Abstract
Associations between respiratory tract infections and asthma inception and exacerbations are well established. Infant respiratory syncytial virus and rhinovirus infections are known to be associated with an increased risk of asthma development, and among children with prevalent asthma, 85% of asthma exacerbations are associated with viral infections. However, the exact nature of this relationship remains unclear. Is the increase in severity of infections an epiphenomenon, meaning respiratory tract infections just appear to be more severe in patients with underlying respiratory disease, or instead a reflection of altered host susceptibility among persons with asthma and atopic disease? The main focus of this review is to summarize the available levels of evidence supporting or refuting the notion that patients with asthma or atopic disease have an altered susceptibility to selected pathogens, as well as discussing the biological mechanism or mechanisms that might explain such associations. Finally, we will outline areas in need of further research because understanding the relationships between infections and asthma has important implications for asthma prevention and treatment, including potential new pathways that might target the host immune response to select pathogens.
Key words: Asthma, viral infections, bacterial infections, allergy, allergic rhinitis, atopic disease, immune function, immune system
Abbreviations used: RSV, Respiratory syncytial virus; TLR, Toll-like receptor
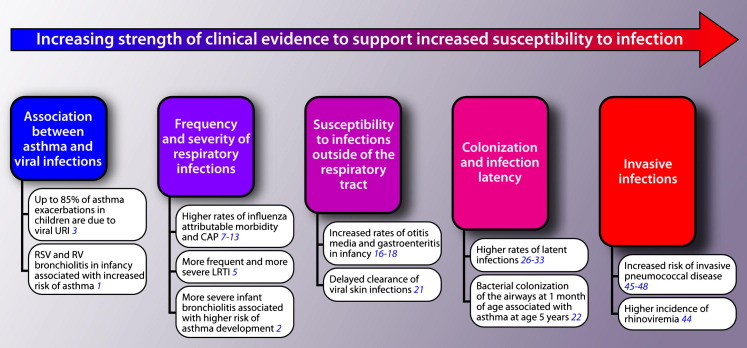
A role for respiratory tract infections in asthma development and exacerbations has been well documented (Fig 1 ). Infections in infancy, including respiratory syncytial virus (RSV) and rhinovirus infections, are known to be associated with an increased risk of asthma development.1 Furthermore, this has been demonstrated to be a dose-response relationship, with severe episodes of infant bronchiolitis increasing the odds of both early childhood asthma and asthma-specific morbidity.2 The same relationship exists between viral respiratory tract infections and asthma exacerbations.3, 4
Fig 1.
Levels of clinical evidence to support an increased risk and host susceptibility to infections in the asthmatic and atopic host. LRTI, Lower respiratory tract infections; RV, rhinovirus; URI, upper respiratory tract infection.
Frequency and severity of viral infections in asthmatic patients
Asthmatic patients have both increased frequency and severity of lower respiratory tract infections compared with subjects without asthma (Fig 1). In a prospective study of rhinovirus infections in cohabitating couples consisting of 1 asthmatic patient and 1 nonasthmatic subject, those with asthma had more frequent lower respiratory tract infections, along with symptoms that were more severe and of greater duration.5 In contrast, asthmatic patients5 and those with atopic disease6 do not appear to have increased frequency, severity, or duration of upper respiratory tract infections.
Asthma has also been identified as a risk factor for influenza-attributable morbidity and community-acquired pneumonia. Influenza-attributable health care use, including both outpatient visits7 and hospitalization rates,8 is higher among asthmatic children compared with healthy children, as are influenza-related complications.9 Population-based surveillance for laboratory-confirmed influenza hospitalizations found children with asthma to account for 32% of influenza-associated hospitalizations during the 2003-2009 influenza seasons and 44% during the 2009 H1N1 pandemic, 4 to 5 times the asthma prevalence rate.8 During the 2009 H1N1 pandemic asthmatic patients compared with nonasthmatic subjects were almost twice as likely to have pneumonia (50% vs 27%) and require care in the intensive care unit (33% vs 19%).9 However, in a study conducted in the United Kingdom, although asthmatic patients were more likely to have severe respiratory distress and require supplemental oxygen, they were half as likely to die or require an advanced level of care compared with nonasthmatic subjects.10 The authors found that the less severe outcomes in asthmatic patients were associated with prior inhaled steroid use and earlier hospital admission. Thus asthmatic patients still might be at an increased risk for influenza-related complications, but because of their medical history, they might be more likely to seek care earlier, resulting in improved outcomes. The risk for community-acquired bacterial and viral pneumonia has been estimated to be at least 2-fold in asthmatic patients compared with that seen in healthy control subjects.11, 12, 13
Asthmatic patients with allergic sensitization appear to have an even greater susceptibility to respiratory tract infections. Children with atopic asthma were found to experience 47% more symptomatic viral illnesses compared with nonatopic asthmatic subjects during the peak virus season (1.19 vs 0.81 per month).14 Allergen-sensitized asthmatic patients also have a higher risk of hospital admission for asthma exacerbations compared with nonsensitized asthmatic patients.15
Susceptibility to infections outside the respiratory tract
The increased susceptibility to infections among asthmatic patients extends beyond the lungs (Fig 1). Children with asthma have increased rates of both otitis media and gastroenteritis during infancy. Increased prevalence of ear infections during infancy was reported by Eldeirawi et al16 among Mexican American asthmatic children (39%) compared with nonasthmatic children (20%); this association was independent of antibiotic use and other infectious history. A similar relationship was noted between otitis media and both asthma and atopic dermatitis in a German birth cohort.17 A cross-sectional study of 26,400 Korean children by Ahn et al18 identified a higher prevalence of acute gastroenteritis during infancy in children later given a diagnosis of asthma. Recall bias is a concern for the studies conducted by Eldeirawi et al16 and Ahn et al18 because they relied on retrospective reporting. In addition, patients with atopic dermatitis are highly susceptible to cutaneous bacterial, viral, and fungal infections, most notably Staphylococcus aureus and herpes simplex virus.19, 20 Delayed therapeutic response times and an increased likelihood of recurrence were identified for genital warts in patients with a history of hay fever, eczema, or asthma.21
Colonization and infection latency in patients with asthma and atopic disease
Bacterial colonization of the airways in infancy is associated with asthma development (Fig 1). Bisgaard et al22 collected hypopharyngeal samples from 321 asymptomatic neonates at 1 month of age and found colonization of the airways with Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis to be associated with the development of asthma by age 5 years. We postulate this colonization is due to an altered immune response that predisposes these infants to early acquisition of these pathogens, although this colonization could also indicate a causal relationship between the pathogen and asthma inception. Increased frequency of colonization with S pneumoniae has also been demonstrated in asthmatic patients. In a cross-sectional, population-based prospective study of 1013 adolescents, asthma was an independent risk factor for nasopharyngeal colonization of S pneumoniae, which was identified in 8.2% of subjects.23 An increased prevalence of bacterial colonization of the skin, primarily with S aureus, is seen in patients with atopic dermatitis24 and occurs in both lesional and clinically normal skin.25
A number of latent infections have been demonstrated to be more common among asthmatic patients, including Mycoplasma pneumoniae,26, 27 Chlamydia pneumoniae,27, 28 adenovirus,29, 30 and rhinovirus.30, 31, 32, 33 We have defined latent infections as asymptomatic bacterial or viral identification after an acute initial infection. As with colonization, we propose that latent infections reflect an altered immune response, although an alternative explanation is that these pathogens might play a role in asthma pathogenesis. Mycoplasma or Chlamydia species have been identified in the airways of 45% and 11% of asthmatic patients, respectively, although Mycoplasma species is found in only 9% of healthy control subjects.27 A positive relationship between Chlamydia pneumoniae–specific secretory IgA antibody levels and asthma exacerbations28 provides further evidence for latent bacterial infection affecting asthma severity. In contrast, Sutherland et al34 identified only 13% of patients with suboptimally controlled asthma to have PCR evidence for M pneumoniae or C pneumoniae on lower airway endobronchial biopsy. Furthermore, the addition of clarithromycin did not improve asthma control, making the clinical relevance of latent atypical bacterial infection unclear. Improved detection methods for atypical respiratory pathogens are needed to more fully characterize the relationship between these pathogens and asthma.
