | + | − | + | − | + | − | + | + | + | + | − | − | + | − | + | − |
| Laiyangcili | P. ussuriensis | + | − | − | − | − | + | + | − | − | − | − | − | + | + | + | − | − | − | − | − | + | + |
| Mili | P. ussuriensis | + | − | − | − | − | − | + | − | − | − | − | − | + | + | + | − | − | − | + | − | − | − |
| Pingli | P. ussuriensis | + | − | + | − | − | − | + | + | + | + | − | − | + | + | + | + | + | + | + | − | + | − |
| Yali | P. ussuriensis | − | − | + | − | − | − | + | − | + | − | + | − | + | + | + | + | − | − | + | − | + | + |
| Zhuzuili | P. ussuriensis | − | − | − | − | − | − | + | − | − | + | + | + | + | + | + | − | + | − | + | − | + | − |
| Bartlett | P. communis | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| La France | P. communis | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Pass Crassane | P. communis | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Precose | P. communis | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Winter Nelis | P. communis | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Alexandrine Douillard | P. communis | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
Band presence and absence are indicated by + and −, respectively.
Fig. 2.
Amplified fragments of RBIP markers TsuRTP001, 002, 003, 004 and 005 in six Japanese pear cultivars.
In Chinese pear, markers TsuRTP007 and TsuRTP014 generated fragments of the same size as found in ‘Hosui’ in all 10 cultivars tested, therefore identifying all 74 of the Japanese and Chinese pear cultivars used in this study. TsuRTP015 also showed amplification in all 10 Chinese pear cultivars. Four markers (TsuRTP002, TsuRTP004, TsuRTP005 and TsuRTP020) showed no amplification in any of the 10 Chinese pear cultivars. In European pear, only one marker (TsuRTP014) amplified fragments in ‘Winter Nelis’ and ‘Alexandrine Douillard’. The other 21 retrotransposon markers showed no amplification in any of the 6 European pear cultivars tested.
Specific marker sets for cultivar differentiation
‘Hosui’ could be differentiated from the other 63 Japanese pear cultivars with 15 different combinations of 3 markers each (for example, TsuRTP001, TsuRTP002 and TsuRTP010). Since we developed RBIP markers from ‘Hosui’, we hoped that ‘Hosui’ could be differentiated by using only one marker. However, the 64 Japanese cultivars examined included many ancestral and offspring cultivars of ‘Hosui’; for example, ‘Nijisseiki’, ‘Kosui’, ‘Chikusui’, ‘Akiakari’ and ‘Akizuki’. Therefore, we found it necessary to use sets of three markers to differentiate ‘Hosui’ from all of the other Japanese cultivars.
The set of 22 RBIP markers could differentiate all 64 Japanese pear cultivars from one another except for three pairs of cultivars: ‘Aikansui’ and ‘Choju’, ‘Hokushin’ and ‘Nijisseiki’ and ‘Shinkou’ and ‘Shinsei’. In addition, marker TsuRTP007 could discriminate the European pear cultivars from the Asian cultivars, because TsuRTP007 (and most of the other RBIP markers) did not generate amplified fragments in any of the European pears, but was amplified in all of the Asian cultivars.