Latent viral infection also appears to be more common among asthmatic patients. Among 50 asymptomatic asthmatic children, adenovirus was found in 78.4% of subjects, rhinovirus in 32.4%, and coronavirus in 2.7%; coinfection with 2 or all 3 viruses was also identified.30 Twenty healthy children were included as control subjects, with only adenovirus detected in 1 nasopharyngeal swab. Results from other29 but not all27, 35 studies have supported an increased prevalence of latent adenovirus infection in asthmatic patients, likely because of varying viral detection techniques and small sample sizes. A high incidence of persistent rhinovirus infection has also been identified in asthmatic patients, with detectable rhinovirus RNA in greater than 40% of asthmatic children 6 weeks after an acute exacerbation.31 Rhinovirus RNA has also been identified in 73% of mucosal biopsy specimens of asymptomatic asthmatic patients compared with only 22% without asthma, with the presence of human rhinovirus significantly associated with lower pulmonary lung function.33 Further studies are needed to investigate the relationship between rhinovirus persistence and asthma disease severity. Because of the finding of successive infections in children with different serotypes of human rhinovirus,36 studies that incorporate genotyping will also be helpful in determining whether what appears to be a latent rhinovirus infection in asthmatic patients is indeed viral persistence or conversely subsequent infections with different serotypes. This differentiation will be critical to furthering our understanding of rhinovirus infection in asthmatic patients and to guide future therapeutic measures, including human rhinovirus-specific vaccines.
Because of the strong relationship between RSV-induced bronchiolitis in infancy and the development of asthma,37 latent RSV infection of asthmatic patients has also been suggested.38 Persistent RSV infection has been documented 100 days after infection in a murine model39 and 5 weeks after infection in a guinea pig model,40 but similar findings have yet to be confirmed in human studies, indicating a key area in need of further research. The ability of RSV to infect41 and persist42 in vitro within human dendritic cells has been demonstrated, and in vivo RSV persistence in human subjects has been suggested by the identification of RNA sequences homologous to the RSV genome in the naive human bone marrow stromal cells of adult and pediatric donors but not the complete virus.43
Invasive infections in patients with asthma and atopic disease
Perhaps the strongest evidence for a relationship between increased infection susceptibility among patients with asthma and atopic disease is the increased rates of invasive disease (Fig 1). In a study of children with respiratory illnesses, rhinoviremia was detected in 25% of children presenting with an asthma exacerbation compared with only 5% of children presenting with other respiratory conditions.44 Among persons 2 to 49 years of age enrolled in Tennessee's Medicaid Program, the average annual incidence rate of invasive pneumococcal disease among asthmatic patients was 3-fold higher compared with that seen in persons without asthma (6.1 vs 2.0 episodes per 10,000).45 Population-based case-control studies conducted in Minnesota46 and Finland47 confirmed these findings, with 17% and 5% of the invasive pneumococcal disease burden attributable to asthma within the respective population studied. An increased risk for serious pneumococcal disease in patients with atopic disease has also been demonstrated.48 Patients with atopic dermatitis are also highly susceptible to widespread disseminated viral infections, including eczema molluscatum, eczema herpeticum, and eczema vaccinatum.49 Interestingly, these conditions are not typically seen in patients with other inflammatory skin conditions, such as psoriasis.50
Potential mechanisms to explain an increased host susceptibility to viral and bacterial infections in patients with asthma and atopic disease
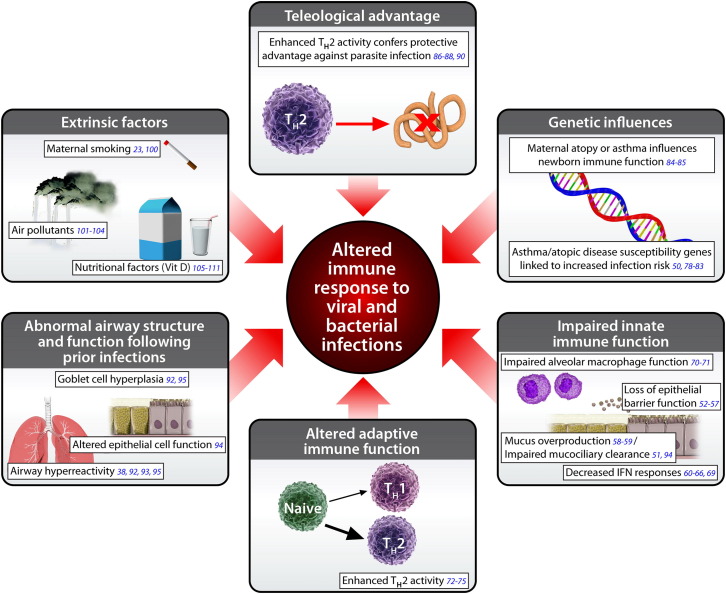
A variety of potential mechanisms have been proposed to explain why patients with asthma and atopic disease appear to have increased susceptibility to select viral and bacterial pathogens (Fig 2 ). Impaired innate immune responses have been observed in patients with asthma and atopic disease, including deficient epithelial cell function, mucus overproduction, decreased interferon responses, and impaired alveolar macrophage function.
Fig 2.
Biological mechanisms that explain an altered immune response to viral and bacterial infections in the asthmatic and atopic host.
Airway epithelium
Epithelial cells play important roles in immune responses, including maintenance of barrier function, mucociliary clearance, production of peptides that have the ability to kill or neutralize microorganisms, and release of chemokines that influence antigen-specific T and B cells; deficiencies in these abilities would be expected to increase a patient's susceptibility to infections.51 Examination of bronchial biopsy specimens from asthmatic patients reveal disrupted tight junctions, an important component of epithelial barrier function, and increased permeability to macromolecules has been demonstrated in epithelial cell cultures.52 A significant association between asthma development and a proteinase inhibitor gene (SPINK5) polymorphism, postulated to impair epithelial barrier function has also been identified within a population of German children.53 The role of atopy in host susceptibility must also be considered because allergens have the potential to interfere with proper epithelial barrier function, and the nasal mucosal changes associated with allergic rhinitis are histologically similar to those seen in the lower airways in asthmatic patients.54 Peptidase allergens disrupt intercellular tight junctions in human bronchial epithelial cell lines, thereby increasing permeability of the airway epithelium,55, 56 and the disruption caused by the dust mite allergen Der p 1 enhanced RSV replication within a human bronchial epithelial cell line.57 These studies shed light on how alteration of the airway epithelia of asthmatic and atopic patients might increase susceptibility to infections.
Mucus
Mucus production is important in handling respiratory pathogens, and there is an increase in both quantity and viscosity in asthmatic patients. Endobronchial biopsy specimens from asthmatic patients demonstrate airway goblet cell hyperplasia with increased numbers of mucus-secreting goblet cells in the epithelium and an increase in the size of the submucosal glands.58 Rhinovirus infection in vivo increases release of the major mucin component of airway mucous secretions, MUC5AC, and in asthmatic patients MUC5AC levels positively correlate with peak viral load.59
Interferon responses
Studies have shown that patients with asthma and atopic disease have a deficient interferon response, with significantly lower levels reported as early as birth60 likely contributing to the future risk for viral respiratory tract infections. An inverse relationship between cord blood IFN-γ responses and the frequency of symptomatic viral respiratory tract infections has been demonstrated within the first year of life.61 Allergen exposure and RSV both reduce INF-γ production compared with RSV infection alone in both murine62 and rat63 models and might thus be synergistic in increasing host susceptibility to infection. The importance of the deficient IFN-γ production is further supported by the modulation of postviral sequelae, including significantly less bronchiolar inflammation and fibrosis, when rats with deficient IFN-γ responses receive exogenous IFN-γ supplementation during acute viral illnesses.64 Deficient IFN-β and IFN-λ responses to infection with rhinovirus have been demonstrated in human bronchial epithelial cells in vitro.65, 66 Furthermore, the amount of IFN-λ production in bronchoalveolar lavage cells infected in vitro with rhinovirus significantly inversely correlated with clinical illness severity of the same subjects infected with rhinovirus in vivo,65 although these findings have not been replicated by others. Bochkov et al67 and Lopez-Souza et al68 demonstrated no difference in interferon responses in bronchial epithelial cells from asthmatic patients in vitro in response to rhinovirus infection compared with those seen in healthy control subjects. More recently, in vitro studies in nasal epithelial cells from asthmatic patients have demonstrated lower production of IFN-λ1 after rhinovirus infection compared with that seen in healthy control epithelium but a higher severity of illness was associated with higher levels of IFN-λ1 production by asthmatic patients in vivo.69 How this relates to human clinical infection risk and illness severity and whether increasing interferon responses would modify morbidity still needs to be elucidated.
Alveolar macrophages
Patients with asthma might also have impaired alveolar macrophage function. Airway macrophages from children with moderate and severe poorly controlled asthma were found to have significantly blunted phagocytosis of S aureus and increased apoptosis.70 Children treated with inhaled corticosteroids were the control group in this study, and although that limits the interpretation, a later study71 also supported impaired alveolar macrophage function with altered airway and intracellular airway macrophage glutathione homeostasis in children with severe asthma compared with children with moderate asthma.
Adaptive immunity
Asthma is characterized by enhanced TH2 activity.72 An increased number of CD4+ T cells, predominantly TH2 cells, are seen in the airways of asthmatic patients, which is in contrast to the TH1 cell predominance seen in healthy airways.73 Delayed postnatal maturation of the immune system, including a delayed transition from a TH2 to TH1 bias, is a risk factor for respiratory tract infections. High IL-5 production by TH2 cells at birth predicts future risk of severe respiratory tract infections in childhood, whereas concomitant IL-10 production by T cells at birth attenuated this risk.74 In a murine model, after infection of the lungs with Chlamydia muridarum, IL-13, a TH2 cytokine, is rapidly produced and promotes susceptibility to infection, possibly related to impairment of macrophage phagocytic function.75 This might explain why allergic asthmatic patients, with a dominant TH2 response and enhanced IL-13 production, would be more susceptible to chlamydial lung infection. Downregulation of Toll-like receptors (TLRs) might be responsible for increased susceptibility of asthmatic patients to Mycoplasma species infection because Mycoplasma species clearance in an allergic murine model has been demonstrated to be due to TLR2 downregulation.76
Impaired adaptive immune responses have been described in those with atopic dermatitis as well. Arkwright et al77 found a significantly lower proportion of children with moderate-to-severe eczema to have adequate antibody responses to pneumococcal vaccination compared with control subjects with isolated recurrent upper respiratory tract infections (17% vs 57%). However, because the children with eczema had no history of severe or recurrent infections with S pneumoniae, the clinical significance of their reduced response to pneumococcal vaccination is uncertain.