Mapping of RBIP markers
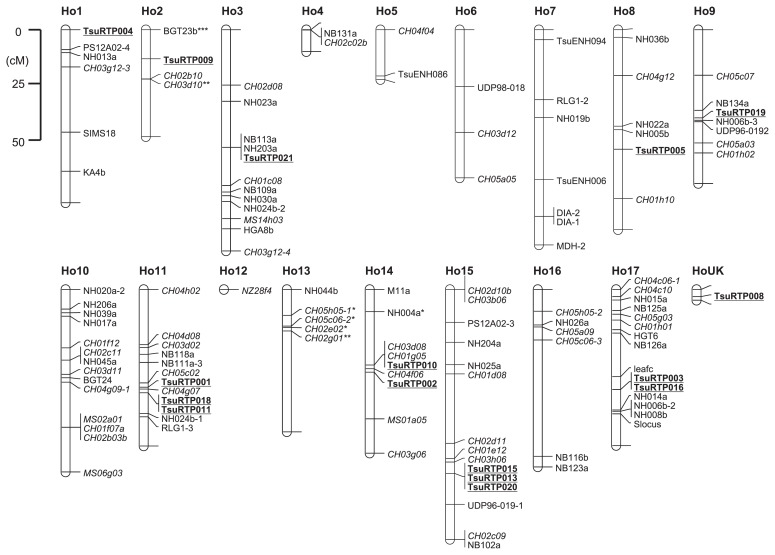
Sixteen RBIP markers showed heterozygous genotypes in ‘Hosui’ and were positioned on a genetic linkage map of ‘Hosui’ constructed from the interspecific cross of ‘Bartlett’ and ‘Hosui’. The other six RBIP markers (TsuRTP006, 007, 012, 014, 017 and 022) showed homozygous genotypes and therefore could not be mapped. The 16 RBIP marker loci were distributed in 10 out of the 18 linkage groups (LGs), including one unknown group. Markers TsuRTP011 and TsuRTP018 mapped to the same position on LG11 and markers, TsuRTP003 and TsuRTP016, mapped to the same position on LG17. Three markers (TsuRTP013, TsuRTP015 and TsuRTP020) were located at the same position on LG15. Since these seven markers were designed from junctions of 5′-LTR sequences and pear genome sequences and the seven pear genome sequences were different from each other, the members of these three sets of RBIP markers were not located at the exact position, although they were very close.
Discussion
We identified pear cultivars by means of polymorphisms in junction regions between LTR retrotransposons and pear genome sequences, which were obtained by 454 sequencing technologies. This method uses primers flanking retrotransposon insertion junctions (consisting of LTR and genome sequences), and scores for the presence and absence of insertions at individual sites. Compared with other retrotransposon-based marker systems such as IRAP (Kalendar et al. 1999), REMAP (Kalendar et al. 1999) and SSAP (Waugh et al. 1997), RBIP can detect polymorphisms produced by the integration of an element at a particular locus (Kalendar et al. 2011). RBIP has been used to study the evolution of Indica and Japonica rice cultivars (Vitte et al. 2004).
Retrotransposon-based marker systems have been used to examine genome evolution within species, and RBIP is sufficiently great to support widespread analyses at the whole-genome level. Active Ty1-copia retrotransposons were isolated in sweet potato; analysis of spontaneous transpositions in locally grown mutant lines derived from ‘Koukei14’ showed that four insertions occurred within 10 ‘Koukei14’ lines used during the past 50 years, suggesting that new insertion sites could be utilized as specific molecular markers for differentiating these lines (Yamashita and Tahara 2006). Yamashita et al. (2008) identified adzuki bean cultivars by RBIP. Here, we showed that three RBIP markers could differentiate ‘Hosui’ from all other Japanese pear cultivars. We believe that the insertion tags will be applicable as efficient cultivar-specific DNA markers.
Several DNA profiling techniques, including RFLP, RAPD, AFLP, S-RNase gene polymorphism, chloroplast DNA polymorphism, SNP and SSR, have been developed and applied to pears and other fruit trees. Among them, SSR markers have become a standard profiling technique because of their co-dominant inheritance, abundance in the genome and high degree of polymorphism. SSR profiling, parentage analysis and evaluation of genetic diversity of pear cultivars and species have been reported (Bao et al. 2007, Kimura et al. 2002, 2003b, Sawamura et al. 2008). However, it has been suggested that the SSR system has several disadvantages, including high mutation rate, and stutter bands. In human pedigrees (Weber and Wong 1993), the average overall mutation rate for 28 di-and tetra-nucleotide SSRs was estimated at about 0.001, which is an exceptionally high rate of mutation. Diwan and Cregan (1997) described that new allele formation at the rate of 2 × 10−4 was observed in soybean mapping population. In contrast, DNA marker systems such as RBIP, IRAP, REMAP and SSAP are retrotransposon based: these methods detect polymorphisms derived from insertion events, and all markers show dominant inheritance. We successfully analyzed the newly developed RBIP markers with multiplex PCR and detected them with a fluorescent DNA sequencer. Detection by agarose gel electrophoresis can be also available for RBIP fragments at about one-tenth the cost compared with the DNA sequencer system.