Genetic influences on immune function development in patients with asthma and atopic disease
Alterations in immune function might be, at least in part, caused by genetic influences because multiple asthma and atopic disease susceptibility genes are functional immune response genes.78, 79 The GABRIEL consortium,80 a large genome-wide association study of asthma, identified a significant association for IL18R1, IL33, HLA-DQ, SMAD3, IL2RB, and ORMDL3. Many of these genes have a multitude of functions, but all have been found to be involved in the TH2 inflammatory response to epithelial damage sustained during trauma or infection. A variety of genetic mutations have been identified in patients with atopic dermatitis that are associated with skin barrier dysfunction. The most commonly identified is a filaggrin mutation present in up to 50% of patients with atopic dermatitis81 and associated with the persistence of atopic dermatitis into adulthood,82 as well as increased asthma severity.83 The downregulation of multiple immune response genes have also been identified in patients with atopic dermatitis.50 It has also been postulated that parental asthma or allergy might influence immune function, with maternal history of atopy or asthma identified as a risk factor for more severe infant human rhinovirus–associated illness.84 Furthermore, lower cytokine responses to innate stimulation by TLR2, TLR3, TLR4, and TLR9 agonists and in vitro stimulation by RSV have been seen in children with a parental history of allergy or asthma.85
Possible teleological explanation for altered immune system in patients with asthma and atopic disease
It has long been debated as to why asthma and atopic diseases have persisted for thousands of years and are prevalent and increasing in most populations. The enhanced TH2 activity seen in patients with asthma and atopic disease might have a teleological basis because it confers a protective advantage against infection with helminthic parasites,86, 87, 88 which were far more common pathogens in past centuries.89 TH2 immune signaling in the lungs of asthmatic patients can have deleterious effects, such as increased eosinophil activity, mucus hypersecretion, and muscle hyperactivity; however, these same immune mechanisms promote helminth expulsion when expressed in the gut in response to parasitic infection.90 Peisong et al88 discovered that children with upregulated TH2 immune signaling caused by an asthma-associated genetic variant of signal transducer and activator of transcription 6 had increased resistance to infection with the helminth Ascaris lumbricoides. A similar relationship between atopy and helminth infection has been described in Venezuelan children86 and in an African adult population.87 Together, these studies support a protective advantage against parasitic infections among those with asthma and atopy. It has also been proposed that the reverse is true; that is, that parasitic exposure reduces the likelihood of the development of asthma and atopic disease.91
Effect of prior infections or latent infection/colonization on immune function in patients with asthma and atopic disease
Other possible mechanisms for increased infection susceptibility include abnormal airway structure and function caused by prior infections and ongoing airway inflammation. Chronic responses to paramyxoviral92 and RSV93 infection have been demonstrated in mice, consisting of airway hyperreactivity and goblet cell hyperplasia persisting for at least 1 year after complete viral clearance and methacholine-induced airway hyperresponsiveness up to 154 days after viral inoculation, respectively. Paramyxovirus infection has also been shown to alter epithelial cell function characterized by decreased airway mucociliary velocity and impaired bacterial clearance.94
Infection latency or colonization might contribute to enhanced susceptibility to infections by promoting ongoing airway inflammation. In a murine model trace levels of parainfluenza virus have been shown to be associated with persistent activation of the natural killer T cell–macrophage innate immune axis and production of IL-13, leading to chronic mucous cell metaplasia and airway hyperreactivity.95 Long-term persistence of RSV and airway hyperresponsiveness and eosinophilia has been demonstrated in the guinea pig lung.38 These findings are notable, but their clinical relevance remains unclear because, despite a likely role for latent parainfluenza and RSV infections in asthmatic patients, persistence of these viruses in human subjects has not been clearly established.
Influence of extrinsic factors on immune function in patients with asthma and atopic disease
Lastly, there are a number of common extrinsic factors that are associated with both increased susceptibility to infection and asthma exacerbations or asthma control, including cigarette smoke, air pollutants, and nutrition. Maternal smoking is a known risk factor for respiratory tract infections96 and the development of asthma97, 98 and atopic disease99 but has more recently also been shown to attenuate neonatal immune function with impaired TLR-mediated immune responses identified in neonates whose mothers smoked during pregnancy.100 Passive smoke exposure has also been shown in adolescents to be associated with higher rates of pneumococcal colonization of the nasopharynx.23
Outdoor air pollutants, including ozone and diesel exhaust, have been implicated in alteration of immune function and increase in infection susceptibility. Exposure to ambient ozone results in the loss of lung epithelial integrity, a key innate immune defense mechanism of the airways.101 Exposure to diesel exhaust increases the susceptibility to influenza virus infection in respiratory epithelial cells both in vitro 102 and in mice in vivo,103 possibly related to the downregulation of antimicrobial host defense molecules, including Clara cell secretory protein and surfactant proteins A and D.104
Nutritional factors, including vitamin D, have also been described to affect immune function. Subclinical vitamin D levels are associated with an increased risk for respiratory tract infections in both infants105, 106 and children.107 Proposed mechanisms by which vitamin D levels modulate immune function include induction of antimicrobial peptide expression,108 downregulation of TLR expression by monocytes,109 and inhibition of T- and B-cell proliferation.110, 111 Some of these effects strengthen the immune system, whereas others actually suppress immune function, making the exact role of vitamin D in the immune system, as well as the ideal circulating levels, still unclear. Randomized controlled trials are ongoing to evaluate the role of vitamin D supplementation during pregnancy and asthma development.112, 113
One must also question whether any of the medications used to treat asthma or atopic disease, most notably corticosteroids, modify infection risk. Fortunately, the use of corticosteroids in asthmatic patients does not appear to be immunosuppressive. The association identified by Talbot et al45 between invasive pneumococcal disease and asthma remained after adjustment for the long-term use of oral corticosteroids. Furthermore, Wos et al33 found no relationship between inhaled corticosteroid dose and rhinovirus presence in the lower airways of patients with bronchial asthma. Nasal and inhaled corticosteroids might actually be protective in patients with allergic rhinitis or asthma by resulting in restitution of the upper and lower airway epithelium, respectively.
Implications for treatment in patients with asthma and atopic disease
The suggestion of altered host susceptibility to specific viral and bacterial pathogens in patients with asthma and atopic disease has important implications for treatment and prevention. Although the current mainstay of therapy is inhaled corticosteroids, which inhibit inflammation, more emphasis might need to be placed on therapies that bolster innate, adaptive, or both immune responses in response to infection or in deterring the long-term consequences of infection.
Other therapies that might prove beneficial include increased vaccination efforts, exogenous type I interferon administration, and glutathione supplementation. As a result of the recently demonstrated increased risk of invasive pneumococcal disease among patients with asthma, pneumococcal vaccination is now recommended for all adult asthmatic patients114 and has been associated with a decrease in asthma-related hospitalizations and asthma-related length of hospital stay.115 Because of the findings of deficient IFN-β production by asthmatic patients, Cakebread et al116 investigated the therapeutic potential of exogenous IFN-β administration on rhinovirus infection in asthmatic patients in primary bronchial epithelial cells from asthmatic donors. Treatment of cells with exogenous IFN-β followed by infection with rhinovirus resulted in a normal antiviral response to rhinovirus, including induction of apoptosis and reduced rhinovirus replication. Intrabronchial delivery of IFN-α in an asthmatic cohort has not been effective because it induced bronchospasm with a significant decrease in FEV1.117 Another treatment option under investigation includes the use of glutathione supplementation in patients with severe asthma because these patients have reduced levels of glutathione and subsequent impairment of airway macrophage function. Ex vivo glutathione supplementation has been demonstrated to significantly improve the phagocytic function of airway macrophages collected from patients with severe asthma.71
Conclusions
The relationship between infections and asthma is still not fully understood. There has been historical debate over whether respiratory tract infections play a causal role or severe infections are merely a marker of those predisposed to asthma. The likelihood is that both are true: hosts with a genetic propensity for asthma are those who also have an altered immune response to specific pathogens, resulting in more severe infection, and these early-life events are additionally causal in asthma development. The shared pathways demonstrated by recent studies support the possibility of altered susceptibility to specific viral and bacterial pathogens within patients with asthma and atopic disease. More research is needed to characterize the innate and adaptive immune responses in patients with asthma and atopic disease to improve control of these diseases, develop treatment strategies for those pathogens for which there are currently no therapies, and someday even to prevent their development.
Footnotes
Disclosure of potential conflict of interest: T. V. Hartert has received grants from the National Institutes of Health, has received consulting fees from the Merck Scientific Advisory Committee, has received fees for participation in review activities for the MedImmune Scientific Advisory Board for the REPORT study, and is employed by Vanderbilt University. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Gern J.E. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J. 2008;27(suppl):S97–S103. doi: 10.1097/INF.0b013e318168b718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll K.N., Wu P., Gebretsadik T., Griffin M.R., Dupont W.D., Mitchel E.F. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corne J.M., Marshall C., Smith S., Schreiber J., Sanderson G., Holgate S.T. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 6.Doyle W.J., Skoner D.P., Fireman P., Seroky J.T., Green I., Ruben F. Rhinovirus 39 infection in allergic and nonallergic subjects. J Allergy Clin Immunol. 1992;89:968–978. doi: 10.1016/0091-6749(92)90219-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E.K., Griffin M.R., Edwards K.M., Weinberg G.A., Szilagyi P.G., Staat M.A. Influenza burden for children with asthma. Pediatrics. 2008;121:1–8. doi: 10.1542/peds.2007-1053. [DOI] [PubMed] [Google Scholar]
- 8.Dawood F.S., Kamimoto L., D'Mello T.A., Reingold A., Gershman K., Meek J. Children with asthma hospitalized with seasonal or pandemic influenza, 2003-2009. Pediatrics. 2011;128:e27–e32. doi: 10.1542/peds.2010-3343. [DOI] [PubMed] [Google Scholar]
- 9.O'Riordan S., Barton M., Yau Y., Read S.E., Allen U., Tran D. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ. 2010;182:39–44. doi: 10.1503/cmaj.091724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semple M., Lim W., Myles P., Enstone J., Openshaw P., Read R. Amsterdam; The Netherlands: 2011 Sep 24-28. Relationship of asthma to outcome in influenza A/H1N1 2009 infection: FLU-CIN cohort study. Poster presented at: European Respiratory Society Annual Congress. [Google Scholar]
- 11.Almirall J., Bolibar I., Serra-Prat M., Roig J., Hospital I., Carandell E. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J. 2008;31:1274–1284. doi: 10.1183/09031936.00095807. [DOI] [PubMed] [Google Scholar]
- 12.Teepe J., Grigoryan L., Verheij T.J. Determinants of community-acquired pneumonia in children and young adults in primary care. Eur Respir J. 2010;35:1113–1117. doi: 10.1183/09031936.00101509. [DOI] [PubMed] [Google Scholar]
- 13.Vinogradova Y., Hippisley-Cox J., Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. 2009;59:e329–e338. doi: 10.3399/bjgp09X472629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olenec J.P., Kim W.K., Lee W.M., Vang F., Pappas T.E., Salazar L.E. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green R.M., Custovic A., Sanderson G., Hunter J., Johnston S.L., Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eldeirawi K., McConnell R., Furner S., Freels S., Stayner L., Hernandez E. Frequent ear infections in infancy and the risk of asthma in Mexican American children. J Asthma. 2010;47:473–477. doi: 10.3109/02770901003759428. [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre E.A., Chen C.M., Herbarth O., Borte M., Schaaf B., Kramer U. Early-life otitis media and incident atopic disease at school age in a birth cohort. Pediatr Infect Dis J. 2010;29:e96–e99. doi: 10.1097/inf.0b013e3181fcd9e8. [DOI] [PubMed] [Google Scholar]
- 18.Ahn K.M., Lee M.S., Hong S.J., Lim D.H., Ahn Y.M., Lee H.R. Fever, use of antibiotics, and acute gastroenteritis during infancy as risk factors for the development of asthma in Korean school-age children. J Asthma. 2005;42:745–750. doi: 10.1080/02770900500308023. [DOI] [PubMed] [Google Scholar]
- 19.Ring J., Abeck D., Neuber K. Atopic eczema: role of microorganisms on the skin surface. Allergy. 1992;47:265–269. doi: 10.1111/j.1398-9995.1992.tb02051.x. [DOI] [PubMed] [Google Scholar]
- 20.Lubbe J. Secondary infections in patients with atopic dermatitis. Am J Clin Dermatol. 2003;4:641–654. doi: 10.2165/00128071-200304090-00006. [DOI] [PubMed] [Google Scholar]