We used nucleotide sequences of 5′- and 3′-LTR regions of the copia-like retrotransposon Ppcrt4 from Japanese pear to identify candidate reads from data set twice the size of the haploid pear genome. The copy number of Ppcrt4-type retrotransposons is estimated to be about 50 to 100 in the pear genome; these copies appear to be distributed on two-thirds of the chromosomes, on the basis of Southern blot and FISH (fluorescence in situ hybridization) analyses (Kim et al. 2011). We identified 17 5′-LTR insertions and 23 3′-LTR insertions, fewer than half of the estimated copy number. We previously found at least 15 distinct copia-like retrotransposon groups in Japanese pear, on the basis of nucleotide sequences of reverse transcriptase domains (Shi et al. 2002). It should be possible to develop additional retrotransposon-based markers by using other types of copia-like retrotransposon groups.
The 22 markers obtained here showed amplification and insertional polymorphism in both Japanese and Chinese pears. However, almost none of them showed amplification in European pear. Based on the band presence or absence of TsuRTP013, TsuRTP015 and TsuRTP020 markers mapped at the same position of LG15, 11 cultivars showed band absence for all three markers (−/−/−, TsuRTP013-/TsuRTP015-/TsuRTP020-). Five, 1, 0, 9, 0, 0 and 54 cultivars showed +/−/−, −/+/−, −/−/+, +/+/−, +/−/+, −/+/+ and +/+/+ of band patterns for the three markers, respectively. This indicates that insertion of TsuRTP013 occurred in pear genome at first and then TsuRTP015 inserted in very close genome region around TsuRTP013 after Asian and European pears were established from common ancestor. Insertion of TsuRTP020 finally took place in very close genome region around TsuRTP013 and TsuRTP015 for ancestor of Japanese pear. Similarly, the insertion process of TsuRTP003 and TsuRTP016 locating on LG17 was examined based on their band patterns. Since −/−, +/−, −/+ and +/+ patterns for TsuRTP003 and TsuRTP016 were observed for 35, 12, 2 and 31 cultivars, insertion of TsuRTP003 occurred followed by insertion of TsuRTP016 in ancestor of Asian pears. Two exceptional native cultivars (Meigetsu and Seiryu) showed −/+ of TsuRTP003/TsuRTP016, which may have originated from mutation or spontaneous hybridization.
The relatively small copy number of Ppcrt4-type retrotransposons was shown in pear, whereas the retrotransposon BARE-1 is present in over 70,000 copies in the barley genome (Kumar et al. 1997, Manninen and Schulman 1993). However, it has been suggested that the Ppcrt4-type retro-transposons might transpose in the genome more than 50 times during approximately 500,000–1,000,000 years of Asian pear evolution.
RBIP markers are distributed widely enough to support genetic mapping projects within the generally narrower germ-plasm of cultivars (Queen et al. 2004). We could place 16 RBIP markers on a genetic linkage map of ‘Hosui’. These markers were distributed in 10 of the linkage groups, in good accordance with the distribution shown in FISH analysis (Kim et al. 2011).
Supplementary Material
Fig. 3.
A genetic linkage map of ‘Hosui’. Linkage groups are designated as Ho1–Ho17 and UK (unknown group). RBIP loci are underlined and in boldface.
Acknowledgments
We thank Drs. T. Matsuyama, H. Fujii, T. Kimura and T. Imai for valuable discussion and suggestions, and also Ms. F. Hosaka, Ms. N. Shigeta and Ms. N. Yagihashi for their technical help. This work is supported by grants-in-aid “New agriculture development genome project” (Genomics for Agricultural Innovation, DD-4040) and “Development of evaluation and management methods for supply of safe, reliable and functional food and farm produce” from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Literature Cited
- Bao L., Chen K., Zhang D., Cao Y., Yamamoto T., Teng Y. (2007) Genetic diversity and similarity of pear (Pyrus L.) cultivars native to East Asia revealed by SSR (simple sequence repeat) markers. Genet. Res. Crop Evol. 54: 959–971 [Google Scholar]