- 21.Stefanaki C., Stefanaki I., Verra P., Hadjivassiliou M., Caroni C., Bethimoutis G. Atopic patients with genital warts have a more protracted clinical course and a greater probability of recurrences. Int J STD AIDS. 2010;21:723–727. doi: 10.1258/ijsa.2010.010225. [DOI] [PubMed] [Google Scholar]
- 22.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bonnelykke K. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 23.Cardozo D.M., Nascimento-Carvalho C.M., Andrade A.L., Silvany-Neto A.M., Daltro C.H., Brandao M.A. Prevalence and risk factors for nasopharyngeal carriage of Streptococcus pneumoniae among adolescents. J Med Microbiol. 2008;57(Pt 2):185–189. doi: 10.1099/jmm.0.47470-0. [DOI] [PubMed] [Google Scholar]
- 24.Higaki S., Morohashi M., Yamagishi T., Hasegawa Y. Comparative study of staphylococci from the skin of atopic dermatitis patients and from healthy subjects. Int J Dermatol. 1999;38:265–269. doi: 10.1046/j.1365-4362.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams J.V., Vowels B.R., Honig P.J., Leyden J.J. S. aureus isolation from the lesions, the hands, and the anterior nares of patients with atopic dermatitis. Pediatr Dermatol. 1998;15:194–198. doi: 10.1046/j.1525-1470.1998.1998015194.x. [DOI] [PubMed] [Google Scholar]
- 26.Kraft M., Cassell G.H., Henson J.E., Watson H., Williamson J., Marmion B.P. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 27.Martin R.J., Kraft M., Chu H.W., Berns E.A., Cassell G.H. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham A.F., Johnston S.L., Julious S.A., Lampe F.C., Ward M.E. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11:345–349. doi: 10.1183/09031936.98.11020345. [DOI] [PubMed] [Google Scholar]
- 29.Macek V., Sorli J., Kopriva S., Marin J. Persistent adenoviral infection and chronic airway obstruction in children. Am J Respir Crit Care Med. 1994;150:7–10. doi: 10.1164/ajrccm.150.1.8025775. [DOI] [PubMed] [Google Scholar]
- 30.Marin J., Jeler-Kacar D., Levstek V., Macek V. Persistence of viruses in upper respiratory tract of children with asthma. J Infect. 2000;41:69–72. doi: 10.1053/jinf.2000.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kling S., Donninger H., Williams Z., Vermeulen J., Weinberg E., Latiff K. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy. 2005;35:672–678. doi: 10.1111/j.1365-2222.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 32.Malmstrom K., Pitkaranta A., Carpen O., Pelkonen A., Malmberg L.P., Turpeinen M. Human rhinovirus in bronchial epithelium of infants with recurrent respiratory symptoms. J Allergy Clin Immunol. 2006;118:591–596. doi: 10.1016/j.jaci.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Wos M., Sanak M., Soja J., Olechnowicz H., Busse W.W., Szczeklik A. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med. 2008;177:1082–1089. doi: 10.1164/rccm.200607-973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland E.R., King T.S., Icitovic N., Ameredes B.T., Bleecker E., Boushey H.A. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126:747–753. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macek V., Dakhama A., Hogg J.C., Green F.H., Rubin B.K., Hegele R.G. PCR detection of viral nucleic acid in fatal asthma: is the lower respiratory tract a reservoir for common viruses? Can Respir J. 1999;6:37–43. doi: 10.1155/1999/938049. [DOI] [PubMed] [Google Scholar]
- 36.Henquell C., Mirand A., Deusebis A.L., Regagnon C., Archimbaud C., Chambon M. Prospective genotyping of human rhinoviruses in children and adults during the winter of 2009-2010. J Clin Virol. 2012;53:280–284. doi: 10.1016/j.jcv.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Sigurs N., Aljassim F., Kjellman B., Robinson P.D., Sigurbergsson F., Bjarnason R. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 38.Bramley A.M., Vitalis T.Z., Wiggs B.R., Hegele R.G. Effects of respiratory syncytial virus persistence on airway responsiveness and inflammation in guinea-pigs. Eur Respir J. 1999;14:1061–1067. doi: 10.1183/09031936.99.14510619. [DOI] [PubMed] [Google Scholar]
- 39.Schwarze J., O'Donnell D.R., Rohwedder A., Openshaw P.J. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med. 2004;169:801–805. doi: 10.1164/rccm.200308-1203OC. [DOI] [PubMed] [Google Scholar]
- 40.Riedel F., Oberdieck B., Streckert H.J., Philippou S., Krusat T., Marek W. Persistence of airway hyperresponsiveness and viral antigen following respiratory syncytial virus bronchiolitis in young guinea-pigs. Eur Respir J. 1997;10:639–645. [PubMed] [Google Scholar]
- 41.Johnson T.R., Johnson C.N., Corbett K.S., Edwards G.C., Graham B.S. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One. 2011;6:e16458. doi: 10.1371/journal.pone.0016458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hobson L., Everard M.L. Persistent of respiratory syncytial virus in human dendritic cells and influence of nitric oxide. Clin Exp Immunol. 2008;151:359–366. doi: 10.1111/j.1365-2249.2007.03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezaee F., Gibson L.F., Piktel D., Othumpangat S., Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol. 2011;45:277–286. doi: 10.1165/rcmb.2010-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xatzipsalti M., Kyrana S., Tsolia M., Psarras S., Bossios A., Laza-Stanca V. Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med. 2005;172:1037–1040. doi: 10.1164/rccm.200502-315OC. [DOI] [PubMed] [Google Scholar]
- 45.Talbot T.R., Hartert T.V., Mitchel E., Halasa N.B., Arbogast P.G., Poehling K.A. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352:2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 46.Juhn Y.J., Kita H., Yawn B.P., Boyce T.G., Yoo K.H., McGree M.E. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol. 2008;122:719–723. doi: 10.1016/j.jaci.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klemets P., Lyytikainen O., Ruutu P., Ollgren J., Kaijalainen T., Leinonen M. Risk of invasive pneumococcal infections among working age adults with asthma. Thorax. 2010;65:698–702. doi: 10.1136/thx.2009.132670. [DOI] [PubMed] [Google Scholar]
- 48.Jung J.A., Kita H., Yawn B.P., Boyce T.G., Yoo K.H., McGree M.E. Increased risk of serious pneumococcal disease in patients with atopic conditions other than asthma. J Allergy Clin Immunol. 2010;125:217–221. doi: 10.1016/j.jaci.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wollenberg A., Rawer H.C., Schauber J. Innate immunity in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:272–281. doi: 10.1007/s12016-010-8227-x. [DOI] [PubMed] [Google Scholar]