- Diwan N., Cregan P.B. (1997) Automated sizing of fluorescent-labeled simple sequence repeat (SSR) markers to assay genetic variation in soybean. Theor. Appl. Genet. 95: 723–733 [Google Scholar]
- Fernandez-Fernandez F., Harvey N.G., James C.M. (2006) Isolation and characterization of polymorphic microsatellite markers from European pear (Pyrus communis L.). Mol. Ecol. Notes 6: 1039–1041 [Google Scholar]
- Flavell A.J., Dunbar E., Anderson R., Pearce S.R., Hartley R., Kumar A. (1992) Tyl-copia group retrotransposons are ubiquitous and heterogeneous in higher plants. Nucleic Acids Res. 20: 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell A.J., Pearce S.R., Kumar A. (1994) Plant transposable elements and the genome. Curr. Opin. Genet. Dev. 4: 838–844 [DOI] [PubMed] [Google Scholar]
- Fujii H., Ogata T., Shimada T., Endo T., Shimizu T., Omura M. (2007) Development of a novel algorithm and the computer program for the identification of minimal marker sets of discriminating DNA markers for efficient cultivar identification. Plant & Animal Genomes XV Conference, P883 [Google Scholar]
- Henderson S.T., Petes T.D. (1992) Instability of simple sequence DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 12: 2749–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirochika H., Hirochika R. (1993) Ty1-copia group retrotransposons as ubiquitous components of plant genomes. Jpn. J. Genet. 68: 35–46 [DOI] [PubMed] [Google Scholar]
- Iketani H., Manabe T., Matsuta N., Akihama T., Hayashi T. (1998) Incongruence between RFLPs of chloroplast DNA and morphological classification in east Asian pear (Pyrus spp.). Genet. Res. Crop Evol. 45: 533–539 [Google Scholar]
- Ishimizu T., Inoue K., Shimonaka M., Saito T., Terai O., Norioka S. (1999) PCR-based method for identifying the S-genotypes of Japanese pear cultivars. Theor. Appl. Genet. 98: 961–967 [Google Scholar]
- Kalendar R., Grob T., Regina M., Suoniemi A., Schulman A.H. (1999) IRAP and REMAP: two new retrotransposon-based DNA fingerprinting techniques. Theor. Appl. Genet. 98: 704–711 [Google Scholar]
- Kalendar R., Flavell A.J., Ellis T.H.N., Sjakste T., Moisy C., Schulman A.H. (2011) Analysis of plant diversity with retrotransposon-based molecular markers. Heredity 106: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Yamamoto M., Hosaka F., Terakami S., Nishitani C., Sawamura Y., Yamane H., Wu J.Z., Matsumoto T., Matsuyama T., et al. (2011) Molecular characterization of novel Ty1-copia-like retrotransposons in pear (Pyrus pyrifolia). Tree Genet. Genomes 7: 845–856 [Google Scholar]
- Kimura T., Shi Y.Z., Shoda M., Kotobuki K., Matsuta N., Hayashi T., Ban Y., Yamamoto T. (2002) Identification of Asian pear varieties by SSR analysis. Breed. Sci. 52: 115–121 [Google Scholar]
- Kimura T., Iketani H., Kotobuki K., Matsuta N., Ban Y., Hayashi T., Yamamoto T. (2003a) Genetic characterization of pear varieties revealed by chloroplast DNA sequences. J. Hort. Sci. Biotech. 78: 241–247 [Google Scholar]
- Kimura T., Sawamura Y., Kotobuki K., Matsuta N., Hayashi T., Ban Y., Yamamoto T. (2003b) Parentage analysis in pear cultivars characterized by SSR markers. J. Jpn. Soc. Hort. Sci. 72: 182–189 [Google Scholar]
- Kumar A., Pearce S.R., McLean K., Harrison G., Heslop-Harrison J.S., Waugh R., Flavell A.J. (1997) The Ty1-copia group of retro-transposons in plants: genomic organisation, evolution, and use as molecular markers. Genetica 100: 205–217 [PubMed] [Google Scholar]
- Manninen I., Schulman A.H. (1993) BARE-1, a copia-like retro-element in barley (Hordeum vulgare L.). Plant. Mol. Biol. 22: 829–846 [DOI] [PubMed] [Google Scholar]
- Monte-Corvo L., Goulao L., Oliveira C. (2001) ISSR analysis of cultivars of pear and suitability of molecular markers for clone discrimination. J. Amer. Soc. Hort. Sci. 127: 262–270 [Google Scholar]
- Nishitani C., Terakami S., Sawamura Y., Takada N., Yamamoto T. (2009) Development of novel EST-SSR markers derived from Japanese pear (Pyrus pyrifolia) Breed. Sci. 59: 391–400 [Google Scholar]
- Noma K., Nakajima R., Ohtsubo H., Ohtsubo E. (1997) RIRE1, a retrotransposon from wild rice Oryza australiensis. Genes Genet. Syst. 72: 131–140 [DOI] [PubMed] [Google Scholar]
- Queen R.A., Gribbon B.M., James C., Jack P., Flavell A.J. (2004) Retrotransposon-based molecular markers for linkage and genetic diversity analysis in wheat. Mol. Genet. Genomics 271: 91–97 [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H.J. (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz, S., Misener S. (eds.) Bioinformatics Methods and Protocols: Methods in Molecular Biology, Humana Press, Totowa, NJ, USA, pp. 365–386 [DOI] [PubMed] [Google Scholar]
- Sawamura Y., Takada N., Yamamoto T., Saito T., Kimura T., Kotobuki K. (2008) Identification of parent-offspring relationships in 55 Japanese pear cultivars using S-RNase allele and SSR markers. J. Jpn. Soc. Hort. Sci. 77: 364–373 [Google Scholar]
- Shi Y.Z., Yamamoto T., Hayashi T. (2002) Characterization of copia-like retrotransposons in pear. J. Jpn. Soc. Hort. Sci. 71: 723–729 [Google Scholar]
- Tahara M., Aoki T., Suzuka S., Yamashita H., Tanaka M., Matsunaga S., Kokumai S. (2004) Isolation of an active element from a high-copy-number family of retrotransposons in the sweetpotato genome. Mol. Gen. Genet. 272: 116–127 [DOI] [PubMed] [Google Scholar]
- Teng Y., Tanabe K., Tamura F., Itai A. (2002) Genetic relationships of Pyrus species and cultivars native to East Asia revealed by randomly amplified polymorphic DNA markers. J. Amer. Soc. Hort. Sci. 127: 262–270 [Google Scholar]
- Terakami S., Kimura T., Nishitani C., Sawamura Y., Saito T., Hirabayashi T., Yamamoto T. (2009) Genetic linkage map of the Japanese pear ‘Housui’ identifying three homozygous genomic regions. J. Jpn. Soc. Hort. Sci. 78: 417–424 [Google Scholar]
- Van Ooijen J.W., Voorrips R.E. (2001) JoinMap 3.0: Software for the calculation of genetic linkage maps. Plant Research International, Wageningen [Google Scholar]
- Vitte C., Ishii T., Lamy F., Brar D., Panaud O. (2004) Genomic paleontology provides evidence for two distinct origins of Asian rice (Oryza sativa L). Mol. Genet. Genomics 272: 504–511 [DOI] [PubMed] [Google Scholar]
- Voorrips R.E. (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 93: 77–78 [DOI] [PubMed] [Google Scholar]
- Waugh R., McLean K., Flavell A.J., Pearce S.R., Kumar A., Thomas B.B.T., Powell W. (1997) Genetic distribution of BARE-1-like retrotransposable elements in the barley genome revealed by sequence-specific amplification polymorphisms (S-SAP). Mol. Gen. Genet. 253: 687–694 [DOI] [PubMed] [Google Scholar]
- Weber J., Wong C. (1993) Mutation of human short tandem repeats. Hum. Mol. Genet. 2: 1123–1128 [DOI] [PubMed] [Google Scholar]
- Yamashita H., Tahara M. (2006) A LINE-type retrotransposon active in meristem stem cells causes heritable transpositions in the sweet potato genome. Plant Mol. Biol. 61: 79–84 [DOI] [PubMed] [Google Scholar]
- Yamashita H., Tahara M., Ooyama Y. (2008) Retrotransposon DNA marker for Azuki cultivar identification. DNA Polymorphism 16: 82–87 [Google Scholar]
- Yamamoto T., Kimura T., Shoda M., Ban Y., Hayashi T., Matsuta N. (2002) Development of microsatellite markers in Japanese pear (Pyrus pyrifolia Nakai). Mol. Ecol. Notes 2: 14–16 [Google Scholar]
- Yamamoto T., Kimura T., Hayashi T., Ban Y. (2006) DNA profiling of fresh and processed fruits in pear. Breed. Sci. 56: 165–171 [Google Scholar]
- Zhao G., Dai H., Chang L., Ma Y., Sun H., He P., Zhang Z. (2010) Isolation of two novel complete Ty1-copia retrotransposons from apple and demonstration of use of derived S-SAP markers for distinguishing bud sports of Malus domestica cv. Fuji. Tree Genet. Genomes 6: 149–159 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.