- 50.Grigoryev D.N., Howell M.D., Watkins T.N., Chen Y.C., Cheadle C., Boguniewicz M. Vaccinia virus-specific molecular signature in atopic dermatitis skin. J Allergy Clin Immunol. 2010;125:153–159. doi: 10.1016/j.jaci.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schleimer R.P., Kato A., Kern R., Kuperman D., Avila P.C. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao C., Puddicombe S.M., Field S., Haywood J., Broughton-Head V., Puxeddu I. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 53.Kabesch M., Carr D., Weiland S.K. von ME. Association between polymorphisms in serine protease inhibitor, kazal type 5 and asthma phenotypes in a large German population sample. Clin Exp Allergy. 2004;34:340–345. doi: 10.1111/j.1365-2222.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 54.Hammad H., Lambrecht B.N. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 55.Wan H., Winton H.L., Soeller C., Tovey E.R., Gruenert D.C., Thompson P.J. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Runswick S., Mitchell T., Davies P., Robinson C., Garrod D.R. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–842. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 57.Foster S., Bedford K.J., Gould M.E., Coward W.R., Hewitt C.R. Respiratory syncytial virus infection and virus-induced inflammation are modified by contaminants of indoor air. Immunology. 2003;108:109–115. doi: 10.1046/j.1365-2567.2003.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ordonez C.L., Khashayar R., Wong H.H., Ferrando R., Wu R., Hyde D.M. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 59.Hewson C.A., Haas J.J., Bartlett N.W., Message S.D., Laza-Stanca V., Kebadze T. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-kappaB and EGFR pathways. Eur Respir J. 2010;36:1425–1435. doi: 10.1183/09031936.00026910. [DOI] [PubMed] [Google Scholar]
- 60.Tang M.L., Kemp A.S., Thorburn J., Hill D.J. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–985. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 61.Copenhaver C.C., Gern J.E., Li Z., Shult P.A., Rosenthal L.A., Mikus L.D. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 62.Peebles R.S., Jr., Sheller J.R., Collins R.D., Jarzecka A.K., Mitchell D.B., Parker R.A. Respiratory syncytial virus infection does not increase allergen-induced type 2 cytokine production, yet increases airway hyperresponsiveness in mice. J Med Virol. 2001;63:178–188. [PubMed] [Google Scholar]
- 63.Hassantoufighi A., Oglesbee M., Richter B.W., Prince G.A., Hemming V., Niewiesk S. Respiratory syncytial virus replication is prolonged by a concomitant allergic response. Clin Exp Immunol. 2007;148:218–229. doi: 10.1111/j.1365-2249.2007.03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorkness R.L., Castleman W.L., Kumar A., Kaplan M.R., Lemanske R.F., Jr. Prevention of chronic postbronchiolitis airway sequelae with IFN-gamma treatment in rats. Am J Respir Crit Care Med. 1999;160:705–710. doi: 10.1164/ajrccm.160.2.9810002. [DOI] [PubMed] [Google Scholar]
- 65.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 66.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bochkov Y.A., Hanson K.M., Keles S., Brockman-Schneider R.A., Jarjour N.N., Gern J.E. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez-Souza N., Favoreto S., Wong H., Ward T., Yagi S., Schnurr D. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller E.K., Hernandez J.Z., Wimmenauer V., Shepherd B.E., Hijano D., Libster R. A mechanistic role for type III IFN-lambda1 in asthma exacerbations mediated by human rhinoviruses. Am J Respir Crit Care Med. 2012;185:508–516. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fitzpatrick A.M., Holguin F., Teague W.G., Brown L.A. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121:1372–1378. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fitzpatrick A.M., Teague W.G., Burwell L., Brown M.S., Brown L.A. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011;69:154–159. doi: 10.1203/PDR.0b013e3182026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyer E.H., DeKruyff R.H., Umetsu D.T. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59:281–292. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
- 73.Robinson D.S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A.M. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 74.Zhang G., Rowe J., Kusel M., Bosco A., McKenna K., de Klerk N. Interleukin-10/interleukin-5 responses at birth predict risk for respiratory infections in children with atopic family history. Am J Respir Crit Care Med. 2009;179:205–211. doi: 10.1164/rccm.200803-438OC. [DOI] [PubMed] [Google Scholar]
- 75.Asquith K.L., Horvat J.C., Kaiko G.E., Carey A.J., Beagley K.W., Hansbro P.M. Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog. 2011;7:e1001339. doi: 10.1371/journal.ppat.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Q., Martin R.J., Lafasto S., Efaw B.J., Rino J.G., Harbeck R.J. Toll-like receptor 2 down-regulation in established mouse allergic lungs contributes to decreased mycoplasma clearance. Am J Respir Crit Care Med. 2008;177:720–729. doi: 10.1164/rccm.200709-1387OC. [DOI] [PubMed] [Google Scholar]
- 77.Arkwright P.D., Patel L., Moran A., Haeney M.R., Ewing C.I., David T.J. Atopic eczema is associated with delayed maturation of the antibody response to pneumococcal vaccine. Clin Exp Immunol. 2000;122:16–19. doi: 10.1046/j.1365-2249.2000.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boguniewicz M., Leung D.Y. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ober C., Yao T.C. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Regan G.M., Irvine A.D. The role of filaggrin in the atopic diathesis. Clin Exp Allergy. 2010;40:965–972. doi: 10.1111/j.1365-2222.2010.03522.x. [DOI] [PubMed] [Google Scholar]
- 82.Barker J.N., Palmer C.N., Zhao Y., Liao H., Hull P.R., Lee S.P. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol. 2007;127:564–567. doi: 10.1038/sj.jid.5700587. [DOI] [PubMed] [Google Scholar]
- 83.Palmer C.N., Ismail T., Lee S.P., Terron-Kwiatkowski A., Zhao Y., Liao H. Filaggrin null mutations are associated with increased asthma severity in children and young adults. J Allergy Clin Immunol. 2007;120:64–68. doi: 10.1016/j.jaci.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Miller E.K., Williams J.V., Gebretsadik T., Carroll K.N., Dupont W.D., Mohamed Y.A. Host and viral factors associated with severity of human rhinovirus-associated infant respiratory tract illness. J Allergy Clin Immunol. 2011;127:883–891. doi: 10.1016/j.jaci.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gold D.R., Bloomberg G.R., Cruikshank W.W., Visness C.M., Schwarz J., Kattan M. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. J Allergy Clin Immunol. 2009;124:1078–1087. doi: 10.1016/j.jaci.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lynch N.R., Hagel I.A., Palenque M.E., Di Prisco M.C., Escudero J.E., Corao L.A. Relationship between helminthic infection and IgE response in atopic and nonatopic children in a tropical environment. J Allergy Clin Immunol. 1998;101:217–221. doi: 10.1016/S0091-6749(98)70386-0. [DOI] [PubMed] [Google Scholar]
- 87.Nyan O.A., Walraven G.E., Banya W.A., Milligan P., Van Der Sande M., Ceesay S.M. Atopy, intestinal helminth infection and total serum IgE in rural and urban adult Gambian communities. Clin Exp Allergy. 2001;31:1672–1678. doi: 10.1046/j.1365-2222.2001.00987.x. [DOI] [PubMed] [Google Scholar]
- 88.Peisong G., Yamasaki A., Mao X.Q., Enomoto T., Feng Z., Gloria-Bottini F. An asthma-associated genetic variant of STAT6 predicts low burden of Ascaris worm infestation. Genes Immun. 2004;5:58–62. doi: 10.1038/sj.gene.6364030. [DOI] [PubMed] [Google Scholar]
- 89.de Silva N.R., Brooker S., Hotez P.J., Montresor A., Engels D., Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Isnard A., Chevillard C. Recent advances in the characterization of genetic factors involved in human susceptibility to infection by schistosomiasis. Curr Genomics. 2008;9:290–300. doi: 10.2174/138920208785133262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flohr C., Quinnell R.J., Britton J. Do helminth parasites protect against atopy and allergic disease? Clin Exp Allergy. 2009;39:20–32. doi: 10.1111/j.1365-2222.2008.03134.x. [DOI] [PubMed] [Google Scholar]
- 92.Walter M.J., Morton J.D., Kajiwara N., Agapov E., Holtzman M.J. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–175. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jafri H.S., Chavez-Bueno S., Mejias A., Gomez A.M., Rios A.M., Nassi S.S. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis. 2004;189:1856–1865. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- 94.Look D.C., Walter M.J., Williamson M.R., Pang L., You Y., Sreshta J.N. Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function. Am J Pathol. 2001;159:2055–2069. doi: 10.1016/S0002-9440(10)63057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim E.Y., Battaile J.T., Patel A.C., You Y., Agapov E., Grayson M.H. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DiFranza J.R., Aligne C.A., Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113(suppl):1007–1015. [PubMed] [Google Scholar]
- 97.Han Y.Y., Lee Y.L., Guo Y.L. Indoor environmental risk factors and seasonal variation of childhood asthma. Pediatr Allergy Immunol. 2009;20:748–756. doi: 10.1111/j.1399-3038.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 98.Karmaus W., Dobai A.L., Ogbuanu I., Arshard S.H., Matthews S., Ewart S. Long-term effects of breastfeeding, maternal smoking during pregnancy, and recurrent lower respiratory tract infections on asthma in children. J Asthma. 2008;45:688–695. doi: 10.1080/02770900802178306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schafer T., Dirschedl P., Kunz B., Ring J., Uberla K. Maternal smoking during pregnancy and lactation increases the risk for atopic eczema in the offspring. J Am Acad Dermatol. 1997;36:550–556. doi: 10.1016/s0190-9622(97)70242-1. [DOI] [PubMed] [Google Scholar]
- 100.Noakes P.S., Hale J., Thomas R., Lane C., Devadason S.G., Prescott S.L. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J. 2006;28:721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- 101.Broeckaert F., Arsalane K., Hermans C., Bergamaschi E., Brustolin A., Mutti A. Serum Clara cell protein: a sensitive biomarker of increased lung epithelium permeability caused by ambient ozone. Environ Health Perspect. 2000;108:533–537. doi: 10.1289/ehp.00108533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaspers I., Ciencewicki J.M., Zhang W., Brighton L.E., Carson J.L., Beck M.A. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005;85:990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- 103.Ciencewicki J., Gowdy K., Krantz Q.T., Linak W.P., Brighton L., Gilmour M.I. Diesel exhaust enhanced susceptibility to influenza infection is associated with decreased surfactant protein expression. Inhal Toxicol. 2007;19:1121–1133. doi: 10.1080/08958370701665426. [DOI] [PubMed] [Google Scholar]
- 104.Gowdy K., Krantz Q.T., Daniels M., Linak W.P., Jaspers I., Gilmour M.I. Modulation of pulmonary inflammatory responses and antimicrobial defenses in mice exposed to diesel exhaust. Toxicol Appl Pharmacol. 2008;229:310–319. doi: 10.1016/j.taap.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 105.Karatekin G., Kaya A., Salihoglu O., Balci H., Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63:473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 106.Morales E., Romieu I., Guerra S., Ballester F., Rebagliato M., Vioque J. Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23:64–71. doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- 107.Wayse V., Yousafzai A., Mogale K., Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 108.Wang T.T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 109.Sadeghi K., Wessner B., Laggner U., Ploder M., Tamandl D., Friedl J. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 110.Chen S., Sims G.P., Chen X.X., Gu Y.Y., Chen S., Lipsky P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 111.Karmali R., Hewison M., Rayment N., Farrow S.M., Brennan A., Katz D.R. 1,25(OH)2D3 regulates c-myc mRNA levels in tonsillar T lymphocytes. Immunology. 1991;74:589–593. [PMC free article] [PubMed] [Google Scholar]
- 112.Brigham and Women's hospital; National Heart, Lung, and Blood Institute. Randomized trial: maternal vitamin D supplementation to prevent childhood asthma (VDAART). Available at: http://clinicaltrials.gov/show/NCT00920621. Accessed February 20, 2012. NLM Identifier: NCT 00920621.
- 113.Copenhagen studies on asthma in childhood. Vitamin D supplementation during pregnancy for prevention of asthma in childhood 2000. Available at: http://clinicaltrials.gov/show/NCT00856947. Accessed February 20, 2012. NLM Identifier: NCT 00856947.
- 114.Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23) MMWR Morb Mortal Wkly Rep. 2010;59:1102–1106. [PubMed] [Google Scholar]
- 115.Ansaldi F., Turello V., Lai P., Bastone G., De L.S., Rosselli R. Effectiveness of a 23-valent polysaccharide vaccine in preventing pneumonia and non-invasive pneumococcal infection in elderly people: a large-scale retrospective cohort study. J Int Med Res. 2005;33:490–500. doi: 10.1177/147323000503300503. [DOI] [PubMed] [Google Scholar]
- 116.Cakebread J.A., Xu Y., Grainge C., Kehagia V., Howarth P.H., Holgate S.T. Exogenous IFN-beta has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011;127:1148–1154. doi: 10.1016/j.jaci.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 117.Krasnowska M., Malolepszy J., Liebhart E., Inglot A.D. Inhaled natural human interferon alpha induces bronchospastic reactions in asthmatics. Arch Immunol Ther Exp (Warsz) 1992;40:75–78. [PubMed] [Google Scholar]